Role of Rare Species on Phytoplankton Size–Abundance Relationships and Size Structure across Different Biogeographical Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Data Collection
2.2. Statistical Analyses
3. Results
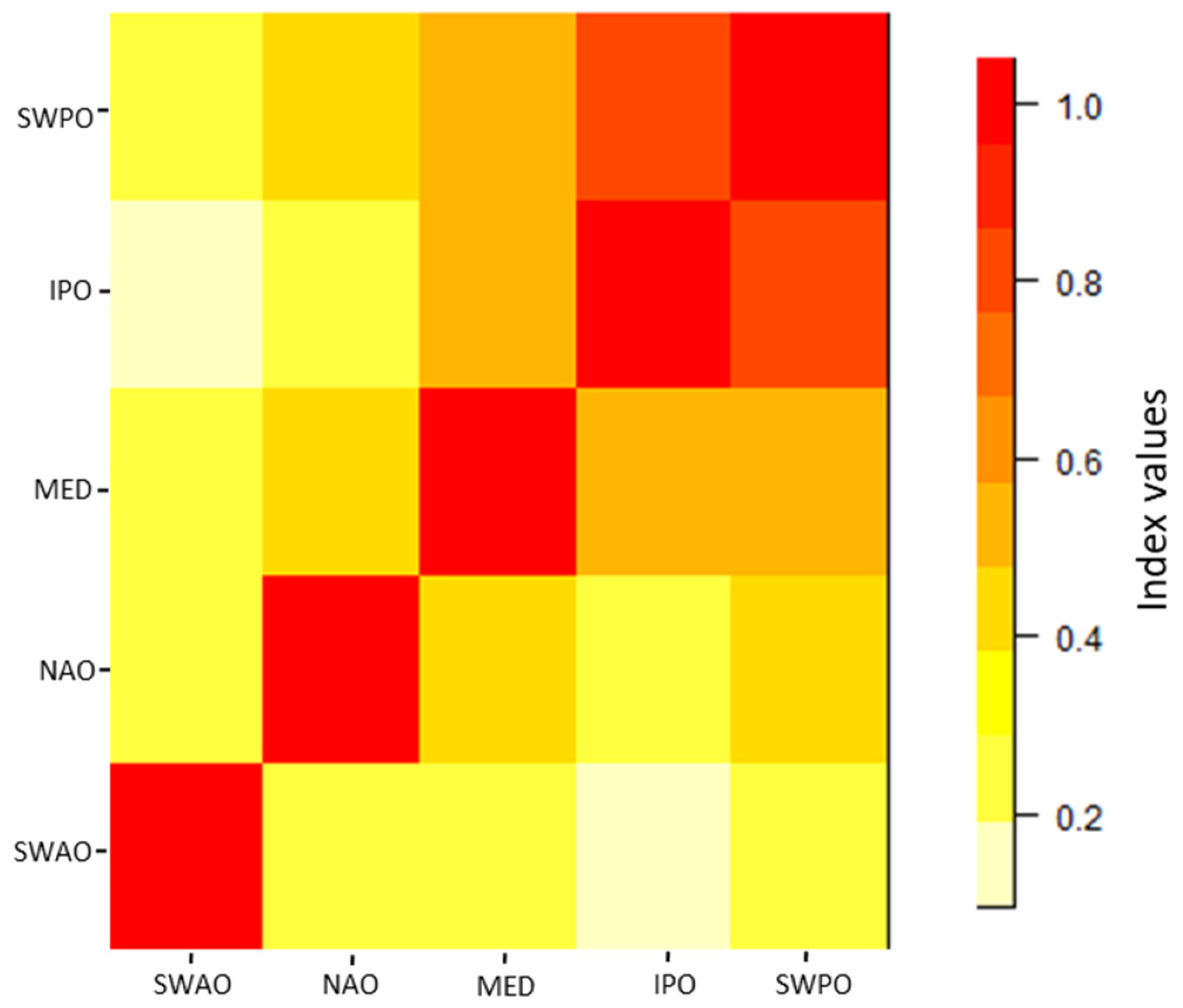
3.1. Global and Local Phytoplankton Size–abundance Relationships
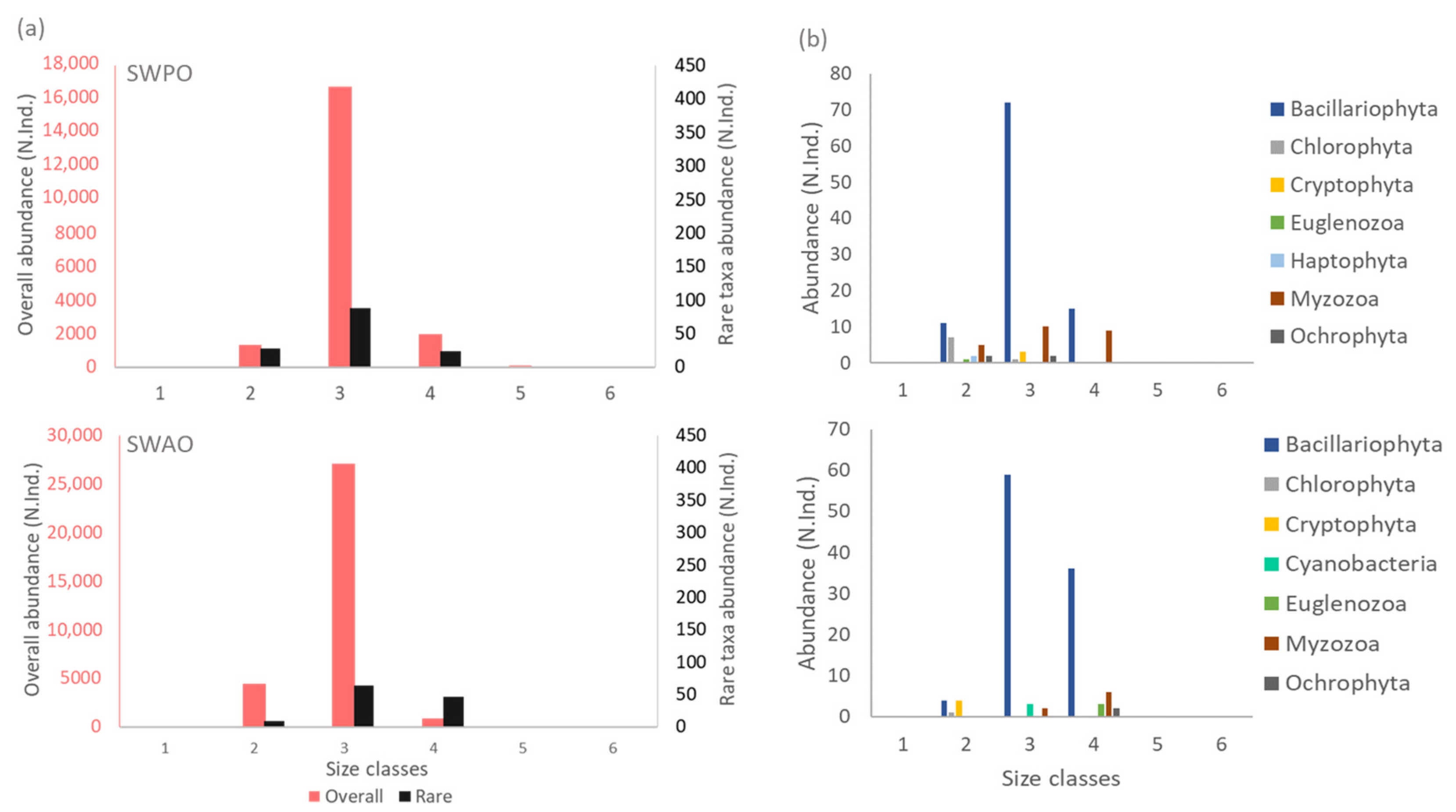
3.2. Taxonomic Diversity and Size Class Distribution of Rare Taxa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Säterberg, T.; Jonsson, T.; Yearsley, J.; Berg, S.; Ebenman, B. A potential role for rare species in ecosystem dynamics. Sci. Rep. 2019, 9, 11107. [Google Scholar] [CrossRef]
- Umaña, M.N.; Mi, X.; Cao, M.; Enquist, B.J.; Hao, Z.; Howe, R.; Iida, Y.; Johnson, D.; Lin, L.; Liu, X. The role of functional uniqueness and spatial aggregation in explaining rarity in trees. Glob. Ecol. Biogeogr. 2017, 26, 777–786. [Google Scholar] [CrossRef]
- Van Schalkwyk, J.; Pryke, J.S.; Samways, M.J. Contribution of common vs. rare species to species diversity patterns in conservation corridors. Ecol. Indic. 2019, 104, 279–288. [Google Scholar] [CrossRef]
- Gaston, K.J.; Lawton, J.H. The population ecology of rare species. J. Fish Biol. 1990, 37, 97–104. [Google Scholar] [CrossRef]
- Chapman, A.S.A.; Tunnicliffe, V.; Bates, A.E. Both rare and common species make unique contributions to functional diversity in an ecosystem unaffected by human activities. Divers. Distrib. 2018, 24, 568–578. [Google Scholar] [CrossRef]
- Ohlemuller, R.; Anderson, B.J.; Araujo, M.B.; Butchart, S.H.M.; Kudrna, O.; Ridgely, R.S.; Thomas, C.D. The coincidence of climatic and species rarity: High risk to small-range species from climate change. Biol. Lett. 2008, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A.; Končeková, L.; Glemnitz, M.; Berger, G.; Pfeffer, H.; Herzon, I. Maintaining and promoting biodiversity. In Sustainable Agriculture; Jakobson, C., Ed.; Baltic University Press: Uppsala, Sweden, 2012. [Google Scholar]
