Abstract
Lavender is an oil-bearing plant, which has long been cultivated for oil, fresh flowers, dried products, and food. Leaf blight disease was observed on ‘Bandera Pink’, which belongs to Lavandula stoechas in Yining County, Xinjiang Uygur Autonomous Region, China. The causal agent of this disease was isolated, and Koch’s postulates were assessed to confirm its pathogenicity. The morphological characteristics of the pathogen were observed, and the LSU, ITS, tef1, and tub2 loci were combined and analyzed. Based on morphological characterization and phylogenetic analyses, the causal agent was identified as a fungal species named Dothiorella sarmentorum. Pathogenicity tests revealed that D. sarmentorum can infect seven varieties of three lavender species. This is the first report of D. sarmentorum causing lavender leaf blight. This study provides a theoretical basis for the diagnosis of disease and the monitoring of disease occurrence and epidemics.
1. Introduction
Lavender (Lavandula, Lamiaceae) contains several commercial species that are cultivated extensively in temperate climates for the extraction of essential oils, as well as traditional medicine, culinary herbs, and ornamental plants [1,2,3,4,5]. Yili Prefecture of Xinjiang is the main cultivation area of lavender in China. At the present time, the lavender cultivation area in the Yili River Valley is 4900 hm2, accounting for 98% of the national lavender cultivation area and more than 90% of the national essential oil production, making it the largest lavender cultivation base in China [6,7].
In field conditions, lavender can be affected by some potential disease factors like fungi and bacteria. The most recognizable root rot of lavender is caused by Fusarium foetens, F. oxysporum, and Meloidogyne javanica [8,9,10]. The Downy mildew disease of lavender is caused by Peronospora belbahrii, and net blotch is caused by Rhizoctonia solani [11,12]. Bacterial speck disease is caused by Xanthomonas hortorum in Korea [13]. In August 2023, lavender leaf blight occurred in Yining County, Xinjiang Uygur Autonomous Region, China.
Dothiorella species are known pathogens on a wide range of hosts, causing environmental or agricultural production losses and seriously affecting healthy tree growth. It caused severe decline of almond trees in orchards in Spain, with disease symptoms including branch collapse, chlorosis of leaves leading to sudden wilting, death, and shoot dieback [14,15]. It also caused the wilting and death of a large number of grapevines in Australia’s wine regions, severely affecting local wine production [16]. Severe stem and branch diseases of hazelnut trees have been observed in several groves in Italy, leading to crown dieback and total tree mortality, with a significant impact on crop production [17]. Dothiorella panicle and shoot blight has been identified as one of the major threats to the Californian pistachio industry [18]. It also causes ulceration and wilting shoot disease in elms in Europe, severely affecting healthy tree growth [19].
The identification of fungal pathogens on the basis of morphological, physiological, and biochemical tests is not precise enough as it is mostly dependent on subtle differences in nutrients, pH, humidity, and environmental acclimatization [20,21]. Thus, polyphasic identification involving morphological characteristics and more than one gene sequence phylogenetic analysis is recommended to remove this predicament [22]. Based on morphology and molecular data, Phillips revived Dothiorella for species with conidia that become brown and 1-septate while they were still attached to the conidiogenous cells; sexual morphs of Dothiorella have pigmented, 1-septate ascospores [23,24]. The research confirmed the presence of D. sarmentorum (Fr.). Spore size and shape are important taxonomic features and valuable criteria for distinguishing the species [25].
The objective of this study was to identify the pathogen causing blight disease on ‘Bandera Pink’ in Xinjiang Uygur Autonomous Region based on morphological characterization and molecular analyses and to compare the differences in the pathogenicity of pathogenic fungi to different varieties of lavender. This study is fundamental for subsequent research on lavender leaf diseases.
