Health Status of Stranded Common Bottlenose Dolphins (Tursiops truncatus) and Contamination by Immunotoxic Pollutants: A Threat to the Pelagos Sanctuary—Western Mediterranean Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Post-Mortem Examination
2.2. Diagnostic Investigations
2.3. Cause of Death Evaluation
2.4. Toxicological Analyses
2.5. Statistical Analysis
3. Results
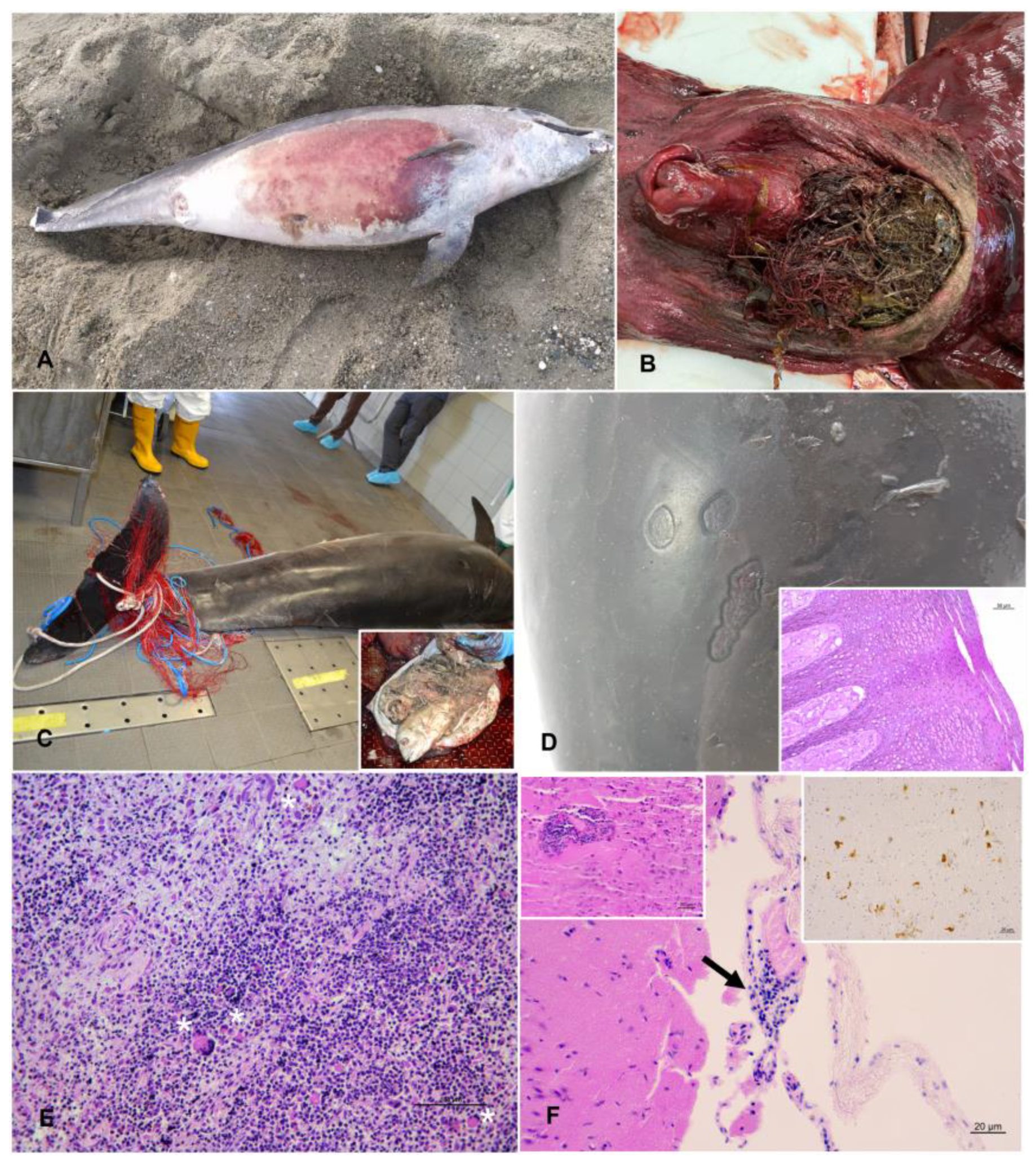
3.1. Post-Mortem Examination
3.2. Diagnostic Investigations
3.2.1. Histopathological-Immunohistochemistry
3.2.2. Bacteriological
3.2.3. Biomolecular
3.2.4. Serological
3.3. Cause of Death Evaluation
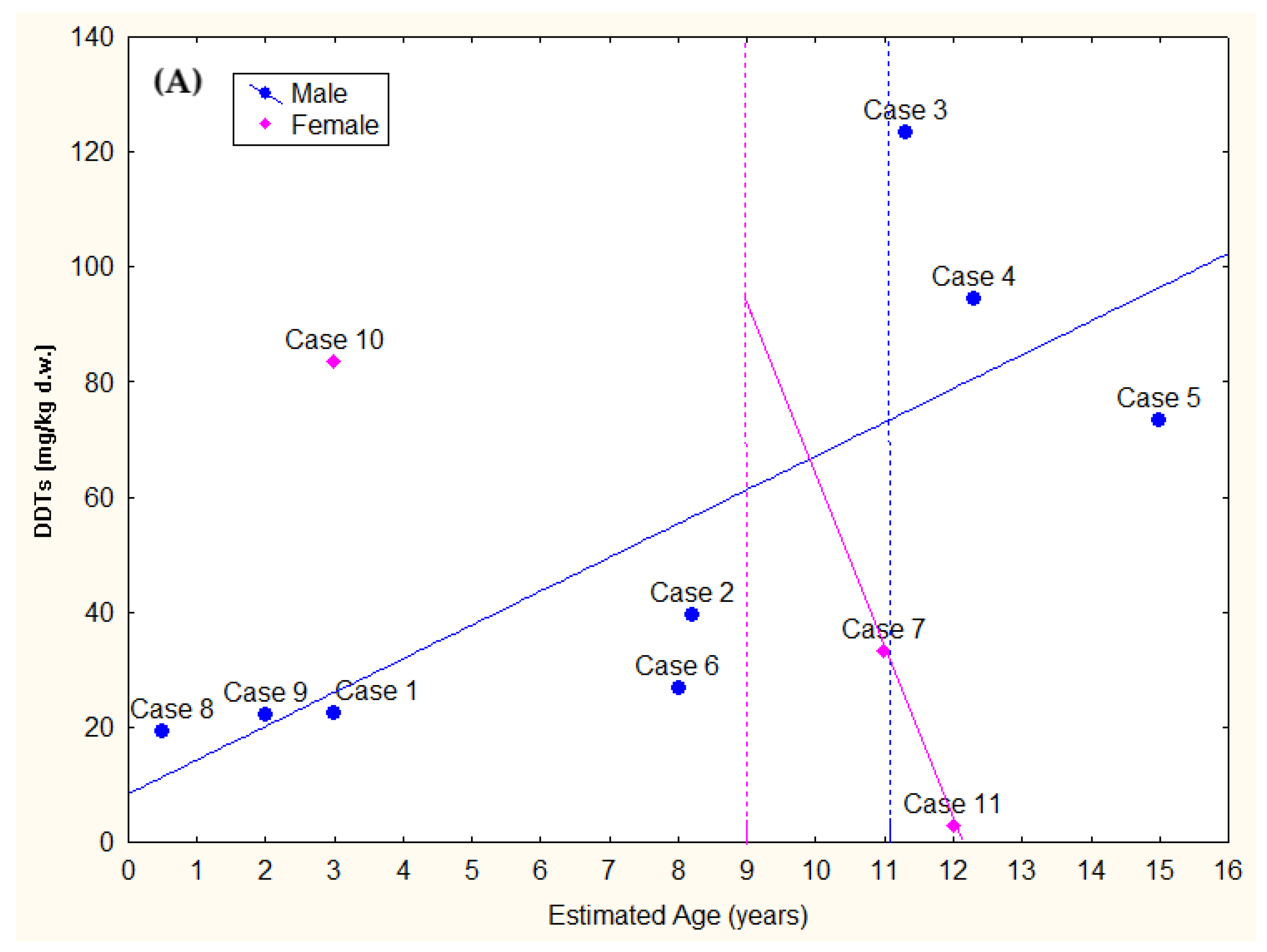
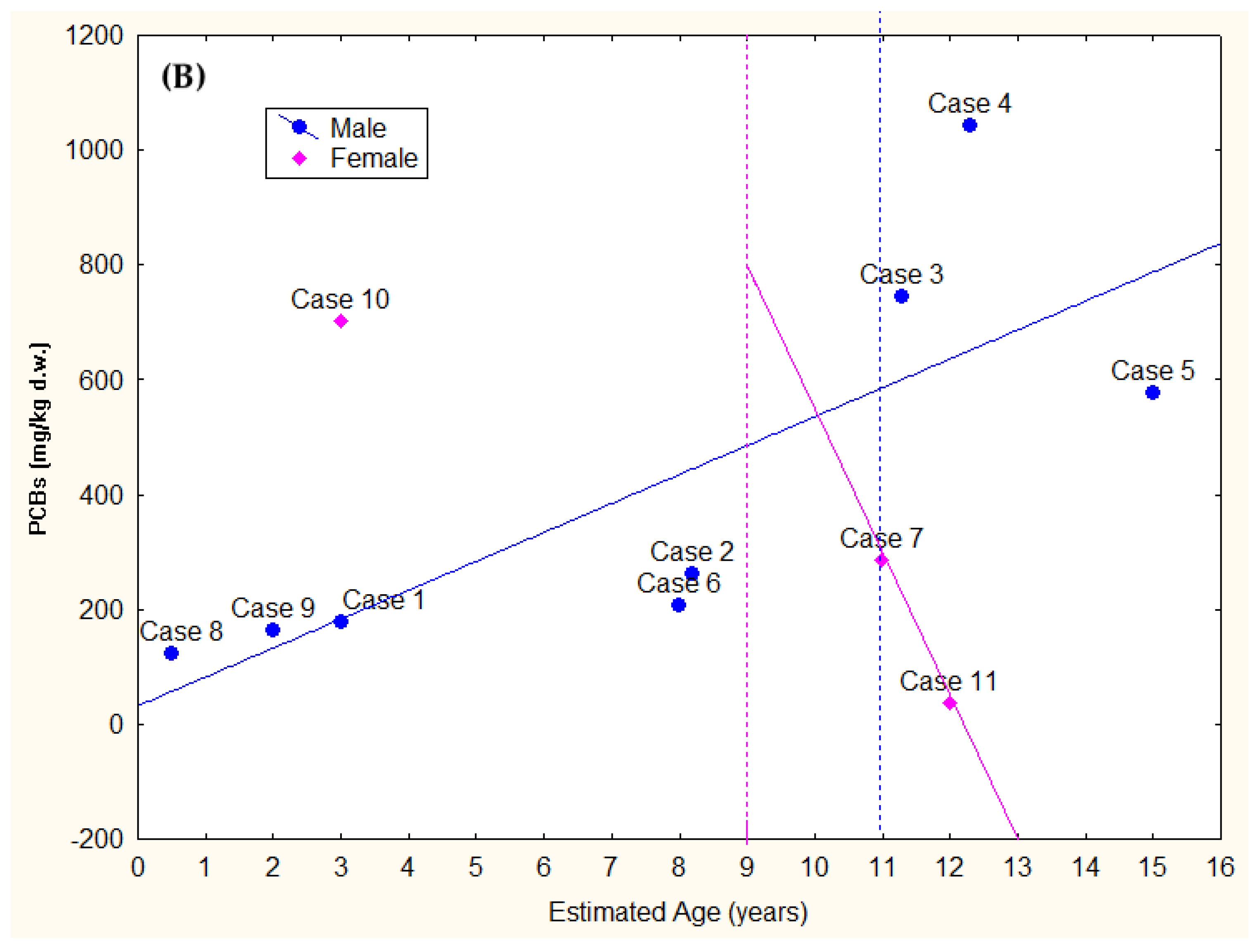
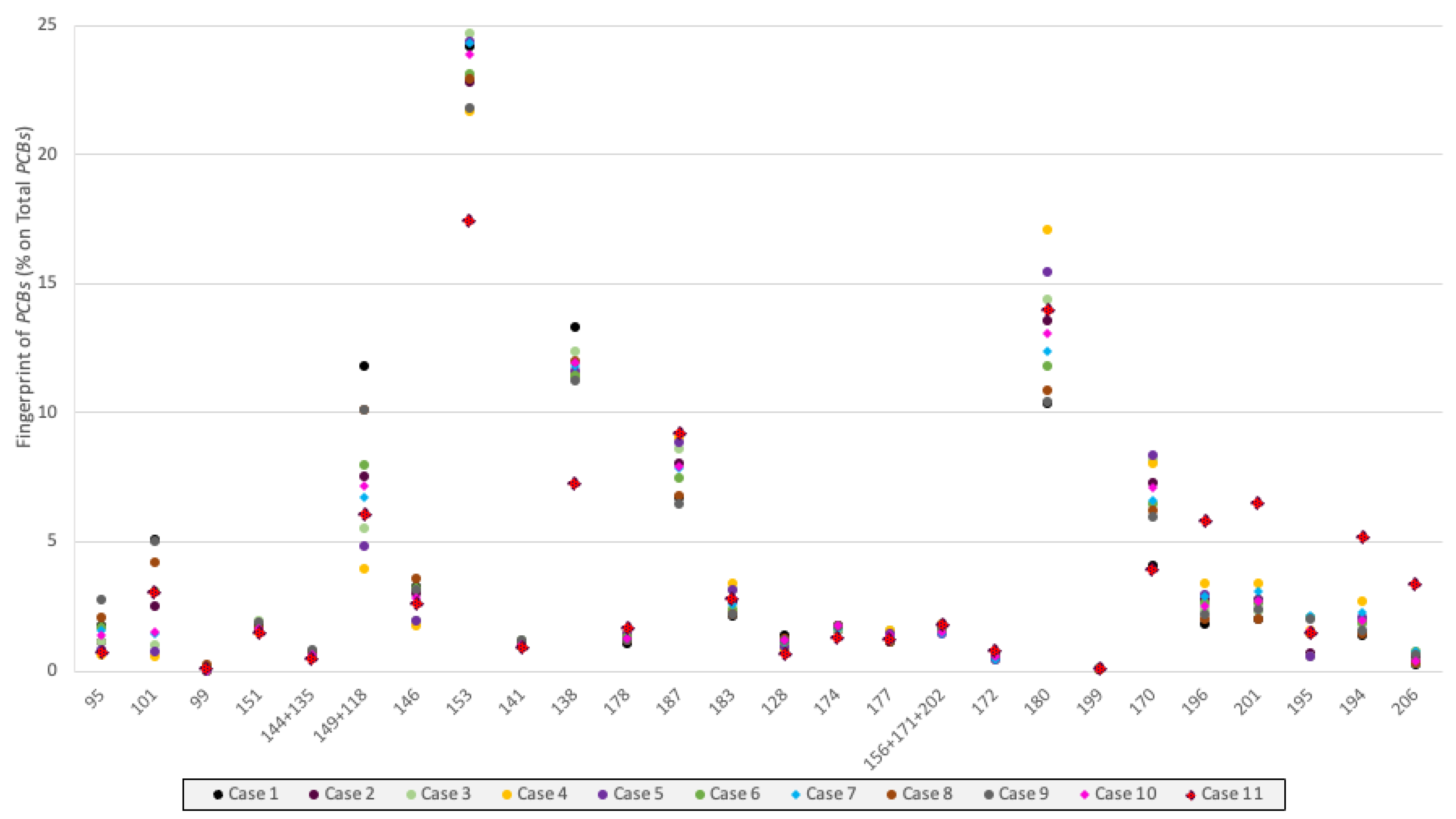
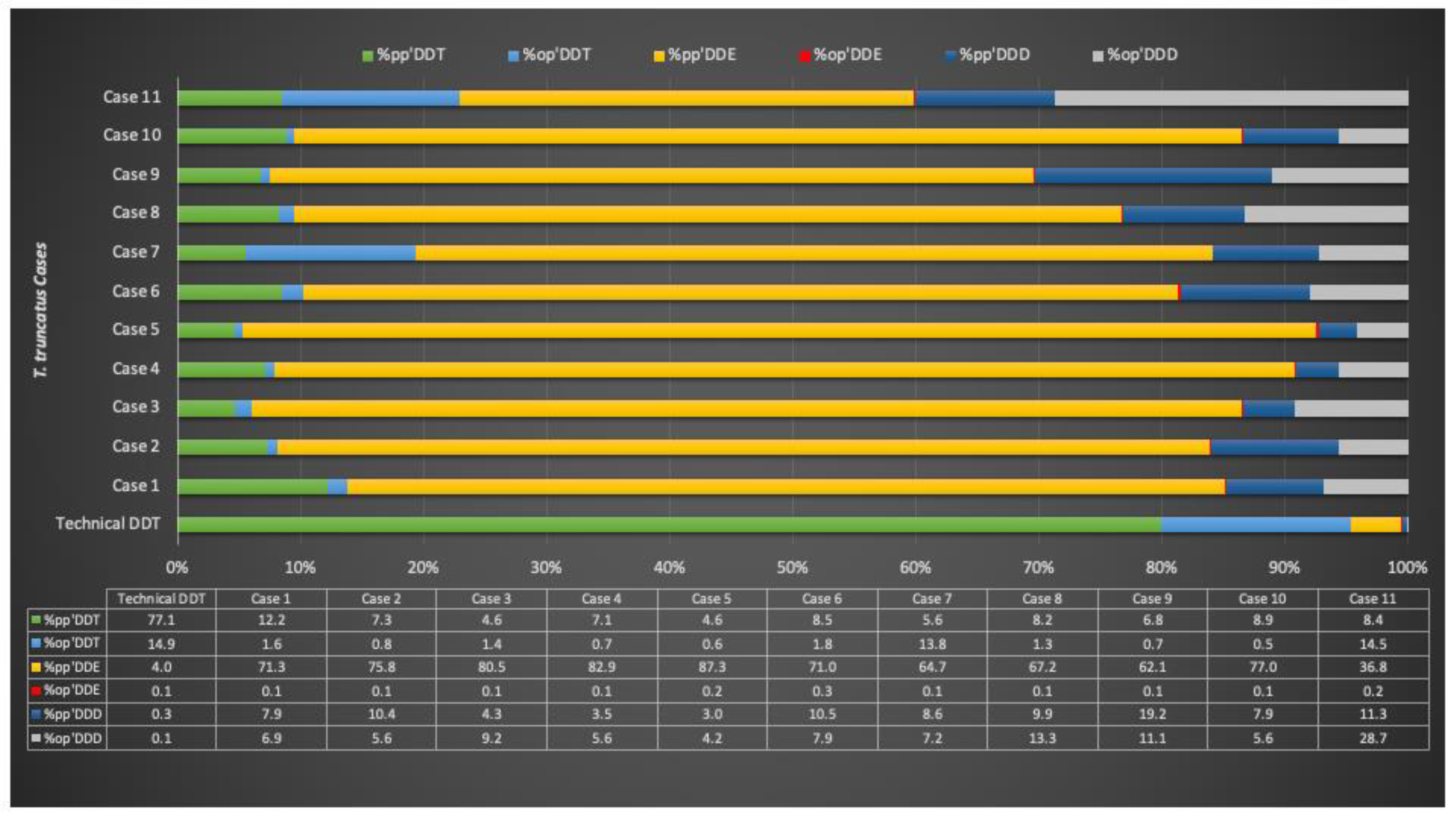
3.4. Toxicological Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguirre, A.A.; Ostfeld, R.; Daszak, P. New Directions in Conservation Medicine: Applied Cases of Ecological Health; Oxford University Press: New York, NY, USA, 2012; ISBN 978-0-19-973147-3. [Google Scholar]
- Giorda, F.; Ballardini, M.; Di Guardo, G.; Pintore, M.D.; Grattarola, C.; Iulini, B.; Mignone, W.; Goria, M.; Serracca, L.; Varello, K.; et al. Postmortem findings in cetaceans found stranded in the Pelagos Sanctuary, Italy, 2007–14. J. Wildl. Dis. 2017, 53, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Bossart, G.D. