Abstract
This study investigates demutational changes in plant communities of phosphorite mines between 2005 and 2021. A total of 23 plant species were initially found in the surveyed areas in 2005, while 68 species were identified in spring 2021. The plant communities were categorized into five different groups, with the older communities (No. I and II) exhibiting richer vegetation and greater diversity. Community No. III comprised various cereals, legumes, orchid plants, and mosses. Dominant species in community No. I included Betula pubescens, Carpinus betulus, Quercus robur, and Picea obovata, while community No. II featured Juniperus communis, Larix sibirica, Pinus sylvestris, and Abies sibirica. Erosion was observed in community No. V on slopes of different ages. The study also found changes in physicochemical indices, such as increased organic matter concentration and decreased pH value (4.9). The basal respiration rate of the pit soil was low (around 0.4 mg CO2/m2/h), and carbonate content ranged from 0.06 to 0.9%. This study presents novel data on the dynamics of species composition, plant community structure, and physicochemical soil parameters in human-altered habitats, thereby contributing to the expansion of our knowledge of vegetation recovery and evolution in such environments.
1. Introduction
In many industrial regions, quarries are an integral part of the landscape, because they provide raw materials—minerals mined by open-pit mining. Over time, if a quarry ceases to be actively mined, it can become a habitat for many living organisms, including rare ones [1]. Quarries are characterized by common features such as:
- (a)
- The heterogeneity of their surfaces, which undergo significant changes due to their increased degradability;
- (b)
- Strongly pronounced unfavorable microclimatic conditions for the optimal existence of organisms; and
- (c)
- As a consequence, the low productivity of such habitats [2].
Remediation failures have been common, despite significant developments in mine remediation technologies over recent decades [3,4,5,6]. However, some aspects are still poorly understood. The study of succession processes offers a framework for understanding the fundamental factors involved in land reclamation and their application in the development of land restoration technologies [7].
In temperate regions, favorable conditions for spontaneous succession provide great potential in remediation processes [8]. In these areas, the main factors controlling ecological succession include regional mesoclimatic differences, landscape factors related to the preserved nearby vegetation, and local factors related to nutrient cycling and soil physicochemical features [9,10]. Serious deficiencies and soil toxicity are often the most severe constraints [11].
Other factors linked to water scarcity and soil erosion can also affect vegetation dynamics [12,13]. In such cases, surface runoff can play a driving role by redistributing soil particles (erosion and sedimentation) and water (soil runoff and moisture) at the gradient scale [14]. On artificial slopes, the emerging development of the soil favors the flow of land and limits the infiltration of rains [15]. In some cases, other runoff flows (rationalized) on the slopes recovered from the top can contribute to soil erosion, negatively impacting plant dynamics [16].
The conventional approach to environmental restoration in quarries involves the re-establishment of quarry vegetation. By restoring the biodiversity of plant communities (phytocenoses), it becomes possible to further enhance the biodiversity of animal communities (zoocenoses) [17]. Even a small number of native plant species contributes to effective quarry restoration [18]. With controlled succession, it might therefore be possible to plant a single plant species over a large area, neglecting the diversity and richness of the community structure that characterizes the quarry’s vegetation. It does not promote plant development, and improves habitat quality on quarry heathland. Further supplementation is required to enhance the understanding of the interrelationships between the physical and chemical properties of soil and the functioning of spontaneously developing vegetation on post-industrial sites [19,20]. Most studies examine the role of soil particle fraction and properties at post-industrial sites during the remediation process [21]. These relationships include regulating water ingress and availability for a specific plant community and the entire ecosystem [22].
Whatever the particular ecological context, the ecological succession in restored habitats is subject to a wide range of contingencies, reducing the predictability of succession [23,24]. Some contingencies initiate particularly important processes in restoring mountain ecosystems. These are remediation errors, changes in legislation, post-mining land use, and the effects of local disturbances that are not accounted for during remediation management [25].
Hydrological malfunctions (mainly due to erosion processes) and contingencies determine vegetation dynamics, given the strong influence of macroclimate and the random nature of mountainous landscapes. Given that the environmental gradient is associated with a continental climate and landscape conservation and the large heterogeneity of remediation treatments, this work aimed to study the changes in plant communities in the Lopatinsky mine, which constitutes the novelty of the work. As mining activities at the Lopatinsky phosphate rock mine did not cease until 1993, the remediation results were rather limited at most sites, requiring research to develop a successful remediation scheme.