2. Materials and Methods
2.1. Sample Collection and Pathogen Isolation
The leaves of symptomatic lavender plants were collected in Yining County (43°94′35.49″ N 81°54′88.12″ E) Xinjiang Uygur Autonomous Region of China in August 2023. The symptomatic leaves were delivered to the laboratory and immersed in 75% ethanol for 30 s, then thoroughly washed in sterile distilled water. Small pieces (2 mm2) with necrotic tissue and healthy parts of the collected leaves were cut using a sterilized blade. Tissue pieces were disinfected by placing them in 3% sodium hypochlorite solution for 3 min, then they were rinsed three times in sterile distilled water and dried naturally on sterilized filter paper. These tissue pieces were incubated on potato dextrose agar (PDA) at 28 °C under 12 h/12 h photoperiod conditions. After 3–4 days, the growing fungal colonies were sub-cultured onto fresh PDA, and pure cultures were obtained by subculturing hyphal tips. After the colony to be cultured had produced conidia, the conidia were gently scraped off the colony using sterile inoculation needles and suspended in 1 mL of sterile distilled water. The suspension of conidia was coated on a PDA culture dish and incubated at 25 °C for 24 h, individual germinated conidia were transferred to fresh PDA plates under a stereomicroscope, and incubation was continued at 25 °C for 36 h for mycelial development to obtain a pure culture strain. Isolates were maintained as a spore suspension in 25% glycerol at −80 °C until ready for use in further studies. Living cultures were deposited in the Pathology Laboratory of the College of forestry and landscape architecture (XJAU).
2.2. DNA Extraction, PCR Amplification, and Sequencing
Total genomic DNA was extracted from 5-day-old mycelium grown on PDA using the CTAB method [26]. The water solution of obtained DNA was kept at −20 °C for further experiments. PCR was run using a BIO-RAD T100TM Thermal Cycler(Bio-Rad Laboratories, Inc., Hercules, CA, USA) to amplify the internal transcribed spacer (ITS) region using primers ITS1/ITS4. The nuclear ribosomal large subunit (LSU), the translation elongation factor 1-alpha (tef1), and the β-tubulin gene (tub2) were amplified using primers LROR/LR5, EF-AF/EF-BR, and BT2A/BT2B (Table 1). All PCR reactions were performed in a 30 µL reaction volume containing 1.5 µL of DNA template, 1.5 µL of each forward and reverse primer, 15 µL of Taq PCR master mix (2×, with blue dye), and 10.5 µL of double-distilled water (dd H2O). The cycling parameters were as follows: a first step of denaturation at 95 °C for 5 min followed by 35 cycles of (i) denaturation at 95 °C for 60 s, (ii) annealing at optimal temperature (55 °C for ITS, tef1, and LSU and 45 °C for tub2) for 80 s, (iii) elongation at 72 °C for 90 s, and a final elongation step of 5 min was applied. PCR products were detected using a 1% agarose gel under 120 V stable voltage, and the electrophoresis time was 25 min. PCR bands were observed in a gel imager to determine whether they were clear and to determine the size of the target bands. PCR products were sent to Shanghai Sangon Bioengineering Co., Ltd. (Shanghai, China) for bidirectional sequencing.

Table 1.
List of primer pair sets used for PCR and sequencing.
2.3. Phylogenetic Analysis
The resulting sequences were edited and assembled by the SeqMan program within the DNASTAR software package (DNASTAR, Madison, WI, USA) and deposited in GenBank (Table 2). For molecular analysis, the ITS, LSU, tef1, and tub2 sequences of the reference isolates of the Dothiorella species were retrieved from the NCBI database (Table 2) and aligned using MEGA 6 software. The phylogenetic analyses of the combined loci were performed using Maximum Likelihood (ML) implemented on the CIPRES Science Gateway portal using RAxML-HPC BlackBox 8.2.10, employing a GTRGAMMA substitution model with 1000 bootstrap replicates.

Table 2.
Taxon names, strain or specimen numbers, and corresponding GenBank accession numbers of the taxa used for the phylogenetic studies. The newly generated sequences are indicated in bold.
