Abstract
Climate change has a direct impact on biodiversity, affecting ecosystems and altering their balance. Many taxa, including insects, are likely to be affected by climate change in terms of geographic distribution. Sarcophagid flies, such as Sarcophaga dux and Sarcophaga haemorrhoidalis, are important flies because of their apparent ecological, forensic, and medical significance. Global habitat suitability varies as a result of climate change. In wildlife management, models that predict species’ spatial distribution are being used more and more, which emphasizes the need for reliable methods to evaluate their accuracy. Consequently, the statistical robustness of maximum entropy was implemented in Maxent to model the current and future global distribution of both flies, involving occurrence data of 155 and 87 points for S. dux and S. haemorrhoidalis, respectively. Based on the Pearson correlation and Jackknife test, five bioclimatic variables were used for current and future predictive models. For future models, two representative concentration pathways (RCPs), 2.6 and 8.5, for 2050 and 2070 were applied. Both statistical parameters, AUC and TSS, were used to assess the resulting models with values equal to 0.80 (±0.01) and 0.9, respectively, for S. dux and equal to 0.86 (±0.01) and 0.92 for S. haemorrhoidalis. The resulting models for S. dux showed high and very high suitability in Europe, Tropical Africa, India, Canada, the United States from Alaska to Florida, Brazil, and Australia. In the case of S. haemorrhoidalis Europe and North and South America displayed low to medium suitability, but North Africa, including Egypt; Tropical Africa; Asia, including Saudi Arabia, India, and China; and Australia showed increased suitability. Decision-makers are put in conflict with their duties to avert destruction in the economic, medical, and ecological sectors by such anticipated models, and use these predictive models as a cornerstone for building a control strategy for such forensically important flies at local spatial scales.
1. Introduction
Sarcophagidae is a family of flies commonly known as flesh flies or Sarcophagid flies [1]. They are found worldwide and are typically medium to large in size, with a gray or black body and three longitudinal stripes on the thorax [1,2]. Flesh flies are a varied group of flies, with over 3000 species documented in the Sarcophagid family [3]. They range in size from medium to huge, with body lengths ranging from a few millimeters to more than a centimeter [4]. Flesh flies are important in recycling because they feed on dead and decomposing animal flesh, including human flesh [5]. They can also infest living animals, such as livestock wounds, and in some cases humans, in the very well-known phenomenon myiasis [4,5]. Furthermore, the presence and development of larvae of particular species of flesh flies are utilized in forensic entomology to establish the time of death of a cadaver [6]. The adults of some species have a very important role in pollination; some species are known to feed on nectar and pollen and can be important pollinators of certain plant species [7].
Sarcophaga dux and Sarcophaga haemorrhoidalis are two widespread species of Sarcophagid flies [8,9]. They dominated most of the old world and many parts of the new world. S. dux is a medium-sized fly, about 8–12 mm in length [8,9]. It has a black and shiny body with bristles on the thorax and abdomen [10]. The wings are translucent with a slight brownish hue. S. dux, like other flesh flies, is a scavenger that feeds on decomposing animal flesh, and its larvae are frequently seen in carrion, increasing its impact on forensic entomology [11]. One intriguing element of S. dux is its mating behavior [12]. Male S. dux flies may frequently congregate in large numbers on exposed surfaces such as rocks or tree trunks to engage in courtship displays in order to attract females [13]. Males undertake a sequence of wing movements and vibrations while generating a pheromone that attracts females to the place [14]. Sarcophaga dux has a wide geographic range, with records from almost all over the world, as the species has been reported in the Afrotropical, Palearctic, and Oriental areas [15].
On the other hand, S. haemorrhoidalis is a very confused species in this family, with a very wide distribution worldwide. Some work in the literature classifies this species as Sarcophaga pernix Harris, 1780 [16]. Adult identification is very difficult, and only males can be identified through genitalia [17]. S. haemorrhoidalis, interestingly, has also been used in medical studies [18]. This species’ larvae have been studied for their capacity to clean wounds and aid healing, and they may have implications in the treatment of chronic wounds [19]. The fly shows veterinary and forensic importance; the larvae have the ability to invade the wound accidentally and could also lead to intestinal myiasis through the uptake of contaminated food [17,18]. The time of its larval development is recorded accurately, and this marks the fly’s importance in determining post mortal intervals [20]. The species has a wide distribution range and is found in various regions around the world [15].
Despite the importance of these two species of Sarcophagidae, there is currently little research on the precise effects of climate change on them and any other Sarcophagid flies, although changes in temperature and precipitation patterns are likely to have a considerable impact on the range and abundance of these flies [21]. Now, climate change is the main challenge to the biological diversity of our planet [22]. Changes in the climate brought on by human activity include burning fossil fuels and clearing forests. These activities cause an increase in greenhouse gas emissions, which raises the atmospheric concentrations of greenhouse gases like carbon dioxide and methane, which trap heat and cause global warming [23]. The rise in temperatures and disturbance in rainfall patterns will highly affect the species’ distribution and abundance [24]. Understanding how species will change their range through space and time will help scientists and decision-makers decrease the impact of global warming on several disciplines, including the medical, social, and environmental sectors [25].