The achievement of this goal requires the following tasks to be accomplished:
- Selecting areas of the Lopatinsky mine with different topographical characteristics to investigate the characteristics of their soils;
- Studying the physical and chemical parameters of the soil cover in the study areas; and
- Analyzing floristic species dominance and changes in plant communities of the Lopatinsky mine.
2. Materials and Methods
2.1. Study Region
This study was conducted in spring–summer periods between 2005 and 2021 (once every 4 years). It took place at permanent monitoring sites located within the Lopatinsky phosphate rock mine region (55°17′44″ N 38°49′57″ E). This area currently represents a giant abandoned phosphate rock deposit near Voskresensk in the Moscow Region (Figure 1). The climate of this area is moderately continental, with relatively hot summers and moderately cold winters. Forest–steppe and steppe dominate the territory. Soils are primarily medium-sized dark.
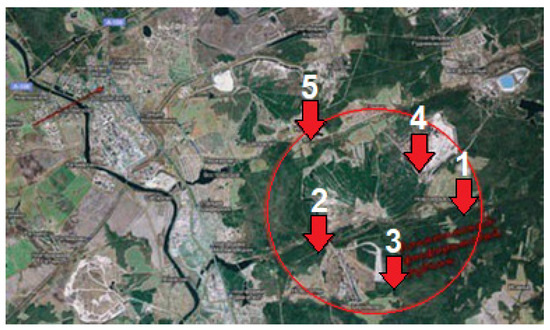
Figure 1.
Four study locations at the Lopatinsky phosphate mine.
The Lopatinsky Mine lies 90 km southeast of Moscow. Phosphate extraction stopped there in 1993. The complex of phosphate mining activities has led to the formation of a typical landscape in this area: long and deep pit troughs interspersed with long and high sand boulders, gradually transforming into flat sandy fields.
2.2. Sampling
Even small changes in topography can have a significant impact on successional processes in soils and vegetation [26,27]. In this regard, sites with different topographic characteristics were selected (Figure 1): plains (No. 1), small enclosed depressions (No. 2), gullies (No. 3), arroyos (No. 4), and slopes (No. 5). In each of them, three experimental plots were established.
Soil material was collected between April and August at random locations near mines (15 soil samples per season, with subsequent averaging). A total of three soil samples per plot were collected every month from April to August, every four years, from a depth of 0 to 15 cm. Before conducting the study, samples were obtained using an auger, drilling 20 mm diameter holes perpendicular to the soil, with a height of 150 mm. Plots of 100 m2 (10 m × 10 m) were laid out to study the flora in each of the ecotypes. The following parameters were taken into account for each plot: (a) plant species composition; (b) soil cover characteristics; (c) total and individual indicators of projective cover for plants in general and species separately. All analyses related to soil cover were carried out under laboratory conditions (study of cover structure, chemical and physical properties). To work with soil, standard sieves with 1.5 mm cells were used, through which sieving was conducted.
Parameters studied in soil samples: pH in water–salt suspension (1:3), skeleton fraction content, granulometric soil composition by pipette method with pyrophosphate peptization of microaggregates [28], rates of aerobic processes (respiration) in the soil, acidometric evaluation of CO2 in carbonates [29].
2.3. Statistical Analysis
The obtained results were processed for validity using MANOVA multivariate analysis of variance using Microsoft Excel and Statistica 10 software packages. For species composition, the similarity of vegetation at different key sites was calculated using the Sorensen-Chekanovsky coefficient [30]. Differences in the obtained results were considered significant at p ≤ 0.05 according to Student’s test.
3. Results
3.1. Characteristics of Demutational Changes in Plant Communities
Since the mining of phosphorites was terminated in 1993, demutational changes involving previously extinct plant species or the introduction of new plant species into the area due to successional processes were examined. In previous research (2005), the floristic composition of the mine site was quite poor, with only 23 species (Figure 2). Already by spring 2021, 68 species had been identified (occurring on more than 20% of the territory’s five vegetated plots).
