Abstract
Marine riparian areas and coastal vegetation are essential and important to the coastal marine ecosystem, although their interactions and functions are still unknown and ignored in marine ecological studies and integrated management planning. In southeastern Brazil, allochthonous resources derived from riparian Atlantic rainforests bordering rocky shores have been observed in abundance together with the shallow subtidal rocky reef benthos. In this study, we used stable isotopes (δ13C and δ15N) to characterize the main components in a benthic trophic web on a shallow tropical rocky shore, to identify the proportional contributions of allochthonous (marine riparian vegetation—MRV) to autochthonous (phytoplankton and algae) inputs and to test which basal food resources contributed most to the marine community on the Atlantic Forest–rocky coast interface. We found eight major food resources and seventeen consumers that we classified into different groups according to their feeding habits and biology. Although the main source of basal resources in the benthic trophic web in the present study remained autochthonous, the allochthonous resources were assimilated by all consumers. MRV is thus an important resource for some primary consumers and it should be included as a potential source of basal resources in marine ecosystems adjacent to marine riparian areas.
1. Introduction
At the border between land and sea, the coastal zone is the region where numerous biological, chemical, physical, geological and meteorological interactions occur and form gradients and where primary production is concentrated [1]. Although terrestrial vegetation, marine phytoplankton and marine macrophytes are parts of distinct environments, they may all represent basal food resources in a coastal marine ecosystem. In some coastal ecosystems, both terrestrially derived (allochthonous) vegetation and autochthonous basal food resources may contribute to the available stock of organic matter. Terrestrial plants have lower energy values and lower protein content (especially dead leaves fallen from the trees) [2,3], while algae have a higher proportion of proteins than plants because they require less fiber for support [4]. Therefore, debris from terrestrial vegetation is less palatable and nutritious to marine species than algae. In mangrove systems, for example, although leaves are much more abundant than other primary resources, their nutritional value is low and they are difficult to digest [5]. Notwithstanding, the bulk of leaves is a significant contribution to the energy budget.
Riparian areas (defined as those bordering water bodies) are highly studied systems as there is a clear linkage between land and stream and freshwater ecosystem functions and wetland ecology [6,7,8,9,10]. In contrast, marine riparian areas, land bordering the sea, have received scant attention [11,12]. When marine–riparian interactions have been studied, they have mainly focused on marine influences on terrestrial systems, such as marine derived nitrogen enrichment by migrating salmon [13], rather than vice versa. It is known that in certain situations input from terrestrial systems, such as mangroves, saltmarshes, etc., to the adjacent marine area can provide connectivity which enhances invertebrate and vertebrate development in the sea [14,15,16]. Although marine riparian systems have not been the subject of much scientific investigation, studies suggest that these systems serve similar functions, regardless of their marine or freshwater context [11]. Like shoreline vegetation, marine riparian areas are essential and important to the nearshore marine ecosystem, however their interactions and functions are still largely unknown and are ignored in marine ecological studies and integrated management planning [11,12,17]. In addition to social benefits, marine riparian areas provide a variety of ecological functions that integrate the marine ecosystem, such as soil and slope stability, sediment control, wildlife habitat, microclimate formation, water quality improvement, nutrient input and fish prey production and habitat structure and shading provision [11].
Although the nutritional quality and palatability of autochthonous resources, such as marine algae, and allochthonous resources, such as terrestrial vegetation detritus, are recognized, studies on the contribution of marine riparian areas to coastal tropical marine systems are still scarce. Although the Atlantic Forest is the second largest rainforest in South America and an important global biome that is home to 35% of Brazil’s biodiversity, with high levels of endemism, few have previously carried out observations of the availability of allochthonous resources derived from Atlantic marine riparian forests [18,19,20,21]. Building upon this, the present study aims to use δ13C and δ15N to characterize the benthic food web in a shallow tropical rocky subtidal coast, estimate the proportional contributions of allochthonous (marine riparian vegetation—MRV) and autochthonous (phytoplankton and algae) food resources to consumers and evaluate which basal food resource contributed the most to the marine community studied at the Atlantic rainforest–coastal interface in Brazil.
2. Materials and Methods
2.1. Study Area
The Ilha Grande Bay (IGB) (22°50′–23°20′ S, 44°00′–44°45′ W) (Figure 1A), located in the south of the state of Rio de Janeiro, Brazil, has a perimeter of approximately 350 km of coastline [22] bordered by the Atlantic rainforest (Figure 1B). This region consists of two bodies of water separated by a constriction between the island and the mainland and is divided into three regions: West Portion, Central Channel and East Portion [23]. The IGB has the largest number of protected areas in the state of Rio de Janeiro [24]. Considered a biodiversity hotspot, IGB has a rich benthic flora and fauna and it is considered a priority area for the conservation of coastal and marine zones [22]. The oceanographic, hydrodynamic and physiographic characteristics of the region and connectivity of coastal ecosystems, input of allochthonous organic matter (Figure 1C,D) and variation of physical and chemical factors are all responsible for its high beta diversity [25] and singular biodiversity [22].
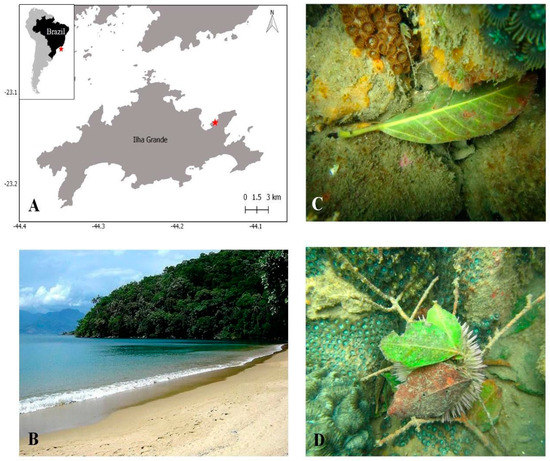
Figure 1.
The study site at Abraãozinho, Ilha Grande Bay (IGB), Brazil: (A) the location of the study region and site (red star); (B) photograph illustrating the marine riparian vegetation formed by Atlantic rainforest adjacent to the rocky shore; (C) a leaf on the reef derived from marine riparian vegetation; (D) the sea urchin (Lythechinus variegatus) exhibiting covering behavior using leaves derived from an adjacent marine riparian area composed of Atlantic rainforest.
The present study was conducted in Spring 2014 on a rocky shore located in Praia do Abraãozinho (23°08′07″ S, 44°09′05″ W), Ilha Grande, IGB. The shallow wave protected rocky shore, which ends in a sand plain at a depth of ≤7 m, has the Atlantic rainforest as its MRV (Figure 1).
2.2. Field Sampling
The species that had their isotopic compositions analyzed in this study were the most abundant at the study site. The relative abundance of the benthos was estimated by SCUBA diving on the rocky shore at 1–3 m depth; we placed replicate quadrats over the reef and estimated cover (sessile) and density (vagile) species of the benthos in each to calculate mean abundances. We identified and selected the taxa or functional groups with the highest mean percentage cover or density for further studies [26].
The most abundant species of primary producers and macroconsumers were collected in December 2014 for stable isotope analysis of C and N. Three replicate individual/colonies of each species were collected, except for the phytoplankton, zooplankton and the ophiuroid Ophiothela mirabilis that, due to their size <1 cm, were collected in greater quantity. For the algae Padina gymnospora and the ophiuroid Ophioderma cinereum, only two replicates were collected. For a uniform isotopic value and low turnover rates, we used different collection methods that made it possible to collect muscle tissue or calcium carbonate-free samples [27,28,29]. The encrusting benthic species were removed with the help of hammer and chisel (corals and bryozoans), scissors (sponges and algae) and spatula (ascidians and anemones). Planktonic organisms were collected with nylon plankton nets (25 µm to collect phytoplankton and 68 µm to collect zooplankton). The sediment samples were collected with the aid of a pot and spatula. Leaf litter from the fallen MRV was also collected underwater near the base of the rocky shore. All samples were identified at species level, except for phytoplankton and zooplankton. All samples were frozen immediately after collection and taken to be sorted and prepared in the laboratory.