- Leitão, R.P.; Zuanon, J.; Villéger, S.; Williams, S.E.; Baraloto, C.; Fortunel, C.; Mendonça, F.P.; Mouillot, D. Rare species contribute disproportionately to the functional structure of species assemblages. Proc. Royal Soc. B 2016, 283, 20160084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, Y.; Wei, G.; Jiao, S. Rare species-driven diversity–ecosystem multifunctionality relationships are promoted by stochastic community assembly. MBio 2022, 13, e00449-22. [Google Scholar] [CrossRef]
- Kondratyeva, A.; Grandcolas, P.; Pavoine, S. Reconciling the concepts and measures of diversity, rarity and originality in ecology and evolution. Biol. Rev. 2019, 94, 1317–1337. [Google Scholar] [CrossRef]
- Boyd, J.N.; Anderson, J.T.; Brzyski, J.; Baskauf, C.; Cruse-Sanders, J. Eco-evolutionary causes and consequences of rarity in plants: A meta-analysis. New Phytol. 2022, 235, 1272–1286. [Google Scholar] [CrossRef]
- Stebbins, G.L. Rarity of plant species: A synthetic viewpoint. Rhodora 1980, 82, 77–86. [Google Scholar]
- Enquist, B.J.; Feng, X.; Boyle, B.; Maitner, B.; Newman, E.A.; Jørgensen, P.M.; Roehrdanz, P.R.; Thiers, B.M.; Burger, J.R.; Corlett, R.T. The commonness of rarity: Global and future distribution of rarity across land plants. Sci. Adv. 2019, 5, eaaz0414. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.J.; Reed, J.M.; Chew, F.S. Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst. 2007, 10, 321–337. [Google Scholar] [CrossRef]
- Hodgson, J.G. Commonness and rarity in British butterflies. J. Appl. Ecol. 1993, 30, 407–427. [Google Scholar] [CrossRef]
- Fattorini, S. Use of insect rarity for biotope prioritisation: The tenebrionid beetles of the Central Apennines (Italy). J. Insect Conserv. 2010, 14, 367–378. [Google Scholar] [CrossRef]
- Booth, J.E.; Gaston, K.J.; Evans, K.L.; Armsworth, P.R. The value of species rarity in biodiversity recreation: A birdwatching example. Biol. Conserv. 2011, 144, 2728–2732. [Google Scholar] [CrossRef]
- Yu, J.; Dobson, F.S. Seven forms of rarity in mammals. J. Biogeogr. 2000, 27, 131–139. [Google Scholar] [CrossRef]
- Loiseau, N.; Mouquet, N.; Casajus, N.; Grenie, M.; Gueguen, M.; Maitner, B.; Mouillot, D.; Ostling, A.; Renaud, J.; Tucker, C.; et al. Global distribution and conservation status of ecologically rare mammal and bird species. Nat. Commun. 2020, 11, 5071. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.G. Are there adequate data to assess how well theories of rarity apply to marine invertebrates? Biodivers. Conserv. 1999, 8, 1295–1318. [Google Scholar] [CrossRef]