Based on environmental needs and occurrence data, species distribution modeling (SDM) is a technique used to forecast the geographic spread of species. In order to create a model that can be used to forecast the likelihood of a species occurring in unsampled places, it is crucial to identify the environmental factors that are significant predictors of a species occurrence [26]. This technique has a wide range of applications, including conservation planning, invasive species management, and climate change adaptation. The distribution of a species may shift over time depending on changes in temperature, precipitation, and other climatic variables [27]. This effect can be modeled using SDM. The Models generated can be used, for instance, to forecast a species’ future distribution under various climate change scenarios, such as those implied by global climate models. The generated maps form an effective method that helps predict how species populations will behave toward climate change [28]. Recently, it was used to evaluate the change that will occur in species distribution as a result of global warming for a wide range of species, from microorganisms to large mammals and birds [29]. Several medical and veterinary important insect pests were also studied using this technique [30]. The development of several modeling tools and statistical methods and the availability of large amounts of accumulative data from species records, in addition to a wide range of generated climatological future scenarios, help scientists predict how our biological future will be and determine what the risk is that our ecosystems will face in the near and far future due to global warming [31,32].
So, the aim of this work is to evaluate the climate change impact on two very widespread Sarcophagid species on the global scale and also to create maps for their current distribution range for the first time.
2. Materials and Methods
2.1. Input Data
Almost all available records of both S. dux and S. haemorrhoidalis were gathered from the literature, either as adults or larval cases [33,34,35,36,37,38]. Moreover, other distribution data from the digital database: The Global Biological Information Facility (GBIF.org https://doi.org/10.15468/dl.xp4www and https://doi.org/10.15468/dl.nbmjwu (accessed on 15 May 2023)) were used for both species. On these data, three filtration stages were carried out. The duplicate records were first deleted [39]. Additionally, data with high spatial uncertainty were removed [40]. Thirdly, to avoid any spatial redundancy, data were spatially rarefied using an ArcGIS algorithm [39,40]. Finally, the remaining georeferenced occurrence points were 155 and 87 points for S. dux and S. haemorrhoidalis, respectively. These points were converted into comma delimited (CSV) format and used to predict the current and future global distribution of S. dux and S. haemorrhoidalis (Figure 1).
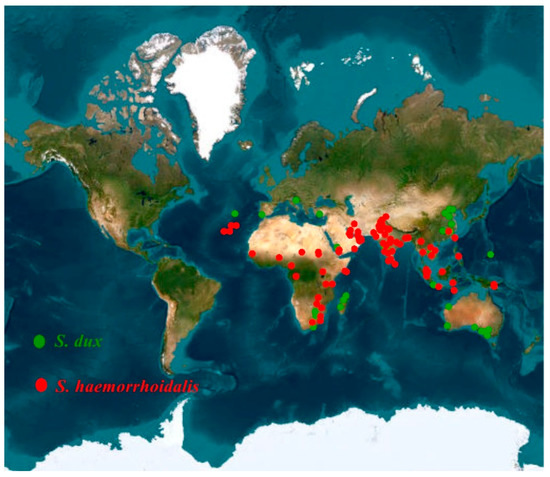
Figure 1.
Distribution of the occurrence records of S. dux and S. haemorrhoidalis.
About nineteen bioclimatic data with a spatial resolution of approximately 5 km2 were downloaded from the WorldClim data (www.worldclim.org, (accessed on 1 April 2023)). Using ArcGIS version 10.7, fifteen bioclimatic variables for current bioclimatic data were transformed into ASCII format to assess the most important biological factors that contribute to the present model of habitat suitability for both S. dux and S. haemorrhoidalis [40]. Bioclimatic variables 8–9 and 18–19 were excluded from the analysis because of known geographic anomalies [41]. Person correlation function with a value equal to (|r| ≥ 0.8) was used to reduce multicollinearity among the fifteen bioclimatic data [41,42]. Consequently, only five bioclimatic layers were used for further analysis.
For future predictions, we used parallel datasets from the WorldClim data (www.worldclim.org, (accessed on 1 April 2023)) of two typical concentration paths (RCPs), 2.6 and 8.5, for the years 2050 (average of estimates for 2041–2060) and 2070 (average of predictions for 2061–2080), respectively, for the global climate model (GCM) [40]. These bioclimatic layers were also changed into ASCII format via ArcGIS v.10.7 [43]. Using the MRI-CGCM3 global climate model, the climatic data for the selected variables were then extended to the years 2050 and 2070 in order to assess how climate change may affect the appropriateness of S. dux and S. haemorrhoidalis habitat in the future. The most recent GCM climate estimates used in the IPCC Fifth Assessment Report contain this information. Moreover, gain and loss of habitat suitability ranges were calculated by subtracting the predicted future maps from the current one using map algebra function implemented in ArcGIS v.10.7 to produce gain and loss maps. Finally, we calculated the predicted areas in kilometers between current and future predictive models via ArcGIS v. 10.7 (Table S1).
