Predicting the Future Distribution of Leucobryum aduncum under Climate Change
Abstract
1. Introduction
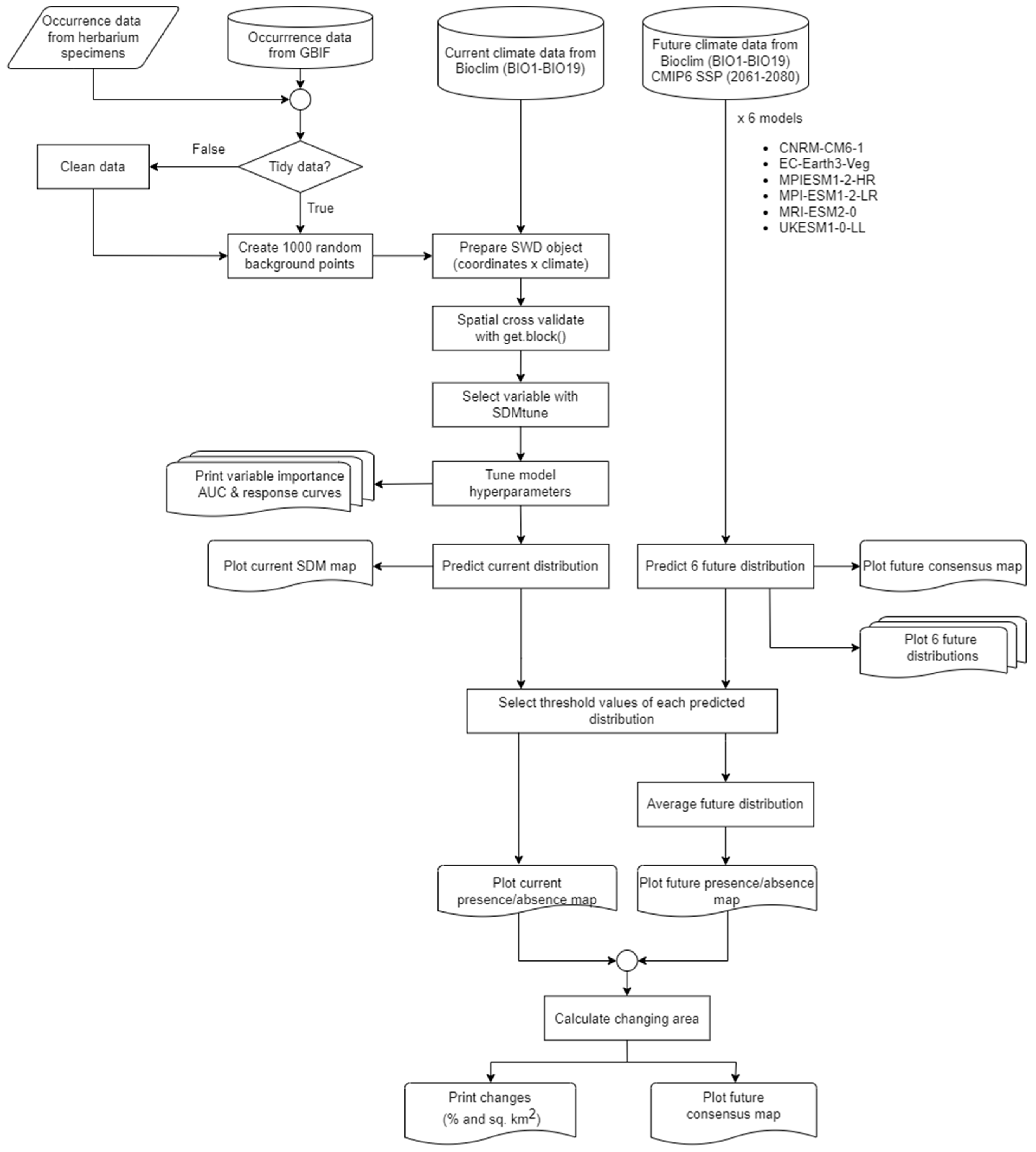
2. Materials and Methods
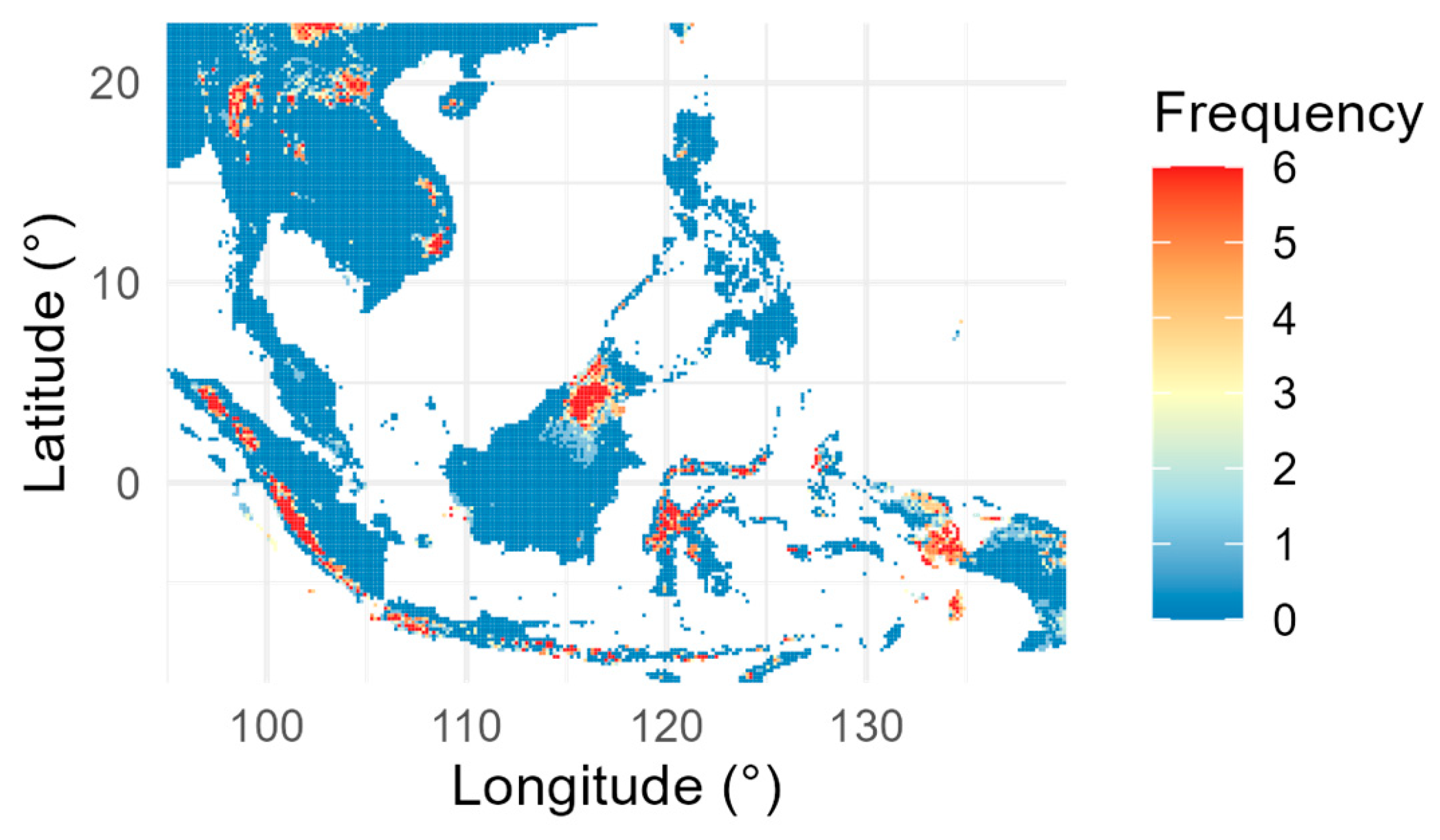
2.1. Data Acquisition
2.2. Current Distribution Modeling
2.3. Predicting Future Distribution
3. Results
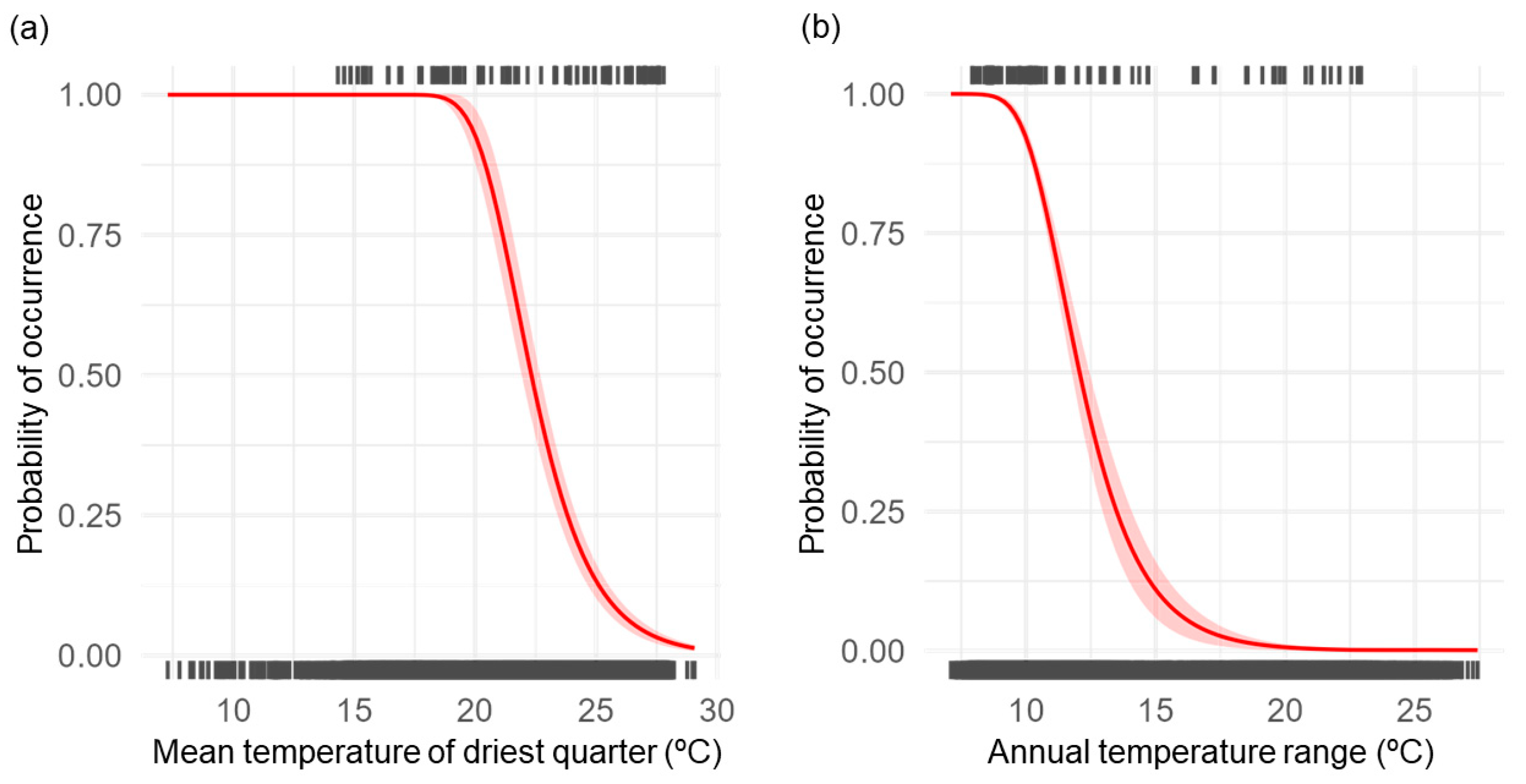
3.1. Selection of Climate Variables
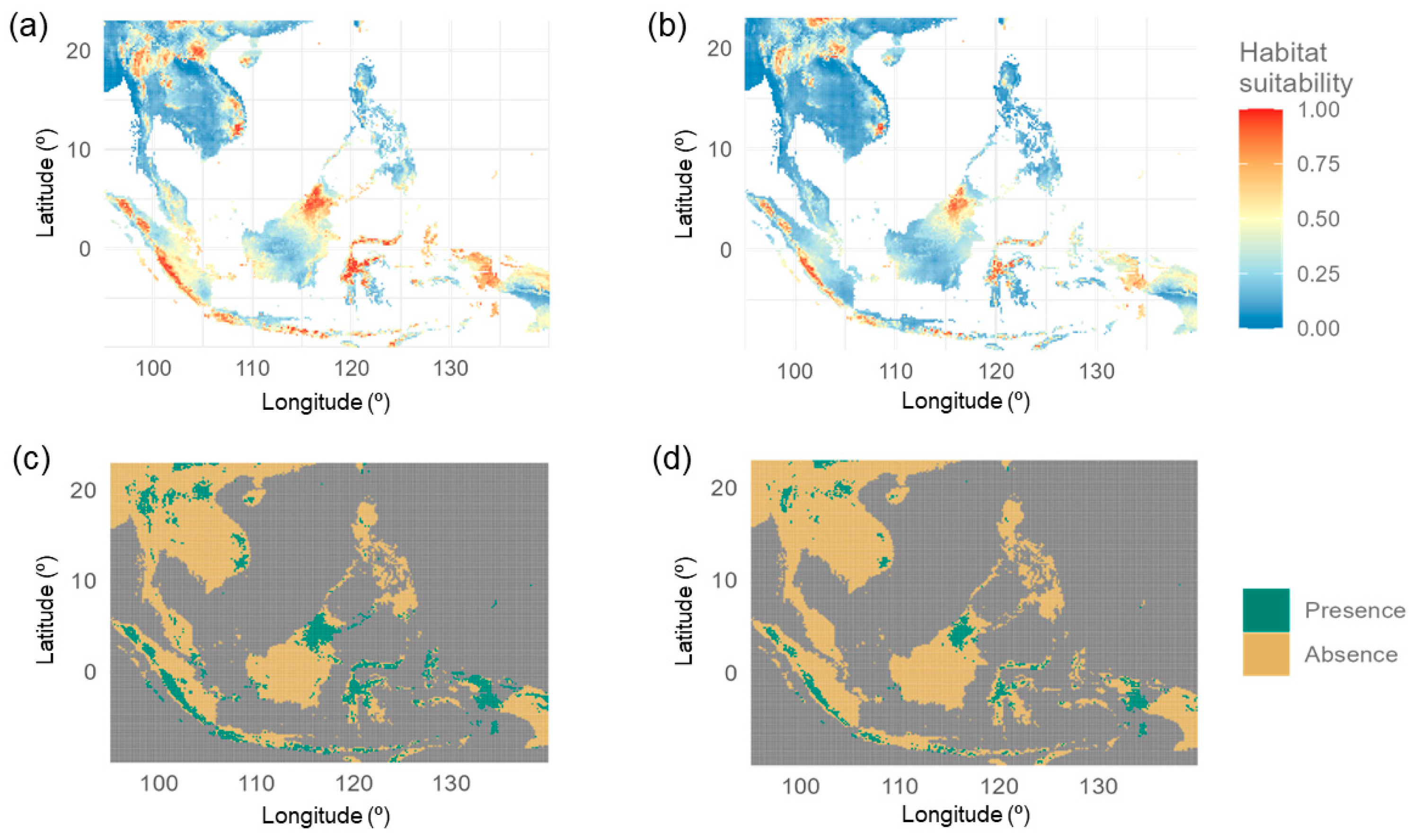
3.2. Current and Future Distribution
3.3. Changes in Future Distribution
4. Discussion
4.1. Predicted Distribution of L. aduncum
4.2. Temperature and L. aduncum Distribution
4.3. Other Important Variables for Bryophyte Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Zhang, Z.; Wang, Z. Bryophyte communities as biomonitors of environmental factors in the Goujiang karst bauxite, southwestern China. Sci. Total Environ. 2015, 538, 270–278. [Google Scholar] [CrossRef]
- Frahm, J.P.; Trustees, S.P. Mosses and Liverworts of Mt. Kinabalu; Sabah Parks Trustees: Kota Kinabalu, Malaysia, 1990. [Google Scholar]
- Kostka, J.E.; Weston, D.J.; Glass, J.B.; Lilleskov, E.A.; Shaw, A.J.; Turetsky, M.R. The Sphagnum microbiome: New insights from an ancient plant lineage. New Phytol. 2016, 211, 57–64. [Google Scholar] [CrossRef]
- Song, L.; Ma, W.-Z.; Yao, Y.-L.; Liu, W.-Y.; Li, S.; Chen, K.; Lu, H.-Z.; Cao, M.; Sun, Z.-H.; Tan, Z.-H.; et al. Bole bryophyte diversity and distribution patterns along three altitudinal gradients in Yunnan, China. J. Veg. Sci. 2015, 26, 576–587. [Google Scholar] [CrossRef]