2.4. Morphology and Culture Characteristics
To study the morphological characteristics of pure isolates, 5 mm (diameter) mycelial plugs from the edge of 5-day-old cultures were transferred to fresh PDA. The plates were incubated at 25 °C in a 12 h/12 h photoperiod. Morphological observation was conducted mainly based on the conidiomata naturally formed on the host tissues, including size, shape, and color. Macromorphological photographs were obtained using a Leica stereomicroscope (M205, Leica, Wetzlar, Germany). Micromorphological observations including the size and shape of conidiophores and conidia were performed using a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan). Twenty conidiomata were sectioned using a sterile scalpel, and then the lengths and widths of 50 randomly chosen conidia were measured. Colony morphology and growth rates were recorded [24].
2.5. Pathogenicity Test
The lavenders used in the experiment were all from the experimental base of the College of Agriculture, Xinjiang Agricultural University. The ‘Bandera Pink’ variety, belonging to Lavandula stoechas, was used to test Koch’s hypothesis; the ‘Blue Spear’, ‘Ellagance Sky’, ‘Avignon Early Blue’, ‘Taikonglan’, and ‘Faguolan’ varieties belonging to L. angustifolia; the Spanish Eyes variety belonging to L. multifida; and the ‘Bandera Pink’ variety belonging to L. stoechas were the different varieties used for the pathogenicity difference test.
Pathogenicity assays were performed on the leaves of lavender seedlings. The strain XJAU XYC-1 was prepared into a spore suspension with a concentration of 1 × 105 cfu/mL for use (the spore concentration was determined using a blood cell counting plate). The spore suspension was sprayed evenly on healthy lavender plants, spraying about 30 mL per plant. Then, a black plastic bag was used for moisturizing for 36 h, and sterile water treatment was sprayed as a control. These seedlings were raised in a growth chamber with a 12 h/12 h photoperiod at a 28 ± 2 °C temperature regime. After 36 h, the black silk plastic bag was removed and the disease incidence was monitored and recorded using the incidence area proportion counting method, which was used to calculate the ratio of the affected area to the total area every 2 days until the 15th day. Furthermore, to confirm Koch’s postulates, re-isolation was performed from lesions and identified based on morphological and molecular characteristics as described above.
2.6. Statistical Analysis
The means and standard errors of the data were calculated using Microsoft Excel formulas. Analysis of variance was used to detect differences among treatments using IBM SPSS Statistics 22.0 and Origin 9.5.
3. Results
3.1. Isolation of the Pathogen
A total of 19 strains of the fungal genus Dothiorella were obtained with similar colony morphology. Sequences of ITS, LSU, tef1, and tub2 were compared and considered to be consistent among the 19 obtained strains. Three strains (XJAU XYC-1, XJAU XYC-2, and XJAU XYC-3) were then randomly selected for the following phylogenetic analysis for species identification.
3.2. Phylogenetic Analysis
The combined dataset of ITS, LSU, tef1, and tub2 loci consisted of 45 strains, with Diplodia subglobosa (CBS 124133) as the outgroup taxon. The final alignment comprised 3152 characters including 512 characters in ITS, 801 characters in LSU, 242 characters in tef1, and 416 characters in tub2. The final ML optimization likelihood value of the best RAxML tree was −8740.98. The topology of our phylogenetic tree is nearly identical to previous publications. Three isolates from the present study formed a clade with CBS 115038 and IMI 63581b named Dothiorella sarmentorum (Figure 1).
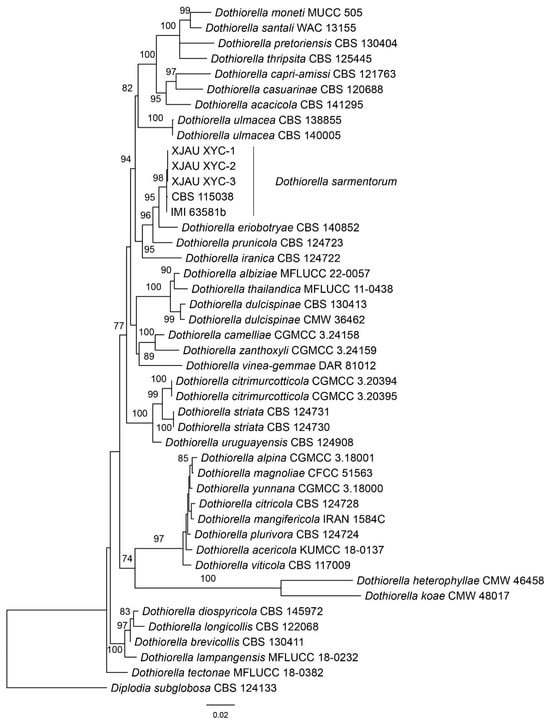
Figure 1.