- Mcclain, C.R. The commonness of rarity in a deep-sea taxon. Oikos 2021, 130, 863–878. [Google Scholar] [CrossRef]
- Schmidt, A.; Feher, G.; Padisák, J. Some rare green algae occurring in the Danube river and its dead-and side-branches in southern Hungary. Biologia-Bratislava 2003, 58, 475–482. [Google Scholar]
- Lenarczyk, J.; Tsarenko, P. Some rare and interesting green algae (Chlorophyta) from subalpine Tatra lakes (High Tatra Mountains, Poland). Oceanol. Hydrobiol. Stud. 2013, 42, 225–232. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Isheva, T.; Ivanov, P.; Uzunov, B.A.; Dimitrova, P. Red List of Bulgarian Algae. II. Microalgae. 2016. Available online: http://hdl.handle.net/10506/1389 (accessed on 11 January 2024).
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.P.M.; Soares, K.W.d.S.; Carvalho, P.d.; Bortolini, J.C. Phytoplankton community diversity, dominance, and rarity: A case study of tropical urban lakes. Acta Limnol. Bras. 2024, 36, e1. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Ringelbelg, J. Characterization of species diversity of phytoplankton assemblages by dominance-diversity curves: With 4 figures and 1 table in the text. Int. Ver. Theor. Angew. Limnol. Verh. 1978, 20, 939–949. [Google Scholar] [CrossRef]
- Gerhard, M.; Mori, C.; Striebel, M. Nonrandom species loss in phytoplankton communities and its effect on ecosystem functioning. Limnol. Oceanogr. 2021, 66, 779–792. [Google Scholar] [CrossRef]
- Ignatiades, L.; Gotsis-Skretas, O. The contribution of rare species to coastal phytoplankton assemblages. Mar. Ecol. 2013, 35, 132–145. [Google Scholar] [CrossRef]
- Crisfield, V.E.; Guillaume Blanchet, F.; Raudsepp-Hearne, C.; Gravel, D. How and why species are rare: Towards an understanding of the ecological causes of rarity. Ecography 2024, 2024, e07037. [Google Scholar] [CrossRef]
- Laraib, M.; Titocci, J.; Rosati, I.; Basset., A. An integrated individual-level trait-based phytoplankton dataset from transitional waters. Sci. Data 2023, 10, 897. [Google Scholar] [CrossRef]