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Centelleghe, C.; Da Dalt, L.; Marsili, L.; Zanetti, R.; Fernandez, A.; Arbelo, M.; Sierra, E.; Castagnaro, M.; Di Guardo, G.; Mazzariol, S. Insights Into Dolphins’ Immunology: Immuno-Phenotypic Study on Mediterranean and Atlantic Stranded Cetaceans. Front. Immunol. 2019, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Di Guardo, G.; Mazzariol, S.; Casini, S. Insights Into Cetacean Immunology: Do Ecological and Biological Factors Make the Difference? Front. Immunol. 2019, 10, 1219. [Google Scholar] [CrossRef]
- Mazzariol, S.; Marcer, F.; Mignone, W.; Serracca, L.; Goria, M.; Marsili, L.; Di Guardo, G.; Casalone, C. Dolphin Morbillivirus and Toxoplasma gondii Coinfection in a Mediterranean Fin Whale (Balaenoptera physalus). BMC Vet. Res. 2012, 8, 20. [Google Scholar] [CrossRef]
- Desforges, J.-P.W.; Sonne, C.; Levin, M.; Siebert, U.; De Guise, S.; Dietz, R. Immunotoxic Effects of Environmental Pollutants in Marine Mammals. Environ. Int. 2016, 86, 126–139. [Google Scholar] [CrossRef]
- Marsili, L.; Jiménez, B.; Borrell, A. Persistent Organic Pollutants in Cetaceans Living in a Hotspot Area: The Mediterranean Sea. In Marine Mammal Ecotoxicology; Fossi, M.C., Panti, C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 185–212. ISBN 978-0-12-812144-3. [Google Scholar]
