Abstract
The distribution of vascular plant species and assemblages existing in beech (Fagus sylvatica L.) forests was compared with the distribution of beech chloroplast DNA (cpDNA) haplotypes, aiming to identify possible interpretable trends of co-occurrence, on a small geographical scale, and to infer the relevant historical factors. Vegetation and genetic (cpSSR) data were collected from 60 plots on Mt. Menikio (northeastern Greece). Classification and ordination analyses were applied on the vegetation data, while on the cpSSR data, diversity measures and genetic structure analyses were employed. A probabilistic co-occurrence analysis was performed on haplotypes and taxa. The results show that a plant biogeographical border exists on Mt. Menikio which, in addition, has acted both as a refugium and as a meeting point of lineages for more than one glacial cycle. Significant associations of co-occurrence between haplotypes and vascular taxa were found but no common distribution patterns between the former and species assemblages were identified. The combined consideration of the distribution profiles of species assemblages, plant species and cpDNA haplotypes (corresponding to the three levels of biodiversity) provides concrete information on historical events, leading to a comprehensive understanding of the evolutionary and biogeographical processes that have shaped specific spatial patterns of biodiversity.
Keywords:
biodiversity; beech; biogeographical border; co-occurrence; cpDNA; refugia; species assemblages 1. Introduction
Biodiversity is being explored at three levels, genetic, species and ecosystem diversity, which are recognized by both the scientific and the political processes (e.g., the UN Convention on Biological Diversity). Among them, genetic and species diversity are considered to co-vary, since they are influenced by the same evolutionary processes (migration, adaptation), shaped by specific ecological and geographical characteristics [1,2,3]. As a consequence, species richness instead of genetic diversity is being frequently used in conservation planning. However, this expectation of parallel influence is not always supported by the empirical data [4]. Taberlet et al. [5], studying the high-mountain flora of the Alps and the Carpathians, showed that species richness and genetic diversity are not correlated and emphasized the need to investigate all three levels of biodiversity in order to better understand the processes affecting them and to design conservation activities. A lack of correlation between species diversity and genetic diversity has been also found for the vascular plants in the Mediterranean basin [6]. Despite the high theoretical and practical importance of a correlation between different biodiversity levels, this issue started gaining increasing importance relatively late, e.g., [7,8,9,10]. Recently, Hrivnak et al. [10] studied the relationship between the genetic variation in black alder (Alnus glutinosa) and the species diversity in the vascular plant communities in black alder riparian forests spreading in the Western Carpathians and in the Pannonian basin.
The content and the coverage of plant communities are differentiated due to ecological factors (e.g., soil, micro- and meso-climate, disturbance regimes) as well as biogeographical ones (macro-climate and species evolutionary and migration history) [11,12]. The same factors may affect the patterns of genetic variants (alleles and haplotypes) within plant species. In particular, the distribution patterns of adaptive genes are influenced by both ecological and biogeographical factors, while neutral genetic diversity is driven mainly by biogeographical ones. cpDNA microsatellite markers are considered as selectively neutral, having a maternal (uniparental) inheritance mode in angiosperms [13], and are widely used to describe the migration history of plant species and their lineages. Common distribution patterns between plant communities and cpDNA haplotypes for a given species have a higher chance of occurring when they both share a common biogeographic history. Similarly, since the plant communities are ephemeral assemblages of species that might not have been present in the past or continue to exist in the future, while the species are more persistent in time, common distribution patterns are more likely to occur between genetic markers and species, rather than between genetic markers and plant communities [14,15]. In particular, species have a much longer history and represent more objectively defined entities compared to plant communities, in particular, the dominant species which have a greater influence on the community structure by regulating habitat properties and species interactions [16,17]. For a given plant community, the distribution of species having common spatial patterns with cpDNA haplotypes should be the result of the postglacial history and of a possible biogeographical influence. Thus, the factors influencing species distribution (biogeography or adaptation) may be described by comparing the spatial patterns of species and cpDNA haplotypes. Congruence between AFLP allele-based and species-based distribution patterns, owned to the Pleistocene glaciations, has been recently shown by Thiel-Egenter et al. [18]. A strong correlation between patterns of species diversity and haplotype variation in coding regions has also been found in island tenebrionid beetle communities [19]. No such comparison has been reported so far using cpDNA haplotypes.
Beech forests constitute an appropriate ground for investigating patterns of co-occurrence between beech cpDNA haplotypes (reflecting postglacial lineages) and plant species. Beech (Fagus sylvatica) is widespread in Europe and forms extensive forests in lowland plains and valleys in the largest part of the continent, while in the southernmost part of its distribution (especially in areas with a Mediterranean macro-climate) it forms island type populations on mountains, at high altitudes [20,21]. Even though its taxonomic status in Europe is not fully elucidated yet, the existence of one species (Fagus sylvatica) with two subspecies (subsp. sylvatica and subsp. orientalis) is widely accepted [22,23,24,25,26,27]. These two taxa are considered to form a contact zone with overlapping distribution ranges in the southeastern part of the Balkan Peninsula [23,24,28,29,30]. The current geographic distribution of beech has been strongly influenced by a series of retractions and expansions during the glacial and interglacial periods in the Quaternary [31,32,33,34], that created various refugia within Greece and a complex admixture of beech lineages [29,35,36,37].
The flora of beech forests has also been influenced by the glacial history of the temperate zone. Willner, Di Pietro, and Bergmeier [38] found that the current distribution of many beech forest species has been limited by postglacial dispersal constraints rather than by their environmental requirements. Numerous understory species of beech forests occur in a restricted part of the beech distribution range in Europe (plant geographical indicators) [38], resulting in a strong geographical gradient of floristic differentiation in the European beech forests, especially towards the south, e.g., [38,39,40]. These areas, where many narrow-range species of beech forest flora occur, coincide well with the potential refugial areas of beech and of other temperate tree species [35]. Large scale studies either of the genetic diversity of beech or of the floristic diversity of its forests have been carried out only recently in Greece [20,34,35,41], where a number of glacial refugia have been identified. On a small geographical scale (Mt. Paggeo), Papageorgiou et al. [36,42] first investigated if sites with different ecological conditions also host different beech lineages and found a relationship between the distribution of a specific plant community (belonging to the Tilio-Acerion phytosociological alliance) and a private beech haplotype, both indicative of a glacial refugium.
Aiming at comparing the distribution of species and assemblages of beech forests and of the beech cpDNA haplotypes on a small geographical scale, towards identifying possible interpretable trends of co-occurrence between them and inferring the relevant historical factors (e.g., migration routes, refugia), we choose Mt. Menikio (N.E. Greece), which has been suggested as a glacial refugium based on genetic data, either on beech, e.g., [32,35,43] or on other plant species, e.g., [44,45]. In addition, a vegetation type belonging to the Tilio-Acerion phytosociological alliance, known to host relict and endemic species that survived the Quaternary glaciations [46,47], has been observed on Mt. Menikio. This mountain is characterized by a diverse topography and has steep slopes and ravines, a topography that fulfills the requirements to have acted as refugium for tree populations during the Quaternary [33,48]. Based on all the above, Mt. Menikio forms a suitable area for testing specific hypotheses concerning the genetic diversity within and among subpopulations of beech and the different assemblages of beech forests and to investigate any patterns of co-occurrence between beech lineages and beech forest species. Thus, an additional aim of this study was to identify possible interpretable trends of co-occurrence between species and assemblages of beech forests and beech glacial lineages on a fine scale on Mt. Menikio, and to infer the relevant historical factors (e.g., migration routes, refugia).
