Abstract
Although growing urbanization has direct negative consequences for local biodiversity, several native species have been observed maintaining populations in urban environments. Understanding which factors influence the ability of native species to persist in urban environments is crucial, both for the study of biological adaptation and of urban planning. The quantification of the proportion of juvenile individuals can be a good proxy for assessing the long-term persistence of urban populations. We present comparative data about spatial and temporal variations in the age-class structure in two suburban and two forest populations of the Cuban endemic lizard Anolis homolechis, obtained during a 20-month survey. We found a four-fold lower proportion of juveniles in the suburban habitat compared to the forest one. There was, however, no evidence for differential female fecundity between the two habitats, as assessed by the proportion of gravid females. Conversely, the rate of tail autotomy (an antipredator behavior) was significantly higher in the suburban juveniles compared to the forest ones, possibly reflecting a higher exposure to predators and, particularly, inter- and intraspecific cannibalism. However, tail loss at initial capture or habitat type had no effect on the probability of recapture of juveniles. We discuss the potential causes and consequences of a modified age-class structure in urbanized environments.
1. Introduction
Growing urbanization is a worldwide phenomenon [1] that has direct negative consequences for local biodiversity [2,3]. Urbanization most often leads to the replacement of specialist species with generalist ones, resulting in biotic homogenization [4,5,6,7,8,9]. In addition, urbanization globally favors alien invasive species to the detriment of native ones [10,11,12,13]. However, several native species have been found to be able to maintain populations, or even flourish, in urban environments [14,15,16,17,18], including some of interest to conservation [19,20,21]. Understanding which factors favor or limit the ability of native species to persist in urban environments is of high importance, both for the study of biological adaptation and urban planning.
So far, research has largely focused on phenotypic differences between urban and rural populations or on phenotypic variation along a gradient of urbanization. A large number of studies, addressing various taxonomic groups, have shown that individuals from urban populations often differ markedly in terms of behavior, physiology, and morphology from their rural counterparts [22,23,24,25,26]. In addition, some studies have provided evidence that species cope with urbanization through behavioral adjustments [27,28] or thanks to preadapted inherent traits [29,30].
Less is known, in contrast, of the variation in demographic success and population dynamics among urban and non-urban populations of native species (see [31,32,33]). Although a precise quantification of demographic parameters in different populations may require an important research effort, the estimation of the age-class distribution, such as the proportion of juvenile individuals, provides a suitable and frequently used proxy, integrating reproduction and early juvenile survival in the population [34,35,36]. Such a parameter is of high importance in demographic and population viability studies [37,38], as well as for species conservation and management [39,40,41]. In particular, differences in age-class structure between populations can be indicative of differences in habitat quality [42] and population trends [43]. Urban and non-urban environments may differ in several dimensions, such as resource availability [44,45], predation risk [46,47], or ambient temperature [48,49], with potential effects on individual growth, age-specific rates of mortality, and fecundity, ultimately affecting age-class structure. Surprisingly, so far, variation in the age-class structure between urban and non-urban populations has received little attention [50,51].
Species of the genus Anolis provide a good biological model to investigate to what extent the age-class structure differs between urban and non-urban populations. This group has been extensively studied, particularly in the insular Caribbean, where species diversity was shown to result from adaptive radiation and adaptation to various ecosystems and microhabitats through morphological and behavioral differentiation [52]. In addition, several Anolis species have successfully adapted to urban and suburban areas [22,53]. Studies of urban anoles have addressed a wide range of topics, such as morphological differentiation [54,55], physiology [56,57], parasite load [58], habitat use [59,60], and behavior [61,62].
Here, we present new and original data about the spatial and temporal variation in the age-class structure among populations of the Cuban endemic A. homolechis. This medium-sized trunk anole is common and widespread in Cuba and can be found in natural habitats as well as anthropized ones [63]. Recently, Vidal et al. (2022), using a nested design, provided evidence for morphological differences between suburban and forest populations of A. homolechis [64]. In addition, the same study reported a significantly male-biased adult sex ratio in suburban populations, whereas the sex ratio was balanced in forest populations. We provide additional analyses on the variation in the proportion of juveniles among individuals captured at the same two suburban and same two forest sites. To document potential sources of variation in the age-class structure between suburban and forest sites, we also examined the variation in the proportion of gravid females and in the rate of tail autotomy in juveniles (as a proxy for predation risk in lizards [65,66,67,68]). As A. homolechis is a seasonal breeder [63], we expected both the proportion of juveniles and that of gravid females to vary between the dry (November–April) and the wet (May–October) season [69,70].
2. Materials and Methods
2.1. Study Sites and Data Collection
Monthly sampling took place at two suburban sites and two forest sites over a 20-month period, from January 2018 to August 2019. The suburban sites were at the limit of Guanajay City and San José de Las Lajas City, both with similar urban development. The natural sites were located in relatively well-preserved forests of the Reserve of the Biosphere Sierra del Rosario and the Natural Protected Landscape Escaleras de Jaruco (see [64,71] for details). The main potential predators of A. homolechis observed in the suburban sites were cats, dogs, domestic chickens, wild birds, and reptiles (including other Anolis species), whereas in the forest sites, they mainly corresponded to birds, reptiles (including other Anolis species), and large arthropods.
During each sampling session, we captured individuals following an established path of about 500 m along tree-lined streets and forest pathways. Captured individuals were sexed, aged, measured for body size (snout-vent length, SVL), and the incidence of tail autotomy was registered as described in [64]. As female A. homolechis typically lay a single-egg clutch [72], we considered the proportion of gravid females in each population as a measure of mean female fecundity. We determined female reproductive status (gravid vs. non-gravid) in the field through belly palpation. We subsequently classified females as adults or juveniles based on the minimal SVL we recorded for a gravid female. Juveniles males were recognized by the presence of postanal scales [73] when visible and distinguished from adult and subadult males by the lack of a development of the dewlap [74]. Following Calsbeek and Irschick (2007), we injected elastomeric implants (Visible Implant Elastomers, Northwest Marine Technology, Inc., Anacortes, WA, USA) under the ventral skin of the limbs of captured individuals [75], combining various colors with the four limbs, to allow for subsequent individual recognition on recapture [52]. Colored marks were not conspicuous, thus minimizing the risk of increased predation or interference with normal behavior during interspecific interactions. After capture and processing, we released the lizards at the exact location where they had been captured.
2.2. Statistical Analysis
As we could not mark some juveniles because of their very small size, we used data on the age-class of individuals at the time of first capture to assess the variation in the proportion of juveniles. To that end, we performed a logistic regression with age-class (juveniles/adult; subadult males were classed as adults) as the response variable and habitat (suburban/forest), site (nested within habitat), season (wet/dry), the interactions between season and habitat, and the interactions between season and site (nested within habitat) as factors.