2.3. Sample Processing for Isotope Analysis
After defrosting the samples, whole collected macroconsumers were screened again to remove the muscle tissue (sea urchins, sea cucumbers, crustaceans and gastropods), the corals had their tissue scraped off, while very small animals, such as ophiuroids, were kept whole and grouped to compose a sample. Sponges, ascidians and anemones had their soft tissue selected. We removed any epiphytes or epizoa of the sampled animals and macroalgae. Sediment samples were filtered with 0.7 µm GF/F filters (Whatman, Maidstone, Kent, UK). All samples were washed with distilled water so that any contaminants that could influence the isotopic compositions were removed. The samples were oven dried for 48 h at 60 °C. After drying, they were macerated and reduced to a fine powder. We acidified the phytoplankton and zooplankton, the calcified macroalgae Jania adhaerens and the ophiuroid O. cinereum before carbon isotopic analysis to remove carbonate [29]. To remove CaCO3 through acidification, 1 mol L−1 of hydrochloric acid (HCl) was added drop by drop using a pipette, until no more CO2 was released. The carbonate radical in the presence of the H+ ion becomes unstable and decomposes, generating CO2, since this structure is more stable, producing the effervescence reaction when the carbonates are attacked by acids. The samples were then inserted in tin capsules, weighed on a digital balance and analyzed for their isotopic compositions of carbon and nitrogen at the Centro de Energia Nuclear na Agricultura da Escola Superior de Agricultura Luiz de Queiroz, São Paulo University, Brazil (CENA-USP).
Stable isotope analyses were performed using a Delta Plus Continuous Flow Isotope Ratio Mass Spectrometer (CF-IRMS, Finnigan MAT, Bremen, Germany) coupled to an elemental analyzer (CE Instruments Wigan, UK). The isotopic composition is expressed in terms of a delta value (δ) in parts per thousand (‰ or ppt) and is obtained according to the formula: δX = [(Rsample/Rstandard) − 1] · 103, where X is 13C or 15N and R the ratio 13C:12C or 15N:14N. The standard material for carbon was the Pee Dee Belamite limestone (PDB) and the standard material for nitrogen was atmospheric air [30]. The deviation standard of isotopic measurements was estimated at 0.09 for δ13C and 0.21 for δ15N by means of repeated measurements of the internal pattern (sugarcane).
2.4. Group Assignment and Data Analyses
Trophic groups were separated according to their possible food resources [30,31,32] (Table 1). Consumers were separated into guilds according to information about their diet, so resources that are not part of consumption were excluded from the analysis (i.e., we excluded resources that are not autotrophic from the analysis of herbivores). To carry out the separation of consumers into trophic groups, we searched for bibliographical references regarding known feeding habits and diet (Table 1). Using the δ13C and δ15N values of producer and consumer species, the contribution of autochthonous and allochthonous food resources to the consumer’s diet were estimated. Assimilations were also estimated by grouping consumers into trophic groups. For mixing model, the simmr package [33] in the R Program [34] was used. The simmr package is designed to infer dietary proportions of organisms consuming various food sources from observations on the stable isotope values taken from the organisms’ tissue samples. The analysis is based on a model that provides a combination of possible solutions that can explain consumer value, incorporating the variability of isotopic values of resources, consumer and fractionation. We assumed the isotopic fractionation of +0.5 ± 0.13‰ for δ13C and +2.3 ± 0.18‰ for δ15N for all animals [35]. We used concentration-dependent models because elemental carbon and nitrogen concentrations varied substantially between food sources [36]. The Brooks–Gelman–Rubin convergence diagnostic provides a numerical convergence summary based on multiple chains (Gelman diagnostic). The Gelman diagnostic values were all close to 1, ensuring satisfactory convergence of the mixing model results (Figures S1 and S2) [33].

Table 1.
Trophic groups analyzed in the present study separated according to their possible food resources.
We used the mean percentage contribution of autochthonous and allochthonous material for each taxon by trophic group to explore the consumption relationships of each group, compared the dietary proportions between MRV and each other food resources and estimated a direct probability that the MRV dietary proportion was larger than that of the other food resources. Three species of macroalgae, P. gymnospora, Cladophora sp. and H. musciformis, were grouped as macroalgae due to the similarity of their isotopic values; however, the calcareous macroalgae J. adhaerens was represented as a separate resource with regard to its isotopic value. Phytoplankton and zooplankton were both combined into what we termed plankton due to overlapping values [51]. Plankton was used for the mixing model of all groups that use both resources. We ran two mixing models for the sea anemone Bunodosoma caissarum, one including the herbivorous gastropod Littorina sp. and the crustacean M. hispidus as two independent food resources and another with both resources combined to reduce the number of resources in the anemone mixing model (“Bunodosoma caissarum model 2”).
3. Results
We identified seven major food resources, among them the MRV, marine sedimentary organic matter, phytoplankton, macroalgae and zooplankton. We also identified seventeen major consumers in the food web, among seven suspension feeders, one mixotroph, two deposit feeders, four herbivores and three omnivores (Table 1 and Table S1). MRV, which represents the allochthonous basal food resource, had the lowest isotopic value of δ13C (mean = −30.0; SD = ± 0.6; n = 3) and had a different signature than autochthonous marine resources, which allowed us to differentiate sources in the consumers. The calcareous algae J. adhaerens presented carbon isotopic values (−8.0; ±0.4; n = 3) that were different from other algae, so it separated from the main algae group (t-test for J. adhaerens and P. gymnospora: t = −18.009, df = 1.601, p = 0.008; J. adhaerens and Cladophora sp.: t = −37.391, df = 3.936, p < 0.0001; J. adhaerens and H. musciformis: = −27.674, df = 3.981, p < 0.0001). Overall, other marine resources showed similar δ13C values, including marine sedimentary organic matter (Figure 2 and Table S1). Food resources had the lowest average δ15N values (p. gymnospora: 4.5, ± 1.9, n = 3; Cladophora sp.: 6.7, ± 0.6, n = 3; H. musciformis: 6.4, ± 0.3, n = 3; J. adhaerens: 7.4, ± 0.3, n = 3), with the exception of phytoplankton and zooplankton (phytoplankton: 9.8 ± 1.9, n = 2; zooplankton: 8.5 ± 2.0, n = 2), and the lowest values were of MRV (terrestrial vegetation: 1.0 ± 0.5, n = 3). The herbivorous gastropod Littorina sp. and the omnivorous crustacean M. hispidus showed heterogeneous isotopic values, especially for the δ15N, consistent with the higher trophic level occupied by this crustacean in relation to the gastropod (Figure 2 and Table S1).
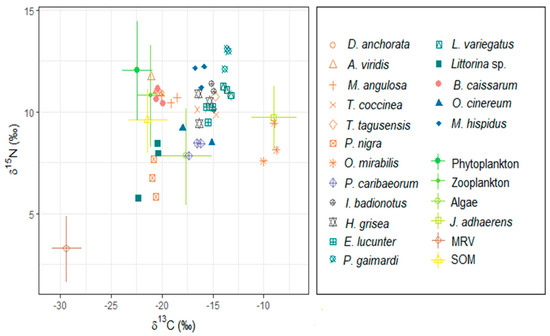
Figure 2.