- Van Bressem, M.F.; Raga, J.A.; Di Guardo, G.; Jepson, P.D.; Duignan, P.J.; Siebert, U.; Barrett, T.; de Oliveira Santos, M.C.; Moreno, I.B.; Siciliano, S.; et al. Emerging Infectious Diseases in Cetaceans Worldwide and the Possible Role of Environmental Stressors. Dis. Aquat. Org. 2009, 86, 143–157. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The Impact of Climate Change on the World’s Marine Ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Sousa, A.; Alves, F.; Dinis, A.; Bentz, J.; Cruz, M.J.; Nunes, J.P. How Vulnerable Are Cetaceans to Climate Change? Developing and Testing a New Index. Ecol. Indic. 2019, 98, 9–18. [Google Scholar] [CrossRef]
- Sanderson, C.E.; Alexander, K.A. Unchartered Waters: Climate Change Likely to Intensify Infectious Disease Outbreaks Causing Mass Mortality Events in Marine Mammals. Glob. Chang. Biol. 2020, 26, 4284–4301. [Google Scholar] [CrossRef]
- Van Weelden, C.; Towers, J.R.; Bosker, T. Impacts of Climate Change on Cetacean Distribution, Habitat and Migration. Clim. Chang. Ecol. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Reed, J.; Harcourt, R.; New, L.; Bilgmann, K. Extreme Effects of Extreme Disturbances: A Simulation Approach to Assess Population Specific Responses. Front. Mar. Sci. 2020, 7, 519845. [Google Scholar] [CrossRef]