2.2. Environmental Niche Modeling
The ecological niche and habitat suitability of S. dux and S. haemorrhoidalis were assessed using the maximum entropy method in Maxent v3.4.3e [44]. The statistical power of this method resulted in robust predictive models [45]. It is widely used due to the capability to create models using only presence records [46]. In modeling studies with small sample numbers, it can also get rid of duplicate data in the same cell [47]. For each of our models, 25% of the records were used for testing, and 75% were used for training [45,46,47]. The maximum background points and iterations permitted were ten thousand and one thousand, respectively [48]. Additionally, a 10-fold cross-validation was carried out, which improved the performance of the model [47,48].
2.3. Model Robustness
The robustness of the resulting models is typically evaluated using the Area Under Curve (AUC) [34]. AUC values above 0.9 are considered to represent excellent model performance, and they range from 0.5 to 1.0 [45]. In order to assess the distribution of the target species, important bioclimatic parameters were found using the Jackknife test [46]. The expected model accuracy was also measured using the True Skill Statistic (TSS) [44]. The TSS value can be anywhere between −1 and 1, with close values to 1 indicating a strong link and values to −1 indicating a weak relationship between the distribution and the predictive model [47,49].
3. Results
3.1. Model Evaluation and Contribution of Environmental Covariates
For both species, the Maxent predictive model achieved a high AUC values of 0.80 (±0.01) for S. dux and 0.86 (±0.01) for S. haemorrhoidalis. The AUC values were often higher in continuous species distribution modeling (SDM) than discontinuous SDM. Additionally, the TSS value was quite high, with values equal to 0.9 and 0.92 for S. dux and S. haemorrhoidalis, respectively, indicating outstanding model performance. TSS readings over 0.5 are often considered acceptable. The percentage contribution of the bioclimatic variables was demonstrated by the Jackknife test of the predictive model (Figure 2a,b, Table 1) . In the case of S. dux, the annual mean temperature (bio 1) was the most effective bioclimatic covariate followed by the min temperature of the coldest month (bio 6), the annual precipitation (bio 12), the mean diurnal range (bio 2), and the precipitation of the driest month (bio 14), respectively. But, for S. haemorrhoidalis, the (bio 1) was the most effective bioclimatic covariate followed by (bio 12), (bio 6), (bio 2), and (bio 14), respectively. Furthermore, the range of the response curves for (bio 1), the most significant environmental covariate, was from 5 °C to 20 °C and from 10 °C to 30 °C for S. dux and S. haemorrhoidalis, respectively (Figure 2c,d).
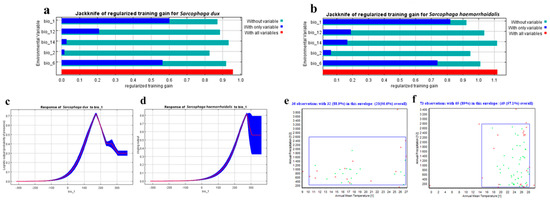
Figure 2.
The Jackknife test of the most important variables (a) for S. dux and (b) for S. haemorrhoidalis. Response curves of the most effective bioclimatic factor (bio 1) in flies’ distribution: (c) for S. dux and (d) for S. haemorrhoidalis. Two-dimensional niche between Annual Temperature (bio 1) and Annual Precipitation (bio 12): (e) for S. dux and (f) for S. haemorrhoidalis.

Table 1.
Relative percentages of bioclimatic covariates used in Maxent to model the current and future habitat suitability of S. dux and S. haemorrhoidalis.
3.2. Two-Dimensional Niche Analysis
The enveloped test was used to generate the two-dimension niche for both S. dux and S. haemorrhoidalis. The most effective bioclimatic variables used in studying these insects are as follow: the annual mean temperature (bio 1) and the annual precipitation (bio 12) (Figure 2e,f). The results indicate the impressive adaptability of both species for different environmental conditions. In S. dux, the annual mean temperature can range from 10 °C to 27 °C and the amount of rain can range from 300 mm to 2600 mm per year. In S. haemorrhoidalis, the annual mean temperature can range from 14 °C to 28 °C and the amount of rain can range from 0 mm to 2700 mm per year.