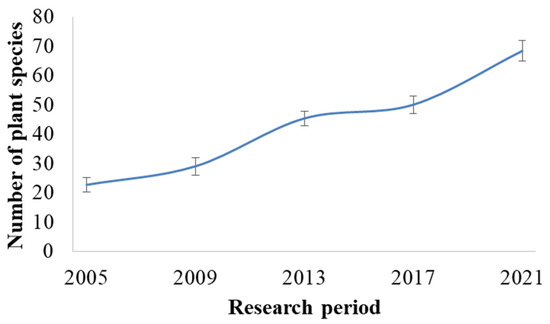
Figure 2.
Changes in vegetation in the area of the Lopatinsky phosphorite mine between 2005 and 2021. The values are presented as mean ± standard deviation (SD).
According to the monitoring results, five different plant communities were identified (Table 1). These plant communities were related to both general natural restoration techniques and environmental factors (mesoclimate and landscape influences), varying widely in terms of plant features. A very sparse (almost bare) and poor community was identified on unsaturated slopes with acid-cover substrates.

Table 1.
Species complex of identified plant communities on the territory of the Lopatinsky phosphorite mine in the spring-summer period 2005–2021.
In the south mining areas with the local climate effects and better-preserved surrounding vegetation, three different vegetation communities were identified on non-vegetated slopes covered with topsoil from abandoned terraced farmland. The three communities differed largely in vegetation cover, diversity, and age. The oldest ones (No. I and II) were more covered and more diverse. The composition of community No. III (slopes ≤ 150 m from natural seed sources) was characterized by different species of Gramineae, Fabaceae and Orchidaceae. At the end of the experiment, nearly all the plant communities on the slopes of the quarry were also represented by a new vegetation component, namely mosses. Throughout the entire experiment, there was the constant presence of five species: Plantago lanceolata, Phagnalon saxatile, Hyparrhenia hirta, Taraxacum officinale, Achillea millefolium. These species demonstrate rapid and multiple distribution and unpretentiousness to weather conditions. The dominant species in plant communities No. I and No. II were: Taraxacum campylodes, Lolium rigidum, Dactylis glomerata, Achillea millefolium. It is worth noting that during the succession processes, the qualitative composition of herbaceous plants has changed the most. More than 20 new species appeared in the first area, 15 appeared in the second, and fewer different plants appeared in the rest due to growth conditions.
Some new plants for certain areas were found in other areas, for example, Lolium rigidum was recorded in No. IV and No. V, and then in No. I. Galium mollugo grew in No. II, and after 16 years it was found in No. I and No. V. Phagnalon saxatile, which initially grew in No. III, spread to No. IV. Two plant communities (No. III and IV) were identified on slopes sown with herbaceous seed mixtures in the western and central mining areas. The mesoclimate is more continental, and the surrounding areas are degraded due to overgrazing. These groups were quite heterogeneous in terms of substrate, as cover and topsoil were used on the slopes where these communities emerged.
All four groups differed in terms of vegetation complexity and erosion-induced degradation. An active exterior surface runoff source primarily caused soil erosion processes on these slopes. A highly erodible community type (No. V) was found on slopes of varying ages. Streamside defenses (channels and berms designed to isolate slopes from mountain paths and banks) were broken or absent in these areas. This community was characterized by shallow plant cover, represented by lichen and mosses (Table 1).
3.2. Physical and Chemical Properties of Soil from the Lopatinsky Phosphorite Mine
Mosses and lichens grew on more acidic soils containing a high level of carbon dioxide; in addition, these were the first plants to grow on poor soils. The acidity of the soil decreased during their growth, which made the environment suitable for herbaceous plants. These plant species, in turn, released carbon dioxide faster, causing the deciduous and coniferous tree species to appear. Soils collected from the slope (plot 5) ended up being the most acidic among the samples, with the acidity ranging from 4 to 5.2, whereas depressions (plot 2) had more alkaline soils, with a pH of 7.5 to 8.3. Accordingly, the concentration of carbon dioxide was inversely proportional to these figures (from 1.5 to 0.3%).
Successional processes in soils occur rather quickly, as can be judged from the results of studying the physical, chemical, and morphological properties of the samples. The minimum pH values were in the upper layers; these values were obtained for the high-mountain sites. All surveyed sites were characterized by a pronounced heterogeneous distribution of fractions in the profile. A large volume of coarse skeletal material containing relatively small fine-grained gravel was found at all sites. The variation in stoniness (from 7.0% in rehabilitated areas to 70% in self-directed funds) indicates a high heterogeneity of conditions in the mine. The results presented in Figure 3 depend heavily on the rehabilitation technique applied. The high CO2 concentrations recorded are a negative consequence of mining, which is most pronounced in carbonate rock. The level of CO2 release into the air can be determined based on the changes occurring with carbonates, as well as the level of respiratory processes in the soil cover (Figure 3).