Phylogenetic tree of Dothiorella generated from the maximum likelihood (ML) analysis based on the combined loci of ITS, LSU, tef1-α, and tub2 sequences.
3.3. Morphological Description of the Pathogen Dothiorella sarmentorum
On the PDA medium, the mycelium formed filamentous colonies. Initially, it was white (Figure 2A), subsequently forming small white colonies, within 4 days. Later, the mycelium turned leaden appressed and became smoke-grey to olivaceous grey at the surface, starting from the center (Figure 2B,C). The mycelium was immersed, consisting of septate, branched, brown, finely verruculose hyphae. Conidiomata that readily formed from the middle of colonies within 20 days were pycnidial, solitary, globose, dark brown to black, immersed in the medium, thick-walled, and up to 400 µm wide (Figure 2D). Conidiophores were hyaline, smooth, rarely branched, and aseptate (Figure 2G,H). The conidiogenous layer with developing conidia was thick and composed of dark brown 5–6-cell layers. Conidiogenous cells were cylindrical to fusiform, hyaline, thin-walled, smooth, and giving rise to periclinal thickenings (Figure 2E). Dark, oval to ovoid, often pigmented ellipsoid conidia were 18.9–24.9 × 8.8–12.1 μm (x = 22.2 × 8.7 μm, n = 60), hyaline, later brown, thick-walled, 1-septate prior to release from conidiogenous cells, and occasionally slightly constricted at the septum with a broadly rounded apex and truncate base (Figure 2F,K).
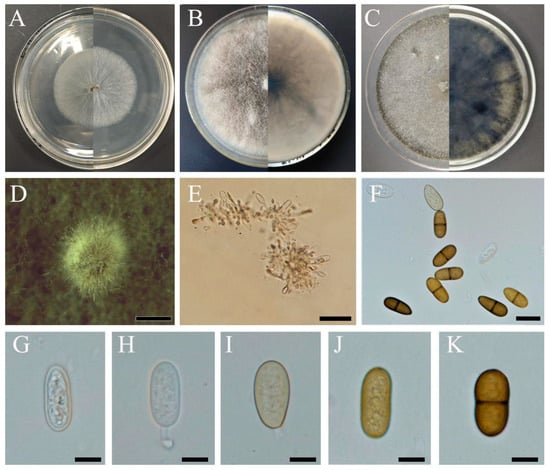
Figure 2.
Morphological characteristics of Dothiorella sarmentorum (XIAU XYC-1). (A–C): colonies of white, later olivaceous grey mycelia after 2 (A), 6 (B), and 10 days (C) of incubation on PDA; (D): conidiomata; (E): conidiogenous cells; (F,K): dark, ovoid, diplodia-like conidia. (G–J): immature conidia. Scale bars: (D) = 2 mm, (E) = 50 μm, (F) = 20 μm, (G–K) = 10 μm.
3.4. Pathogenicity
Seedlings of ‘Bandera Pink’ inoculated with Dothiorella sarmentorum (XJAU XYC-1) developed symptoms that were the same as those observed in the field. The lavender leaves turned black and curled, and disease spots spread through the stems 4 d after inoculation (Figure 3D–F). The whole lavender plant was curled, withered, and necrotic, and the lavender roots were black 10 d after inoculation (Figure 3G–I,K,M). The negative control was sprayed with sterile water on healthy lavender plants and did not develop disease symptoms (Figure 3A–C,J,L). The fungus was reisolated from symptomatic leaves and identified as D. sarmentorum based on morphological characteristics and DNA sequence data, thus fulfilling Koch’s postulates.