In order to assess factors affecting the probability of females being gravid or not, we build a general linear mixed model (GLMM) for binomial data, with females’ identity as a random factor (as some females were recaptured more than once) and habitat, site (nested within habitat), and season as explanatory variables. Because body size may influence the percentage of gravid females in lizard populations [76,77], we added the covariable SVL to the model. Second- and third-order interactions were also included in the model.
We tested the effect of habitat, site (nested within habitat), and season on juveniles’ tail autotomy rate (presence/absence of evidence of tail breakage at the first capture) using a logistic regression model. SVL was included in the model as a proxy for the age of individuals, as older individuals should have experienced more predation attempts [78,79].
Finally, to assess the effect of urbanization on juvenile apparent survival, we used a logistic regression with the probability of whether or not a juvenile was recaptured as a response variable and habitat and site (nested within habitat) as explanatory variables. We excluded juveniles that were too small to be marked and those captured for the first time during the last sampling session from the analysis. We added tail autotomy rate and SVL to the model as explanatory variables, both at the first capture, as tail autotomy is supposed to affect survival [80] and SVL has a positive effect on recapture probability [81]. Second- and third-order interactions were also included in the model.
Linearity was confirmed for all models by inspecting diagnostic graphs of residuals and fitted values. All models were simplified using a backward elimination of non-significant variables, and the results from the simplified models are presented. When the simplest model was the null one, the values for all tested explanatory variables in the additive model are provided. Confidence intervals for means were computed through bootstrapping (10,000 simulations). All analyses were conducted using the R programming language, version 4.0. [82]. The results of the tests were considered significant at the 0.05 level.
3. Results
We captured 168 juveniles and 1048 adults (16.03% of juveniles), distributed between suburban (22 juveniles and 449 adults) and forest habitats (146 juveniles and 599 adults), and wet (86 juveniles and 586 adults) and dry seasons (82 juveniles and 462 adults) (see Appendix A for sample size per site). Habitat (likelihood ratio χ21,1214 = 68.39, p < 0.001) and season (χ21,1214 = 7.96, p = 0.005) were retained in the simplest model as significant effects on the proportion of juveniles captured, but not site (nested within habitat). The differences corresponded to a four-fold decrease in the proportion of juveniles in suburban habitats (4.67%) compared to forest habitats (19.59%) and about a 18% increase in the dry season (15.07%) compared to the wet season (12.79%; Figure 1). Removing the 11 smallest and unmarked juveniles from the data set did not affect the model selection and results.
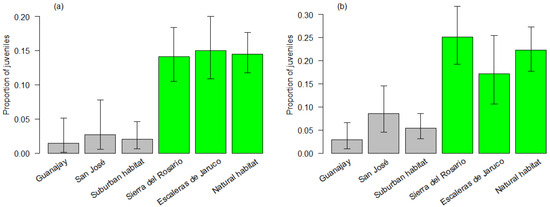
Figure 1.
Proportions of juvenile Anolis homolechis captured in each sampled site and habitat type (gray: suburban habitat, green: forest habitat) during (a) the wet season (May–October) and (b) the dry season (November–April), from January 2018 to August 2019. Whiskers show confidence intervals (95%) for each proportion.
Table 1 shows the reproductive status of 307 different females, of which 50 individuals were captured on more than one occasion (2–5 recaptures). The simplest model retained season (GLMM: likelihood ratio χ21,363 = 42.12, p < 0.001) and SVL (χ21,363 = 26.02, p < 0.001) as significant effects, but not habitat and site (nested within habitat), thus indicating that urbanization and site characteristics did not affect females’ gravidity. The probability of females being gravid increased during the wet season (wet vs. dry season: slope = 1.84, confidence interval, CIslope 0.91 to 2.77) and with female body size (SVL: slope = 0.24, CIslope 0.08 to 0.40).

Table 1.
Variation in the percentage of gravid individuals (%G) among female Anolis homolechis captured in suburban and forest sites during the wet (May–October) and dry (November–April) seasons from January 2018 to August 2019 (N = sample size; n = number of different individuals).
We observed tail autotomy in 31 juveniles. The probability of tail loss was significantly affected by habitat (likelihood ratio χ21,166 = 8.69, p = 0.003) and SVL (χ21,166 = 4.67, p = 0.03), whereas site (nested within habitat) and season were not retained in the simplest model. Overall, 36.36% (8 of 22) of the suburban juveniles experienced tail autotomy compared to 15.75% (23 of 146) in the forest juveniles (Figure 2). Contrary to our expectation, tail autotomy decreased with increasing size in juveniles (SVL: slope = −0.10, CIslope −0.20 to −0.01).
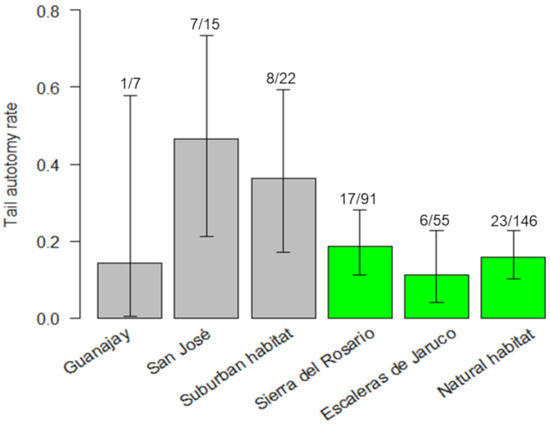
Figure 2.
Proportions of juvenile Anolis homolechis with evidence of tail autotomy for each sampled site and habitat type (gray: suburban habitat, green: forest habitat). Whiskers show confidence intervals (95%) for each proportion. Values above bars show the number of individuals with autotomized tails over the total number of juveniles.
We recaptured 24 juveniles out of 142 marked before the last sampling session. None of the tested explanatory variables had an effect on the probability of recapture (likelihood ratio test, habitat: χ21,140 = 0.01, p = 0.90; site (nested within habitat): χ22,140 = 0.56, p = 0.75; tail autotomy: χ21,140 = 0.21, p = 0.65; SVL: χ21,140 = 0.11, p = 0.74).
4. Discussion
This is, to the best of our knowledge, the first study to document the variation in the proportion of juveniles between urbanized and natural populations of anoles. We observed a significantly and consistent lower proportion of juveniles in the suburban populations of A. homolechis compared to the forest ones. Using a nested design allowed us to quantitatively assess the effect of urbanization, independently of chance events or differences that may exist between sites, with no relation to their degree of urbanization [83]. We are therefore confident that the marked difference between the two habitats is ecologically meaningful.