Isotopic coordinates of δ15N and δ13C of sources and consumers from a tropical rocky shore at Ilha Grande Bay, Brazil. For resources, bars indicate SD around the mean. Each consumer has a color corresponding to the trophic group: orange—suspension feeder; purple—mixotroph; gray—deposit feeder; green—herbivores; pink—omnivores I; blue—omnivores II. Taxa code: MRV—marine riparian vegetation; SOM—marine sedimentary organic matter. For more details on trophic groups, see Table 1 and Table S1.
The solitary ascidian P. nigra had the lowest δ15N mean value (6.8 ± 0.9, n = 3), followed by the ophiuroid O. mirabilis (8.4 ± 1.0, n = 3) and the sponge M. angulosa (10.6 ± 0.2, n = 3). The highest δ15N mean value was of the sponge A. viridis (11.3 ± 0.4, n = 3) (Table S1). Of all the resources, plankton was the most assimilated by the suspension feeders, marine sponges D. anchorata, A. viridis and M. angulosa and ascidia P. nigra. The calcareous algae J. adhaerens was the resource most assimilated by Tubastraea corals and by the ophiuroid O. mirabilis. MRV was assimilated by all consumers and the ascidian P. nigra was the suspension feeder that most assimilated MRV, its second most assimilated resource, the first being plankton. The suspension feeders that had the lowest average MRV assimilation were the corals Tubastraea (Figure 3 and Table S2). The resource most assimilated by mixotroph P. caribaeorum was the calcareous algae J. adhaerens, followed by plankton and algae. MRV was the least assimilated resource (Figure 4 and Table S2).
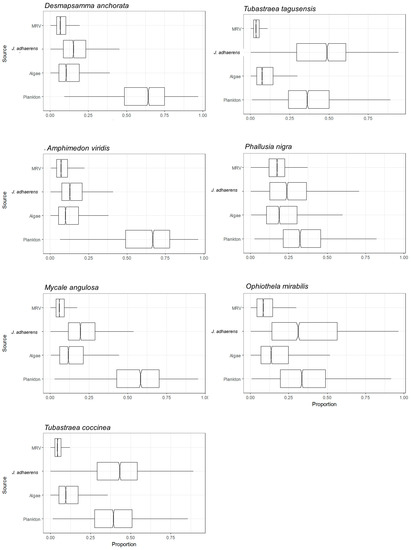
Figure 3.
Assimilation web of suspension feeder species estimated by mixing model of C and N stable isotopes from a tropical rocky shore at Abraãozinho, Ilha Grande Bay, Brazil. Taxa code: MRV—marine riparian vegetation.
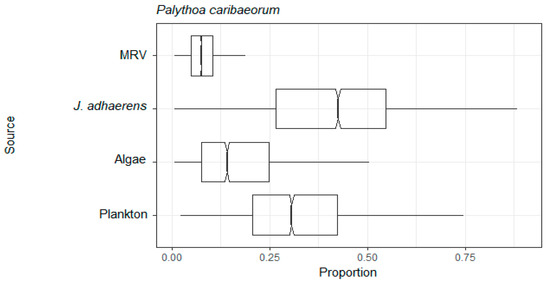
Figure 4.
Assimilation web of mixotroph Palythoa caribaeorum estimated by mixing model of C and N stable isotopes from a tropical rocky shore at Abraãozinho, Ilha Grande Bay, Brazil. Taxa code: MRV = marine riparian vegetation.
The assimilation of different resources was similar for consumers of the deposit feeding group, where the most assimilated resource was calcareous algae J. adhaerens, followed by sedimentary organic matter. MRV was the least assimilated resource (Figure 5 and Table S2). In the herbivore group, the least assimilated resource for sea urchins was MRV. They had the highest assimilation of the calcareous algae J. adhaerens, followed by algae, while for the gastropod Littorina sp., the most assimilated resource was algae (Figure 6 and Table S2). The average δ15N value of B. caissarum (omnivore group I) was greater than all potential resources, except for the crustacean M. hispidus (Figure 7 and Table S2). Even when combining the potential resources of gastropod Littorina sp. and crustacean M. hispidus to the mixing model of the B. caissarum, the percentages of assimilation remained similar to the mixing model with these two potential resources separated. Plankton was the resource most assimilated by the omnivore group I. The average assimilation of MRV and algae were higher than that of J. adhaerens, which were the least assimilated resources (Figure 7 and Table S2). Consumers in omnivore group II had a very similar proportional resource assimilation. The most assimilated resource in this group was the calcareous algae J. adhaerens. MRV was the least assimilated resource. For the crab, the gastropod Littorina sp. was the second most assimilated resource, and for the brittle star, algae was second most consumed resource, followed by the gastropod (Figure 8 and Table S2). The results of the mixing model by trophic groups (Table S3) showed that the average MRV assimilation varied from 4–8% for suspension feeders, deposit feeders, mixotrophs, omnivores II and herbivores to 11% for herbivores and for omnivores I. The mean MRV assimilation by trophic groups obtained from the mixing models by species varied from 7–10% for suspension feeders, mixotrophs and deposit feeders to 11–12% for herbivores and omnivores (Table S2).
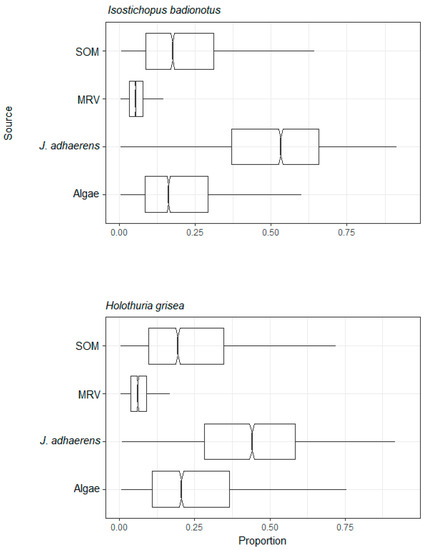
Figure 5.
Assimilation web of deposit feeder species estimated by mixing model of C and N stable isotopes from a tropical rocky shore at Abraãozinho, Ilha Grande Bay, Brazil. Taxa code: MRV = marine riparian vegetation; SOM = marine sedimentary organic matter.
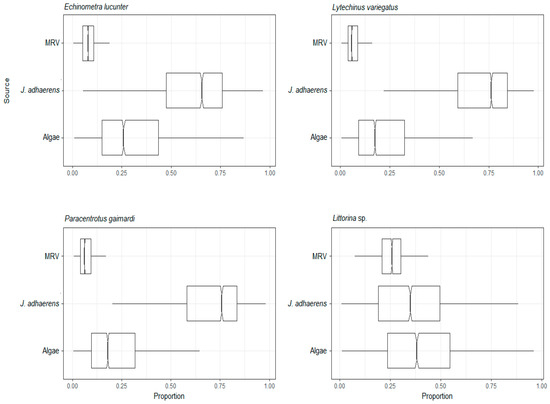
Figure 6.
Assimilation web of herbivorous species estimated by mixing model of C and N stable isotopes from a tropical rocky shore at Abraãozinho, Ilha Grande Bay, Brazil. Taxa code: MRV = marine riparian vegetation.
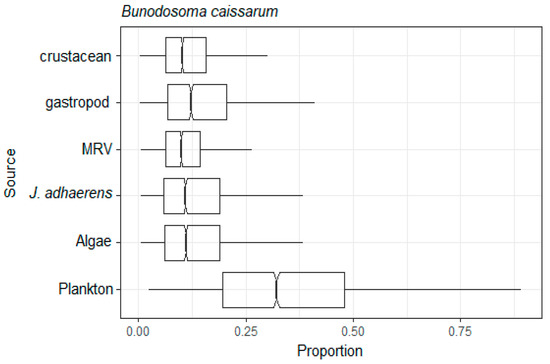
Figure 7.