- Fossi, M.C.; Marsili, L.; Casini, S.; Bucalossi, D. Development of New-Tools to Investigate Toxicological Hazard Due to Endocrine Disruptor Organochlorines and Emerging Contaminants in Mediterranean Cetaceans. Mar. Environ. Res. 2006, 62, S200–S204. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Larson, S. A Review of Organochlorine Contaminants in Nearshore Marine Mammal Predators. J. Environ. Anal. Toxicol. 2016, 6, 370. [Google Scholar] [CrossRef]
- Neri, A.; Sartor, P.; Voliani, A.; Mancusi, C.; Marsili, L. Diet of Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821), in the Northwestern Mediterranean Sea. Diversity 2023, 15, 21. [Google Scholar] [CrossRef]
- Jones, K.C.; de Voogt, P. Persistent Organic Pollutants (POPs): State of the Science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Dron, J.; Wafo, E.; Boissery, P.; Dhermain, F.; Bouchoucha, M.; Chamaret, P.; Lafitte, D. Trends of Banned Pesticides and PCBs in Different Tissues of Striped Dolphins (Stenella coeruleoalba) Stranded in the Northwestern Mediterranean Reflect Changing Contamination Patterns. Mar. Pollut. Bull. 2022, 174, 113198. [Google Scholar] [CrossRef]
- Borrell, A.; Aguilar, A.; Tornero, V.; Sequeira, M.; Fernandez, G.; Alıs, S. Organochlorine Compounds and Stable Isotopes Indicate Bottlenose Dolphin Subpopulation Structure around the Iberian Peninsula. Environ. Int. 2006, 32, 516–523. [Google Scholar] [CrossRef]