We observed only a slight, albeit significant, seasonal variation in the proportion of juveniles, with, surprisingly, higher values in the dry season compared to the wet season. Unfortunately, little information is available on the temporal variation in the proportion of juveniles in other Anolis species, such that comparisons with previous studies are very limited. Opposite to our results, Andrews and Wright (1994) reported a higher abundance of juveniles during the wet season than during the dry season in the neotropical and relatively short-lived A. limifrons [84]. However, patterns of rainfall in our study area (northwestern Cuba) are characterized by heavier rainfall during the dry season over the last 40 years compared to other parts of the country [85], such that the contrast between the dry and wet season might be less pronounced there. Future studies are, however, necessary to better document the seasonal variation in age-class structure among populations of Anolis species, particularly in relation with climate change.
The observed difference in age-class structure between the suburban and forest populations is open to alternative explanations. On the one hand, it may result from a reduced adult survivorship in forest populations compared to suburban ones. However, the results from a capture–mark–recapture study conducted at the same sites failed to show any significant effect of habitat on adult survival rate [81]. On the other hand, the differences in age-class structure may reflect habitat-related differences in female fecundity and/or in juvenile mortality. Whereas we did not detect any significant effect of habitat type on the proportion of gravid females, there was a marked seasonal effect on female fecundity, with a much higher proportion of gravid females during the wet season compared to the dry season, as observed in other anoles species [86,87,88]. Nevertheless, this did not translate into a higher proportion of juveniles during the wet season. This may be due to the longer incubation time with relatively cooler temperatures during the wet season or to the fact that that newly hatched juveniles are more difficult to detect in the field. In addition, juvenile quality, and hence survival, improves late in the wet season (see [89]), possibly contributing to the higher proportion of juveniles in the following months corresponding to the dry season.
The difference in age-class structure between the two habitats might then be caused by higher predation risk in the suburban sites. Suburban habitats, with reduced vegetation cover and more impervious surfaces than natural areas [90,91,92], are less complex than natural habitats and may therefore offer fewer refuges for prey, making juveniles particularly exposed to predation risk. Accordingly, the proportion of juvenile A. homolechis showing tail autotomy in the suburban habitat was more than twice that in the forest habitat. However, interpreting the significance of tail loss in relation to predation pressure and survival is not straightforward. Balakrishna et al. (2021) observed that although urban males of the tropical lizard Psamnophilus dorsalis had greater tail loss than rural males, predation risk (assessed from incidences of attacks on artificial models of lizards) did not differ between the two habitats [68]. On the other hand, Koenig et al. (2002) found that domestic cats killed mainly juvenile lizards, especially following parturition, in suburban populations of bluetongue lizards, Tiliqua scincoides [93]. Bateman and Fleming (2011) argued, however, that tail autotomy in the brown anole A. sagrei would not necessarily reflect predation rate but rather the ability of individuals to escape predation attempts by more or less efficient predators [94]. The study was based on observations of the rate of tail loss between sites with pet cats (fed by pet owners and supposedly less efficient), sites with feral cats (supposedly more efficient), and sites with no cats. Accordingly, we did not record the presence of feral or domestic cats at our forest study sites, whereas they were relatively abundant at the two suburban sites. However, unlike central Florida where the study by Bateman and Fleming (2011) took place [94], domestic cats in Cuba are rarely fed by pet owners and do actively hunt for prey. Moreover, the rates of tail autotomy in the juvenile A. homolechis observed in the present study were well above that reported by Bateman and Fleming (2011) for A. sagrei, in which tail loss was observed in only 1% of juveniles [94]. In addition, contrary to our expectations, the rate of tail loss was negatively related to the body size of juveniles, possibly indicative of attacks by predators of a much smaller size than cats, such as large arthropods [95,96,97], snakes, or other lizard species [98]. Capizzi et al. (2008) observed an increasing proportion of lizards in the diet of two Mediterranean snakes along a gradient of habitat alteration [99], and, recently, Rodríguez-Cabrera and Hernández Gómez (2021) provided evidence for predation by urban snakes upon anoles in Cuba [100]. However, the frequency of encounters with snakes and large arthropods during our field study was higher in the forest habitat than in the suburban one.
The lower proportion of juveniles in the suburban environment could actually be related to more intense cannibalism in the suburban environment. Both inter- and intraspecific cannibalism on juveniles have been evidenced in several anole species [101,102,103], including A. homolechis (A. Vidal, personal observation). For instance, juveniles of native anole species tend to be disproportionately rare in areas where the invasive A. sagrei is abundant [101]. Interestingly, we noted a higher abundance of A. sagrei in the suburban sites compared with the forest ones during our study. As prey diversity might be lower for reptiles in urbanized environments [104], cannibalism may constitute an alternative foraging strategy for adult anoles at our suburban study sites. Indeed, resource availability often determines the intensity of cannibalism, with potential consequences on the age-class structure, particularly in the case of size-dependent cannibalism [105]. In addition, cannibalism might be an adaptive strategy in populations invading new environments [106] (see also [107]). Further investigations into the variation in levels of inter- and intraspecific cannibalism between urban and non-urban populations of Anolis species are needed to test this hypothesis.
More to the point, irrespective of its origin, tail autotomy can incur fitness costs to lizards, such as increased vulnerability to predators, possibly due to reduced locomotor performance [108,109]. This was not directly confirmed in the present study, as we failed to find any influence of tail loss or habitat on the probability of recapturing marked juveniles. This negative result should, however, be taken with caution, as we were unable to mark the smallest juveniles (which could be more vulnerable to predation attempts, as suggested by the negative relationship between tail loss and body size of juveniles) and because the probability of recapture is affected by both survival and permanent emigration outside of the study area. Juveniles with autotomized tails may thus have a lower probability of survival such that juveniles with intact tails (because they have fled from predators or because they have not encountered predators) have a higher survival probability and, thus, and can grow to larger sizes. A more detailed study of juvenile survival in A. homolechis relying on capture–mark–recapture analysis with weekly or shorter intervals between capture sessions would be more appropriate to test for the effect of tail loss and habitat on juvenile survival.