Figure 3.
Leaf disease symptoms observed in lavender seedlings after Dothiorella sarmentorum fungal inoculation. (A–C): negative control—leaves sprayed with sterile water. (D–F): Dothiorella sarmentorum conidial suspension was spray-inoculated onto seedlings of lavender; (G–I): symptoms of disease 4 d after inoculation; (G–I): symptoms of disease 10 d after inoculation. (J): negative control—the whole plant is healthy; (K): treatment—the whole plant has withered; (L): negative control—the roots of plants are brown; (M): treatment: the roots of plants are black.
Dothiorella sarmentorum showed different degrees of pathogenicity on ‘Blue Spear’, ‘Ellagance Sky’, ‘Spanish Eyes’, ‘Bandera Pink’, ‘Avignon Early Blue’, ‘Taikonglan’, and ‘Faguolan’ seedlings (Figure 4 and Figure 5). The average disease incidence that developed on inoculated ‘Blue Spear’, ‘Ellagance Sky’, ‘Spanish Eyes’, ‘Bandera Pink’, ‘Avignon Early’ Blue’, ‘Taikonglan’, and ‘Faguolan’ seedlings were 38.96%, 41.46%, 16.38%, 31.78%, 11.62%, 7.99%, and 10.66%, respectively, 15 days after inoculation (Figure 4).
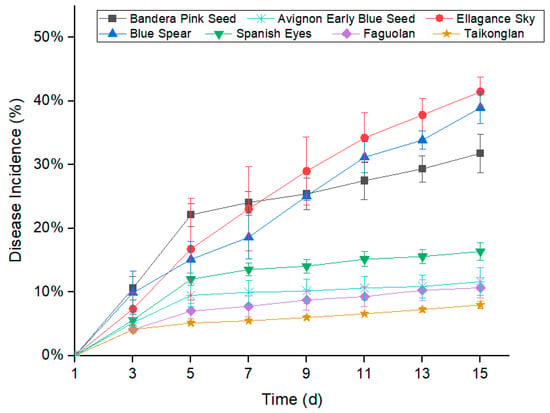
Figure 4.
Disease incidence observed in different varieties of lavender after Dothiorella sarmentorum inoculation. Analysis of variance of disease incidence that developed on Blue Spear, Ellagance Sky, Spanish Eyes, Bandera Pink, Avignon Early Blue, Taikonglan, and Faguolan seedlings following inoculation with Dothiorella sarmentorum. Values shown are means ± the standard errors.


Figure 5.
Leaf disease symptoms observed in different varieties of lavender after Dothiorella sarmentorum inoculation. Leaf disease symptoms on (A–A3) Bandera Pink; (B–B3) Ellagance Sky; (C–C3) Blue Spear; (D–D3) Spanish Eyes; (E–E3) Faguolan; (F–F3) Taikonglan; and (G–G3) Avignon Early Blue lavender seedlings. (A–G) the negative control—leaves sprayed with sterile water (control—CK); the Dothiorella sarmentorum spore suspension was spray-inoculated onto seedlings of lavender. (A1–G1): 3 d after inoculation; (A2–G2): the overall image of 15 d after inoculation; (A3–G3): the partial image of 15 d after inoculation.
4. Discussion
According to morphological and molecular analyses as well as pathogenicity tests, we demonstrated that the dieback symptoms observed on lavender (Lavandula stoechas) plants in China are due to D. sarmentorum. To our knowledge, this is the first report of D. sarmentorum as a new pathogen of L. stoechas. The pathogenicity test confirmed that D. sarmentorum is a pathogenic fungus that can also infect L. angustifolia and L. multifida. There are variations in the pathogenicity of different varieties of lavender.