3.3. Current Habitat Suitability Models
The predictive current global distribution of S. dux and S. haemorrhoidalis agrees with the present-day occurrence of both flies. Our predictive maps showed that S. dux and S. haemorrhoidalis are found in different parts of the world (Figure 3a,b). In Europe, S. dux showed very high suitability from Portugal and Spain in the east, along with France, the UK, Germany, and Italy, to Ukraine and the eastern borders of Russia in the west. While S. haemorrhoidalis showed medium suitability, especially in France, Germany, Italy, and Spain (Figure 3a,b). Asia, India, Thailand, Vietnam, and southern parts of Saudi Arabia showed high and very high suitability for S. dux, but China and Saudi Arabia showed very high suitability for S. haemorrhoidalis. Also, Malaysia, the Philippines, and Indonesia showed medium habitat suitability in the case of S. dux and very high suitability in the case of S. haemorrhoidalis (Figure 3a,b). Tropical Africa showed medium to very high suitability, and North Africa, including Northern parts of Egypt, Algeria, Tunisia, and Morocco, showed high suitability for both species (Figure 3a,b). In North America, S. dux showed high and very high suitability across Canada and the United States, from Alaska to Florida, while S. haemorrhoidalis showed low suitability. Southern parts of Brazil and northern parts of Argentina showed high to very high suitability for S. dux, whereas Colombia, Venezuela, and Brazil showed high habitat suitability for S. haemorrhoidalis (Figure 3a,b). Generally, our models showed high and very high suitability for both fly species in the overall Australian territories, except the southern parts showed low suitability for S. haemorrhoidalis (Figure 3a,b). Finally, a niche overlapping map illustrated the common range shared by both flies (Figure S1), and changes in the areas in kilometers illustrated the differences between current and future global predictive models (Table S1).
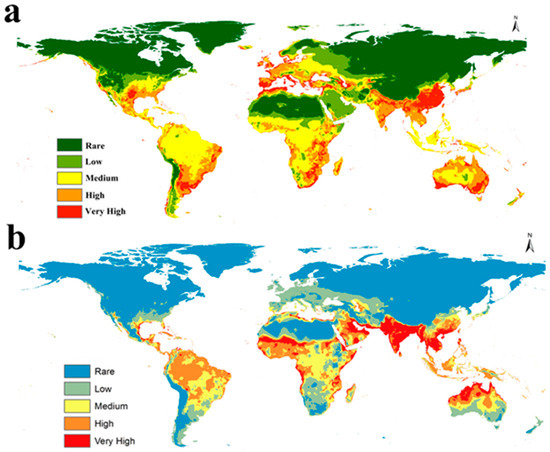
Figure 3.
Predicted current potential global distribution: (a) for S. dux and (b) for S. haemorrhoidalis.
3.4. Future Habitat Suitability Models
The produced Maxent models for the global potential distributions of S. dux and S. haemorrhoidalis under future climate change scenarios RCP 2.6 and RCP 8.5 for the years 2050 and 2070 are illustrated in Figure 4. In Europe, our predictive models showed an ascending increase in habitat suitability in the future for S. dux from RCP 2.6 2050 to RCP 8.5 2070, especially in Russia (Figure 4a–d), while the habitat suitability for S. haemorrhoidalis showed no remarkable change in the future RCPs except RCP 8.5 2070, which assures an increase in suitability in Italy, Spain, and Turkey (Figure 4e–h). In Asia, future models of S. dux showed a decrease in suitability in Western Saudi Arabia, Iraq, Kuwait, and Iran (Figure 4a–d), while S. haemorrhoidalis showed an increase in habitat suitability in the western coast of Saudi Arabia (Figure 4e–h, Table S1).
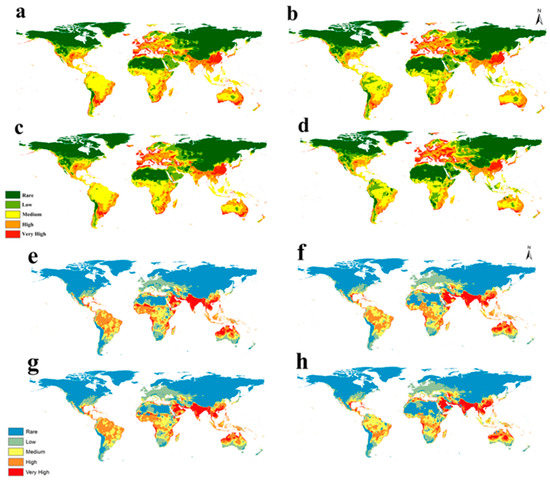
Figure 4.
Predicted global future distribution of under two RCPs: (a) RCP 2.6 in 2050, (b) RCP 8.5 in 2050, (c) RCP 2.6 in 2070, and (d) RCP 8.5 in 2070 for S. dux; (e) RCP 2.6 in 2050, (f) RCP 8.5 in 2050, (g) RCP 2.6 in 2070, and (h) RCP 8.5 in 2070 for S. haemorrhoidalis.
In Africa, the calibration maps of S. dux showed a loss in suitability in Zambia, Botswana, and Namibia and a gain in Niger and Mali (Figure 5a–d), but S. haemorrhoidalis showed a high loss of suitability in Northern Africa, including Egypt, Tunisia, and Morocco, and West Africa, including Mauritania and Senegal (Figure 5e–h). In North America, S. dux showed an increase in suitability in the eastern parts of the United States (Figure 5a–d), whereas S. haemorrhoidalis showed a gain in suitability in the south of the United States (Figure 5e–h). South America showed an increase in suitability for S. haemorrhoidalis in Southern Brazil in RCP 8.5 2070. Finally, S. dux showed a loss of suitability in Australia; however, S. haemorrhoidalis showed a gain in middle Australia (Figure 5a–h).