2. Materials and Methods
2.1. Study Area
Mt. Menikio lies at the southwestern part of a mountainous landscape in northeastern Greece and its highest peak is 1963 m a.s.l. (Figure 1a). It occupies an area of ca. 380 km2 characterized by topographic and bioclimatic diversity, while geologically, it is composed mainly of marbles and schists, and sporadically of gneiss. Fagus sylvatica is the dominant species on this mountain.
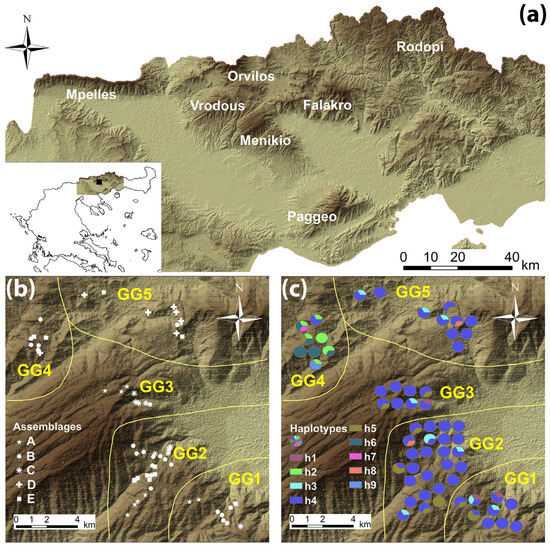
Figure 1.
Map of the study area showing Mt. Menikio and the mountains of northeast Greece (a), the distribution of the species assemblages on Mt. Menikio (b), the distribution of the haplotypes on Mt. Menikio (c). A 3D view of the ASTER GDEM (ASTER-GDEM, 2011) is presented as the background of the maps. Yellow lines (b,c) indicate the distinction of the five geographical groups (GGs). In the insert map (a), the geographical location of Mt. Menikio is indicated by a black box.
2.2. Sampling Scheme
Sixty plots, each of an area of 400 m2, were chosen within the Fagus sylvatica forests of Mt. Menikio, representing the whole distribution of the species on this mountain as well as all the different environmental and growing conditions. Altitude, geological substrate, soil properties, exposition and topography were considered as indicators to distinguish these different conditions, within which the plots were sampled randomly. In each of the 60 plots, all vascular plants were recorded and their coverage was estimated using the 9-grade Braun-Blanquet scale [49,50]. The taxon identification of the vascular plants was carried out using relevant flora [51,52,53,54,55,56,57,58,59], while their nomenclature follows Dimopoulos et al. [27,60,61].
In each of the 60 plots, three beech individuals were sampled for DNA analysis. These individuals were at least 50 m apart from each other and were located within or adjacent to the edges of the vegetation plots, lying in the same environmental and growing conditions as the plots.
2.3. DNA Analyses
DNA was extracted from leaf material following the protocol of Doyle and Doyle [62] with minor modifications.
Three chloroplast microsatellites (cpSSRs) were employed (ccmp4, ccmp7 and ccmp10) [63], which have been broadly used to describe cpDNA variation and to characterize postglacial lineages of beech in Greece [29,35,36], Europe, e.g., [43,64,65,66] and Iran [67]. Amplification mix and conditions followed Weising and Gardner [63]. Allele separation and identification was performed in a LI-COR 4200L sequencer (LI-COR Inc., Lincoln, NE, USA), using IRD-800 labeled primers (VBC Biotech, Vienna, Austria) and the software LICOR SAGAGT 2.1 with internal size standards as well as control beech samples from a previous study [35].
2.4. Vegetation Data Analyses
Plots were classified to distinguish assemblages representing different ecological and/or spatial groupings. Different classification techniques were employed, such as cluster analysis with different agglomerative methods and distance measures, TWINSPAN and k-means [68,69,70]. The cluster analysis with the flexible beta method (b = −0.25) and Bray–Curtis distance, gave the best interpretable results and thus was chosen and applied using the Vegan 2.5-7 [71] and Cluster 2.1.2 [72] packages in R version 4.3.2. Diagnostic species were determined using the algorithm of Tsiripidis, Bergmeier, Fotiadis and Dimopoulos [41]. Phi coefficient [73] was calculated for the groups that were positively differentiated by taxa against those negatively differentiated or not differentiated. Only the taxa having a phi coefficient higher than 0.3 were considered as diagnostic. Groups of plots were ordered according to the hierarchy of a new cluster analysis performed with the flexible beta (b = −0.25) and the Bray–Curtis distance measure, but this time using the synoptic table of relative frequencies of taxa in the vegetation clusters as the dataset. Non-metric multidimensional scaling (NMDS) analysis was applied to interpret the floristic differentiation among the vegetation units, using the R package Vegan 2.5.-7 [71]. In the classification and ordination analyses, taxa occurring in three or less plots were omitted, prior to the analysis, to reduce noise.
2.5. Genetic Data Analyses
Haplotypes were assigned based on the combination of the three cpSSRs alleles. Given the high topographic diversity of Mt. Menikio, in carrying out the analyses, the study area was split into five geographical subdivisions (called herein “geographical groups, GGs”, GG1 to GG5; see Figure 1b,c). These GGs are separated by geographical distance and/or topographical features (such as gorges and valleys), that can act as migration barriers, also taking into account the zonal vegetation pattern of Mt Menikio [74]. Patterns of genetic diversity and differentiation were considered within and among the five GGs. Frequencies of haplotypes were calculated for each GG. Diversity within the GGs was quantified using the number of different haplotypes n(a) and the effective number of haplotypes n(e). A minimum spanning tree of haplotypes was calculated, based on the pairwise distances in fragment length of the different haplotypes, using the algorithm of Rohlf [75] as employed in Arlequin 3.01 [76] and visualized with HapStar [77].
The presence of a phylogeographic structure was checked by comparing if Nst (the differentiation among populations taking into account haplotype differences) was significantly higher than Gst (the differentiation among populations without taking into account haplotype differences), employing the PERMUT&CpSSR v.2.0 software [78] and 10,000 permutations. Analysis of molecular variance—AMOVA [79]—was employed to describe the genetic structure of the studied populations in the five GGs. Another AMOVA was performed in the vegetation assemblages distinguished by the cluster analysis, in order to check for any cpDNA structure in them. All AMOVAs were based on haplotype identity and phylogenetic dissimilarity between haplotypes and carried out using Arlequin 3.01 [76], while significance was conducted with 10,000 permutations.
A Bayesian model was used to further investigate the genetic structure of the studied individuals, by employing BAPS [80]. A non-spatial model [81] was used for the delineation of genetic clusters based on cpDNA haplotypes and a spatial one for the description of their geographical pattern [82].
2.6. Co-Occurrence between Haplotypes and Taxa
For checking any significant relationship of co-occurrence between beech haplotypes and the taxa recorded in the vegetation plots, a probabilistic species co-occurrence analysis was run using the package “cooccur” in R [83]. This analysis returns the probabilities of two objects (in our case taxa vs. haplotypes or haplotypes vs. haplotypes) to co-occur or not to co-occur by random, while assuming that their distribution in space is random and independent. In the cases where the probability of co-occurrence of two objects by random is equal or lower than 0.05, then these objects are considered to have a statistically significant positive association, while in the cases where the probability of two objects not to co-occur by random is equal or lower than 0.05, then these objects are considered to have a statistically significant negative association. Taxa and haplotypes recorded in three or less plots were not considered.
3. Results
3.1. Vegetation Data Classification
Five assemblages (A, B, C, D and E) were distinguished through cluster analysis (Figure 1b). The synoptic table of taxa relative frequencies in the five assemblages is presented in Table S1 (in the Supplementary Materials), where the diagnostic (differential) taxa of the five assemblages are also shown.