Dothiorella sarmentorum has been recorded in several countries around the world, and it can not only cause tree canker disease as a pathogenic bacterium but also parasitize 66 plant tissues such as Salicaceae and Rosaceae as endophytic fungi [24]. D. sarmentorum is a cosmopolitan species and has been isolated from 34 different host species including Malus, Menispermum, Prunus, Pyrus, and Ulmus genera. Molecular studies have described D. sarmentorum in 17 woody hosts [22,31]. However, there are relatively few reports on the diseases that D. sarmentorum causes in herbaceous plants. This study further confirms that lavender is a new host of D. sarmentorum, which is of great significance for understanding the source of this pathogen in nature. Considering that the lavender leaf spot disease caused by D. sarmentorum has the characteristics of a large lesion area, strong destructive power, and rapid development, it has the risk of widespread transmission and epidemics. Therefore, it is necessary to strengthen the knowledge about the ecology, epidemiology, biogeography, and infection biology of this pathogen that represents a serious threat to the lavender industry.
5. Conclusions
Dothiorella sarmentorum is an emerging pathogen causing L. angustifolia leaf spot in Yining County, Xinjiang, China. The pathogenicity test confirmed that D. sarmentorum is a pathogenic fungus that can also infect L. stoechas and L. pinnata. The data obtained in this study should provide a theoretical basis for monitoring and preventing this disease. The potential impact of D. sarmentorum on lavender production in this area of China warrants further investigation to determine potential disease management strategies.
Author Contributions
Conceptualization, R.M.; methodology, R.M.; software, M.L. and C.L.; validation, M.L. and C.L.; formal analysis, W.S. and A.W.; investigation, A.W., and W.S.; resources, A.W., M.L. and X.S.; data curation, C.L., M.L. and R.M.; writing—original draft preparation, C.L.; writing—review and editing, R.M.; visualization, W.S.; supervision, R.M. and X.S.; project administration, R.M.; funding acquisition, R.M. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31960316, and the Key Research and development program of Xinjiang Province, grant number 2022B02036-1.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cavanagh, H.M.; Wilkinson, J.M. Lavender essential oil: A review. Aust. Infect. Control. 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Bradley, B.F.; Starkey, N.J.; Brown, S.L.; Lea, R.W. Anxiolytic effects of Lavandula angustifolia odour on the Mongolian gerbil elevated plus maze. J. Ethnopharmacol. 2007, 111, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Prusinowska, R.; Smigielski, K.B. Composition, biological properties and therapeutic effects of Lavender (Lavandula angustifolia). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Zhen, S.; Burnett, S.E. Effects of substrate volumetric water content on English lavender morphology and photosynthesis. HortScience 2015, 50, 909–915. [Google Scholar] [CrossRef]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The effects of lavender essential oil on wound healing: A review of the current evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and Taxane Production by Taxomyces andreanae, an Endophytic Fungus of Pacific Yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Tang, S.M.; Ran, B.; Zhu, L.; Dong, S.; Luo, W.; Zhang, X.; Huang, X. Evaluation of Cold Tolerance of Different Lavender Varieties. Chin. Wild Plant Resour. 2023, 42, 8–15. [Google Scholar] [CrossRef]
- Jun, X.W.; Guo, B.J.; Xing, W. First report of Fusarium foetens causing root rot on lavender (Lavandula angustifolia) in China. J. Plant Pathol. 2023, 105, 1173–1174. [Google Scholar] [CrossRef]
- Garibaldi, A.; Bertetti, D.; Pensa, P.; Ortu, G.; Gullino, M.L. First Report of Fusarium oxysporum Causing Wilt on Allard’s Lavender (Lavandula x allardii) in Italy. Plant Dis. 2015, 99, 1868. [Google Scholar] [CrossRef]