- Sandhi, A.; Landberg, T.; Greger, M. Phytofiltration of arsenic by aquatic moss (Warnstorfia fluitans). Environ. Pollut. 2018, 237, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Aranda, S.C.; Gabriel, R.; Borges, P.A.V.; Santos, A.M.C.; De Azevedo, E.B.; Patiño, J.; Hortal, J.; Lobo, J.M. Geographical, temporal and environmental determinants of bryophyte species richness in the Macaronesian Islands. PLoS ONE 2014, 9, e101786. [Google Scholar] [CrossRef] [PubMed]
- Bes, M.; Corbera, J.; Sayol, F.; Bagaria, G.; Jover, M.; Preece, C.; Viza, A.; Sabater, F.; Fernández-Martínez, M. On the influence of water conductivity, pH and climate on bryophyte assemblages in Catalan semi-natural springs. J. Bryol. 2018, 40, 149–158. [Google Scholar] [CrossRef]
- Chen, S.-B.; Ferry Slik, J.W.; Gao, J.; Mao, L.-F.; Bi, M.-J.; Shen, M.-W.; Zhou, K.-X. Latitudinal diversity gradients in bryophytes and woody plants: Roles of temperature and water availability. J. Syst. Evol. 2015, 53, 535–545. [Google Scholar] [CrossRef]
- Medina, N.G.; Albertos, B.; Lara, F.; Mazimpaka, V.; Garilleti, R.; Draper, D.; Hortal, J. Species richness of epiphytic bryophytes: Drivers across scales on the edge of the Mediterranean. Ecography 2014, 37, 80–93. [Google Scholar] [CrossRef]
- Yamaguchi, T. A revision of the genus Leucobryum (Musci) in Asia. J. Hattori Bot. Lab. 1993, 73, 1–123. [Google Scholar] [CrossRef]
- Sukkharak, P.; Chantanaorrapint, S. Bryophyte studies in Thailand: Past, present, and future. Cryptogam. Bryol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Bang-Juan, L.; He, S. Leucobryaceae. In Moss Flora of China; Chien, G., Crosby, M., He, S., Eds.; Missouri Botanical Garden: St. Louis, MO, USA, 1999; Volume 1. [Google Scholar]