- Gonzalvo, J.; Lauriano, G.; Hammond, P.S.; Viaud-Martinez, K.A.; Fossi, M.C.; Natoli, A.; Marsili, L. Chapter Nine—The Gulf of Ambracia’s Common Bottlenose Dolphins, Tursiops truncatus: A Highly Dense and yet Threatened Population. In Advances in Marine Biology; Notarbartolo Di Sciara, G., Podestà, M., Curry, B.E., Eds.; Mediterranean Marine Mammal Ecology and Conservation; Academic Press: Cambridge, MA, USA, 2016; Volume 75, pp. 259–296. [Google Scholar]
- Jepson, P.D.; Deaville, R.; Barber, J.L.; Aguilar, À.; Borrell, A.; Murphy, S.; Barry, J.; Brownlow, A.; Barnett, J.; Berrow, S.; et al. PCB Pollution Continues to Impact Populations of Orcas and Other Dolphins in European Waters. Sci. Rep. 2016, 6, 18573. [Google Scholar] [CrossRef]
- Genov, T.; Jepson, P.D.; Barber, J.L.; Hace, A.; Gaspari, S.; Centrih, T.; Lesjak, J.; Kotnjek, P. Linking Organochlorine Contaminants with Demographic Parameters in Free-Ranging Common Bottlenose Dolphins from the Northern Adriatic Sea. Sci. Total Environ. 2019, 657, 200–212. [Google Scholar] [CrossRef]
- Wafo, E.; Sarrazin, L.; Diana, C.; Dhermain, F.; Schembri, T.; Lagadec, V.; Pecchia, M.; Rebouillon, P. Accumulation and Distribution of Organochlorines (PCBs and DDTs) in Various Organs of Stenella coeruleoalba and a Tursiops truncatus from Mediterranean Littoral Environment (France). Sci. Total Environ. 