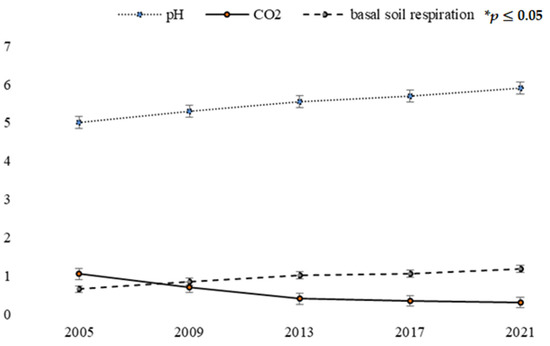
Figure 3.
Average physical and chemical properties of soil samples from the Lopatinsky phosphorite mine: pH, CO2 (%) and basal soil respiration rate (mg CO2/m2/h) in 2005–2021. The values are presented as mean ± standard deviation (SD). * values are statistically significant at p ≤ 0.05.
Carbon dioxide emissions are replaced by sequestration in soil and vegetation development. The latter’s rate can be estimated by the content of organic carbon in the soil. The basal respiration rate of the quarry soil was extremely low (about 0.4 mg CO2/m2/h). The main sources of carbon dioxide in the air are weathered carbonates, and the content of CO2 varied from 0.06 to 0.9%.
4. Discussion
Vegetation change is a normal process of succession in all ecosystems, including those affected by adverse natural or human influences. The primary plants that occur during this process are mosses and lichens, sometimes grasses, which begin to populate poor soils, thereby improving their fertility, as in the case of plots 3 to 5. Based on this research, the aforementioned plots were arguably in the first states of the succession process. Plot 2 was populated predominantly by coniferous trees, which signifies the transition process. There were deciduous trees detected in plot 1, which suggests a considerably advanced succession process and biotope renewal [31]. It follows from this that slopes are slower to regenerate, probably due to high levels of soil erosion. Plains and depressions are more suitable for plants, as evidenced by the rapid growth of tree species.
In the Lopatinsky phosphorite mine region, the demutational structure of plant communities is mainly determined by the initial mountain slope conditions and the climate-related environmental gradient. Similar results have been obtained for European anthropogenic habitats, where initial conditions of altered areas (particularly soil pH) and mesoclimatic differences have been found to determine succession patterns [8].
For soil chemistry, the temporal progression of the succession was linked to soil pH levels (Figure 3). Vegetation migration, settlement, and species replacement in acidic areas were severely restricted (Figure 2). This is likely due to the absence of relevant species in nearby propagation sources (which grow on major soils) and the occasional toxicity associated with extremely acidic substrates.
Similar natural recovery stresses related to soil acidity and toxicity have often been found in mining operations [11]. At the Lopatinsky mine, this problem primarily affects abandoned mines that operated until their final closure in 1993. To date, local mining companies have not applied a substrate selection procedure to cover weathered surfaces.
In the absence of significant stresses due to soil characteristics, the climate and well-preserved surrounding vegetation have been responsible for various succession patterns. However, other factors may also play a role in differentiating successions and subsequent community transitions identified at the mine sites under review. These slopes were restored with high-quality topsoil and surrounded by preserved patches of forest with the European hornbeam (Carpinus betulus L.), English oak (Quercus robur L.), Siberian spruce (Picea obovata Ledeb.), and a diverse shrub community. It is especially important for restored mine sites, where the community structure is generally limited by long distances from seed sources by reducing the dispersal capacity of species [32,33].
These grass mixes have usually been used to simply “green the slopes” in order to meet legal requirements regarding soil erosion and water quality control. As a result, the succession was suspended on most of these vegetated slopes, resulting in a persistent herbaceous community (No. 3) heavily dominated by Fabaceae and grasses. Similarly, it has commonly been reported that revegetation by fast-growing herbaceous species may impede long-term vegetation development due to competition from spontaneous colonizers [34]. This result highlights the need to modify the composition of vegetation seed mixes using native species. These mixes should include some successful colonizing shrub species that may be limited by the availability and remoteness of natural propagule sources.