Assimilation web of the omnivore Bunodossoma caissarum estimated by mixing model of C and N stable isotopes from a tropical rocky shore at Abraãozinho, Ilha Grande Bay, Brazil. Taxa code: MRV = marine riparian vegetation.
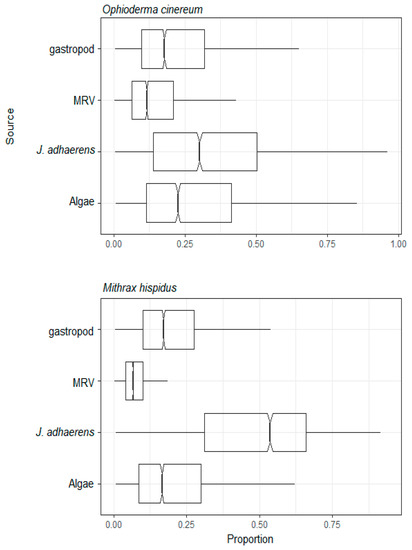
Figure 8.
Assimilation web of omnivores group II estimated by mixing model of C and N stable isotopes from a tropical rocky shore at Abraãozinho, Ilha Grande Bay, Brazil. Taxa code: MRV = marine riparian vegetation.
4. Discussion
This study provides novel insights into the organization and trophic structure of food webs on tropical rocky shores, the identification and relative importance of different basal resources, the contribution of marine riparian vegetation in marine systems at the land–sea interface and trophic niche organization within such communities. All the resources and consumers considered in the present study are quite abundant along the studied tropical rocky shores, which justifies the consideration of each one as a potential food resource [22].
MRV, which represents allochthonous resource, had a lower isotopic value of δ13C than marine resources. We confirmed the lowest values of δ13C in MRV, similar to results found in other studies, including δ13C values from Atlantic rainforest tree leaves [52,53,54,55,56]. The entry of terrestrial organic matter has important consequences for the dynamics of benthic coastal communities [57,58,59]. Depending on its origin, organic matter which may be imported from the terrestrial environment or locally produced by primary producers differs substantially in its biochemical composition and availability to consumers. The exchange of organic matter across ecosystem boundaries has important consequences for the availability of matter and energy, as it contributes to sediment deposition and mineralization and serves as food for the fauna [60]. In contrast to MRV, algae commonly present the highest δ13C values among primary producers [52,53].
As major primary producers on rocky reefs, we expected macroalgae (here those other than Jania adhaerens, which is treated below) to be highly assimilated by some consumers, especially due to the palatability, nutritional value and abundance that make macroalgae an important and highly available resource. For example, in flat and shallow rocky reefs in temperate regions, benthic macrophytes such as kelps may dominate organic matter input [61,62,63]. In fact, macroalgae matter was assimilated to some extent by most consumers but higher assimilation proportions were only found in the herbivorous gastropod Littorina sp. (see below) and the sea urchin Echinometra lucunter. This fact might explain why macroalgae were assimilated in greater proportion by E. lucunter than in the other sea urchins. This species is a rock-borer, often inhabiting burrows scraped with its spines and teeth on rocky substrata [64], which limits its range [65]. It thus depends mainly on a supply of a wide range of larger drifting fragments of algae that are caught up in the holes on the incoming tide or actively caught [66,67].
The present study suggests that although MRV is not the main source of energy for consumers under the waves, it can be consumed to some extent by animals, albeit in different proportions. The results (mean and dispersion) of the mixing model by trophic groups were similar to those of the mixing models by species. The latter was more informative for explaining the differences in assimilation between species of the same trophic group. The primary marine sources of organic matter are phytoplankton, macroalgae and marine magnoliophytes, the first being the dominant resource in oceanic regions. Organic matter in the sea can also be of terrestrial origin, transported to the sea by continental drainage or wind [68]. The southeastern coast of Brazil is characterized by its proximity to the Atlantic rainforest, which contributes debris from terrestrial vegetation to the nearshore coastal ecosystem, especially during the rainy season [69]. In fact, Atlantic rainforest MRV has been estimated to have a mean litterfall of 20–125 g dry weight.m−2.month−1 [70], the upper limit being similar for mangroves in the region [71]. On the studied rocky reefs, large particles of MRV (leaves, twigs, fruits, flowers and their fragments) mainly accumulate (or are most visible) on the bottom nearby the interface with the sandy plain. This material is thus available for mobile herbivores/detritivores, and as it decomposes into smaller fragments, it may also become re-suspended in the water column as particulate organic matter [72]. The large solitary ascidian P. nigra and the gastropod Littorina sp., even though they are part of different trophic guilds, had higher average assimilation of MRV than assimilation of the other resources. Ascidians are suspension feeding organisms while Littorina sp. is a predominantly herbivorous gastropod. However, littorinid snails are known to also consume both angiosperm detritus and the fungi colonizing dead plants, making refractory angiosperm material available as feces to other organisms [73] as well as browsing directly on the surface of rock, inadvertently ingesting detritus [74]. Algae have a higher proportion of protein than plants [4] and although they were a clearly important resource for Littorina sp., in our study, MRV still made up one quarter of their assimilation despite the low nutritional quality and palatability.
Suspension feeders have morphological structures capable of capturing suspended particles as a potential source of food, either items large enough to be seized individually or smaller particles obtained in sufficient quantity through filter feeding [69,75]. Four of the seven suspension feeders, the ascidian and all the sponges had plankton as their main assimilated resource. Plankton was also the main resource for the anemone B. caissarum. The second resource most assimilated by the suspension feeders was the articulated calcareous algae J. adhaerens.
Jania adhaerens was a major and key resource for all three herbivorous sea urchins, the suspension feeding corals Tubastraea and brittle star O. mirabilis, the mixotrophic P. caribaeorum, the deposit feeding sea cucumbers, the omnivorous crab M. hispidus and brittle star O. cinereum. J. adhaerens is a major component of the intertidal to shallow subtidal benthos of the region [76,77] and it is an important component of the algal turf. Algal turfs are a typically low algae layer, several mm to cm high [78], which are a highly productive algal group in the tropics [79,80]. These turfs are ubiquitous on rocky reefs and shores throughout the region [76] and known to cover 29–65% of the shallow subtidal benthos in IGB [81,82]. This species has also been reported as one of the main foods consumed by herbivorous sea urchins [83], as was the case here for the three studied sea urchins. As well as the sea urchins, other vagile invertebrates such as Mithracidae crabs are known to be adapted to consume a wide variety of algae, including calcareous species that commonly co-occur in these habitats [50,84]. In the case of Mithracidae, some species of these crabs are cleaners of fouling algae from hosts to which they associate to escape predation. In the case of mixotrophic P. caribaeorum and the other suspension feeders, it is known that epithelial cells of the coralline red algae in general and J. adhaerens specifically constantly become senescent, are shed and replaced [85], a mechanism that promotes better nutrient uptake and epiphyte avoidance. This, together with breakage due to wave action, are probably major mechanisms that are responsible for making a supply of ≥ single-cell-sized particles of J. adherens available as particulate material in the water column and accessible to suspension feeders.
In isotopic space, as shown in Figure 2, the δ13C values of the sedimentary organic matter were central when compared to the range which the other resources presented (from Jania adhaerens to MRV), which may suggest a contribution from multiple sources for the organic matter in the sediment. Marine sediment is usually partially composed of marine derived organic matter but may include terrestrially derived organic matter as well. Terrestrially derived organic matter is higher in marine sediments near the coast, and even seabed sediments deposited in areas remote of the continents may also contain a mixture of organic matter derived from marine and terrestrial processes [86]. It is interesting to note that despite this the deposit feeding sea cucumbers, which inhabit the base of the reef at the sand–rock interface and consume large amounts of sedimentary organic matter, predominantly derived their resources from Jania adhaerens, which is a highly abundant part of the turf community [22].