- Brown, J.H.; Maurer, B.A. Evolution of species assemblages: Effects of energetic constraints and species dynamics on the diversification of the North American avifauna. Am. Nat. 1987, 130, 1–17. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M. Global scale macroecology: Interactions between population size, geographic range size and body size in the Anseriformes. J. Anim. Ecol. 1996, 65, 701–714. [Google Scholar] [CrossRef]
- Gjoni, V.; Glazier, D.S. A perspective on body size and abundance relationships across ecological communities. Biology 2020, 9, 42. [Google Scholar] [CrossRef]
- Belgrano, A.; Allen, A.P.; Enquist, B.J.; Gillooly, J.F. Allometric scaling of maximum population density: A common rule for marine phytoplankton and terrestrial plants. Ecol. Lett. 2002, 5, 611–613. [Google Scholar] [CrossRef]
- Basset, A.; Sangiorgio, F.; Pinna, M. Monitoring with benthic macroinvertebrates: Advantages and disadvantages of body size descriptors. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, S43–S58. [Google Scholar] [CrossRef]
- Shokri, M.; Cozzoli, F.; Vignes, F.; Bertoli, M.; Pizzul, E.; Basset, A. Metabolic rate and climate change across latitudes: Evidence of mass-dependent responses in aquatic amphipods. J. Exp. Biol. 2022, 225, jeb244842. [Google Scholar] [CrossRef]
- Damuth, J. Population density and body size in mammals. Nature 1981, 290, 699–700. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, P.; Marañón, E.; Harbour, D.; Harris, R.P. Invariant scaling of phytoplankton abundance and cell size in contrasting marine environments. Ecol. Lett. 2006, 9, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.M.; Gaston, K.J. Who is rare? Artefacts and complexities of rarity determination. In The Biology of Rarity: Causes and Consequences of Rare—Common Differences; Springer: Dordrecht, The Netherlands, 1997; pp. 48–60. [Google Scholar]
- Roselli., L.; Litchman, E.; Stanca, E.; Cozzoli, F.; Basset, A. Individual trait variation in phytoplankton communities across multiple spatial scales. J. Plankton Res. 2017, 39, 577–588. [Google Scholar] [CrossRef]