Independently of predation, other factors may contribute to explaining the lower proportion of juveniles in the suburban environment. For instance, urbanized environments often have reduced canopy cover and more heat-absorbing surfaces, resulting in higher mean ambient and ground temperatures and higher maximum temperatures in urban areas compared to natural ones, including tropical areas [110], with potential effects on reproductive success. Indeed, Tiatragul et al. (2019) showed that suburban nest sites of anoles were warmer and drier with greater thermal variance than forest ones [111]. Recent evidence in A. sagrei [112] suggests that the so-called “urban heat island effect” [48] may increase egg mortality and alter embryonic development in urban anoles, although its ultimate effect on the age-class structure has not been addressed yet. Similarly, pathogen transmission among reptiles previously occupying natural habitats could be enhanced by urbanization [22,113]. For instance, wall lizards, Podarcis muralis, show higher parasite loads in urban areas compared to in rural areas [114]. Although evidence from a wide range of host taxa suggests that juveniles are almost always more susceptible to pathogens than adults [115], the consequences of pathogen transmission on the age-structure of urban populations of vertebrates has received little attention.
The generality of the contrasted age-class structure between urban and non-urban populations of anoles, as well as its causes and consequences, deserves further consideration. Differences in age-class structure may have numerous implications, particularly in terms of population dynamics and stability [38]. In that respect, comparing the proportion of juveniles between urban and non-urban populations of Anolis species and other lizard species may contribute to a better understanding of the ability of native species to persist in urbanized areas.
Author Contributions
Conceptualization, A.V. and F.C.; Data curation, A.V.; Formal analysis, A.V. and F.C.; Funding acquisition F.C.; Investigation A.V.; Methodology, A.V. and F.C.; Project administration, A.V.; Resources, A.V.; Software, A.V.; Supervision, F.C.; Visualization, A.V.; Writing—original draft, A.V. and F.C.; Writing—review and editing, A.V. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Caribaea Initiative (www.Caribaea.org), through a PhD fellowship to A.V., and through the projects “Conservación y uso sostenible de la biodiversidad biológica en los ecosistemas montañosos Guamuhaya y Guaniguanico bajo un enfoque paisajístico” (code: P211LH005-008) and “Colecciones Zoológicas, su conversación y manejo III, del Programa Ramal de Ciencia y Técnica, Diversidad Biológica (2018–2020)”, both managed by the Instituto de Ecología y Sistemática.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this published paper.
Acknowledgments
We thank the Administration of the Sierra del Rosario Biosphere Reserve and the Ranch of Lázaro Medina and Miriam Llanes in Escaleras de Jaruco for allowing us a free stay in their facilities during the surveys. We also thank Manuel Iturriaga, Adonis González Carralero, Carlos Hernández Peraza, Héctor M. Díaz Perdomo, Alejandro García Montano, Yaira López Hurtado, Hansel Caballero Silva, Anaisa Cajigas Gandia, Rachel Batista Alvarez, Claudia Vega Catalá, J. Deyvis Viera García, Armando R. Longueira Loyola, Maylín Rodríguez Rubial, Gustavo Blanco Vale, and Tatiana Homar García for their help with the captures. Access to restricted areas of Sierra del Rosario, as well as the capture, marking, and handling of animals, were carried out with the agreement of the Director of the Sierra del Rosario Biosphere Reserve, Fidel Hernández Figueroa, and the Environmental License 2019/01 of the Oficina de Regulación y Seguridad Ambiental, Ministerio de Ciencia, Tecnología y Medio Ambiente of Cuba. We thank two anonymous referees for useful and constructive comments on an earlier version.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Age-class and the percentage of juveniles (%J) of Anolis homolechis captured in suburban and forest sites during dry (November–April) and wet season (May–October) from January 2018 to August 2019.
| Wet Season | Dry Season | Total | ||||||||
| Juveniles | Adults | %J | Juveniles | Adults | %J | Juveniles | Adults | %J | ||
| Suburban sites | ||||||||||
| Guanajay | 2 | 97 | 2.02 | 5 | 137 | 3.52 | 7 | 234 | 2.90 | |
| San José de las Lajas | 3 | 96 | 3.03 | 12 | 119 | 9.16 | 15 | 215 | 6.52 | |
| Forest sites | ||||||||||
| Sierra del Rosario | 43 | 217 | 16.53 | 48 | 128 | 27.27 | 91 | 345 | 20.87 | |
| Escaleras de Jaruco | 38 | 176 | 17.76 | 17 | 78 | 17.89 | 55 | 254 | 17.80 | |
References
- Angel, S.; Parent, J.; Civco, D.L.; Blei, A.; Potere, D. The Dimensions of Global Urban Expansion: Estimates and Projections for All Countries, 2000–2050. Prog. Plan. 2011, 75, 53–107. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, Biodiversity, and Conservation. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Elmqvist, T.; Fragkias, M.; Goodness, J.; Güneralp, B.; Marcotullio, P.J.; McDonald, R.I.; Parnell, S.; Schewenius, M.; Sendstad, M.; Seto, K.C.; et al. Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities; Springer: New York, NY, USA, 2013; ISBN 9789400770881. [Google Scholar]
- McKinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 7, 247–260. [Google Scholar] [CrossRef]