Considering that the omnivorous sea anemone B. caissarum can consume invertebrates, we considered six potential resources [47]. However, other omnivores such as the crab M. hispidus had a higher δ15N value than B. caissarum, which indicates that this resource is being consumed in a low proportion. An animal’s 15N:14N ratio is more enriched by 15N than by its food resource, since nitrogen compounds with 14N are excreted more quickly than those with 15N [87]. We determined that plankton was the major resource most assimilated by B. caissarum but it is important to highlight that for this species the MRV and macroalage also had higher average assimilation than autochthonous resources such as J. adhaerens.
The mixotroph (the mat forming zoantharian P. caribaeorum) obtained higher proportions of resources from the three autochthonous resources than the allochthonous one. This species is also ubiquitous on rocky reefs and shores throughout the region [88] and is known to cover 26–47% of the shallow subtidal benthos in IGB [82,83]. However, as a zooxanthellate organism, it is unclear how much of its energy is obtained from autotrophy rather than heterotrophy through the feeding on suspended material. Certainly, in corals, some of the products of photosynthesis are transferred to the animal host and in some cases, these can provide the majority of the host’s carbon requirements (60–70% for reef corals [89,90]). However, in a comparative study, Sebens (1977) [91] noted that P. caribaeorum had more food items than other zoantharians and so the contribution of heterotrophy in P. caribaeorum might be somewhat higher.
We estimate that the contribution from the marine riparian vegetation bordering the tropical rocky shore we studied was pervasive, species specific and part of the resources assimilated by benthic consumers. However, this study represents a single observation and was not replicated spatially and temporally, as it was only carried out in the summer season, which hinders the ability to infer seasonal relationships in general. We would recommend further studies of this type be carried out to better examine the generality of our results over space and in systems with other benthic species and community structures. Other coastal ecosystems such as mangroves, estuaries and sandy beaches also receive debris from bordering terrestrial vegetation. Our results corroborate that the contribution of autochthonous resources seems to be the main source in coastal zones [54,92], mangroves [53,93,94,95,96], estuaries [52,97] and sandy beaches [20], even though consumers depend on more than one source [54,98]. Most marine riparian areas still lack studies that show the contribution of their vegetation to the marine environment. It should also be considered that multiple resources (the number of sources greater than the number of isotopic types + 1) can influence the robustness of the assimilation results, since all solutions of the assimilated proportion are viable, the contributions of the most commonly used source are underestimated, while the contributions of less used sources are overestimated [31]. We emphasize therefore that the minimum and maximum MRV contributions for consumers were interpreted here, and the variation in the percentage contribution of each resource was reported with the credibility intervals.
In summary, this study provided new information on the trophic organization of the main components of the benthos along the riparian area including coastal vegetation, which are integral and important parts of the coastal marine ecosystem. The results indicate that among the autochthonous resources, algal turfs appear to be the more important basal resource but are not the only components that are part of the basal resources in the benthic food web. Allochthonous resources such as marine riparian vegetation (MRV) were assimilated by all consumers, were an important resource for some primary consumers and should be considered as a potential source of basal resources in other marine ecosystems adjacent to marine riparian areas. Furthermore, we suggest more research should be focused on the marine riparian–shore interface, including the composition of the bottom nearby the interface with the sandy plain and the MOP, as better knowledge will have implications for coastal management, our understanding of nutrient sources and coastal food chain structure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15060725/s1, Table S1: Mean values and standard deviation (SD) of isotopic carbon (δ13C) and nitrogen (δ15N) ratios of food resources and consumers on a tropical rocky reef adjacent a marine riparian area at Ilha Grande Bay, Brazil; Table S2. Mean of assimilation (%) of each resource per taxon on a shallow tropical rocky shore at Ilha Grande Bay, Brazil; Table S3. Mean of assimilation (%) of each resource per trophic group on a shallow tropical rocky shore at Ilha Grande Bay, Brazil. Figure S1: Diagnostic plots from the R simmr mixing model package with the result of the model fit for each species. Figure S2: Diagnostic plots from the R simmr mixing model package with the result of the model fit for each trophic group.
Author Contributions
Conceptualization, L.M.P.-T., V.N.-L. and J.C.C.; methodology, L.M.P.-T. and V.N.-L.; formal analysis, L.M.P.-T. and V.N.-L.; investigation, L.M.P.-T., V.N.-L. and J.C.C.; resources, V.N.-L. and J.C.C.; data curation, L.M.P.-T.; writing—original draft preparation, L.M.P.-T.; writing—review and editing, L.M.P.-T., V.N.-L. and J.C.C.; funding acquisition, J.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Ciências do Mar grant number 1137/2010; Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro grant number E-26/202.493/2019 and Universidade do Estado do Rio de Janeiro grant number PAPD-UERJ/2019. The APC was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico grant number 313698/2021-0.
Institutional Review Board Statement
The study was conducted in accordance with license number 005/2009 approved by Instituto Estadual do Ambiente—INEA on 18 December 2007.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
LMPT acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) that provided a PhD scholarship. We thank Amanda Guilherme da Silva, Juliana Magalhães Araujo and Mariana Pinto Aguiar for their support in the field.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Anthony, K.R.N.; Fabricius, K.E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 2000, 252, 221–253. [Google Scholar] [CrossRef]
- Boyd, C.E. Amino acid, protein, and caloric content of vascular aquatic macrophytes. Ecology 1970, 51, 902–906. Available online: https://www.jstor.org/stable/1933986 (accessed on 10 January 2023). [CrossRef]
- Platt, T.; Irwin, B. Caloric content of phytoplankton. Limnol. Oceanogr. 1973, 18, 306–310. [Google Scholar] [CrossRef]
- Bowen, S.H.; Lutz, E.V.; Ahlgren, M.O. Dietary protein and energy as determinants of food quality: Trophic strategies compared. Ecology 1995, 76, 899–907. [Google Scholar] [CrossRef]
- Ashton, E.C. Mangrove sesarmid crab feeding experiments in Peninsular Malaysia. J. Exp. Mar. Biol. Ecol. 2002, 273, 97–119. [Google Scholar] [CrossRef]
- Peterjohn, W.T.; Correll, D.L. Nutrient Dynamics in an Agricultural Watershed: Observations on the Role of a Riparian Forest. Ecology 1984, 65, 1466–1475. [Google Scholar] [CrossRef]
- Pusey, B.J.; Arthington, A.H. Importance of the riparian zone to the conservation and management of freshwater fish: A review. Mar. Freshw. Res. 2003, 54, 1–16. [Google Scholar] [CrossRef]
- Sandin, L.; Solimini, A.G. Freshwater ecosystem structure–function relationships: From theory to application. Freshw. Biol. 2009, 54, 2017–2024. [Google Scholar] [CrossRef]
- Neres-Lima, V.; Machado-Silva, F.; Baptista, D.F.; Oliveira, R.B.S.; Andrade, P.M.; Oliveira, A.F.; Sasada-Sato, C.Y.; Silva-Junior, E.F.; Feijó-Lima, R.; Angelini, R.; et al. Allochthonous and autochthonous carbon flows to secondary production in tropical forested streams. Freshw. Biol. 2017, 62, 1012–1023. [Google Scholar] [CrossRef]
- Tromboni, F.; Lourenço-Amorim, C.; Neres-Lima, V.; Thomas, S.A.; Silva-Araújo, M.; Feijó-Lima, R.; Silva-Júnior, E.F.; Healtherly, T., II; Moulton, T.P.; Zandonà, E. Conversion of tropical forests to agriculture alters the accrual, stoichiometry, nutrient limitation, and taxonomic composition of stream periphyton. J. Int. Rev. Hydrobiol. 2019, 104, 116–126. [Google Scholar] [CrossRef]
- Brennan, J.S.; Culverwell, H. Marine Riparian: An Assessment of Riparian Functions in Marine Ecosystems, 1st ed.; Washington Sea Grant Program: Seattle, WA, USA, 2005. [Google Scholar]
- Brennan, J.S. Marine Riparian Vegetation Communities of Puget Sound (No. TR-2007-02); Seattle District, U.S. Army Corps of Engineers: Seattle, WA, USA, 2007.