Assemblage A represents calcicolous beech forests, differentiated by typical calcicolous species. It is not thermophilus, as the species that are indicators of warm conditions have low frequencies.
Assemblage B represents acidic beech forests, growing on siliceous substrates. It hosts typical species growing in beech forests, but species of oak forests are also rather frequent, indicating a comparatively warmer character.
Assemblage C includes plots that are transitional between beech and ravine forests. It grows on calcareous substrates, in topographic conditions that provide high soil moisture (mainly on colluvial soils).
Assemblage D represents thermophilous and mesic beech forests of northeastern Greece, having a floristic affinity with the ecologically similar beech forests of the Rodopi massif and other mountains of northeastern Greece as is shown through its differential species, such as Pulmonaria rubra, Symphytum tuberosum and Carex digitata [10].
Finally, assemblage E represents thermophilous and acidic beech forests. It is mainly differentiated from the other assemblages, by the lack of calcicolous species, as well as of many species of beech and oak forests.
Regarding the spatial distribution of the assemblages on the mountain, assemblages A and C are found in the central and southern part of the mountain (GG1, GG2 and GG3), assemblage B is spread all over the mountain, assemblage D is found only in the northern part (GG4 and GG5) while assemblage E is found both in the northern and the southern parts (GG1, GG2, GG4 and GG5) but not in the central one (GG3) (Figure 1b).
3.2. Vegetation Data Ordination
The first NMDS axis discriminates mainly assemblage E (Figure 2). Thermophilous and acidophilous taxa occur in the right part of the diagram, while the left part of the diagram includes calcicolous and moist-demanding taxa. Therefore, the first NMDS axis seems to represent a gradient related to moisture and soil pH (both are decreasing towards the right part of the axis).
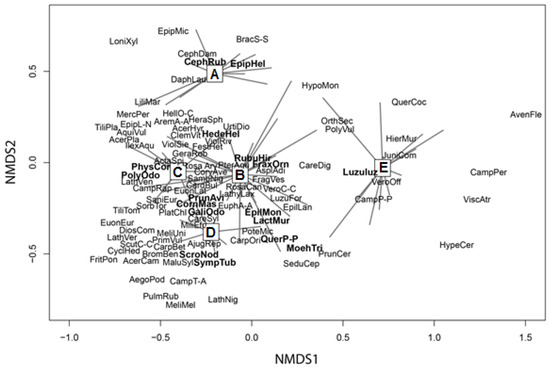
Figure 2.
Ordination diagram of the first two NMDS axes. The spiders present the distribution of the plots of the five assemblages in the ordination space. Only the differential species are shown in the diagram (regular fonts) and those having a significant association (co-occurrence) with haplotypes (bold fonts). Most of the latter are also differential except Lactuca muralis, Moehringia trinervia and Scrophularia nodosa. For the taxa abbreviations see Table S1 (in Supplementary Materials).
The second NMDS axis separates mainly assemblage A from D, while the other assemblages are located in the central part of the axis. This axis may represent a phytogeographical gradient, because the taxa located in its upper part are more common in the beech forests of central Greece (e.g., Daphne laureola, Ilex aquifolium) and indicate a warmer (sub-Mediterranean) climate, while the taxa occurring in its lower part are characteristic of the beech forests of northern Greece (e.g., Sympytum tuberosum, Pulmonaria rubra), and indicate a more continental climate.
3.3. Haplotype Diversity
All three cpSSRs used were polymorphic and nine different beech haplotypes (h1–h9) were derived on Mt. Menikio (Supplementary Materials, Table S2). Haplotype h4 dominated all GGs besides GG4, where the private haplotypes h6 and h2 were the most frequent ones (Table 1). GG4 hosted the highest number of haplotypes and showed the highest value of genetic diversity (Table 1). The minimum spanning tree (Figure 3) showed three clusters: one including h2 and h9, a second including h6 and h7 and a third main cluster including the rest of the haplotypes. The haplotypes h2, h6, h7 and h9 were found exclusively in GG4 (Figure 1c, Table 1). Haplotype h4 was the most common in the third cluster, differing in one mutation step with h1, h3, h5 and h8.

Table 1.
Haplotype frequencies and diversity measures for the five geographical groups (GGs) of Fagus sylvatica on Mt. Menikio (N: number of individuals; n(a): number of haplotypes; n(e): effective number of haplotypes).
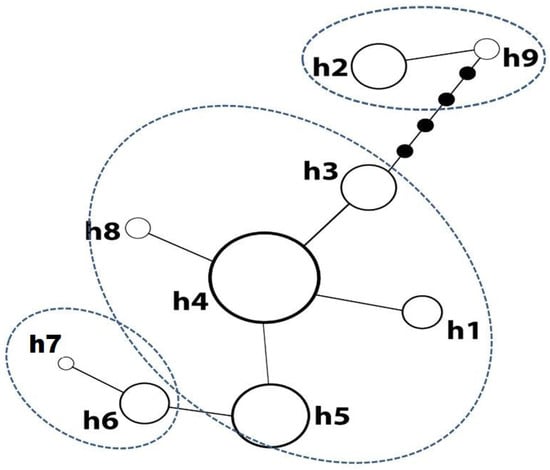
Figure 3.
Minimum spanning network of the nine haplotypes described, based on pairwise differences in fragment length.
The Bayesian inference of the haplotypes in each plot classified the plots into six genetic clusters (Figure 4).
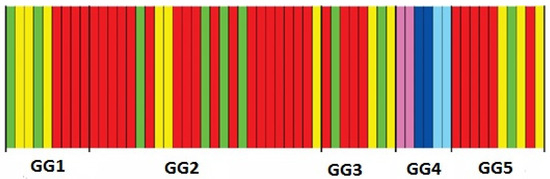
Figure 4.
Plots clustered according to the Bayesian inference of tree haplotypes (BAPS). Plots are ordered according to their geographical groups (GGs) (shown below the figure) and each plot color represents a different genetic cluster (BAPS).
Three clusters were observed in GG4 exclusively, while three additional clusters were common in the other four GGs. The total differentiation among the 60 plots was high (Gst = 0.347), higher than the one among the five GGs (Gst = 0.213). In both cases, the Nst values were higher than the relevant Gst values (Nst = 0.405 and Nst = 0.251, respectively). Even though the divergence between Gst and Nst was not significant, it was, however, an indication that a phylogeographic signal was present in the plots and GGs of this study, indicating a genetically structured population. In the AMOVA analysis of the five GGs (Supplementary Materials, Table S3), most of the total haplotype diversity was found within plots (43.86%), while a high percentage was classified among GGs as well (35.22%). Diversity among plots within GGs reached 20.92%. The AMOVA describing the structure of haplotype diversity in the five species assemblages did not show a significant differentiation among them, with Φst being negative (Supplementary Materials, Table S4).
The spatial Bayesian model suggested a clear geographical pattern of haplotype diversity: a small cluster with the plots of GG4 only and a larger one with all the other plots and GGs (Figure 5a).
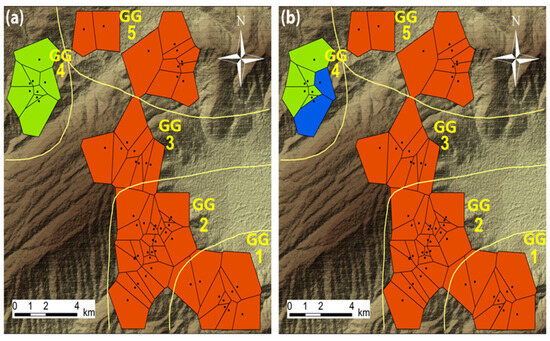
Figure 5.