- Durante, G.; Stanca, E.; Roselli, L.; Basset, A. Phytoplankton composition in six Northern Scotland lagoons (Orkney Islands). Transit. Water. Bull. 2013, 7, 159–174. [Google Scholar]
- Roselli, L.; Stanca, E.; Ludovisi, A.; Durante, G.; Souza, J.S.D.; Dural, M.; Alp, T.; Bulent, S.; Gjoni, V.; Ghinis, S. Multi-scale biodiverity patterns in phytoplankton from coastal lagoons: The Eastern Mediterranean. Transit. Water. Bull. 2013, 7, 202–219. [Google Scholar]
- Stanca, E.; Roselli, L.; Cellamare, M.; Basset, A. Phytoplankton composition in the coastal magnetic island lagoon, western pacific ocean (Australia). Transit. Water. Bull. 2013, 7, 145–158. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im text und auf 1 tafel. Verh.-Int. Ver. Theor. Angew. Limnol. Mitte. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Sournia, A. Introduction, Cyanophycées, Dictyochophycées, Dinophycées et Raphidophycées. 1986. Available online: https://cir.nii.ac.jp/crid/1130000797027661568 (accessed on 3 March 2010).
- Round, F.E.; Crawford, R.M.; Mann, D.G. Biology and morphology of the genera. In The Diatom; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Tomas, C.R. Identifying Marine Phytoplankton; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Phytoplankton Virtual Research Environment VRE. Available online: https://www.phytovre.lifewatchitaly.eu/vre/shapes-groups/ (accessed on 1 March 2011).
- World Register of Marine Species WoRMS. Available online: https://www.marinespecies (accessed on 9 April 2013).
- Vadrucci, M.R.; Cabrini, M.; Basset, A. Biovolume determination of phytoplankton guilds in transitional water ecosystems of Mediterranean Ecoregion. Trans. Water. Bull. 2007, 1, 83–102. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Techn. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Morisita, M. Measuring of the Dispersion of individuals and analysis of the distributional patterns. Mem. Fac. Sci. Kyushu Univ. Ser. E 1959, 3, 65–80. [Google Scholar]
- Blanco, J.M.; Echeverria, F.; García, C.M. Dealing with size-spectra: Some conceptual and mathematical problems. Sci. Mar. 1994, 58, 17–29. [Google Scholar]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 March 2022).