- Helfield, J.M.; Naiman, R.J. Keystone interactions: Salmon and bear in riparian forests of Alaska. Ecosystems 2006, 9, 167–180. [Google Scholar] [CrossRef]
- Bruno, D.O.; Riccialdelli, L.; Botto, F.; Acha, E.M. Organic matter sources for fish larvae and juveniles in a marine-estuarine interface (Mar Chiquita lagoon, Argentina). Environ. Biol. Fishes 2017, 100, 1609–1622. [Google Scholar] [CrossRef]
- Buelow, C.; Sheaves, M. A birds-eye view of biological connectivity in mangrove systems. Estuar. Coast. Shelf Sci. 2015, 152, 33–43. [Google Scholar] [CrossRef]
- Taylor, M.D.; Gaston, T.F.; Raoult, V. The economic value of fisheries harvest supported by saltmarsh and mangrove productivity in two Australian estuaries. Ecol. Indic. 2018, 84, 701–709. [Google Scholar] [CrossRef]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N.; et al. Global overview of ecosystem services provided by riparian vegetation. Bioscience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Oliveira, A.E.S.; Kurtz, B.C.; Creed, J.C. Fitossociologia e produção de serrapilheira em um trecho de Mata Atlântica, no Município de Angra dos Reis, RJ. BioFarm 2008, 2, 1–19. [Google Scholar]
- Colombini, I.; Chelazzi, L.; Gibson, R.N.; Atkinson, R.J.A. Influence of marine allochthonous input on sandy beach communities. Oceanogr. Mar. Biol. Annu. Rev. 2003, 41, 115–159. [Google Scholar]
- Garcia, A.M.; Oliveira, M.C.L.M.; Odebrecht, C.; Colling, J.L.A.; Vieira, J.P.; Rodrigues, F.L.; Bastos, R.F. Allochthonous versus atochthonous organic matter sustaining macroconsumers in a subtropical sandy beach revealed by stable isotopes. Mar. Biol. Res. 2019, 15, 241–258. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Martensen, A.C.; Metzger, J.P.; Tabarelli, M.; Scarano, F.; Fortin, M.J. The Brazilian Atlantic Forest: A shrinking biodiversity hotspot. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 405–425. [Google Scholar]
- Creed, J.C.; Pires, D.O.; Figueiredo, M.D.O. Biodiversidade Marinha da Baía da Ilha Grande, 1st ed.; Ministério do Meio Ambiente—MMA: Brasilia, Brazil, 2007; pp. 43–54.
- Mahiques, M.M.; Furtado, V.V. Utilização da análise dos componentes principais na caracterização dos sedimentos de superfície de fundo da baia da ilha grande (rj). Bol. Inst. Oceanogr. 1989, 37, 1–19. [Google Scholar] [CrossRef]
- INEA. Superintendência Regional Baía da Ilha Grande (Supbig). 2020. Available online: http://www.inea.rj.gov.br/Portal/MegaDropDown/Regionais/BaiadaIlhaGrande/index.htm&lang=PT-BR#/UnidadesdeConservacao (accessed on 10 March 2023).
- Carlos-Júnior, L.A.; Spencer, M.; Neves, D.M.; Moulton, T.P.; Pires, D.D.O.; Castro, C.B.; Ventura, C.R.R.; Ferreira, C.E.L.; Serejo, C.S.; Oigman-Pszczol, S.; et al. Rarity and beta diversity assessment as tools for guiding conservation strategies in marine tropical subtidal communities. Divers. Distrib. 2019, 25, 743–757. [Google Scholar] [CrossRef]
- McIntyre, A.D.; Eleftheriou, A. Methods for the Study of Marine Benthos, 1st ed.; Blackwell Science: Oxford, UK, 2005; pp. 1–42. [Google Scholar]
- Cerling, T.E.; Ayliffe, L.K.; Dearing, M.D.; Ehleringer, J.R.; Passey, B.H.; Podlesak, D.W.; Torregrossa, A.; West, A.G. Determining biological tissue turnover using stable isotopes: The reaction progress variable. Oecologia 2007, 151, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Cabanellas-Reboredo, M.; Deudero, S.; Blanco, A. Stable-isotope signatures (δ13C and δ15N) of different tissues of Pinna nobilis Linnaeus, 1758 (Bivalvia): Isotopic variations among tissues and between seasons. J. Molluscan Stud. 2009, 75, 343–349. [Google Scholar] [CrossRef]
- Pires-Teixeira, L.M.; Neres-Lima, V.; Creed, J.C. Is acidification of samples for isotopic analysis of carbon and nitrogen necessary for shoreline marine species? Mar. Freshw. Res. 2020, 72, 256–262. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology, 1st ed.; Springer: New York, NY, USA, 2006; pp. 40–75. [Google Scholar]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef]
- McCormack, S.A.; Trebilco, R.; Melbourne-Thomas, J.; Blanchard, J.L.; Fulton, E.A.; Constable, A. Using stable isotope data to advance marine food web modelling. Rev. Fish Biol. Fish. 2019, 29, 277–296. [Google Scholar] [CrossRef]
- Parnell, A. Simmr: A Stable Isotope Mixing Model; R Package Version 0.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://CRAN.R-project.org/package=simmr (accessed on 6 March 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 6 March 2023).