- Norhazrina, N.; Nadirah, I.; Syazwana, N.; Asyifaa, S.; Maideen, H.M.K. The moss flora of Ulu Gombak Forest Reserve, Gombak, Selangor, Malaysia. The 2018 UKM FST Postgraduate Colloquium. In Proceedings of the Universiti Kebangsaan Malaysia, Faculty of Science and Technology 2018 Postgraduate Colloquium, Selangor, Malaysia, 4–6 April 2018. [Google Scholar]
- Tiwutanon, P.; Chaiyasut, K.; Lumbsch, H.T.; Kraichak, E. Resurrection of Leucobryumscalare Müll.Hal. ex M.Fleisch. (Bryophyta, Leucobryaceae) based on phylogenetic and morphometric evidence. PhytoKeys 2023, 222, 27–47. [Google Scholar] [CrossRef]
- Kou, J.; Wang, T.; Yu, F.; Sun, Y.; Feng, C.; Shao, X. The moss genus Didymodon as an indicator of climate change on the Tibetan Plateau. Ecol. Indic. 2020, 113, 106204. [Google Scholar] [CrossRef]
- Wen, A.; Wu, T.; Zhu, X.; Li, R.; Wu, X.; Chen, J.; Qiao, Y.; Ni, J.; Ma, W.; Li, X.; et al. Changes in the spatial distribution of Bryophytes on the Qinghai–Tibet Plateau under CMIP6 future projections. Environ. Earth Sci. 2021, 81, 15. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Jagerbrand, A.K.; Erfanian, M.B.; Chen, S.; Sun, S.Q.; Molau, U. Bryophyte cover and richness decline after 18 years of experimental warming in alpine Sweden. AoB Plants 2020, 12, plaa061. [Google Scholar] [CrossRef]
- Zanatta, F.; Engler, R.; Collart, F.; Broennimann, O.; Mateo, R.G.; Papp, B.; Munoz, J.; Baurain, D.; Guisan, A.; Vanderpoorten, A. Bryophytes are predicted to lag behind future climate change despite their high dispersal capacities. Nat. Commun. 2020, 11, 5601. [Google Scholar] [CrossRef] [PubMed]