- Irwin, A.J.; Finkel, Z.V.; Schofield, O.M.; Falkowski, P.G. Scaling-up from nutrient physiology to the size-structure of phytoplankton communities. J. Plankton Res. 2006, 28, 459–471. [Google Scholar] [CrossRef]
- Cermeno, P.; de Vargas, C.; Abrantes, F.; Falkowski, P.G. Phytoplankton biogeography and community stability in the ocean. PLoS ONE 2010, 5, e10037. [Google Scholar] [CrossRef]
- Ignatiades, L.; Gotsis-Skretas, O.; Pagou, K.; Krasakopoulou, E. Diversification of phytoplankton community structure and related parameters along a large-scale longitudinal east–west transect of the Mediterranean Sea. J. Plankton Res. 2009, 31, 411–428. [Google Scholar] [CrossRef]
- Gaston, K.J. Rarity; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Rabinowitz, D.; Cairns, S.; Dillon, T. Seven forms of rarity and their frequency in the flora of the British Isles. In Conservation Biology: The Science of Scarcity and Diversity; Soule, M.E., Ed.; Sinauer Associates: Sunderland, MA, USA, 1986. [Google Scholar]
- Reed, J.M. A system for ranking conservation priorities for neotropical migrant birds based on relative susceptibility to extinction. In Ecology and Conservation of Neotropical Migrant Landbirds; Hangan, J.M., Johnston, D.W., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1992. [Google Scholar]
- Kunin, W.E.; Gaston, K.J. The Biology of Rarity: Causes and Consequences of Rare—Common Differences; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ricotta, C.; Godefroid, S.; Celesti-Grapow, L. Common species have lower taxonomic diversity evidence from the urban floras of Brussels and Rome. Divers. Distrib. 2008, 14, 530–537. [Google Scholar] [CrossRef]
- Padisák, J.; Hajnal, É.; Krienitz, L.; Lakner, J.; Üveges, V. Rarity, ecological memory, rate of floral change in phytoplankton—And the mystery of the Red Cock. Hydrobiologia 2010, 653, 45–64. [Google Scholar] [CrossRef]
- Cao, Y.; Williams, D.D.; Williams, N.E. How important are rare species in aquatic community ecology and bioassessment? Limnol. Oceanogr. 1998, 43, 1403–1409. [Google Scholar] [CrossRef]
- White, E.P.; Ernest, S.K.M.; Kerkhoff, A.J.; Enquist, B.J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007, 22, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, F.; Shokri, M.; Boulamail, S.; Marrocco, V.; Vignes, F.; Basset, A. The size dependency of foraging behaviour: An empirical test performed on aquatic amphipods. Oecologia 2022, 199, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Sabetta, L.; Fiocca, A.; Margheriti, L.; Vignes, F.; Basset, A.; Mangoni, O.; Carrada, G.C.; Ruggieri, N.; Ianni, C. Body size–abundance distributions of nano-and micro-phytoplankton guilds in coastal marine ecosystems. Estuar. Coast. Shelf Sci. 2005, 63, 645–663. [Google Scholar] [CrossRef]
- Fox, B.J.; Brown, J.H. Assembly rules for functional groups in North American desert rodent communities. Oikos 1993, 67, 358–370. [Google Scholar] [CrossRef]