Spatial distribution of clustered plots, representing the best clustering solution, using a Voronoi tessellation (BAPS) (a) and using a fixed number of clusters for plots (K = 3) (b). Yellow lines as well as the background of maps are as in Figure 1.
Due to the fact that the haplotype tree suggested three different groups of haplotypes, the spatial model was run also for K = 3. A similar clustering was produced with GG4 splitting further into two sub-clusters (Figure 5b), with haplotypes corresponding to the two groups described by the haplotype tree; h2 and h9 in the one and h6 and h7 in the other.
3.4. Haplotypes and Taxa Co-Occurrence
Significant associations of co-occurrence either between haplotypes or between haplotypes and taxa were explored (Table 2). Haplotypes h2 and h4 had the highest number of cases with positive or negative associations. Haplotype h2 was negatively associated with h4 and thermophilous species (Fraxinus ornus, Hedera helix and Physospermum cornubiense) and positively associated with mesophilous species (Galium odoratum and Prunus avium), with Quercus petraea ssp. polycarpa and with Lactuca muralis (a species of deciduous broadleaved forests). Haplotype h3 was significantly associated with only three species; positively with the mesophilous species Carpinus betulus and Polygonatum odoratum, and negatively with the acidophilous species Luzula luzuloides. Haplotype h4 was negatively associated with h6, with typical species of beech forests and with species of more mesic conditions (Epilobium montanum, Galium odoratum, Prunus avium, Scrophularia nodosa and Symphytum tuberosum), while it was positively associated with thermophilous or calcicolous species (Cephalanthera rubra, Fraxinus ornus, Hedera helix). Haplotype h5 was negatively associated with only one species, Moehringia trinervia, which occurs sporadically within beech forests, especially on sites disturbed by wood cuttings. Finally, h6 was negatively associated with the somewhat thermophilous species Cornus mas and Hedera helix, that occur mainly in oak forests, and positively associated with the mesophilous species Galium odoratum and Prunus avium.

Table 2.
Haplotypes having a significant, positive or negative association with taxa or other haplotypes of Fagus sylvatica on Mt. Menikio. Third column: observed number of plots having both entities (haplotypes and taxa or other haplotypes); fourth column: expected number of plots having both entities; fifth column: the probability that two entities would co-occur at a frequency less than the observed number of co-occurrence sites if they were distributed independently of one another; and sixth column: the probability that two entities would co-occur at a frequency greater than the observed one. Statistically significant probabilities at p-value ≤ 0.05 are marked with bold typescript.
4. Discussion
The identification of a glacial refugium is of great importance for taxonomists, paleobotanists, sociologists, geneticists and conservationists. Studying the genetic and vegetation structure within the refugium provides essential data in attempting to decipher the evolutionary processes that took place there, as well as in undertaking the appropriate measures for the conservation of the plant species and of the genetic resources hosted in the refugium.
In the present study, we chose a mountain with a complex relief located in northeastern Greece (Mt. Menikio), suggested to have functioned as a glacial refugium, where we studied the distribution and structure of neutral genetic markers and of the assemblages and flora taxa in Fagus sylvatica forests, which is the dominating plant species and whose forests cover a great part of this mountain. Apart from comparing the distribution patterns of the vegetation assemblages and of the genetic traits, we checked for associations of co-occurrence between haplotypes as well as between haplotypes and the vascular taxa forming the plant assemblages.
The presence of assemblage C in the central part of the mountain points to its function as a refugium, since it is transitional to the ravine forests of Tilio-Acerion. The Tilio-Acerion ravine forests grow within ravines or on steep slopes, on deep colluvial soils and for these reasons are characterized by environmental conditions (moist and relatively warm) that may have provided a near-stable environment during the glaciations, buffering the extreme effects of Quaternary climate variability and contributing to the survival of residual plant populations [33,36,47]. The refugial function of the above sites is also indicated by the presence of the following taxa: Corylus colurna, Carpinus betulus, Taxus baccata, Haberlea rhodopensis and Fraxinus excelsior. They are all rare species in Greece and they occur almost exclusively in the southernmost part of the Balkan Peninsula within ravine forests [44,46].
The results of vegetation classification and ordination show certain indications on the existence of both ecological and plant geographical gradients on Mt. Menikio, which may constitute a transition zone from the Mediterranean to a more continental climate. The main gradient of vegetation differentiation on the mountain concerns soil acidity and moisture and based on this gradient, assemblage E is strongly differentiated, ecologically and floristically, from the other assemblages. On the other hand, assemblages A and C are present only in the central and southern part while assemblage D is restricted exclusively to the northern part of the mountain. The restricted occurrence of assemblage C in three localities may be attributed to the special site conditions required for the development of this assemblage, namely ravines or steep north-exposed slopes on calcareous substrate.
Assemblage D is floristically and ecologically very similar to the Melittis melissophyllum subsp. albida-Fagus sylvatica community described by Tsiripidis et al. [84] from the Rodopi mountain range and found to be restricted in the northeastern-most part of Greece according to Tsiripidis et al. [12]. Assemblage D is differentiated against the rest of the communities in the study area by species such as Pulmonaria rubra, Symphytum tuberosum and Carex digitata, which were found to differentiate beech forests of Rodopi (also of Mt. Voras for Pulmonaria rubra) against the more thermophilous beech forests of mountains of a lower latitude in Greece [12]. On the other hand, assemblage A is differentiated, along with assemblage C, by the species Hedera helix and Daphne laureola, which were also found as geographical indicator species of the more thermophilous beech forests of central Greece [12]. Assemblage A is floristically related more to the calcareous beech forests of central Greece than those of the Rodopi mountain range according to the vegetation table of Tsiripidis et al. [10]. On the basis of the above-described differentiation between assemblages A and D as well their extreme distribution along the second NMDS axis, we interpreted the latter axis as a plant geographical gradient that represents a transition from a species pool with more Mediterranean elements in the southern part of the study area to a species pool with elements of more northern regions of Balkan Peninsula and Europe in the northernmost part of the study area. Perhaps the border between the Mediterranean and the Continental biogeographical areas, which is currently set along the Greek–Bulgarian borders [85], is actually located to the south, including the northern part of Mt. Menikio. However, it should be noted that the whole study area may comprise a transition zone with complex ecological and geographical gradients. The complexity is further enhanced by the fact that assemblages B and E, which grow on acidic soils and are distributed all over the study area, resemble more the acidophilous forests of the Rodopi mountain range than the acidophilous forests of Orthilio secundae-Fagetum sylvaticae of central Greece [12,20]. We assume that the above-mentioned complexity is caused by the combined effect of geography/topography and of geology. Specifically, at the warmer south- and sea-facing slopes of Mt. Menikio and in the warmer micro-climate of sites with calcareous substrate, the species and assemblages of more southern, and thus Mediterranean, origin occur, while on the northern slopes of the mountain that face more continental regions and in the cooler micro-climate of sites with an acidic substrate, species and assemblages of more northern and thus continental origin appear. However, in sites with an acidic substrate at the central or southern part of the study area, species of both Mediterranean and continental origin are encountered, according to the effect of other ecological parameters on the micro-climate, such as the altitude, topography and vegetation structure. Such an interpretation fits with the nature of a transition gradient along which the probability of occurrence of some species gradually decreases while that of other species increases progressively.
The differentiation of the northwestern part of the mountain is illustrated in the haplotype distribution, namely, the presence of haplotypes h2, h6, h7 and h9 only in GG4 (along with a minor presence of h4 and h5 which were common to all GGs).