- Wierzcholska, S.; Dyderski, M.K.; Jagodziński, A.M. Potential distribution of an epiphytic bryophyte depends on climate and forest continuity. Glob. Planet. Change 2020, 193, 103270. [Google Scholar] [CrossRef]
- Cong, M.; Jian, M.; Xu, Y.; Tang, L.; Li, J.; Yang, W.; Zhu, Y. Geographical distribution and migration routes of the medical bryophyte, Climacium dendroides, under climate warming in China. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2021, 156, 663–670. [Google Scholar] [CrossRef]
- Désamoré, A.; Laenen, B.; Stech, M.; Papp, B.; Hedenäs, L.; Mateo, R.G.; Vanderpoorten, A. How do temperate bryophytes face the challenge of a changing environment? Lessons from the past and predictions for the future. Glob. Change Biol. 2012, 18, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Merinero, S.; Dahlberg, C.J.; Ehrlen, J.; Hylander, K. Intraspecific variation influences performance of moss transplants along microclimate gradients. Ecology 2020, 101, e02999. [Google Scholar] [CrossRef] [PubMed]
- Cerrejón, C.; Valeria, O.; Mansuy, N.; Barbé, M.; Fenton, N.J. Predictive mapping of bryophyte richness patterns in boreal forests using species distribution models and remote sensing data. Ecol. Indic. 2020, 119, 106826. [Google Scholar] [CrossRef]
- Kruijer, H.J.D.; Raes, N.; Stech, M. Modelling the distribution of the moss species Hypopterygium tamarisci (Hypopterygiaceae, Bryophyta) in Central and South America. Nova Hedwig. 2010, 91, 399–420. [Google Scholar] [CrossRef]
- Enroth, J. The Bryophytes of Sabah (North Borneo) with Special Reference to the BRYOTROP Transect of Mount Kinabalu. IV. Leucobryaceae (Bryopsida). Willdenowia 1989, 18, 529–554. [Google Scholar]