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From Patterns to Emerging Processes in Mechanistic Urban Ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sol, D.; González-Lagos, C.; Moreira, D.; Maspons, J.; Lapiedra, O. Urbanisation Tolerance and the Loss of Avian Diversity. Ecol. Lett. 2014, 17, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Devictor, V.; Julliard, R.; Couvet, D.; Lee, A.; Jiguet, F. Functional Homogenization Effect of Urbanization on Bird Communities. Conserv. Biol. 2007, 21, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Luck, G.W.; Smallbone, L.T. The Impact of Urbanization on Taxonomic and Functional Similarity among Bird Communities. J. Biogeogr. 2011, 38, 894–906. [Google Scholar] [CrossRef]
- Knop, E. Biotic Homogenization of Three Insect Groups Due to Urbanization. Glob. Chang. Biol. 2016, 22, 228–236. [Google Scholar] [CrossRef]
- Chace, J.F.; Walsh, J.J. Urban Effects on Native Avifauna: A Review. Landsc. Urban Plan. 2006, 74, 46–69. [Google Scholar] [CrossRef]
- van Rensburg, B.J.; Peacock, D.S.; Robertson, M.P. Biotic Homogenization and Alien Bird Species along an Urban Gradient in South Africa. Landsc. Urban Plan. 2009, 92, 233–241. [Google Scholar] [CrossRef]
- Gaertner, M.; Wilson, J.R.U.; Cadotte, M.W.; MacIvor, J.S.; Zenni, R.D.; Richardson, D.M. Non-Native Species in Urban Environments: Patterns, Processes, Impacts and Challenges. Biol. Invasions 2017, 19, 3461–3469. [Google Scholar] [CrossRef]
- Santana Marques, P.; Resende Manna, L.; Clara Frauendorf, T.; Zandonà, E.; Mazzoni, R.; El-Sabaawi, R. Urbanization Can Increase the Invasive Potential of Alien Species. J. Anim. Ecol. 2020, 89, 2345–2355. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, S.; Batabyal, A.; Thaker, M. Dining in the City: Dietary Shifts in Indian Rock Agamas across an Urban-Rural Landscape. J. Herpetol. 2016, 50, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.; Rocha, F.L.; Cordeiro-Estrela, P. Bats in Urban Areas of Brazil: Roosts, Food Resources and Parasites in Disturbed Environments. Urban Ecosyst. 2017, 20, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Rebolo-Ifrán, N.; Tella, J.L.; Carrete, M. Urban Conservation Hotspots: Predation Release Allows the Grassland-Specialist Burrowing Owl to Perform Better in the City. Sci. Rep. 2017, 7, 3527. [Google Scholar] [CrossRef]
- Suri, J.; Sumasgutner, P.; Hellard, É.; Koeslag, A.; Amar, A. Stability in Prey Abundance May Buffer Black Sparrowhawks Accipiter Melanoleucus from Health Impacts of Urbanization. Ibis 2017, 159, 38–54. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Lan, S.; Zhang, Q.; Chen, S. Common Blackbirds Turdus Merula Use Anthropogenic Structures as Nesting Sites in an Urbanized Landscape. Curr. Zool. 2015, 61, 435–443. [Google Scholar] [CrossRef]
- Exantus, J.M.; Beaune, D.; Cézilly, F. The Relevance of Urban Agroforestry and Urban Remnant Forest for Avian Diversity in a Densely-Populated Developing Country: The Case of Port-Au-Prince, Haiti. Urban For. Urban Green. 2021, 63, 127217. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities Are Hotspots for Threatened Species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Soanes, K.; Lentini, P.E. When Cities Are the Last Chance for Saving Species. Front. Ecol. Environ. 2019, 17, 225–231. [Google Scholar] [CrossRef]
- French, S.S.; Webb, A.C.; Hudson, S.B.; Virgin, E.E. Town and Country Reptiles: A Review of Reptilian Responses to Urbanization. Integr. Comp. Biol. 2018, 58, 948–966. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, K.; Gallo, T. Behavior Change in Urban Mammals: A Systematic Review. Front. Ecol. Evol. 2020, 8, 576665. [Google Scholar] [CrossRef]
- Gomez, G.S.M.Y.; Van Dyck, H. Ecotypic Differentiation between Urban and Rural Populations of the Grasshopper Chorthippus brunneus Relative to Climate and Habitat Fragmentation. Oecologia 2012, 169, 125–133. [Google Scholar] [CrossRef]
- Eggenberger, H.; Frey, D.; Pellissier, L.; Ghazoul, J.; Fontana, S.; Moretti, M. Urban Bumblebees Are Smaller and More Phenotypically Diverse than Their Rural Counterparts. J. Anim. Ecol. 2019, 88, 1522–1533. [Google Scholar] [CrossRef]
- Patankar, S.; Jambhekar, R.; Suryawanshi, K.R.; Nagendra, H. Which Traits Influence Bird Survival in the City? A Review. Land 2021, 10, 92. [Google Scholar] [CrossRef]
- Lowry, H.; Lill, A.; Wong, B.B.M. Behavioural Responses of Wildlife to Urban. Biol. Rev. 2012, 88, 537–549. [Google Scholar] [CrossRef]
- Moule, H.; Michelangeli, M.; Thompson, M.B.; Chapple, D.G. The Influence of Urbanization on the Behaviour of an Australian Lizard and the Presence of an Activity-Exploratory Behavioural Syndrome. J. Zool. 2016, 298, 103–111. [Google Scholar] [CrossRef]
- Walsh, S.; Goulet, C.T.; Wong, B.B.M.; Chapple, D.G. Inherent Behavioural Traits Enable a Widespread Lizard to Cope with Urban Life. J. Zool. 2018, 306, 189–196. [Google Scholar] [CrossRef]
- Garitano-Zavala, Á.; Calbimonte, R.; Esteve-Herraiz, G. The Behavioral Responses of the Chiguanco Thrush to Urbanization in a Neotropical City Comes from Preadapted Behavioral Traits. Front. Ecol. Evol. 2022, 10, 830902. [Google Scholar] [CrossRef]
- McGowan, K.J. Demographic and Behavioral Comparisons of Suburban and Rural American Crows. In Avian Ecology and Conservation in an Urbanizing World; Marluff, J.M., Bowman, R., Donelly, R., Eds.; Kluwer Academic Press: Norwell, MA, USA, 2001; pp. 365–381. [Google Scholar]