Regarding the haplotype distribution, the most frequent one on Mt. Menikio was h4, which is also the most common throughout Greece, having its expansion center putatively in a nearby region [29,35]. Haplotype h1 was found in low frequencies in Mt. Menikio and in central Greece [35]. Haplotype h2 has been frequent in the east Rodopi massif (northeastern Greece) and is considered to have originated from a local glacial refugium [35], while haplotype h3 has been found mainly on the nearby-located Mt. Paggeo, originating from a local refugium [36]. Haplotype h5 has been found in low frequencies in several locations within Greece [35] and it may be considered as a relict haplotype. Haplotypes h6 and h7, that were found in this study only in GG4, are frequent in northeastern Greece and Turkey [29,35,64] and are considered as lineages originating from F. sylvatica ssp. οrientalis. Haplotypes h8 and h9 are reported in this study for the first time. The presence of unique haplotypes indicates that Mt. Menikio has acted as a refugium while the presence of haplotypes of different distribution and origin (widespread and relict) indicates that Mt. Menikio has acted as a meeting point of lineages for more than one glacial cycle. Similarly, the presence of different beech postglacial lineages on different parts of the same mountain has been described in northeastern Greece [36], southwestern France [48] and southern Italy [86]. This indicates that in mountainous areas, on the rear edge of a temperate tree species [87], glacial migration has happened repeatedly both in latitude and altitude and has caused complex fine-scale diversity patterns. Furthermore, the haplotype distribution on Mt. Menikio in relation to its complex relief (that includes gorges and small isolated valleys in lower altitudes) as well as the presence of relict vegetation types and plant species, indicates the existence of several small-scale glacial refugia on this mountain, rather than a large one. This pattern (“refugia within refugia”) has been suggested by Gomez and Lunt [88] as occurring commonly for plant and animal species in the Iberian Peninsula as well, and it may have been caused due to the climate fluctuations and the repeated migration of species in the southern European peninsulas.
Besides GG4, all other GGs of Mt. Menikio, located on the eastern, central and southern part of the mountain, showed a typical minor polymorphism, with h4 being common and the other closely related haplotypes being very rare. This is a typical diversity profile expected in populations that derive from closely located glacial refugia, since the rare haplotypes are not eliminated by genetic drift that usually occurs at subsequent migration events in long distances [89]. The two frequent haplotypes in GG4 (h2 and h6) have probably migrated from the east, originating from a secondary glacial refugium with haplotypes of eastern origin located in close proximity to Mt. Menikio. Indeed, the indication for such a refugium in the enclosed valley occurring between the northwestern part of Menikio and Mt. Vrodous comes from the presence of a unique, large population of Geranium versicolor around this valley. This species is a good plant geographical indicator, characterizing beech forests in southern Italy and the western Pindos massif (western Greece) [20,40], where it is common, compared to its rare presence in northeastern Greece [90].
The spatial differentiation of haplotypes on Mt. Menikio partly coincides with the geographical differentiation of vegetation between the northern and southern parts of the mountain. While the latter seems to be the result of a transition from a Mediterranean to a more continental climate, the biogeographical separation of the cpDNA haplotype distribution in the northwestern part of the mountain is probably the outcome of differences in postglacial migration patterns of beech lineages. Indeed, no common distribution patterns of the beech cpDNA haplotypes and of the species assemblages were found in the study area (Table S4). That is because species assemblages are formed through the response of individual species to ecological and geographical gradients and are considered as ephemeral structures [14,91]. The local scale of this study renders the ecological gradients as more important for the vegetation differentiation [11,12] and indeed the first NMDS axis represents such a gradient (Figure 2). Thus, we did not observe any common distribution pattern between the beech cpDNA haplotypes and the vegetation assemblages because the former are distributed on the basis of biogeographical factors, while the latter are formed primarily on the basis of ecological factors and secondarily on biogeographical ones.
The five haplotypes (h2, h3, h4, h5 and h6) included in the analysis of co-occurrence showed significant associations with a relatively high number of species (16 in total). Five of them (Epilobium montanum, Galium odoratum, Moehringia trinervia, Scrophularia nodosa and Symphytum tuberosum) are considered typical for beech forests [38], while five additional species have been reported as playing a geographical differential role in the distribution of beech forests in Greece [12]. Specifically, Symphytum tuberosum and Luzula luzuloides differentiate all ecological groups of beech forests of northeastern Greece (mainly Rodopi massif), Prunus avium differentiates the beech forests of calcareous and warm sites in northeastern Greece and Hedera helix and Polygonatum odoratum differentiates the beech forests of warm sites in central Greece. However, among these species, only Symphytum tuberosum is found restricted in northern Mt. Menikio, while Hedera helix is very common in the southern part and very rare in the northern part of the mountain.
The species–haplotype associations found in this study lead to the conclusion that an association between the distribution of species and haplotypes may be either communal (direct) or individualistic (indirect). Communal positive associations concern the cases where species and haplotypes have followed common (or significantly different in the case of a negative association) glacial migration routes or have survived in the same (or different in the case of a negative association) refugial areas, namely when species and haplotypes share a common glacial history. In our study, such cases are the negative association of haplotype h4 with Symphytum tuberosum, caused by the different postglacial migration routes, and the positive association of haplotype h3 with Carpinus betulus showing persistence in the same micro-refugial areas. Carpinus betulus occurs in the southern Balkan Peninsula mainly within putative refugia in gorges [46], while h3 is a rare haplotype located in a gorge on Mt. Menikio and also in a gorge on Mt. Paggeo [36], indicating that it may represent a relict lineage that has survived into micro-refugia in gorges. Based on these cases, communal associations in the distribution of plant species and beech haplotypes indicate the biogeographical significance of both.
However, a species-haplotype association can also be individualistic, namely of an indirect nature. In these cases, both the distribution of species and of haplotypes may have been affected by the same biogeographical processes (e.g., climate, proximity to refugia, existence of a barrier), without (haplotypes and species) sharing necessarily a common postglacial history. Such cases of individualistic relationships are the negative association of haplotypes h2 and h6 with thermophilous species, the positive association of haplotype h4 with thermophilous species, as well as the negative association of the haplotype h4 with mesic species (indicating a continental climate). These species are not geographical indicators, but their distribution in the study area indicates the existence of biogeographical borders (in our case, a differentiation in the climate and the intense relief of Mt. Menikio). This border may have also affected the migration of beech glacial lineages, by acting as a barrier in the migration of certain lineages and in their settling in specific refugia.
Overall, our results obtained from studying the distribution patterns of a set of species and cpDNA haplotypes revealed interpretable distribution patterns about historical factors, such as migration routes and refugia of species and populations. cpDNA haplotypes in Fagus have a maternal inheritance mode, they do not recombine and they migrate by seed dispersal. Species are also dispersed by seeds. Thus, the same historical factors may result in similar distribution patterns in haplotypes and species [18], e.g., some species may have spatially congruent refugia in cases of similar ecological preferences or similar discontinuous ranges [92]. However, in this work, we found cases of communal as well as of individualistic associations between species and haplotypes. The former may be the result of the same historical factor(s), while the latter are practically incongruent to theoretical expectations and may have been caused by an individualistic response to particular changes in the climate [93]. Since cpSSR haplotypes are considered as selectively neutral, their distribution patterns are expected to be the outcome of migration history and not of ecological factors. Thus, the combined investigation of cpDNA haplotypes and species distribution patterns can help to discriminate between the effects of ecological and historical factors in the present day distribution of species and reveal additional information about the postglacial migration history of species and the existence of refugia in an area. The differentiation and distribution of plant assemblages also reflect historical factors and thus can provide important information in parallel to genetic markers, especially cpDNA haplotypes. However, no congruence should be expected between current ecological conditions and neutral markers, except in cases when assemblages indicate adequate conditions for hosting relict species/populations, or indicate a plant geographical differentiation.