- Ligrone, R. Studies on the leaf structure in some species of Leucobryaceae. III. Leucobryum sanctum (Brid.) Hampe and Leucobryum sp. (Leucobryeae). J. Bryol. 2013, 13, 411–416. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G.; Warton, D. blockCV: An r package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Methods Ecol. Evol. 2018, 10, 225–232. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P.; McPherson, J. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2023, 13, 2. [Google Scholar]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R package to tune and evaluate species distribution models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, S.; Luo, H.; Zhi, X.; Wang, H. Future changes in precipitation extremes over Southeast Asia: Insights from CMIP6 multi-model ensemble. Environ. Res. Lett. 2021, 16, 024013. [Google Scholar] [CrossRef]
- Iqbal, Z.; Shahid, S.; Ahmed, K.; Ismail, T.; Ziarh, G.F.; Chung, E.-S.; Wang, X. Evaluation of CMIP6 GCM rainfall in mainland Southeast Asia. Atmos. Res. 2021, 254, 105525. [Google Scholar] [CrossRef]
- Kamworapan, S.; Bich Thao, P.T.; Gheewala, S.H.; Pimonsree, S.; Prueksakorn, K. Evaluation of CMIP6 GCMs for simulations of temperature over Thailand and nearby areas in the early 21st century. Heliyon 2021, 7, e08263. [Google Scholar] [CrossRef]
- Supharatid, S.; Aribarg, T.; Nafung, J. Bias-corrected CMIP6 climate model projection over Southeast Asia. Theor. Appl. Climatol. 2022, 147, 669–690. [Google Scholar] [CrossRef]
- Fricko, O.; Havlik, P.; Rogelj, J.; Klimont, Z.; Gusti, M.; Johnson, N.; Kolp, P.; Strubegger, M.; Valin, H.; Amann, M.; et al. The marker quantification of the Shared Socioeconomic Pathway 2: A middle-of-the-road scenario for the 21st century. Glob. Environ. Change 2017, 42, 251–267. [Google Scholar] [CrossRef]