- Evans, B.S.; Ryder, T.B.; Reitsma, R.; Hurlbert, A.H.; Marra, P.P. Characterizing Avian Survival along a Rural-to-Urban Land Use Gradient. Ecology 2015, 96, 1631–1640. [Google Scholar] [CrossRef]
- Gould, N.P.; Powell, R.; Olfenbuttel, C.; Deperno, C.S. Growth and Reproduction by Young Urban and Rural Black Bears. J. Mammal. 2021, 102, 1165–1173. [Google Scholar] [CrossRef]
- Tkadlec, E.; Zejda, J. Small Rodent Population Fluctuations: The Effects of Age Structure and Seasonality. Evol. Ecol. 1998, 12, 191–210. [Google Scholar] [CrossRef]
- Hille, S.M.; Rödel, H.G. Small-Scale Altitudinal Effects on Reproduction in Bank Voles. Mamm. Biol. 2014, 79, 90–95. [Google Scholar] [CrossRef]
- Nolet, B.A.; Schreven, K.H.T.; Boom, M.P.; Lameris, T.K. Contrasting Effects of the Onset of Spring on Reproductive Success of Arctic-Nesting Geese. Auk 2019, 137, ukz063. [Google Scholar] [CrossRef]
- Ruggiero, L.F.; Hayward, G.D.; Squires, J.R. Viability Analysis in Biological Evaluations: Concepts of Population Viability Analysis, Biological Population, and Ecological Scale. Conserv. Biol. 1994, 8, 364–372. [Google Scholar] [CrossRef]
- Hoy, S.R.; MacNulty, D.R.; Smith, D.W.; Stahler, D.R.; Lambin, X.; Peterson, R.O.; Ruprecht, J.S.; Vucetich, J.A. Fluctuations in Age Structure and Their Variable Influence on Population Growth. Funct. Ecol. 2020, 34, 203–216. [Google Scholar] [CrossRef]
- Maguire, L.A.; Wilhere, G.F.; Dong, Q. Population Viability Analysis for Red-Cockaded Woodpeckers in the Georgia Piedmont. J. Wildl. Manag. 1995, 59, 533–542. [Google Scholar] [CrossRef]
- Luís, C.; Rebelo, R.; Brito, J.C.; Godinho, R.; Paulo, O.S.; Crespo, E.G. Age Structure in Lacerta Schreiberi from Portugal. Amphib. Reptil. 2004, 25, 336–343. [Google Scholar]
- Dubey, S.; Sinsch, U.; Dehling, M.J.; Chevalley, M.; Shine, R. Population Demography of an Endangered Lizard, the Blue Mountains Water Skink. BMC Ecol. 2013, 13, 4. [Google Scholar] [CrossRef]
- Bruton, M.J.; McAlpine, C.A.; Maron, M. Regrowth Woodlands Are Valuable Habitat for Reptile Communities. Biol. Conserv. 2013, 165, 95–103. [Google Scholar] [CrossRef]
- Tutterow, A.M.; Graeter, G.J.; Pittman, S.E. Bog Turtle Demographics within the Southern Population. Copeia 2017, 105, 293–300. [Google Scholar] [CrossRef]
- Harvey, J.A.; Chernicky, K.; Simons, S.R.; Verrett, T.B.; Chaves, J.A.; Knutie, S.A. Urban Living Influences the Nesting Success of Darwin’s Finches in the Galápagos Islands. Ecol. Evol. 2021, 11, 5038–5048. [Google Scholar] [CrossRef]
- Hubert, P.; Julliard, R.; Biagianti, S.; Poulle, M.L. Ecological Factors Driving the Higher Hedgehog (Erinaceus europeaus) Density in an Urban Area Compared to the Adjacent Rural Area. Landsc. Urban Plan. 2011, 103, 34–43. [Google Scholar] [CrossRef]
- Fischer, J.D.; Cleeton, S.H.; Lyons, T.P.; Miller, J.R. Urbanization and the Predation Paradox: The Role of Trophic Dynamics in Structuring Vertebrate Communities. Bioscience 2012, 62, 809–818. [Google Scholar] [CrossRef]
- Lehrer, E.W.; Schooley, R.L.; Whittington, J.K. Survival and Antipredator Behavior of Woodchucks (Marmota monax) along an Urban-Agricultural Gradient. Can. J. Zool. 2012, 90, 12–21. [Google Scholar] [CrossRef]
- Arnfield, A.J. Two Decades of Urban Climate Research: A Review of Turbulence, Exchanges of Energy and Water, and the Urban Heat Island. Int. J. Climatol. 2003, 23, 1–26. [Google Scholar] [CrossRef]
- Rizwan, A.M.; Dennis, L.Y.C.; Liu, C. A Review on the Generation, Determination and Mitigation of Urban Heat Island. J. Environ. Sci. 2008, 20, 120–128. [Google Scholar] [CrossRef]
- Kozlovsky, D.Y.; Jarjour, C.A.; Morand-Ferron, J. Urbanization Is Associated with Differences in Age Class Structure in Black-Capped Chickadees (Poecile atricapillus). Urban Ecosyst. 2021, 24, 405–416. [Google Scholar] [CrossRef]
- Gómez Villafañe, I.E.; Cavia, R.; Vadell, M.V.; Suárez, O.V.; Busch, M. Differences in Population Parameters of Rattus Norvegicus in Urban and Rural Habitats of Central Argentina. Mammalia 2013, 77, 187–193. [Google Scholar] [CrossRef]
- Losos, J.B. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles; University of California Press: Berkeley, CA, USA, 2009; ISBN 9780520943735. [Google Scholar]
- Powell, R.; Henderson, R.W. Urban Herpetology in the West Indies. In Urban Herpetology; Mitchell, J.C., Jung Brown, R.E., Bartholomew, B., Eds.; Society for the Study of Amphibians and Reptiles: Salt Lake City, UT, USA, 2008; pp. 389–404. [Google Scholar]
- Irschick, D.J.; Carlisle, E.; Elstrott, J.; Ramos, M.; Buckley, C.; Vanhooydonck, B.; Meyers, J.; Herrel, A. A Comparison of Habitat Use, Morphology, Clinging Performance and Escape Behaviour among Two Divergent Green Anole Lizard (Anolis carolinensis) Populations. Biol. J. Linn. Soc. 2005, 85, 223–234. [Google Scholar] [CrossRef]