The Balkan Peninsula has served as a refugial area during the last as well as during previous glacial cycles and numerous refugia have been identified across the peninsula. However, apart from the refugial locations, the scientific research has not gone much deeper into the structure of the species (dominant or other) within the refugia regarding genetic, biogeographical, ecological or other traits, a fact that hinders both the understanding of the relevant processes and the adoption of the most appropriate conservation measures. Aleksic, Piotti, Geburek and Vendramin [94] have gone through a whole-population genetic characterization of a refugial Picea omorika population in the Balkans, identifying indeed a complex structure within this population and emphasizing the need to go one step further than the traditional sampling and follow sampling schemes leading towards a whole population genetic characterization. In the present study, the combined consideration of the distribution profiles of the species assemblages in beech forests, of the plant species within the assemblages and of the cpDNA haplotypes of the dominant tree (namely beech), following a comparatively dense sampling within a refugial area, have contributed to a concrete understanding of the biogeographical processes that have shaped specific spatial patterns of biodiversity. To our knowledge, there have been no studies so far using cpDNA haplotypes for comparing the distribution patterns of genetic variants and species.
Finally, it should be noted that this study is on a local scale and concerns an area which is a glacial refugium and represents a transition zone between two biogeographical regions. Furthermore, the genetic analyses concern a widespread dominant species (beech) and selectively neutral DNA markers. On a larger scale, Hrivnak et al. [10] did not find any relationship between the genetic variation in the dominant species (alder) and the species diversity of the plant communities. Additional studies at various scales, concerning species with different life or migration histories, are needed in order to investigate further the usefulness of the combined analyses of DNA markers, species and assemblages for answering biogeographical questions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16030152/s1, Table S1: synoptic table of taxa relative frequencies in the five vegetation assemblages of Fagus sylvatica on Mt. Menikio (A to E); Table S2: fragments’ length (in base pairs), assignment and frequencies of the Fagus sylvatica haplotypes identified by three polymorphic microsatellites (ccmp10, ccmp7, ccmp4) on Mt. Menikio; Table S3: analysis of molecular variance (AMOVA) for geographic groups (GGs) of Fagus sylvatica on Mt. Menikio using haplotype distance based on fragment length differences; Table S4: analysis of molecular variance (AMOVA) for plant assemblages of Fagus sylvatica on Mt. Menikio using haplotype distance based on fragment length differences.
Author Contributions
I.T., A.C.P. and A.D.D. conceived of the idea; I.T., S.S. and A.D.D. collected the samples; S.S. and I.T. identified the plant specimens; S.S. carried out the lab analyses; I.T., S.S., A.C.P. and A.D.D. carried out the data analyses; I.T., A.C.P. and A.D.D. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Postgraduate Programme “Conservation of Biodiversity and Sustainable Exploitation of Native Plants (BNP)” (School of Biology, Aristotle University of Thessaloniki), while partial funding of the sampling came from the Aristotle University of Thessaloniki.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors kindly thank Nikoleta Karaiskou for her contribution to the SSR analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vellend, M. Species diversity and genetic diversity: Parallel processes and correlated patterns. Am. Nat. 2005, 166, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M.; Geber, M.A. Connections between species diversity and genetic diversity. Ecol. Lett. 2005, 8, 767–781. [Google Scholar] [CrossRef]
- Vellend, M.; Lajoie, G.; Bourret, A.; Murria, C.; Kembel, S.W.; Garant, D. Drawing ecological inferences from coincident patterns of population- and community-level biodiversity. Mol. Ecol. 2014, 23, 2890–2901. [Google Scholar] [CrossRef]
- Lamy, T.; Laroche, F.; David, P.; MAssol, F.; Jarne, P. The contribution of species-genetic diversity correlations to the understanding of community assembly rules. Oikos 2017, 126, 759–771. [Google Scholar] [CrossRef]
- Taberlet, P.; Zimmermann, N.E.; Englisch, T.; Tribsch, A.; Holderegger, R.; Alvarez, N.; NIklfeld, H.; Coldea, G.; Mirek, Z.; Moilanen, A.; et al. Genetic diversity in widespread species is not congruent with species richness in alpine plant communities. Ecol. Lett. 2012, 15, 1439–1448. [Google Scholar] [CrossRef]
- Fady, B.; Conord, C. Macroecological patterns of species and genetic diversity in vascular plants of the Mediterranean basin. Divers. Distrib. 2010, 16, 53–64. [Google Scholar] [CrossRef]
- Puscas, M.; Choler, P.; Taberlet, P. No positive correlation between species and genetic diversity in European alpine grasslands dominated by Carex curvula. Divers. Distrib. 2008, 14, 852–861. [Google Scholar] [CrossRef]
- Odat, N.; Hellwig, F.H.; Jetschke, G.; Fischer, M. On the relationship between plant species diversity and genetic diversity of Plantago lanceolata (Plantaginaceae) within and between grassland communities. J. Plant Ecol. 2010, 3, 41–48. [Google Scholar] [CrossRef]
- Wehenkel, C.; Bergmann, F.; Gregorius, H.R. Is there a trade-off between species diversity and genetic diversity in forest tree communities? Plant Ecol. 2006, 185, 151–161. [Google Scholar] [CrossRef]
- Hrivnák, M.; Krajmerová, D.; Hrivnák, R.; Slezák, M.; Kochjarová, J.; Jarolímek, I.; Gömöry, D. Interplay between tree genetic variation, plant community composition and environment in forest communities dominated by black alder (Alnus glutinosa (L.) Gaertn.). Perspect. Plant Ecol. 2023, 60, 125748. [Google Scholar] [CrossRef]
- Knollová, I.; Chytrý, M. Oak-hornbeam of the Czech Republic: Geographical and ecological approaches to vegetation classification. Preslia 2004, 76, 291–311. [Google Scholar]
- Tsiripidis, I.; Bergmeier, E.; Dimopoulos, P. Geographical and ecological differentiation in Greek Fagus forest vegetation. J. Veg. Sci. 2007, 18, 743–750. [Google Scholar] [CrossRef]
- Ouborg, N.J.; Piquot, Y.; Van Groenendael, J.M. Population genetics, molecular markers and the study of dispersal in plants. J. Ecol. 1999, 87, 551–568. [Google Scholar] [CrossRef]
- Huntley, B. How plants respond to climate change: Migration rates, individualism and the consequences for plant communities. Ann. Bot. 1991, 67, 15–22. [Google Scholar] [CrossRef]
- Prentice, I.C.; Jolly, D.; BIOME 6000 participants. Mid-Holocene and glacial-maximum vegetation geography of the northern continents and Africa. J. Biogeogr. 2000, 27, 507–519. [Google Scholar] [CrossRef]
- Zacharias, M.A.; Roff, J.C. Use of focal species in marine conservation and management: A review and critique. Aquat. Conserv. 2001, 11, 59–76. [Google Scholar] [CrossRef]