Successional processes occurring with the soil cover are primarily related to the dynamics of physical, biochemical, and chemical changes, as well as changes occurring within the underlying carbonate rock strata. The intensive weathering of limestone debris contributes to an important content of fine soils (the only exception being a stony bottom), increasing the water content and fertility [35]. These authors found that an increase in the concentration of organic matter, followed by acidification, is the main factor that determines soil formation processes. These processes resulted in the formation of horizons with maximum depth and maximum organic carbon content in the cumulative ecotype with optimum wetting characteristics and physical conditions that characterize the substrate. Also noted is the decreasing difference over time between the soil layers below the surface and the deeper soil layers, which may be due to the increasing concentration of organic matter occurring over time [36].
The present study results are similar to those of other researchers, who have found increases in biodiversity in abandoned quarries in different climatic zones and geographic locations. In particular, quarries can serve as refuges for some animal species as well as habitats for plants [37,38]. This is due to the fact that there is a diversity of landscape types in quarries, thus expanding the number of unoccupied ecological niches. In other words, quarries that do not work are a factor in increasing biodiversity [1].
The demutational changes in vegetation in different types of soils and rocks inherent in different quarries were investigated in detail. Much of the research has focused on vegetation succession [39,40]; some studies have investigated changes in soil cover [41]. However, there are few comprehensive observations on the re-establishment of land cover and connectivity of these components. The appearance of mycorrhizal plants, which include orchids and legumes, was also noted for the areas studied. This is characteristic of natural regeneration processes, but can also be observed under unfavorable conditions, and may contribute to rapid primary succession [42,43].
5. Conclusions
This document describes the results of phosphorus mine surveys to determine demutational changes in plant communities over the spring–summer 2005–2021 period. Plot Nos. 1 and 2 were better covered and more diverse; community No. 3 (slopes ≤ 150 m from natural seed sources) was characterized by different species of grasses, legumes, and orchids; the bulk of the vegetation on the quarry slopes was represented with mosses; a highly erodible community type (No. 5) was characterized by shallow vegetation of algae, lichens, and mosses. The basal respiration rate of the pit soil was extremely low (about 0.4 mg CO2/m2/h). The primary sources of carbon dioxide emissions into the air were decayed carbonates. The content of CO2 in carbonate varies from 0.06 to 0.9%. Scientific studies have shown that a significant portion of the mine can be recovered through self-mediation.
Author Contributions
Conceptualization, methodology, software, validation, writing—review and editing, supervision, D.G.; formal analysis, investigation, resources, data curation, writing—original draft preparation, visualization, project administration, I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Talento, K.; Amado, M.; Kullberg, J.C. Quarries: From abandoned to renewed places. Land 2020, 9, 136. [Google Scholar] [CrossRef]
- Gull, A.; Ahmad Lone, A.; Ul Islam Wani, N. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; BoD—Books on Demand: Hamburg, Germany, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Calzada Olvera, B. Innovation in mining: What are the challenges and opportunities along the value chain for Latin American suppliers? Miner Econ. 2022, 35, 35–51. [Google Scholar] [CrossRef]