- Winchell, K.M.; Reynolds, R.G.; Prado-Irwin, S.R.; Puente-Rolón, A.R.; Revell, L.J. Phenotypic Shifts in Urban Areas in the Tropical Lizard Anolis cristatellus. Evolution 2016, 70, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M.; Warner, D.A. Thermal Tolerance in the Urban Heat Island: Thermal Sensitivity Varies Ontogenetically and Differs between Embryos of Two Sympatric Ectotherms. J. Exp. Biol. 2019, 222, jeb210708. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Staton, S.C.; Winchell, K.M.; Rochette, N.C.; Fredette, J.; Maayan, I.; Schweizer, R.M.; Catchen, J. Parallel Selection on Thermal Physiology Facilitates Repeated Adaptation of City Lizards to Urban Heat Islands. Nat. Ecol. Evol. 2020, 4, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Thawley, C.J.; Moniz, H.A.; Merritt, A.J.; Battles, A.C.; Michaelides, S.N.; Kolbe, J.J. Urbanization Affects Body Size and Parasitism but Not Thermal Preferences in Anolis Lizards. J. Urban Ecol. 2019, 5, juy031. [Google Scholar] [CrossRef]
- Kolbe, J.J.; Battles, A.C.; Avilés-Rodríguez, K.J. City Slickers: Poor Performance Does Not Deter Anolis Lizards from Using Artificial Substrates in Human-Modified Habitats. Funct. Ecol. 2016, 30, 1418–1429. [Google Scholar] [CrossRef]
- Winchell, K.M.; Carlen, E.J.; Puente-Rolón, A.R.; Revell, L.J. Divergent Habitat Use of Two Urban Lizard Species. Ecol. Evol. 2018, 8, 25–35. [Google Scholar] [CrossRef]
- Chejanovski, Z.A.; Avilés-Rodríguez, K.J.; Lapiedra, O.; Preisser, E.L.; Kolbe, J.J. An Experimental Evaluation of Foraging Decisions in Urban and Natural Forest Populations of Anolis Lizards. Urban Ecosyst. 2017, 20, 1011–1018. [Google Scholar] [CrossRef]
- Lapiedra, O.; Chejanovski, Z.; Kolbe, J.J. Urbanization and Biological Invasion Shape Animal Personalities. Glob. Chang. Biol. 2017, 23, 592–603. [Google Scholar] [CrossRef]
- Rodríguez Schettino, L. The Iguanid Lizards of Cuba; University of Florida Press: Gainesville, FL, USA, 1999. [Google Scholar]
- Vidal, A.; Iturriaga, M.; Mancina, C.A.; Cézilly, F. Differences in Sex Ratio, Tail Autotomy, Body Size and Body Condition between Suburban and Forest Populations of the Cuban Endemic Lizard Anolis homolechis. Urban Ecosyst. 2022, 25, 1711–1723. [Google Scholar] [CrossRef]
- Arnold, E.N. Caudal Autotomy as a Defense. Biol. Reptil. 1987, 16, 235–273. [Google Scholar]
- Diego-Rasilla, F.J. Influence of Predation Pressure on the Escape Behaviour of Podarcis muralis Lizards. Behav. Process. 2003, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.E.; Pérez-Mellado, V.; Vitt, L.J. Ease and Effectiveness of Costly Autotomy Vary with Predation Intensity among Lizard Populations. J. Zool. 2004, 262, 243–255. [Google Scholar] [CrossRef]
- Balakrishna, S.; Amdekar, M.S.; Thaker, M. Morphological Divergence, Tail Loss, and Predation Risk in Urban Lizards. Urban Ecosyst. 2021, 24, 1391–1398. [Google Scholar] [CrossRef]
- Ortíz Bultó, L.P.; Pérez Rodríguez, A.; Rivero Valencia, A.; León Vega, N.; Díaz Gonzalez, M.; Pérez Carrera, A. Assessment of Human Health Vulnerability to Climate Variability and Change in Cuba. Environ. Health Perspect. 2006, 114, 1942–1949. [Google Scholar] [CrossRef]
- Almaguer, M.; Aira, M.-J.; Rodríguez-Rajo, F.J.; Rojas, T. Temporal Dynamics of Airborne Fungi in Havana (Cuba) during Dry and Rainy Seasons: Influence of Meteorological Parameters. Integr. J. Biometeorol. 2013, 58, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Pradel, R.; Cézilly, F. Do Suburban Populations of Lizards Behave Differently from Forest Ones? An Analysis of Perch Height, Time Budget, and Display Rate in the Cuban Endemic Anolis homolechis. Diversity 2023, 15, 261. [Google Scholar] [CrossRef]
- Meiri, S.; Avila, L.; Bauer, A.M.; Chapple, D.G.; Das, I.; Doan, T.M.; Doughty, P.; Ellis, R.; Grismer, L.; Kraus, F.; et al. The Global Diversity and Distribution of Lizard Clutch Sizes. Glob. Ecol. Biogeogr. 2020, 29, 1515–1530. [Google Scholar] [CrossRef]
- Barbour, T.; Ramsden, C.T. The Herpetology of Cuba. Mem. Museum Comp. Zool. 1919, 42, 71–213. [Google Scholar] [CrossRef][Green Version]
- Williams, E.E.; Rand, A.S. Species Recognition, Dewlap Function, and Faunal Size. Am. Zool. 1977, 17, 261–270. [Google Scholar] [CrossRef]
- Calsbeek, R.; Irschick, D.J. The Quick and the Dead: Correlational Selection on Morphology, Performance, and Habitat Use in Island Lizards. Evolution 2007, 61, 2493–2503. [Google Scholar] [CrossRef]
- Allan, G.M.; Prelypchan, C.J.; Gregory, P.T. Population Profile of an Introduced Species, the Common Wall Lizard (Podarcis muralis), on Vancouver Island, Canada. Can. J. Zool. 2006, 84, 51–57. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Zhang, Z.; Xu, X. Sexual Size Dimorphism and Female Reproduction in the White-Striped Grass Lizard Takydromus wolteri. Curr. Zool. 2012, 58, 236–243. [Google Scholar] [CrossRef]
- Lovely, K.R.; Mahler, D.L.; Revell, L.J. The Rate and Pattern of Tail Autotomy in Five Species of Puerto Rican Anoles. Evol. Ecol. Res. 2010, 12, 67–88. [Google Scholar]
- Tyler, A.R.K.; Winchell, K.M.; Revell, L.J. Tails of the City: Caudal Autotomy in the Tropical Lizard, Anolis cristatellus, in Urban and Natural Areas of Puerto Rico. J. Herpetol. 2016, 50, 435–441. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. To Cut a Long Tail Short: A Review of Lizard Caudal Autotomy Studies Carried out over the Last 20 Years. J. Zool. 2009, 277, 1–14. [Google Scholar] [CrossRef]
- Vidal, A.; Cézilly, F.; Pradel, R. Contemporary Survival Selection Fails to Explain Observed Patterns of Phenotypic Divergence between Suburban and Forest Populations of the Cuban Endemic Lizard, Anolis homolechis. in preparation.
- R CoreTeam. R: A Language and Environment for Statistical Computing. 2023. Available online: http://www.R-project.org (accessed on 20 December 2023).