- Fox, B.J.; Kirkland, G.L., Jr. An assembly rule for functional groups applied to North American soricid communities. J. Mammal. 1992, 73, 491–503. [Google Scholar] [CrossRef]
- Morris, D.W.; Knight, T.W. Can consumer-resource dynamics explain patterns of guild assembly? Am. Nat. 1996, 147, 558–575. [Google Scholar] [CrossRef]
- Zhu, W.; Wan, L.; Zhao, L. Effect of nutrient level on phytoplankton community structure in different water bodies. J. Environ. Sci. 2010, 22, 32–39. [Google Scholar] [CrossRef]
- Litchman, E. Resource competition and the ecological success of phytoplankton. In Evolution of Primary Producers in the Sea; Falkowski, P.G., Knoll, A.H., Eds.; Academic Press: Burlington, MA, USA, 2007. [Google Scholar]
- Kostryukova, A.M.; Krupnova, T.G.; Mashkova, I.V.; Gavrillkina, S.V.; Egorov, N.O. Phytoplankton diversity in three lakes of South Ural, Russia. Biodiversitas J. Biol. Divers. 2018, 19, 1459–1467. [Google Scholar] [CrossRef]
- Arhonditsis, G.; Karydis, M.; Tsirtsis, G. Analysis of phytoplankton community structure using similarity indices: A new methodology for discriminating among eutrophication levels in coastal marine ecosystems. Environ. Manag. 2003, 31, 0619–0632. [Google Scholar] [CrossRef] [PubMed]
- Kruk, C.; Huszar, V.L.; Peeters, E.T.; Bonilla, S.; Costa, L.; Lürling, M.; Reynolds, C.S.; Scheffer, M. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- Weithoff, G. The concepts of plant functional types and functional diversity in lake phytoplankton: A new understanding of phytoplankton ecology. Freshw. Biol. 2003, 48, 1669–1675. [Google Scholar] [CrossRef]
- Bergkemper, V.; Stadler, P.; Weisse, T. Moderate weather extremes alter phytoplankton diversity-a microcosm study. Freshw. Biol. 2018, 63, 1211–1224. [Google Scholar] [CrossRef]
- Santos, J.B.O.; Brasil, J.; Huszar, V.L.M. Responses of functional and taxonomic phytoplankton diversity to environmental gradients in subtropical and tropical reservoirs. Front. Environ. Sci. 2022, 10, 899571. [Google Scholar] [CrossRef]
- Santos, A.M.C.; Carneiro, F.M.; Cianciaruso, M.V. Predicting productivity in tropical reservoirs: The roles of phytoplankton taxonomic and functional diversity. Ecol. Indic. 2015, 48, 428–435. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, D.; Chen, L.; Giesy, J.P.; Zhang, W.; Yuan, C.; Ni, L.; Shen, H.; Xie, P. Light, but not nutrients, drives seasonal congruence of taxonomic and functional diversity of phytoplankton in a eutrophic highland lake in China. Front. Plant Sci. 2020, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Graco-Roza, C.; Soininen, J.; Correa, G.; Pacheco, F.S.; Miranda, M.; Domingos, P.; Marinho, M.M. Functional rather than taxonomic diversity reveals changes in the phytoplankton community of a large dammed river. Ecol. Indic. 2021, 121, 107048. [Google Scholar] [CrossRef]



| GSAR | Thresholds | Slope | 95%CI | Intercept | df | r2 |
|---|---|---|---|---|---|---|
| 100 | −0.38 | −0.50 to −0.26 | 174 | 304 | 0.11 | |
| 99 ** | −0.60 | −0.71 to −0.48 | 2540 | 173 | 0.38 | |
| 95 | −0.72 | −0.84 to −0.60 | 17,804 | 88 | 0.61 | |