- Belote, R.T.; Carroll, C.; Martinuzzi, S.; Michalak, J.; Williams, J.W.; Williamson, M.A.; Aplet, G.H. Assessing agreement among alternative climate change projections to inform conservation recommendations in the contiguous United States. Sci. Rep. 2018, 8, 9441. [Google Scholar] [CrossRef]
- Sporn, S.G.; Bos, M.M.; Hoffstätter-Müncheberg, M.; Kessler, M.; Gradstein, S.R. Microclimate determines community composition but not richness of epiphytic understory bryophytes of rainforest and cacao agroforests in Indonesia. Funct. Plant Biol. 2009, 36, 171–179. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Lindblad, K.E.M.; Björk, R.G.; Alatalo, J.M.; Molau, U. Bryophyte and Lichen Diversity Under Simulated Environmental Change Compared with Observed Variation in Unmanipulated Alpine Tundra. Biodivers. Conserv. 2006, 15, 4453–4475. [Google Scholar] [CrossRef]
- Lang, S.I.; Cornelissen, J.H.C.; Shaver, G.R.; Ahrens, M.; Callaghan, T.V.; Molau, U.; Ter Braak, C.J.F.; Hölzer, A.; Aerts, R. Arctic warming on two continents has consistent negative effects on lichen diversity and mixed effects on bryophyte diversity. Glob. Change Biol. 2012, 18, 1096–1107. [Google Scholar] [CrossRef]
- Glime, J.M. Chapter 7—Water Relations. In Bryophyte Ecology Volume 1: Physiological Ecology; Michigan Technological University: Houghton, MI, USA, 2017. [Google Scholar]
- Bates, J.W. Growth of Leucobryum glaucum cushions In a Berkshire oakwood. J. Bryol. 1989, 15, 785–791. [Google Scholar] [CrossRef]