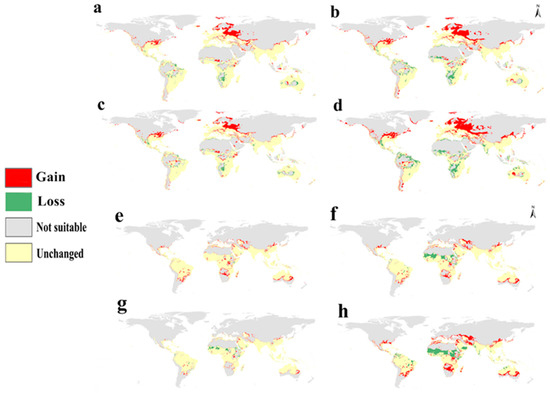
Figure 5.
Calibration maps showing gain and loss in habitat suitability through the four future scenarios against the current status: (a) RCP 2.6 in 2050, (b) RCP 8.5 in 2050, (c) RCP 2.6 in 2070, and (d) RCP 8.5 in 2070 for S. dux; (e) RCP 2.6 in 2050, (f) RCP 8.5 in 2050, (g) RCP 2.6 in 2070, and (h) RCP 8.5 in 2070 for S. haemorrhoidalis.
4. Discussion
Climate change endangers ecosystems and species distribution in a variety of ways, including by causing severe droughts in some regions, more frequent and intense storms and floods in other regions, and habitat loss and fragmentation [50]. Moreover, climate change may provide new opportunities for invasive species to establish and thrive in new locations, potentially resulting in native species’ displacement and changes in ecosystem functions [51]. Furthermore, many species are predicted to face increased extinction risks as a result of climate change, particularly those that are already threatened or have limited ranges [52]. For these reasons, the prediction of how species range will be in the future is considered an important need by scientists and decision-makers to mitigate climate change or decrease its effect on our planet [53].
The present work forms a step in this direction, as it shows how the future will be for two very important and widespread flesh flies on the global scale. The current distribution of the two species is very compatible with their real situation on the ground (Figure 3a,b). S. dux is distributed through southern Asia, southern Europe, parts of the Middle East, sub-Saharan Africa, and southern North America. Also, many parts of South America show high habitat suitability for this species, although this has not been recorded there before, and this information is very critical, as if S. dux accidentally enters this region, this will create a very serious invasive species with a highly suitable habitat (Figure 3a). On the other hand, S. haemorrhoidalis appears to have a large range through tropical and subtropical habitats around the world (Figure 3b). Unlike its cousin, S. dux, this species is not common in Europe or large parts of North America. The generated current maps of the two species will be very helpful, especially for forensic sectors throughout the world, as they will help investigators identify these species’ ranges.
The accuracy of the generated maps is considered to be very high, either for the AUC test or the TSS test, for the two species, even when compared to several previous studies that have dealt with other dipterous species [39,43]. AUC and TSS are both helpful metrics for assessing the effectiveness of SDMs, but they each have unique advantages and disadvantages. Although the AUC is a widely used metric in the literature, it has drawn criticism for being impervious to changes in prevalence and failing to account for the model’s specificity [54]. In contrast, the TSS considers both sensitivity and specificity and is less sensitive to changes in prevalence, although it is less frequently used in the literature and may be trickier to interpret [55].
Bio 1 (annual mean temperature) forms the most effective bioclimatic variable in the distribution of the two species, but each one has a completely different pattern toward this variable (Figure 2). S. dux has a wide range of temperatures that it could live in, while S. haemorrhoidalis has a very narrow range of temperatures that it could live in. This is very similar to several previously studied species, especially in a very similar family such as Calliphoridae. The species Old world screw worm Chrysomya bezziana has a very narrow temperature range, while the species of the same genus Chrysomya albiceps has a wide temperature range [29,34]. Precipitation-related variables such as bio 12 (annual precipitation) have a very low impact on the two species’ distribution and have a small contribution to the generated model (Figure 2). This could be due to the variety of habitats that the two species live in, from the heavy rain forests of the Indonesian tropics to the extremely dry deserts of Arabia.
The importance of temperature and humidity in the life cycles of these two species has been discussed previously in several works. S. haemorrhoidalis larvae require warm temperatures for accelerated growth and development, and temperature has a significant impact on how the species develops. The larvae of S. haemorrhoidalis developed most quickly in lab experiments at temperatures between 24 °C and 30 °C. It also experiences higher mortality due to high temperatures, with larvae and pupae being especially susceptible to heat stress [56]. In the same way, temperature has a significant impact on S. dux growth, just like it does on S. haemorrhoidalis. S. dux larvae developed most quickly in lab tests at temperatures between 25 °C and 30 °C. S. dux larvae were discovered to be relatively heat-tolerant, in contrast to S. haemorrhoidalis, with some studies finding effective development at temperatures as high as 38 °C, but this is not supported by the results generated here [57].