- Oliveira, S.A.; Dlugos, D.M.; Agudelo, P.; Jeffers, S.N. First report of Meloidogyne javanica pathogenic on hybrid lavender (Lavandula ×intermedia) in the United States. Plant Dis. 2022, 106, 335. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Afroz, T.; Kim, S.B. First report of web blight on lavender caused by Rhizoctonia solani AG-1-IB in Korea. Plant Dis. 2020, 104, 2518. [Google Scholar] [CrossRef]
- Marco, T.; Anthony, B.; Sebastian, P. Downy mildew of lavender caused by Peronospora belbahrii in Israel. Mycol. Prog. 2020, 19, 1537–1543. [Google Scholar] [CrossRef]
- Rotondo, F.; Testen, A.L.; Horvat, M.M.; Roman-Reyna, V.; Klass, T.L.; Jacobs, J.M.; Miller, S.A. First report of Xanthomonas hortorum causing bacterial leaf spot of lavender (Lavandula × intermedia) in Ohio. Plant dis. 2021, 105, 484. [Google Scholar] [CrossRef] [PubMed]
- Gramaje, D.; Agustí-Brisach, C.; Pérez-Sierra, A.; Moralejo, E.; Olmo, D.; Mostert, L.; Damm, U.; Armengol, J. Fungal trunk pathogens associated with wood decay of almond trees on Mallorca (Spain). Persoonia 2012, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.M.; Úrbez-Torres, J.R.; Trouillas, F.P. Dothiorella and Spencermartinsia, new species and records from grapevines in Australia, Australas. Plant Path. 2015, 44, 43–56. [Google Scholar] [CrossRef]
- Pitt, W.M.; Huang, R.; Steel, C.C.; Savocchia, S. Identification, distribution and current taxonomy of Botryosphaeriaceae species associated with grapevine decline in New South Wales and South Australia. Aust. J. Grape Wine R. 2010, 16, 258–271. [Google Scholar] [CrossRef]
- Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Franceschini, A.; Alves, A.; Abdollahzadeh, J.; Phillips, A.J.L. Phylogeny, morphology and pathogenicity of Botryosphaeriaceae, Diatrypaceae and Gnomoniaceae associated with branch diseases of hazelnut in Sardinia (Italy). Eur. J. Plant Patho. 2016, 146, 259–279. [Google Scholar] [CrossRef]
- Chen, S.F.; Morgan, D.P.; Michailides, T.J. Botryosphaeriaceae and Diaporthaceae associated with panicle and shoot blight of pistachio in California, USA. Fungal Divers. 2014, 67, 157–179. [Google Scholar] [CrossRef]
- Jürisoo, L.; Adamson, K.; Padari, A.; Drenkhan, R. Health of elms and Dutch elm disease in Estonia. Eur. J. Plant Patho. 2019, 154, 823–841. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wu, S.W. Application and progress in fungal taxonomy and nomenclature by molecular systematics. Prog. Microbiol. Immunol. 2015, 43, 48–53. [Google Scholar] [CrossRef]
- Mukuma, C. Morphological and Molecular Identification and Characterization of Dry Bean Fungal Root Rot Pathogens in Zambia. M.Sc. Thesis, University of Nebraska, Lincoln, NE, USA, 2016. [Google Scholar]
- Dar, G.J.; Nazir, R.; Wani, S.A.; Farooq, S. Isolation, molecular characterization and first report of Dothiorella gregaria associated with fruit rot of walnuts of Jammu and Kashmir, India, Microb. Pathog. 2023, 175, 105989. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Correia, A.; Lugue, J. Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 2005, 97, 513–529. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [PubMed]
- Ivanová, H. Identification and characterization of the fungus Dothiorella sarmentorum on necrotic shoots of declining ash in Slovakia. Folia Oecologica 2018, 45, 53–57. [Google Scholar] [CrossRef]
- Fan, X.L.; Du, Z.; Liang, Y.M.; Tian, C.M. Melanconis (Melanconidaceae) associated with Betula spp. in China. Mycol. Prog. 2016, 15, 1–9. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pcr Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycete. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Camporesi, E.; Hyde, K.D.; Phillips, A.J.L.; Fu, C.Y.; Yan, J.Y.; Li, X.H. Dothiorella species associated with woody hosts in Italy. Mycosphere 2016, 7, 51–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).