- Číhal, L.; Kaláb, O.; Plášek, V. Modeling the distribution of rare and interesting moss species of the family Orthotrichaceae (Bryophyta) in Tajikistan and Kyrgyzstan. Acta Soc. Bot. Pol. 2017, 86, 1–15. [Google Scholar] [CrossRef][Green Version]
- Vanderpoorten, A.; Goffinet, B. Physiological ecology. In Introduction to Bryophytes; Cambridge University Press: Cambridge, UK, 2009; pp. 185–213. [Google Scholar]
- Proctor, M.C.F. Physiological ecology. In Bryophyte Biology, 2nd ed.; Shaw, A.J., Goffinet, B., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 237–268. [Google Scholar]
- Rydin, H. Population and community ecology of bryophytes. In Bryophyte Biology, 2nd ed.; Shaw, A.J., Goffinet, B., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 393–444. [Google Scholar]
- Glime, J.M. Chapter 10—Temperature. In Bryophyte Ecology Volume 1: Physiological Ecology; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Gignac, L.D. Bryophytes as Predictors of Climate Change. In Bryophyte Ecology and Climate Change; Stark, L.R., Slack, N.G., Tuba, Z., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 461–482. [Google Scholar]

| Abbreviation | Variable | Permutation Importance | SD |
|---|---|---|---|
| bio9 | Mean temperature of the driest quarter | 34.98 | 5.54 |
| bio7 | Annual Temperature range (bio5–bio6) | 32.50 | 4.14 |
| bio18 | Precipitation of the warmest quarter | 9.93 | 5.31 |
| bio2 | Mean diurnal range (mean of monthly (max temp.–min temp.)) | 8.08 | 3.36 |
| bio12 | Annual precipitation | 7.70 | 2.98 |
| bio5 | Max temperature of the warmest month | 6.88 | 2.63 |
| Frequency | Area (km2) | Percentage |
|---|---|---|