The future situation for the two species shows completely different results. S. haemorrhoidalis will not be in a significantly different position from its current situation, while the worst climate change scenario of 2070 shows a high degree of habitat loss, especially in tropical Africa (Figure 4e,f and Figure 5e–h). S. dux, on the other hand, will increase and expand through different parts of its range, especially in Europe, in all studied scenarios. Such changes in the S. dux range will surely affect the surrounding ecosystem that it lives in (Figure 4a–d and Figure 5a–d).
Climate change may cause changes in the interactions of Sarcophagid flies with other species [6]. Changes in temperature and precipitation patterns could impact the timing and success of other decomposer species, such as bacteria and fungus, which play essential roles in carrion decomposition [12]. Changes in precipitation patterns may alter the availability and decomposition of carrion by flesh flies, which are scavengers that feed on dead animal flesh [18]. For example, if precipitation becomes more intermittent or intense, it may change the rate at which carcasses decompose, thereby decreasing the availability of food for flesh flies. These changes may have an impact on the availability and quality of food for flesh flies, potentially leading to population decreases [58,59].
5. Conclusions
The present work provides valuable insights into the potential impacts of climate change on insect populations and highlights the need for continued research and monitoring in this area. By better understanding the effects of climate change on insect populations, we can develop more effective strategies for dealing with such changes and help mitigate the impacts of climate change on our planet’s biodiversity. It is clear that climate change has a significant influence on the global potential distribution of Sarcophaga dux and Sarcophaga haemorrhoidalis. The maximum entropy model was used to predict the current and future distributions of these flies and illustrate that their potential habitat suitability is likely to shift in response to changing climatic conditions. These findings have important implications for wildlife management and conservation efforts, as well as for forensic and medical applications of these flies. Overall, this study highlights the need for reliable methods to evaluate the accuracy of species distribution models in the face of ongoing climate change. Furthermore, the study demonstrates the utility of maximum entropy modeling as a tool for predicting species distribution under changing climatic conditions. This approach can be used to identify areas of high conservation value as well as inform management decisions related to invasive species and habitat restoration. However, such models are subject to certain limitations and uncertainties, and further research is needed to improve their accuracy and reliability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15080903/s1, Figure S1: Niche overlapping between S. dux and S. haemorrhoidalis; Table S1: Comparison between current and future distribution area of S. dux and S. haemorrhoidalis based on natural Jenks map classification.
Author Contributions
Conceptualization, E.M.H. and M.G.N.; methodology, E.M.H., M.G.N. and A.A.A.-K.; software, A.A.A.-K. and M.G.N.; validation, E.M.H. and M.G.N.; formal analysis, E.M.H., A.A.A.-K. and M.G.N.; investigation, A.A.A.-K. and M.G.N.; resources, M.G.N. and E.M.H.; data curation, A.A.A.-K., E.M.H. and M.G.N.; writing—original draft preparation, E.M.H., M.G.N. and A.A.A.-K.; writing—review and editing, E.M.H., M.G.N. and A.A.A.-K.; visualization, E.M.H.; supervision, M.G.N.; project administration, A.A.A.-K., M.G.N. and E.M.H.; funding acquisition, A.A.A.-K. All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University for supporting this research through sabbatical leave program.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Princess Nourah bint Abdulrahman University for supporting this research through sabbatical leave program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pape, T.; Blagoderov, V.; Mostovski, M.B. Order Diptera Linnaeus, 1758. Zootaxa 2011, 3148, 222–229. [Google Scholar] [CrossRef]
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Hall, M.J.R.; Wall, R.; Stevens, J.R. Traumatic Myiasis: A Neglected Disease in a Changing World. Annu. Rev. Entomol. 2016, 61, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, A.; Pape, T. Sarcophagidae (Diptera) of Thailand: A Review of Current Knowledge. Zootaxa 2012, 3423, 1–73. [Google Scholar] [CrossRef]