- Li, Q.-M.; Cai, C.-N.; Xu, W.-M.; Cao, M.; Sha, L.-Q.; Lin, L.-X.; He, T.-H. Adaptive genetic diversity of dominant species contributes to species co-existence and community assembly. Plant Divers. 2022, 44, 271–278. [Google Scholar] [CrossRef]
- Thiel-Egenter, C.; Alvarez, N.; Holderegger, R.; Tribsch, A.; Englisch, T.; Wohlgemuth, T.; Colli, L.; Gaudeul, M.; Gielly, L.; Jogan, N.; et al. Break zones in the distributions of alleles and species in alpine plants. J. Biogeogr. 2011, 38, 772–782. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Anastasiou, I.; Spagopoulou, F.; Stalimerou, M.; Terzopoulou, S.; Legakis, A.; Vogler, A.P. Testing the species--genetic diversity correlation in the Aegean archipelago: Toward a haplotype-based macroecology? Am. Nat. 2011, 178, 241–255. [Google Scholar] [CrossRef]
- Bergmeier, E.; Dimopoulos, P. Fagus sylvatica forest vegetation in Greece: Syntaxonomy and gradient analysis. J. Veg. Sci. 2001, 12, 109–126. [Google Scholar] [CrossRef]
- Bohn, U.; Gollub, G.; Hettwer, C.; Neuhäuslová, Z.; Raus, T.; Schlüter, H.; Weber, H. (Eds.) Map of the Natural Vegetation of Europe. Interactive CD-ROM. Explanatory Text, Legend, Maps. Scale 1:2.500.000; Landwirtschaftsverlag: Münster, Germany, 2004. [Google Scholar]
- Aldén, B. Fagus L. In Mountain Flora of Greece 1; Strid, A., Ed.; Cambridge University Press: Cambridge UK, 1986; pp. 51–52. [Google Scholar]
- Akeroyd, J.A. Fagus L. In Flora Europaea, Vol. 1, Psilotaceae to Plataneceae, 2nd ed.; Tutin, T.G., Heywood, V.H., Burges, N.A., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; p. 72. [Google Scholar]
- Christensen, K.I. Fagus L. In Flora Hellenica Vol. 1; Strid, A., Tan, K., Eds.; Koeltz: Königstein, Germany, 1997; pp. 40–41. [Google Scholar]
- Denk, T.; Grimm, G.; Stögerer, K.; Langer, M.; Hemleben, V. The evolutionary history of Fagus in western Eurasia: Evidence from genes, morphology and the fossil record. Plant Syst. Evol. 2002, 232, 213–236. [Google Scholar] [CrossRef]
- Denk, T. Phylogeny of Fagus L. (Fagaceae) based on morphological data. Plant Syst. Evol. 2003, 240, 55–81. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany, 2013. [Google Scholar]
- Tsiripidis, I.; Athanasiadis, N. Contribution to the knowledge of the vascular flora of NE Greece: Floristic composition of the beech (Fagus sylvatica L.) forests in the Greek Rodopi. Willdenowia 2003, 33, 273–297. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Vidalis, A.; Gailing, O.; Tsiripidis, I.; Hatziskakis, S.; Boutsios, S.; Galatsidas, S.; Finkeldey, R. Genetic variation of beech (Fagus sylvatica L.) in Rodopi (N.E. Greece). Eur. J. For. Res. 2008, 127, 81–88. [Google Scholar] [CrossRef]
- Cardoni, S.; Piredda, R.; Denk, T.; Grimm, G.W.; Papageorgiou, A.C.; Schulze, E.D.; Scoppola, A.; Salehi Shanjani, P.; Suyama, Y.; Tomaru, N.; et al. 5S-IGS rDNA in wind-pollinated trees (Fagus L.) encapsulates 55 million years of reticulate evolution and hybrid origins of modern species. Plant J. 2022, 109, 909–926. [Google Scholar] [CrossRef]
- Tinner, W.; Lotter, A.F. Holocene expansions of Fagus sylvatica and Abies alba in Central Europe: Where are we after eight decades of debate? Quat. Sci. Rev. 2006, 25, 526–549. [Google Scholar] [CrossRef]
- Magri, D.; Vendramin, G.G.; Comps, B.; Dupanloup, I.; Geburek, T.; Gömöry, D.; Latgalowa, M.; Litt, T.; Paule, L.; Roure, J.M.; et al. A new scenario for the Quaternary history of European beech populations: Paleobotanical evidence and genetic consequences. New Phytol. 2006, 171, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Tzedakis, P.C.; Lawson, I.T.; Frogley, M.R.; Hewitt, G.M.; Preece, R.C. Buffered tree population changes in a Quaternary refugium: Evolutionary implications. Science 2002, 297, 2044–2047. [Google Scholar] [CrossRef] [PubMed]
- Tsipidou, O.; Leinemann, L.; Korakis, G.; Finkeldey, R.; Gailing, O.; Papageorgiou, A.C. Fine-Scale Spatial Patterns of the Genetic Diversity of European Beech (Fagus sylvatica L.) around a Mountainous Glacial Refugium in the SW Balkans. Forests 2021, 12, 725. [Google Scholar] [CrossRef]
- Hatziskakis, S.; Papageorgiou, A.C.; Gailing, O.; Finkeldey, R. High chloroplast haplotype diversity in Greek populations of beech (Fagus sylvatica L.). Plant Biol. 2009, 11, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.C.; Tsiripidis, I.; Mouratidis, T.; Hatziskakis, S.; Gailling, O.; Eliades, N.-G.H.; Vidalis, A.; Drouzas, A.D.; Finkeldey, R. Complex fine-scale phylogeographical patterns in a putative refugial region for Fagus sylvatica (Fagaceae). Bot. J. Linn. Soc. 2014, 174, 516–528. [Google Scholar] [CrossRef][Green Version]
- Müller, M.; Lopez, P.A.; Papageorgiou, A.C.; Tsiripidis, I.; Gailing, O. Indications of genetic admixture in the transition zone between Fagus sylvatica L. and Fagus sylvatica ssp. orientalis Greut. & Burd. Diversity 2019, 11, 90. [Google Scholar] [CrossRef]
- Willner, W.; Di Pietro, R.; Bergmeier, E. Phytogeographical evidence for post-glacial dispersal limitation of European beech forest species. Ecography 2009, 32, 1011–1018. [Google Scholar] [CrossRef]
- Marinšek, A.; Šilc, U.; Čarni, A. Geographical and ecological differentiation of Fagus forest vegetation in SE Europe. Appl. Veg. Sci. 2013, 16, 131–147. [Google Scholar] [CrossRef]
- Willner, W.; Jiménez-Alfaro, B.; Agrillo, E.; Biurrun, I.; Campos, J.A.; Cami, A.; Casella, L.; Csiky, J.; Gusterevska, R.; Didukh, Y.P.; et al. Classification of European beech forests: A Gordian Knot? App. Veg. Sci. 2017, 20, 494–512. [Google Scholar] [CrossRef]
- Tsiripidis, I.; Bergmeier, E.; Fotiadis, G.; Dimopoulos, P. A new algorithm for the determination of differential taxa. J. Veg. Sci. 2009, 20, 233–240. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Varsamis, G.; Tsiripidis, I.; Dimitrakopoulos, P.G.; Papageorgiou, A.C. Patterns of leaf morphological traits of beech (Fagus sylvatica L.) along an altitudinal gradient. Forests 2021, 12, 1297. [Google Scholar] [CrossRef]
- Manolis, A. Genetic Diversity of Beech (Fagus sylvatica) in East Rodopi. Master’s Thesis, Department of Forestry, Environmental Management and Natural Resources, Democritus University of Thrace, Orestiada, Greece, 2011. [Google Scholar]
- Petrova, G.; Moyankova, D.; Nishii, K.; Forrest, L.; Tsiripidis, I.; Drouzas, A.D.; Djlianov, D.; Möller, M. The European paleoendemic Haberlea rhodopensis (Gesneriaceae) has an oligocene origin and a pleistocene diversification and occurs in a long-persisting refugial area in southeastern Europe. Int. J. Plant Sci. 2015, 176, 499–514. [Google Scholar] [CrossRef]
- Drouzas, A.D.; Charitonidou, M.; Tsiftsis, S. Chloroplast DNA variation in Epipactis atrorubens populations from northern Greece. Bot. Lett. 2017, 164, 55–62. [Google Scholar] [CrossRef]
- Košir, P.; Čarni, A.; Di Pietro, R. Classification and phytogeographical differentiation of broad-leaved ravine forests in southeastern Europe. J. Veg. Sci. 2008, 19, 331–342. [Google Scholar] [CrossRef]
- Mastrogianni, A.; Kallimanis, A.S.; Chytrý, M.; Tsiripidis, I. Phylogenetic diversity patterns in forests of a putative refugial area in Greece: A community level analysis. For. Ecol. Manag. 2019, 446, 226–237. [Google Scholar] [CrossRef]