- Hurlbert, S.H. Pseudoreplication and the Design of Ecological Field Experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Andrews, R.M.; Wright, S.J. Long-Term Population Fluctuations of a Tropical Lizard: A Test of Causality. In Lizard Ecology; Vitt, L.J., Pianka, E.R., Eds.; Princeton University Press: Princeton, NJ, USA, 1994; pp. 267–285. [Google Scholar]
- Alvarez-Socorro, G.; Fernández-Alvarez, J.C.; Sorí, R.; Pérez-Alarcón, A.; Nieto, R.; Gimeno, L. Space-Time Assessment of Extreme Precipitation in Cuba between 1980 and 2019 from Multi-Source Weighted-Ensemble Precipitation Dataset. Atmosphere 2021, 12, 995. [Google Scholar] [CrossRef]
- Jenssen, T.A.; Nunez, S.C. Male and Female Reproductive Cycles of the Jamaican Lizard, Anolis opalinus. Copeia 1994, 3, 767–780. [Google Scholar] [CrossRef]
- Ramírez-Bautista, A.; Vitt, L.J. Reproduction in the Lizard Anolis nebulosus (Polychrotidae) from the Pacific Coast of Mexico. Herpetologica 1997, 53, 423–431. [Google Scholar]
- Domínguez, M.; Sanz, A.; Almaguer, N.; Chávez, J. Seasonal Reproduction in Males of the Cuban Lizard Anolis lucius (Polychrotidae). Bol. Soc. Herpetol. Mex 2006, 14, 1–19. [Google Scholar]
- Pearson, P.R.; Warner, D.A. Early Hatching Enhances Survival despite Beneficial Phenotypic Effects of Lateseason Developmental Environments. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180256. [Google Scholar] [CrossRef]
- Forman, R.T.T. Urban Ecology: Science of Cities; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Stroud, J.T.; Colom, M.; Ferrer, P.; Palermo, N.; Vargas, V.; Cavallini, M.; Lopez, J.; Jones, I. Behavioral Shifts with Urbanization May Facilitate Biological Invasion of a Widespread Lizard. Urban Ecosyst. 2019, 22, 425–434. [Google Scholar] [CrossRef]
- Hahs, A.K.; McDonnell, M.J.; McCarthy, M.A.; Vesk, P.A.; Corlett, R.T.; Norton, B.A.; Clemants, S.E.; Duncan, R.P.; Thompson, K.; Schwartz, M.W.; et al. A Global Synthesis of Plant Extinction Rates in Urban Areas. Ecol. Lett. 2009, 12, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.; Shine, R.; Glenn, S. The Dangers of Life in the City: Patterns of Activity, Injury and Mortality in Suburban Lizards (Tiliqua scincoides). J. Herpetol. 2002, 36, 62–68. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Frequency of Tail Loss Reflects Variation in Predation Levels, Predator Efficiency, and the Behaviour of Three Populations of Brown Anoles. Biol. J. Linn. Soc. 2011, 103, 648–656. [Google Scholar] [CrossRef][Green Version]
- Jehle, R.; Franz, A.; Kapfer, M.; Schramm, H.; Tunner, H.G. Lizards as Prey of Arthropods: Praying Mantis Mantis religiosa (Linnaeus, 1758) Feeds on Juvenile Sand Lizard Lacerta agilis Linnaeus, 1758. Herpetozoa 1996, 9, 157–159. [Google Scholar]
- Hernández, E.F.; Rodríguez-Cabrera, T.M. Predation on a Cuban Brown Anole, Anolis sagrei (Dactyloidae), by a Spider, Cupiennius Cubae (Ctenidae), in the Cienfuegos Botanical Garden, South-Central Cuba. Reptil. Amphib. 2014, 21, 98–99. [Google Scholar] [CrossRef]
- Maffei, F.; Ubaid, F.K.; Jim, J. Predation of Herps by Spiders (Araneae) in the Brazilian Cerrado. Herpetol. Notes 2010, 3, 167–170. [Google Scholar]
- Losos, J.B.; Schoener, T.W.; Spiller, D.A. Predator-Induced Behaviour Shifts and Natural Selection in Field-Experimental Lizard Populations. Nature 2004, 432, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, D.; Capula, M.; Rugiero, L.; Luiselli, L. Dietary Patterns of Two Sympatric Mediterranean Snakes along a Gradient of Habitat Alteration. Herpetol. J. 2008, 18, 141–146. [Google Scholar]
- Rodríguez-Cabrera, T.M.; Hernández Gómez, A. New Prey Records for Two Snakes of the Genus Tropidophis (Tropidophiidae) from Urban Habitats in La Habana, Cuba. Reptil. Amphib. 2021, 28, 512–515. [Google Scholar] [CrossRef]
- Gerber, G.P.; Echternacht, A.C. Evidence for Asymmetrical Intraguild Predation between Native and Introduced Anolis Lizards. Oecologia 2000, 124, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Cates, C.D.; Delaney, D.M.; Buckelew, A.M.; Durso, A.M.; French, S.S.; Reedy, A.M.; Warner, D.A. Anolis sagrei (Brown anole). Cannibalism. Herpetol. Rev. 2014, 45, 491. [Google Scholar]
- De Armas, L.F. Predation of Cuban Brown Anoles, Anolis sagrei (Squamata: Dactyloidae), by House Sparrows, Passer Domesticus (Aves: Passeriformes), and an Annotated List of Lizards Preyed upon by House Sparrows. Reptil. Amphib. 2021, 28, 432–434. [Google Scholar] [CrossRef]
- Wolfe, A.K.; Bateman, P.W.; Fleming, P.A. Does Urbanization Influence the Diet of a Large Snake? Curr. Zool. 2018, 64, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Claessen, D.; de Roos, A.M.; Persson, L. Population Dynamic Theory of Size-Dependent Cannibalism. Proc. R. Soc. Lond. B 2004, 271, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Cupul-Magaña, F.G.; González-Santillán, E.; Rodríguez-López, E.; Bueno-Villegas, J.; Verdín-Huerta, L.E. First Record of Parental Care in the Scolopendrid Centipede Hemiscolopendra marginata (Say, 1821) from Mexico (Scolopendromorpha: Scolopendridae). Pan-Pac. Entomol. 2018, 94, 1–3. [Google Scholar] [CrossRef]
- DeVore, J.L.; Crossland, M.R.; Shine, R.; Ducatez, S. The Evolution of Targeted Cannibalism and Cannibal-Induced Defenses in Invasive Populations of Cane Toads. Proc. Natl. Acad. Sci. USA 2021, 118, e2100765118. [Google Scholar] [CrossRef]
- Fox, S.F.; Mccoy, K. The Effects of Tail Loss on Survival, Growth, Reproduction, and Sex Ratio of Offspring in the Lizard Uta stansburiana in the Field. Oecologia 2000, 122, 327–334. [Google Scholar] [CrossRef]
- Downes, S.; Shine, R. Why Does Tail Loss Increase a Lizard’s Later Vulnerability to Snake Predators? Ecology 2001, 82, 1293–1303. [Google Scholar] [CrossRef]
- Marcotullio, P.J.; Keßler, C.; Quintero Gonzalez, R.; Schmeltz, M. Urban Growth and Heat in Tropical Climates. Front. Ecol. Evol. 2021, 9, 616626. [Google Scholar] [CrossRef]
- Tiatragul, S.; Hall, J.M.; Pavlik, N.G.; Warner, D.A. Lizard Nest Environments Differ between Suburban and Forest Habitats. Biol. J. Linn. Soc. 2019, 126, 392–403. [Google Scholar] [CrossRef]
- Hall, J.M.; Warner, D.A. Thermal Spikes from the Urban Heat Island Increase Mortality and Alter Physiology of Lizard Embryos. J. Exp. Biol. 2018, 221, jeb181552. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.A.; Altizer, S. Urbanization and the Ecology of Wildlife Diseases. Trends Ecol. Evol. Evol. 2020, 22, 95–102. [Google Scholar] [CrossRef]
- Lazić, M.M.; Carretero, M.A.; Živković, U.; Crnobrnja-isailović, J. City Life Has Fitness Costs: Reduced Body Condition and Increased Parasite Load in Urban Common Wall Lizards, Podarcis muralis. Salamandra 2017, 53, 10–17. [Google Scholar]
- Ashby, B.; Bruns, E. The Evolution of Juvenile Susceptibility to Infectious Disease. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180844. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).