- Mello-Patiu, C.A.; Vargas, H.A. The Importance of Flies in Pollination. J. Pollinat. Ecol. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Whitmore, D.; Wall, R. Mites and Insects in Wound Healing: An Update and Review. Trauma 2018, 20, 3–17. [Google Scholar] [CrossRef]
- Yeates, D.K.; Wiegmann, B.M. Congruence and Controversy: Toward a Higher-Level Phylogeny of Diptera. Annu. Rev. Entomol. 1999, 44, 397–428. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, L.; Zhang, M.; Chu, H.; Cao, J.; Li, K.; Zhang, R. The Mitochondrial Genome of Sarcophaga dux (Diptera: Sarcophagidae), a Species with High Invasion Ability. Mitochondrial DNA Part B 2019, 4, 2284–2285. [Google Scholar] [CrossRef]
- Ebejer, M.J.; Falzon, V. A Review of the Sarcophagidae (Diptera) of Malta, with the First Record of Sarcophaga (Liopygia) ruficornis from the Maltese Islands. J. Entomol. Res. Soc. 2018, 20, 23–35. [Google Scholar]
- Hall, M.J.R.; Wall, R. Myiasis of Humans and Domestic Animals. Adv. Parasitol. 1995, 35, 257–334. [Google Scholar] [CrossRef]
- Makhubo, B.G.; Githure, J.I. Diversity and Distribution of Sarcophagid Flies (Diptera: Sarcophagidae) in Nairobi and Its Environs, Kenya. J. Insect Sci. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Sukontason, K.; Narongchai, P.; Kanchai, C.; Vichairat, K.; Sribanditmongkol, P.; Bhoopat, T.; Kurahashi, H.; Chockjamsai, M.; Piangjai, S.; Bunchu, N.; et al. Forensic entomology cases in Thailand: A review of cases from 2000 to 2006. Parasitol. Res. 2007, 101, 1417–1423. [Google Scholar] [CrossRef]
- Wells, J.D.; Greenberg, B. Courtship Behavior of the Flesh Flies (Diptera: Sarcophagidae). J. Insect Behav. 1992, 5, 185–199. [Google Scholar] [CrossRef]
- Sherman, R.A.; Hall, M.J.R.; Thomas, S. Medicinal Maggots: An Ancient Remedy for Some Contemporary Afflictions. Annu. Rev. Entomol. 2000, 45, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Barták, M.; Khrokalo, L.; Verves, Y. New records, synonyms and combinations for oriental Sarcophagidae (Diptera), with updated checklists for Cambodia, India, Taiwan, Thailand and Vietnam. J. Asia Pacific Entomol. 2019, 22, 44–55. [Google Scholar]
- Bhattacharjee, D.; Halder, S. Medical Maggots: An Ancient Remedy for Wound Healing. J. Egypt. Soc. Parasitol. 2017, 47, 647–651. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic Entomology in Criminal Investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Grassberger, M.; Reiter, C. Effect of Temperature on Lucilia sericata (Diptera: Calliphoridae) Development with Special Reference to the Isomegalen- and Isomorphen-Diagram. Forensic Sci. Int. 2002, 128, 177–182. [Google Scholar] [CrossRef]
- Marino, A.; Putzolu, M.; Mazzarello, V.; Dessy, L.A. Maggot Therapy for the Treatment of Diabetic Foot Ulcers: A Meta-Analysis. J. Wound Care 2016, 25, 139–146. [Google Scholar] [CrossRef]
- Jordaens, K.; Sonet, G.; Richet, R.; Dupont, E.; Braet, Y. A Molecular Phylogenetic Analysis of Sarcophagidae (Diptera) and the Evolution of Larval Myiasis within the Calyptratae. Int. J. Parasitol. 2012, 42, 353–363. [Google Scholar] [CrossRef]
- Czajka, M.A.; Leather, S.R. A Review of Pest Control Strategies and Their Potential for Use in Sustainable and Ecologically Sound Pest Management. Crop Prot. 2016, 83, 37–45. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. In Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Nica, A.; Popescu, A.; Ibanescu, D.C. Human influence on the climate system. Curr. Trends Nat. Sci. 2019, 8, 209–215. [Google Scholar]
- Kassim, N.F.; Yaakop, S.; Idris, A.B. Effect of Temperature on Development of Sarcophaga (Liosarcophaga) dux Thomson (Diptera: Sarcophagidae) in Malaysia. Trop. Biomed. 2014, 31, 283–291. [Google Scholar]
- Matuszewski, S. An Initial Study of Insect Succession and Carrion Decomposition in Various Forest Habitats of Central Europe. Forensic Sci. Int. 2013, 231, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Wang, G.; Huey, R.B. Global metabolic impacts of recent climate warming. Nature 2010, 467, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Duarte, C.M.; Poloczanska, E.; Richardson, A.J.; Singer, M.C. Overstretching attribution. Nat. Clim. Chang. 2011, 1, 2–4. [Google Scholar] [CrossRef]
- Cox, J.S.H. The Role of Geographic Information Systems and Spatial Analysis in Area-Wide Vector Control Programm. In Area-wideControl of Insect Pests; Springer: Dordrecht, The Netherlands, 2007; pp. 199–209. [Google Scholar]
- Bässler, C.; Heilmann-Clausen, J.; Karasch, P.; Brandl, R.; Halbwachs, H. Climate Change Disproportionately Increases Habitat Loss for Rare Species in Fragmented Landscapes. Glob. Chang. Biol. 2020, 26, 3604–3619. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Yang, D. Global Warming Threatens Biodiversity and Ecosystem Services: A Review. J. Clean. Prod. 2022, 334, 130470. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Guo, X.; Zhang, Y.; Wang, Z. Climate Change and the Distributional Dynamics of the Housefly (Musca domestica) in China. Environ. Monit. Assess. 2020, 192, 761. [Google Scholar] [CrossRef]