- McCutchan, J.H., Jr.; Lewis, W.M., Jr.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Phillips, D.L.; Koch, P.L. Incorporating concentration dependence in stable isotope mixing models. Oecologia 2002, 130, 114–125. [Google Scholar] [CrossRef]
- Leys, S.P.; Eerkes-Medrano, D.I. Feeding in a calcareous sponge: Particle uptake by pseudopodia. Biol. Bull. 2006, 211, 157–171. [Google Scholar] [CrossRef]
- Wanick, R.C.; Vieira, R.P.; Santelli, R.E.; Paranhos, R.P.D.R.; Coutinho, C.C. Screening for cultivable species of marine sponges (porifera) in an ecologically inspired ex situ system. J. Fish. Sci. 2015, 9, 5. [Google Scholar]
- Dharan, D.T.; Prasad, G. Food and feeding of Phallusia nigra, Savigny, 1816. Int. J. Zool. Stud. 2018, 3, 261–264. [Google Scholar]
- Hendler, G. The feeding biology of Ophioderma brevispinum (Ophiuroidea: Echinodermata). In Echinoderms: Proceedings of the International Echinoderm Conference, Tampa, FL, USA, 17 September 1982; Balkema: Rotterdam, The Netherland, 1982; pp. 21–27. [Google Scholar]
- Mantelatto, M.C.; Vidon, L.F.; Silveira, R.B.; Menegola, C.; Da Rocha, R.M.; Creed, J.C.; Mantelatto, M.C.; Vidon, L.F.; Silveira, R.B.; Menegola, C.; et al. Host species of the non-indigenous brittle star Ophiothela mirabilis (Echinodermata: Ophiuroidea): An invasive generalist in Brazil? Mar. Biodivers. Rec. 2016, 9, 8. [Google Scholar] [CrossRef]
- Turon, X.; Codina, M.; Tarjuelo, I.; Uriz, M.J.; Becerro, M.A. Mass recruitment of Ophiothrix fragilis (Ophiuroidea) on sponges: Settlement patterns and post-settlement dynamics. Mar. Ecol. Prog. Ser. 2000, 200, 201–212. [Google Scholar] [CrossRef]
- Santana, E.F.C.; Alves, A.L.; Santos, A.D.M.; Maria Da Gloria, G.S.; Perez, C.D.; Gomes, P.B. Trophic ecology of the zoanthid Palythoa caribaeorum (Cnidaria: Anthozoa) on tropical reefs. J. Mar. Biol. Assoc. UK 2015, 95, 301–309. [Google Scholar] [CrossRef]
- Sloan, N.A.; Von Bodungen, B. Distribution and feeding of the sea cucumber Isostichopus badionotus in relation to shelter and sediment criteria of the Bermuda platform. Mar. Ecol. Prog. Ser. 1980, 2, 257–264. [Google Scholar] [CrossRef]
- Calderon, E.N.; Zilberberg, C.; Pavia, P.C. The possible role of Echinometra lucunter (Echinodermata: Echinoidea) in the local distribution of Darwinella sp. (Porifera: Dendroceratida) in Arraial do Cabo, Rio de Janeiro State, Brazil. Porifera Res. Biodivers. Innov. Sustain. 2007, 28, 211–217. [Google Scholar]
- Granado, I.; Caballero, P. Feeding rates of Littorina striata and Osilinus atratus in relation to nutritional quality and chemical defenses in seaweeds. Mar. Biol. 2001, 138, 1213. [Google Scholar] [CrossRef]
- Moraes, F.; Chagas-Júnior, A. Border between two worlds: The first record of sea anemone feeding on centipede. Int. J. Myriap. 2009, 2, 215. [Google Scholar] [CrossRef]
- Del Valle, J.C.; Acuña, F.H.; Mañanes, A.A.L. Digestive flexibility in response to environmental salinity and temperature in the non-symbiotic sea anemone Bunodosoma zamponii. Hydrobiologia 2015, 759, 189–199. [Google Scholar] [CrossRef]
- Tewes, R.P. The Ecology and Feeding Biology of Ophioderma cinereum in a Mangrove Environment. Ph.D. Thesis, Department of Biology, University of Missouri-Kansas City, Kansas, MO, USA, 1984. [Google Scholar]
- Dubiaski-Silva, J.; Masunari, S. Natural diet of fish and crabs associated with the phytal community of Sargassum cymosum C. Agardh, 1820 (Phaeophyta, Fucales) at Ponta das Garoupas, Bombinhas, Santa Catarina State, Brazil. J. Nat. Hist. 2008, 42, 1907–1922. [Google Scholar] [CrossRef]
- Phillips, D.L.; Newsome, S.D.; Gregg, J.W. Combining sources in stable isotope mixing models: Alternative methods. Oecologia 2005, 144, 520–527. [Google Scholar] [CrossRef]
- Santos, E.P.; Condini, M.V.; Santos, A.C.A.; Alvarez, H.M.; de Moraes, L.E.; Garcia, A.F.S.; Garcia, A.M. Spatio-temporal changes in basal food source assimilation by fish assemblages in a large tropical bay in the SW Atlantic Ocean. Estuaries Coasts 2020, 43, 894–908. [Google Scholar] [CrossRef]
- Loneragan, N.R.; Bunn, S.E.; Kellaway, D.M. Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary? A multiple stable-isotope study. Mar. Biol. 1997, 130, 289–300. [Google Scholar] [CrossRef]
- Corbisier, T.N.; Soares, L.S.H.; Petti, M.A.V.; Muto, E.Y.; Silva, M.H.C.; McClelland, J.; Valiela, I.J.A.E. Use of isotopic signatures to assess the food web in a tropical shallow marine ecosystem of Southeastern Brazil. Aquat. Ecol. 2006, 40, 381–390. [Google Scholar] [CrossRef]
- Brito, E.F.; Moulton, T.P.; De Souza, M.L.; Bunn, S.E. Stable isotope analysis indicates microalgae as the predominant food source of fauna in a coastal forest stream, south-east Brazil. Austral Ecol. 2006, 31, 623–633. [Google Scholar] [CrossRef]
- Neres-Lima, V.; Brito, E.F.; Krsulović, F.A.M.; Detweiler, A.M.; Hershey, A.E.; Moulton, T.P. High importance of autochthonous basal food source for the food web of a Brazilian tropical stream regardless of shading. Int. Rev. Hydrobiol. 2016, 101, 132–142. [Google Scholar] [CrossRef]
- Machado-Silva, F.; Neres-Lima, V.; Oliveira, A.F.; Moulton, T.P. Forest cover controls the nitrogen and carbon stable isotopes of rivers. Sci. Total. Environ. 2022, 817, 152784. [Google Scholar] [CrossRef]
- Herman, P.M.; Middelburg, J.J.; Widdows, J.; Lucas, C.H.; Heip, C.H. Stable isotopes as trophic tracers: Combining field sampling and manipulative labelling of food resources for macrobenthos. Mar. Ecol. Prog. Ser. 2000, 204, 79–92. [Google Scholar] [CrossRef]
- Salen-Picard, C.; Arlhac, D. Long-term changes in a Mediterranean benthic community: Relationships between the polychaete assemblages and hydrological variations of the Rhône river. Estuaries 2002, 25, 1121–1130. [Google Scholar] [CrossRef]
- Salen-Picard, C.; Darnaude, A.M.; Arlhac, D.; Harmelin-Vivien, M.L. Fluctuations of macrobenthic populations: A link between climate-driven river run-off and sole fishery yields in the Gulf of Lions. Oecologia 2002, 133, 380–388. [Google Scholar] [CrossRef]
- Duggins, D.O.; Simenstad, C.A.; Estes, J.A. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 1989, 245, 170–173. [Google Scholar] [CrossRef]
- Fredriksen, S. Food web studies in a Norwegian kelp forest based on stable isotope (δ13C and δ15N) analysis. Mar. Ecol. Prog. Ser. 2003, 260, 71–81. [Google Scholar] [CrossRef]
- Mann, K.H. Production and use of detritus in various freshwater, estuarine, and coastal marine ecosystems. Limnol. Oceanogr. 1988, 33, 910–930. [Google Scholar] [CrossRef]
- Lima, E.J.B.; Gomes, P.B.; Souza, J.R.B. Reproductive biology of Echinometra lucunter (Echinodermata: Echinoidea) in a northeast Brazilian sandstone reef. An. Acad. Bras. Cienc. 2009, 81, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Schoppe, S.; Werding, B. The boreholes of the sea urchin genus Echinometra (Echinodermata: Echinoidea: Echinometridae) as a microhabitat in tropical South America. Mar. Ecol. 1996, 17, 181–186. [Google Scholar] [CrossRef]