2005, 348, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Shoham-Frider, E.; Kress, N.; Wynne, D.; Scheinin, A.; Roditi-Elsar, M.; Kerem, D. Persistent Organochlorine Pollutants and Heavy Metals in Tissues of Common Bottlenose Dolphin (Tursiops truncatus) from the Levantine Basin of the Eastern Mediterranean. Chemosphere 2009, 77, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Romanić, S.H.; Holcer, D.; Lazar, B.; Klinčić, D.; Mackelworth, P.; Fortuna, C.M. Organochlorine Contaminants in Tissues of Common Bottlenose Dolphins Tursiops truncatus from the Northeastern Part of the Adriatic Sea. Environ. Toxicol. Pharmacol. 2014, 38, 469–479. [Google Scholar] [CrossRef]
- Grattarola, C.; Giorda, F.; Iulini, B.; Pintore, M.D.; Pautasso, A.; Zoppi, S.; Goria, M.; Romano, A.; Peletto, S.; Varello, K.; et al. Meningoencephalitis and Listeria monocytogenes, Toxoplasma gondii and Brucella spp. Coinfection in a Dolphin in Italy. Dis. Aquat. Org. 2016, 118, 169–174. [Google Scholar] [CrossRef] [PubMed]
- C.Re.Di.Ma. Reports Strandings. C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2016); Open Science Framework: Impera, Italy, 2016. [Google Scholar]
- C.Re.Di.Ma. Reports Strandings. C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2017); Open Science Framework: Impera, Italy, 2017. [Google Scholar]
- C.Re.Di.Ma. Reports Strandings. C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2018); Open Science Framework: Impera, Italy, 2018. [Google Scholar]