- Qi, Z.; Han, Y.; Afrane, S.; Liu, X.; Zhang, M.; Crittenden, J.; Chen, J.L.; Mao, G. Patent mining on soil pollution remediation technology from the perspective of technological trajectory. Environ. Pollut. 2023, 316, 120661. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Huang, W.; Han, H.; Xu, C. Pollution control of wastewater from the coal chemical industry in China: Environmental management policy and technical standards. Renew. Sust. Energ. Rev. 2021, 143, 110883. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, J.; Chen, F.; Li, X.; Zhang, Q.; Yang, Y. Adaptive development of soil bacterial communities to ecological processes caused by mining activities in the Loess Plateau, China. Microorganisms 2020, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; Zedler, J.B.; Falk, D.A. Foundations of Restoration Ecology; Island Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Robinson, D.A.; Panagos, P.; Borrelli, P.; Jones, A.; Montanarella, L.; Tye, A.; Obst, C.G. Soil natural capital in Europe; a framework for state and change assessment. Sci. Rep. 2017, 7, 6706. [Google Scholar] [CrossRef] [PubMed]
- Pickett, S.T.A.; Collins, S.L.; Armesto, J.J. A hierarchical consideration of causes and mechanisms of succession. Vegetatio 1987, 69, 109–114. [Google Scholar] [CrossRef]
- Li, J.; Nie, M.; Powell, J.R.; Bissett, A.; Pendall, E. Soil physico-chemical properties are critical for predicting carbon storage and nutrient availability across Australia. Environ. Res. Lett. 2020, 15, 094088. [Google Scholar] [CrossRef]
- González-Martínez, A.; de Simón-Martín, M.; López, R.; Táboas-Fernández, R.; Bernardo-Sánchez, A. Remediation of potential toxic elements from wastes and soils: Analysis and energy prospects. Sustainability 2019, 11, 3307. [Google Scholar] [CrossRef]
- Easdale, M.H.; Fariña, C.; Hara, S.; León, N.P.; Umaña, F.; Tittonell, P.; Bruzzone, O. Trend-cycles of vegetation dynamics as a tool for land degradation assessment and monitoring. Ecol. Indic. 2019, 107, 105545. [Google Scholar] [CrossRef]
- Kogo, B.K.; Kumar, L.; Koech, R. Impact of land use/cover changes on soil erosion in Western Kenya. Sustainability 2020, 12, 9740. [Google Scholar] [CrossRef]
- Szabó, J.A.; Centeri, C.; Keller, B.; Hatvani, I.G.; Szalai, Z.; Dobos, E.; Jakab, J. The use of various rainfall simulators in the determination of the driving forces of changes in sediment concentration and clay enrichment. Water 2020, 12, 2856. [Google Scholar] [CrossRef]
- Morbidelli, R.; Corradini, C.; Saltalippi, C.; Flammini, A.; Dari, J.; Govindaraju, R. Rainfall infiltration modeling: A review. Water 2018, 10, 1873. [Google Scholar] [CrossRef]
- Mohammed, S.; Al-Ebraheem, A.; Holb, I.J.; Alsafadi, K.; Dikkeh, M.; Pham, Q.B.; Szabo, S. Soil management effects on soil water erosion and runoff in Central Syria— A comparative evaluation of general linear model and random forest regression. Water 2020, 12, 2529. [Google Scholar] [CrossRef]
- Bingemer, J.; Pfeiffer, M.; Hohberg, K. First 12 years of tardigrade succession in the young soils of a quickly evolving ecosystem. Zool. J. Linn. Soc. 2020, 188, 887–899. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Liu, X. Index system to evaluate the quarries ecological restoration. Sustainability 2018, 10, 619. [Google Scholar] [CrossRef]
- Pourbabaei, H.; Salehi, A.; Ebrahimi, S.S.; Khodaparast, F. Variations of soil physicochemical properties and vegetation cover under different altitudinal gradient, western Hyrcanean forest, north of Iran. J. For. Sci. 2020, 66, 159–169. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Sierka, E.; Dyderski, M.K.; Bierza, W.; Magurno, F.; Besenyei, L.; Błońska, A.; Ryś, K.; Jagodziński, A.M.; Woźniak, G. Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Halecki, W.; Klatka, S. Application of soil productivity index after eight years of soil reclamation with sewage sludge Amendments. Environ. Manag. 2021, 67, 822–832. [Google Scholar] [CrossRef]
- Fischer, C.; Tischer, J.; Roscher, C.; Eisenhauer, N.; Ravenek, J.; Gleixner, G.; Hildebrandt, A. Plant species diversity affects infiltration capacity in an experimental grassland through changes in soil properties. Plant Soil 2014, 397, 1–16. [Google Scholar] [CrossRef]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef]
- Grainger, M.J. An Evaluation of Coastal Dune Forest Restoration in Northern KwaZulu-Natal, South Africa. Doctoral dissertation, University of Pretoria, Pretoria, South Africa, 2011. [Google Scholar]