- Asgaard, U.; Bromley, R.G. Echinometrid sea urchins, their trophic styles and corresponding bioerosion. In Current Developments in Bioerosion; Springer: Berlin/Heidelberg, Germany, 2008; pp. 279–303. [Google Scholar]
- Reyes-Luján, J.; Barrios, J.; Arrieche, D.; Zapata-Vívenes, E.; Salgado, W.; Lodeiros, C. Dieta del erizo negro Echinometra lucunter (Echinometra: Echinoidea) en el Nororiente de Venezuela. Rev. Biol. Trop. 2015, 63, 233–242. [Google Scholar]
- Barnes, R.S.K.; Hughes, R.N. An Introduction to Marine Ecology, 3rd ed.; John Wiley & Sons: Oxford, UK, 1999; pp. 222–237. [Google Scholar]
- Mahiques, M.M.; Tessler, M.G.; Furtado, V.V. Characterization of energy gradient in enclosed bays of Ubatuba region, south-eastern Brazil. Estuar. Coast. Shelf Sci. 1998, 47, 431–446. [Google Scholar] [CrossRef]
- Oliveira, A.E.S.; Creed, J.C. Mollusca, Bivalvia, Isognomon bicolor (CB Adams 1845): Distribution extension. Check List 2008, 4, 386–388. [Google Scholar] [CrossRef]
- Bernini, E.; Rezende, C.E. Litterfall in a mangrove in Southeast Brazil. Pan-Am. J. Aquat. Sci. 2010, 5, 508–519. [Google Scholar]
- França, J.S.; Gregório, R.S.; de Paula, J.D.A.; Júnior, J.F.G.; Ferreira, F.A.; Callisto, M. Composition and dynamics of allochthonous organic matter inputs and benthic stock in a Brazilian stream. Mar. Freshw. Res. 2009, 60, 990–998. [Google Scholar] [CrossRef]
- McQuaid, C.D. Biology of the gastropod family Littorinidae. II. Role in the ecology of intertidal and shallow marine ecosystems. Oceanogr. Mar. Biol. 1996, 34, 263–302. [Google Scholar]
- Norton, T.A.; Hawkins, S.J.; Manley, N.L.; Williams, G.A.; Watson, D.C. Scraping a living: A review of littorinid grazing. Hydrobiologia 1990, 193, 117–138. [Google Scholar] [CrossRef]
- Gili, J.M.; Coma, R. Benthic suspension feeders: Their paramount role in littoral marine food webs. Trends Ecol. Evol. 1998, 13, 316–321. [Google Scholar] [CrossRef]
- Figueiredo, M.O.; Tâmega, F.T.S. Macroalgas marinhas. In Biodiversidade Marinha da Baía da Ilha Grande, 1st ed.; Creed, J.C., Pires, D.O., Figueiredo, M.D.O., Eds.; Ministério do Meio Ambiente—MMA: Brasília, Brazil, 2007; pp. 152–180. [Google Scholar]
- Mantelatto, M.C.; Carlos-Júnior, L.A.; Côrrêa, C.; Cardoso, C.F.d.L.; Creed, J.C. Depth-related drivers of benthic community structure on shallow subtidal rocky reefs. Estuar. Coast. Shelf Sci. 2022, 266, 107743. [Google Scholar] [CrossRef]
- Connell, S.; Foster; Airoldi, L. What are algal turfs? Towards a better description of turfs. Mar. Ecol. Prog. Ser. 2014, 495, 299–307. [Google Scholar] [CrossRef]
- Klumpp, D.W.; McKinnon, A.D. Community structure, biomass and productivity of epilithic algal communities on the Great Barrier Reef: Dynamics at different spatial scales. Mar. Ecol. Prog. Ser. 1992, 86, 77–89. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Bellwood, D.R. Sediments ratchet-down coral reef algal turf productivity. Sci. Total Environ. 2020, 713, 136709. [Google Scholar] [CrossRef]
- Lages, B.G.; Fleury, B.G.; Menegola, C.; Creed, J.C. Change in tropical rocky shore communities due to an alien coral invasion. Mar. Ecol. Prog. Ser. 2011, 438, 85–96. [Google Scholar] [CrossRef]
- Mantelatto, M.C.; Fleury, B.G.; Menegola, C.; Creed, J.C. Cost–benefit of different methods for monitoring invasive corals on tropical rocky reefs in the southwest Atlantic. J. Exp. Mar. Biol. Ecol. 2013, 449, 129–134. [Google Scholar] [CrossRef]
- Loma, T.L.; Conand, C.; Harmelin-Vivien, M.; Ballesteros, E. Food selectivity of Tripneustes gratilla (L.) (Echinodermata: Echinoidea) in oligotrophic and nutrient-enriched coral reefs at La Reunion (Indian Ocean). Bull. Mar. Sci. 2002, 70, 927–938. [Google Scholar]
- Stachowicz, J.J.; Hay, M.E. Facultative mutualism between an herbivorous crab and a coralline alga: Advantages of eating noxious seaweeds. Oecologia 1996, 105, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Pueschel, C.M.; Judson, B.L.; Wegeberg, S. Decalcification during epithallial cell turnover in Jania adhaerens (Corallinales, Rhodophyta). Phycologia 2005, 44, 156–162. [Google Scholar] [CrossRef]
- Westerhausen, L.; Poynter, J.; Eglinton, G.; Erlenkeuser, H.; Sarnthein, M. Marine and terrigenous origin of organic matter in modern sediments of the equatorial East Atlantic: The σ13C and molecular record. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 1087–1121. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Mantelatto, M.C.; Oliveira, A.E.S.; Menegola, C.; Casares, F.A.; Creed, J.C. Depth and grazing intensity are the main drivers of subtidal hardground benthic community structure on tropical south Atlantic reefs. Mar. Ecol. 2020, 00, e12586. [Google Scholar] [CrossRef]
- Muscatine, L.; McCloskey, L.R.; Marian, R.E. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol. Oceanogr. 1981, 26, 601–661. [Google Scholar] [CrossRef]
- Tanner, J.E. Consequences of density-dependent heterotrophic feeding for a partial autotroph. Mar. Ecol. Prog. Ser. 2002, 227, 293–304. [Google Scholar] [CrossRef]
- Sebens, K.P. Autotrophic and heterotrophic nutrition of coral reef zoanthids. In Proceedings of the 3rd International Coral Reef Symposium, Miami, FL, USA, 3 May 1977; Volume 1, pp. 397–404. [Google Scholar]
- Matsuura, Y.; Wada, E. Carbon and nitrogen stable isotope ratios in marine organic matters of the coastal ecosystem in Ubatuba, southern Brazil. Cienc. Cult. 1994, 46, 141–146. [Google Scholar]
- Stoner, A.W.; Zimmerman, R.J. Food pathways associated with penaeid shrimps in a mangrove-fringed estuary. Fish. Bull. 1988, 86, 543–552. [Google Scholar]
- Marguillier, S.; Van der Velde, G.; Dehairs, F.; Hemminga, M.A.; Rajagopal, S. Trophic relationships in an interlinked mangrove-seagrass ecosystem as traced by delta13C and delta15N. Mar. Ecol. Prog. Ser. 1997, 151, 115–121. [Google Scholar] [CrossRef]
- Claudino, M.C.; Pessanha, A.L.M.; Araújo, F.G.; Garcia, A.M. Trophic connectivity and basal food sources sustaining tropical aquatic consumers along a mangrove to ocean gradient. Estuar. Coast. Shelf Sci. 2015, 167, 45–55. [Google Scholar] [CrossRef]
- Bouillon, S.; Connolly, R.M. Carbon exchange among tropical coastal ecosystems. In Ecological Connectivity among Tropical Coastal Ecosystems, 1st ed.; Springer: Dordrecht, Belgium, 2009; pp. 45–70. [Google Scholar]
- Deegan, L.A.; Garritt, R.H. Evidence for spatial variability in estuarine food webs. Mar. Ecol. Prog. Ser. 1997, 147, 31–47. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Thomas, F.; Sergent, L.; Duxbury, M. Identification of trophic interactions within an estuarine food web (northern New Zealand) using fatty acid biomarkers and stable isotopes. Estuar. Coast. Shelf Sci. 2006, 70, 271–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).