- C.Re.Di.Ma. Reports Strandings. C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2019); Open Science Framework: Impera, Italy, 2019. [Google Scholar]
- C.Re.Di.Ma. Reports Strandings. C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2020); Open Science Framework: Impera, Italy, 2020. [Google Scholar]
- C.Re.Di.Ma. Reports Strandings. C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2021); Open Science Framework: Impera, Italy, 2021. [Google Scholar]
- Grattarola, C.; Gallina, S.; Giorda, F.; Pautasso, A.; Ballardini, M.; Iulini, B.; Varello, K.; Goria, M.; Peletto, S.; Masoero, L.; et al. First Report of Salmonella 1,4,[5],12:I:- In Free-Ranging Striped Dolphins (Stenella coeruleoalba), Italy. Sci. Rep. 2019, 9, 6061. [Google Scholar] [CrossRef]
- Giorda, F.; Di Guardo, G.; Varello, K.; Pautasso, A.; Sierra, E.; Pintore, M.D.; Grattarola, C.; Colella, E.M.; Berio, E.; Goria, M.; et al. Retrospective Immunohistochemical Investigation on Dolphin Morbillivirus Infection by Comparing the Performance of Heterologous Monoclonal and Polyclonal Antibodies—Short Communication. Acta Vet. Hung. 2021, 69, 204–210. [Google Scholar] [CrossRef]