- Kuter, N. Reclamation of degraded landscapes due to opencast mining. In Advances in Landscape Architecture; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Kim, D.; Kupfer, J.A. Tri-variate relationships among vegetation, soil, and topography along gradients of fluvial biogeomorphic succession. PLoS ONE 2016, 11, e0163223. [Google Scholar] [CrossRef] [PubMed]
- Biber, P.; Seifert, S.; Zaplata, M.K.; Schaaf, W.; Pretzsch, H.; Fischer, A. Relationships between a substrate, surface characteristics, and vegetation in an initial ecosystem. Biogeosciences 2013, 10, 8283–8303. [Google Scholar] [CrossRef]
- Beretta, A.N.; Silbermann, A.V.; Paladino, L.; Torres, D.; Bassahun, D.; Musselli, R.; García-Lamohte, A. Soil texture analyses using a hydrometer: Modification of the Bouyoucos method. Cienc. Investig. Agrar. 2014, 41, 25–26. [Google Scholar] [CrossRef]
- Dettori, R.; Donadio, D. Carbon dioxide, bicarbonate and carbonate ions in aqueous solutions under deep Earth conditions. Phys. Chem. Chem. Phys. 2020, 22, 10717–10725. [Google Scholar] [CrossRef] [PubMed]
- de Smith, M.J. Statistical Analysis Handbook: A Comprehensive Handbook of Statistical Concepts, Techniques and Software Tools; The Winchelsea Press, Drumlin Security Ltd.: Edinburgh, Scotland, 2018. [Google Scholar]
- Chen, F.; Yang, Y.; Mi, J.; Liu, R.; Hou, H.; Zhang, S. Effects of vegetation pattern and spontaneous succession on remediation of potential toxic metal-polluted soil in mine dumps. Sustainability 2019, 11, 397. [Google Scholar] [CrossRef]
- Jordano, P. What is long-distance dispersal? And a taxonomy of dispersal events. J. Ecol. 2016, 105, 75–84. [Google Scholar] [CrossRef]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Kingsford, R.T. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 2011, 99, 1299–1307. [Google Scholar] [CrossRef]
- Hagen, D.; Evju, M. Using short-term monitoring data to achieve goals in a large-scale restoration. Ecol. Soc. 2013, 18, 29. [Google Scholar] [CrossRef]
- Nguemezi, C.; Tematio, P.; Yemefack, M.; Tsozue, D.; Silatsa, T. Soil quality and soil fertility status in major soil groups at the Tombel area, South-West Cameroon. Heliyon 2020, 6, e03432. [Google Scholar] [CrossRef]
- Poirier, V.; Basile-Doelsch, I.; Balesdent, J.; Borschneck, D.; Whalen, J.K.; Angers, D.A. Organo-mineral interactions are more important for organic matter retention in subsoil than topsoil. Soil Syst. 2020, 4, 4. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Isbell, F.; Purves, D.W.; Loreau, M.; Hector, A. Understanding the value of plant diversity for ecosystem functioning through niche theory. Proc. Biol. Sci. 2016, 283, 20160536. [Google Scholar] [CrossRef] [PubMed]
- Zaplata, M.K.; Dullau, S. Applying ecological succession theory to birds in solar parks: An approach to address protection and planning. Land 2022, 11, 718. [Google Scholar] [CrossRef]
- Garófano-Gómez, V.; Metz, M.; Egger, G.; Díaz-Redondo, M.; Hortobágyi, B.; Geerling, G.; Corenblit, D.; Steiger, J. Vegetation succession processes and fluvial dynamics of a mobile temperate riparian ecosystem: The lower Allier River (France). Biogéomorphologie 2017, 23, 187–202. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, E.; Lee, E.; Lee, S.; Cho, K.; Lee, Y.; Chung, S.; Jeong, H.; You, Y. Characteristics of vegetation succession on the Pinus thunbergii forests in warm temperate regions, Jeju Island, South Korea. J. Ecol. Environ. 2019, 43, 44. [Google Scholar] [CrossRef]
- Egli, M.; Hunt, A.G.; Dahms, D.; Raab, G.; Derungs, C.; Raimondi, S.; Yu, F. Prediction of soil formation as a function of age using the percolation theory approach. Front. Environ. Sci. 2018, 6, 108. [Google Scholar] [CrossRef]
- Krüger, C.; Kohout, P.; Janoušková, M.; Püschel, D.; Frouz, J.; Rydlová, J. Plant communities rather than soil properties structure arbuscular mycorrhizal fungal communities along primary succession on a mine spoil. Front. Microbiol. 2017, 8, 719. [Google Scholar] [CrossRef]
- Zaplata, M.K.; Winter, S.; Fischer, A.; Kollmann, J.; Ulrich, W. Species-driven phases and increasing structure in early-successional plant communities. Am. Nat. 2013, 181, E17–E27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).