| 0 | 3,803,472.00 | 82.49 |
| 1 | 215,050.40 | 4.66 |
| 2 | 120,246.20 | 2.61 |
| 3 | 81,702.23 | 1.77 |
| 4 | 91,205.94 | 1.98 |
| 5 | 109,874.70 | 2.38 |
| 6 | 189,227.40 | 4.10 |
| Class | Current Distribution | Future Distribution | ||
|---|---|---|---|---|
| km2 | Percentage | km2 | Percentage | |
| Absence | 3,805,821.00 | 82.54 | 4,198,314.00 | 91.07 |
| Presence | 804,957.50 | 17.46 | 411,563.30 | 8.93 |
| Class | Area | Percentage of Area Compared to Current Presence | |
|---|---|---|---|
| km2 | Percentage | ||
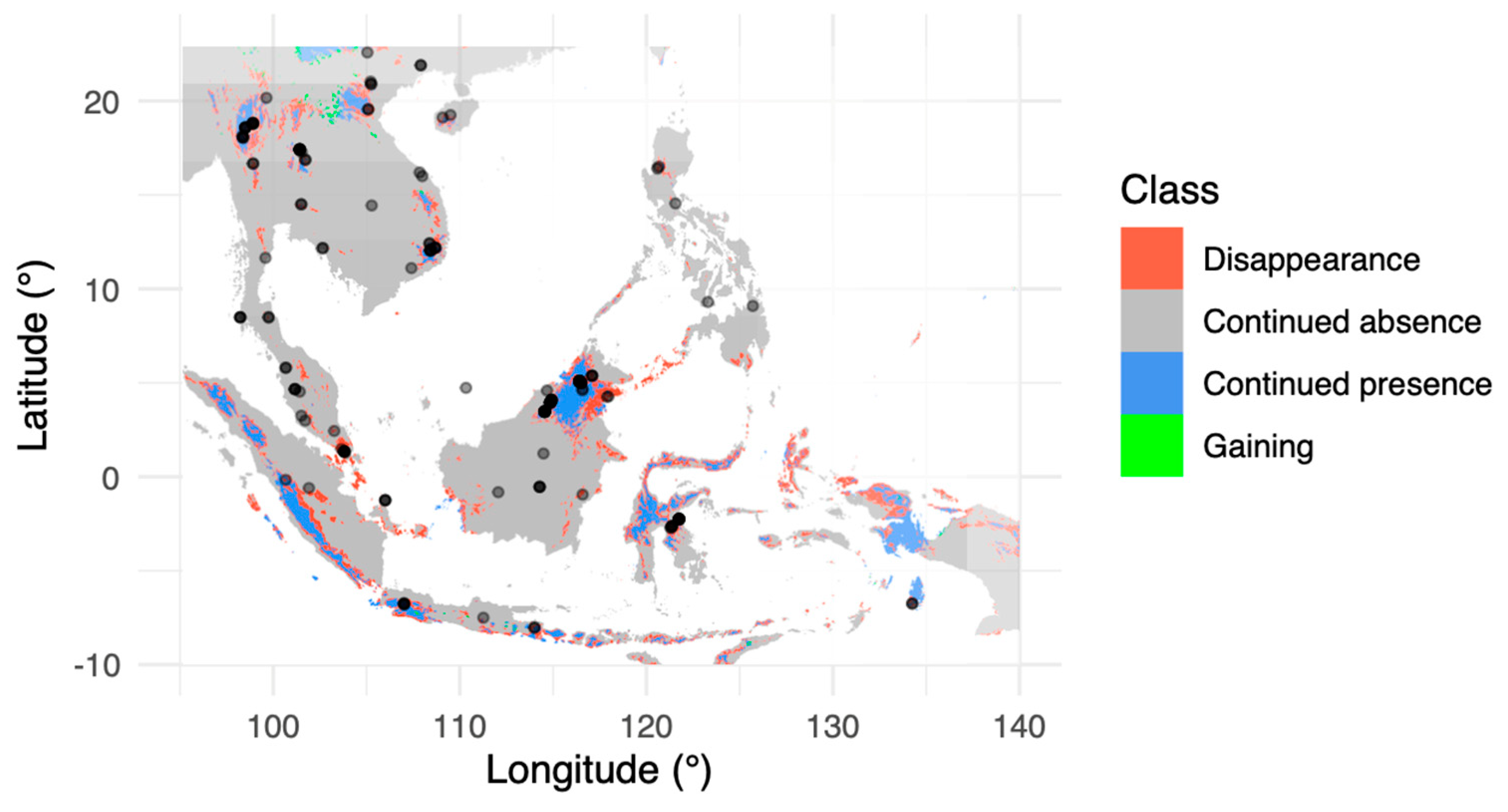
| disappearance | 403,669.80 | 8.76 | 50.16 |
| continued absence | 3,794,644.00 | 82.32 | - |
| continued presence | 401,130.30 | 8.70 | 49.84 |
| gaining | 10,432.99 | 0.23 | 1.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chawengkul, P.; Tiwutanon, P.; Sanevas, N.; Kraichak, E. Predicting the Future Distribution of Leucobryum aduncum under Climate Change. Diversity 2024, 16, 125. https://doi.org/10.3390/d16020125
Chawengkul P, Tiwutanon P, Sanevas N, Kraichak E. Predicting the Future Distribution of Leucobryum aduncum under Climate Change. Diversity. 2024; 16(2):125. https://doi.org/10.3390/d16020125
Chicago/Turabian StyleChawengkul, Puwadol, Patsakorn Tiwutanon, Nuttha Sanevas, and Ekaphan Kraichak. 2024. "Predicting the Future Distribution of Leucobryum aduncum under Climate Change" Diversity 16, no. 2: 125. https://doi.org/10.3390/d16020125
APA StyleChawengkul, P., Tiwutanon, P., Sanevas, N., & Kraichak, E. (2024). Predicting the Future Distribution of Leucobryum aduncum under Climate Change. Diversity, 16(2), 125. https://doi.org/10.3390/d16020125