- Sgrò, C.M.; Lowe, A.J.; Hoffmann, A.A. Building Resilience to Climate Change in Australia’s Biodiversity Hotspots. Trends Ecol. Evol. 2023, 38, 1–3. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Sanit, S.; Klong-Klaew, T.; Tomberlin, J.K.; Sukontason, K. Sarcophaga (Liosarcophaga) dux (Diptera: Sarcophagidae): A flesh fly species of medical importance. Biol. Res. 2014, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Mahato, S. Intra-puparial development of flesh fly Sarcophaga dux (Thomson) (Diptera, Sarcophagidae). Curr. Sci. 2016, 25, 1063–1070. [Google Scholar] [CrossRef]
- Braverman, I.; Dano, I.; Saah, D.; Gapany, B. Aural myiasis caused by flesh fly larva, Sarcophaga haemorrhoidalis. J. Oto. 1994, 23, 204–205. [Google Scholar]
- Abdel-Hafeez, E.H.; Mohamed, R.M.; Belal, U.S.; Atiya, A.M.; Takamoto, M.; Aosai, F. Human wound myiasis caused by Phormia regina and Sarcophaga haemorrhoidalis in Minia Governorate, Egypt. Parasitol. Res. 2015, 114, 3703–3709. [Google Scholar] [CrossRef]
- Haseman, L. Sarcophaga haemorrhoidalis Larvae as Parasites of the Human Intestine (Dipt.). Entomol. News. 1917, 28, 343. [Google Scholar]
- Ndueze, O.U.; Noutcha, M.A.; Umeozor, O.C.; Okiwelu, S.N. Arthropods associated with wildlife carcasses in lowland rainforest, Rivers State, Nigeria. Europ. J. Exper. Biol. 2013, 3, 111–114. [Google Scholar]
- Hosni, E.M.; Nasser, M.; Al-Ashaal, S.; Rady, M.H.; Kenawy, M.A. Modeling current and future global distribution of Chrysomya bezziana under changing climate. Sci. Rep. 2020, 10, 4947. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shaara, H.; Alashaal, S.A.; Hosni, E.M.; Nasser, M.G.; Ansari, M.J.; Alharbi, S.A. Modeling the Invasion of the Large Hive Beetle, Oplostomus fuligineus, into North Africa and South Europe under a Changing Climate. Insects 2021, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Lira-Noriega, A.; Medina-Vogel, G.; Peterson, A.T. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas: Use of Maxent and Niche A to assure strict model transference. Geospat. Health 2014, 9, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hosni, E.M.; Nasser, M.; Al-Khalaf, A.A.; Al-Shammery, K.A.; Al-Ashaal, S.; Soliman, D. Invasion of the Land of Samurai: Potential Spread of Old-World Screwworm to Japan under Climate Change. Diversity 2022, 14, 99. [Google Scholar] [CrossRef]
- Hosni, E.M.; Al-Khalaf, A.A.; Nasser, M.G.; Abou-Shaara, H.F.; Radwan, M.H. Modeling the Potential Global Distribution of Honeybee Pest, Galleria mellonella under Changing Climate. Insects 2022, 13, 484. [Google Scholar] [CrossRef]
- Hosni, E.M.; Al-Khalaf, A.A.; Naguib, R.M.; Afify, A.E.; Abdalgawad, A.A.; Faltas, E.M.; Hassan, M.A.; Mahmoud, M.A.; Naeem, O.M.; Hassan, Y.M.; et al. Evaluation of Climate Change Impacts on the Global Distribution of the Calliphorid Fly Chrysomya albiceps Using GIS. Diversity 2022, 14, 578. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 20 March 2020).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar]
- Elith, J.H.; Graham, C.P.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. Pervasive Human-Driven Decline of Life on Earth Points to the Need for Transformative Change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating Extinction Risk from Climate Change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Ekström, J.; Åkerrén Ögren, J.; Sjöblom, T. Exact Probability Distribution for the ROC Area under Curve. Cancers 2023, 15, 1788. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar]
- Zehra, N.; Mishra, V. An entomotoxicological study on the influence of house hold toxins on the colonization of carrion by Sarcophaga haemorrhoidalis. J. Environ. Sci. Toxicol. Food Technol. 2020, 14, 40–43. [Google Scholar]
- Zhang, X.; Li, Y.; Shang, Y.; Ren, L.; Chen, W.; Wang, S.; Guo, Y. Development of Sarcophaga dux (diptera: Sarcophagidae) at constant temperatures and differential gene expression for age estimation of the pupae. J. Therm. Biol. 2020, 93, 102735. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Sahlén, G.; Rämert, B. The Effects of Climate Change on Forensic Entomology: A Review. J. Forensic Sci. 2020, 65, 1030–1036. [Google Scholar] [CrossRef]
- Benbow, M.E.; Fjong, S.; Nguyen, J.; Tomberlin, J.K. Microbial Ecology of Carrion Decomposition in the Context of Climate Change. Front. Ecol. Evol. 2020, 8, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).