- De Lafontaine, G.; Ducousso, A.; Lefèvre, S.; Magnanou, E.; Petit, R.J. Stronger spatial genetic structure in recolonized areas than in refugia in the European beech. Mol. Ecol. 2013, 22, 4397–4412. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. In Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964. [Google Scholar]
- Wilmanns, O. Ökologische Pflanzensoziologie, 4th ed.; Aufl. Quelle & Meyer: Heidelberg, Germany, 1989. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea; Cambridge University Press: Cambridge, UK, 1968; Volume 2. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea; Cambridge University Press: Cambridge, UK, 1972; Volume 3. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea; Cambridge University Press: Cambridge, UK, 1976; Volume 4. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea; Cambridge University Press: Cambridge, UK, 1980; Volume 5. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 1. [Google Scholar]
- Strid, A. (Ed.) Mountain Flora of Greece; Cambridge University Press: Cambridge, UK, 1986; Volume 1. [Google Scholar]
- Strid, A.; Tan, K. (Eds.) Flora Hellenica; Koeltz: Königstein, Germany, 1997; Volume 1. [Google Scholar]
- Strid, A.; Tan, K. (Eds.) Flora Hellenica; Koeltz: Königstein, Germany, 2002; Volume 2. [Google Scholar]
- Strid, A.; Tan, K. (Eds.) Mountain Flora of Greece; Edinburgh University Press: Edinburgh, UK, 1991; Volume 2. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Supplement. Willdenowia 2016, 46, 301–348. [Google Scholar] [CrossRef]
- Flora of Greece Web: Vascular Plants of Greece. An Annotated Checklist. Available online: https://portal.cybertaxonomy.org/flora-greece (accessed on 15 December 2023).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Weising, K.; Gardner, R.C. A set of conserved PCR primers for the analysis of simple sequence repeat polymorphisms in chloroplast genomes of dicotyledonous angiosperms. Genome 1999, 42, 9–19. [Google Scholar] [CrossRef]
- Gailing, O.; von Wuehlisch, G. Nuclear markers (AFLPs) and chloroplast microsatellites differ between Fagus sylvatica and Fagus orientalis. Silvae Genet. 2004, 53, 105–110. [Google Scholar] [CrossRef]
- Vettori, C.; Paffetti, D.; Paule, L.; Giannini, R. Identification of the Fagus sylvatica L. and Fagus orientalis lipsky species and intraspecific variability. For. Genet. 2004, 11, 223–230. [Google Scholar]
- Travaglini, D.; Paffetti, D.; Bianchi, L.; Bottacci, A.; Bottalico, F.; Giovannini, G.; Maltoni, A.; Nocentini, S.; Vettori, C.; Calamini, G. Characterization, structure and genetic dating of an old-growth beech-fir forest in the northern Apennines (Italy). Plant Biosyst. 2012, 146, 175–188. [Google Scholar] [CrossRef]
- Shanjani, P.S.; Vendramin, G.G.; Calagari, M. Genetic diversity and differentiation of Fagus orientalis Lipsky in Hyrcanian forests revealed by nuclear and chloroplast microsatellite markers. Conserv. Genet 2010, 11, 2321–2331. [Google Scholar] [CrossRef]
- Hill, M.O. TWINSPAN—A Fortran Program for Arranging Multivariate Data in an Ordered Two Way Table by Classification of the Individuals and the Attributes; Ecology & Systematics, Cornell University: Ithaca, NY, USA, 1979. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; English ed.; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MJM Press: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Oksanen, J.; Guillaume, F.B.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 September 2023).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1.2. 2021. Available online: https://CRAN.R-project.org/package=cluster (accessed on 20 September 2023).
- Chytrý, M.; Tichý, L.; Holt, J.; Botta-Dukát, J. Determination of diagnostic species with statistical fidelity measures. J. Veg. Sci. 2002, 13, 79–90. [Google Scholar] [CrossRef]
- Karagiannakidou, V.; Kokkini, S. The flora of Mount Menikion in North East Greece. Phyton 1987, 27, 267–283. [Google Scholar]
- Rohlf, F.J. A probabilistic minimum spanning tree algorithm. Inf. Process. Lett. 1978, 7, 44–48. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin version 3.0: An integrated software package for population genetics data analysis. Evol. Bioinf. Online 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Teacher, A.G.F.; Griffiths, D.J. HapStar: Automated haplotype network layout and visualisation. Mol. Ecol. Res. 2010, 11, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Pons, O.; Petit, R.J. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 1996, 144, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Corander, J.; Waldmann, P.; Sillanpää, M.J. Bayesian analysis of genetic differentiation between populations. Genetics 2003, 163, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Connor, T.R.; Sirén, J.; Aanensen, D.M.; Corander, J. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol. Biol. Evol. 2013, 30, 1224–1228. [Google Scholar] [CrossRef]
- Corander, J.; Sirén, J.; Arjas, E. Bayesian Spatial Modelling of Genetic Population Structure. Comput. Stat. 2008, 23, 111–129. [Google Scholar] [CrossRef]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D.; Athanasiadis, N. Classification and gradient analysis of the beech forest vegetation of the southern Rodopi (Northeast Greece). Folia Geobot 2007, 42, 249–270. [Google Scholar] [CrossRef]
- European Environment Agency. Biogeographic Regions in Europe; European Environment Agency: Copenhagen, Denmark, 2012.
- Figliuolo, G. Landscape Genetics of Fagus sylvatica in One of Its Glacial Refuge Areas. In Wild Plants: Identification Uses, and Conservation; Davis, R.E., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 149–177. [Google Scholar]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matter. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Lunt, D. Refugia within Refugia: Patterns of Phylogeographic Concordance in the Iberian Peninsula. In Phylogeography of Southern European Refugia; Weiss, S., Ferrand, N., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 155–188. [Google Scholar]
- Petit, R.J.; Aguinagalde, I.; de Beaulieu, J.-L.; Bittkau, C.; Brewer, S.; Cheddadi, R.; Ennos, R.; Fineschi, S.; Griver, D.; Vendramin, G.G. Glacial refugia: Hotspots but not melting pots of genetic diversity. Science 2003, 300, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- Strid, A. (University of Copenhagen, Copenhagen, Denmark). Personal communication, 2009.
- van der Maarel, E.; Franklin, J. Vegetation Ecology: Historical Notes and Outline. In Vegetation Ecology, 2nd ed.; van der Maarel, E., Franklin, J., Eds.; Wiley-Blackwell: Chicester, UK, 2013; pp. 1–27. [Google Scholar]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalén, L. Refugia revisited: Individualistic responses of species in space and time. Proc. R. Soc. B Biol. Sci. 2010, 277, 661–671. [Google Scholar] [CrossRef]
- Stewart, J.R. The progressive effect of the individualistic response of species to Quaternary climate change: An analysis of British mammalian faunas. Quat. Sci. Rev. 2008, 27, 2499–2508. [Google Scholar] [CrossRef]
- Aleksić, J.M.; Piotti, A.; Geburek, T.; Vendramin, G.G. Exploring and conserving a “microcosm”: Whole-population genetic characterization within a refugial area of the endemic, relict conifer Picea omorika. Conserv. Genet. 2017, 18, 777–788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).