| 90 | −0.71 | −0.84 to −0.57 | 30,634 | 58 | 0.66 | |
| 75 | −0.59 | −0.76 to −0.41 | 39,909 | 25 | 0.65 | |
| LSAR | ||||||
| SWPO | 100 | −0.31 | −0.54 to −0.09 | 68 | 113 | 0.06 |
| 99 ** | −0.85 | −1.04 to −0.66 | 6650 | 66 | 0.55 | |
| 95 | −0.93 | −1.11 to −0.76 | 23,516 | 42 | 0.73 | |
| 90 | −0.91 | −1.10 to −0.73 | 34,108 | 28 | 0.78 | |
| 75 | −0.94 | −1.14 to −0.74 | 90,925 | 12 | 0.90 | |
| SWAO | 100 | 0.64 | −0.92 to −0.37 | 508 | 96 | 0.18 |
| 99 * | −1.09 | −1.31 to −0.87 | 23,895 | 59 | 0.61 | |
| 95 | −1.17 | −1.40 to −0.93 | 96,070 | 32 | 0.76 | |
| 90 | −1.20 | −1.46 to −0.94 | 206,798 | 20 | 0.82 | |
| 75 | −0.72 | −1.34 to −0.09 | 57,019 | 6 | 0.56 | |
| MED | 100 | −0.36 | −0.55 to −0.17 | 93 | 109 | 0.11 |
| 99 | −0.54 | −0.75 to −0.33 | 970 | 60 | 0.31 | |
| 95 | −0.46 | −0.66 to −0.25 | 2898 | 29 | 0.42 | |
| 90 | −0.52 | −0.77 to −0.31 | 6113 | 20 | 0.57 | |
| 75 * | −0.19 | −0.32 to −0.07 | 3202 | 10 | 0.55 | |
| IPO | 100 | −0.43 | −0.65 to −0.20 | 164 | 109 | 0.11 |
| 99 | −0.54 | −0.77 to −0.30 | 1335 | 56 | 0.27 | |
| 95 | −0.77 | −1.06 to −0.49 | 37,501 | 22 | 0.59 | |
| 90 | −0.65 | −0.97 to −0.33 | 39,592 | 12 | 0.62 | |
| 75 | −0.71 | −1.12 to −0.30 | 142,217 | 5 | 0.79 | |
| NAO | 100 | −0.30 | −0.48 to −0.11 | 83 | 122 | 0.07 |
| 99 | −0.47 | −0.63 to −0.31 | 514 | 84 | 0.29 | |
| 95 | −0.53 | −0.69 to −0.36 | 1485 | 54 | 0.43 | |
| 90 | −0.59 | −0.77 to −0.41 | 3354 | 36 | 0.55 | |
| 75 | −0.53 | −0.75 to −0.31 | 7315 | 14 | 0.66 | |
| Shannon-Diversity Index | Global | SWPO | SWAO | MED | IPO | NAO |
|---|---|---|---|---|---|---|
| Overall | 5.08 | 4.03 | 3.38 | 4.28 | 3.30 | 4.75 |
| After 1% | 5.02 | 3.97 | 3.35 | 4.19 | 3.26 | 4.68 |
| After 5% | 4.78 | 3.74 | 3.20 | 3.98 | 3.17 | 4.24 |
| After 10% | 4.58 | 3.60 | 3.15 | 3.75 | 3.07 | 3.91 |
| After 25% | 3.90 | na | na | na | na | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laraib, M.; Titocci, J.; Giannakourou, A.; Reizopoulou, S.; Basset, A. Role of Rare Species on Phytoplankton Size–Abundance Relationships and Size Structure across Different Biogeographical Areas. Diversity 2024, 16, 98. https://doi.org/10.3390/d16020098
Laraib M, Titocci J, Giannakourou A, Reizopoulou S, Basset A. Role of Rare Species on Phytoplankton Size–Abundance Relationships and Size Structure across Different Biogeographical Areas. Diversity. 2024; 16(2):98. https://doi.org/10.3390/d16020098
Chicago/Turabian StyleLaraib, Maira, Jessica Titocci, Antonia Giannakourou, Sofia Reizopoulou, and Alberto Basset. 2024. "Role of Rare Species on Phytoplankton Size–Abundance Relationships and Size Structure across Different Biogeographical Areas" Diversity 16, no. 2: 98. https://doi.org/10.3390/d16020098
APA StyleLaraib, M., Titocci, J., Giannakourou, A., Reizopoulou, S., & Basset, A. (2024). Role of Rare Species on Phytoplankton Size–Abundance Relationships and Size Structure across Different Biogeographical Areas. Diversity, 16(2), 98. https://doi.org/10.3390/d16020098