- Romani-Cremaschi, U.; Zoppi, S.; Mattioda, V.; Audino, T.; Marsili, L.; Varello, K.; Iulini, B.; Marra, C.; Zoccola, R.; Battistini, R.; et al. Morganella Morganii Septicemia and Concurrent Renal Crassicaudiasis in a Cuvier’s Beaked Whale (Ziphius cavirostris) Stranded in Italy. Front. Mar. Sci. 2023, 9, 1058724. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale della Repubblica Italiana. Available online: https://www.mase.gov.it/sites/default/files/archivio/normativa/legge_11102001_391_ratifica_accordo_santuario_mammiferi_marini.pdf (accessed on 19 January 2023).
- Pelagos Sanctuary. Available online: https://www.sanctuaire-pelagos.org/en/human-activities/coastal-urbanization (accessed on 20 March 2023).
- Panti, C.; Spinsanti, G.; Marsili, L.; Casini, S.; Frati, F.; Fossi, M.C. Ecotoxicological Diagnosis of Striped Dolphin (Stenella coeruleoalba) from the Mediterranean Basin by Skin Biopsy and Gene Expression Approach. Ecotoxicology 2011, 20, 1791–1800. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Marsili, L.; Maltese, S.; Spinsanti, G.; Casini, S.; Caliani, I.; Gaspari, S.; Muñoz-Arnanz, J.; Jimenez, B.; et al. The Pelagos Sanctuary for Mediterranean Marine Mammals: Marine Protected Area (MPA) or Marine Polluted Area? The Case Study of the Striped Dolphin (Stenella coeruleoalba). Mar. Pollut. Bull. 2013, 70, 64–72. [Google Scholar] [CrossRef]
- IJsseldijk, L.; Brownlow, A.; Mazzariol, S. Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling; ACCOBAMS ASCOBANS: Rotterdam, The Netherlands, 2019. [Google Scholar]
- Tanaka, H.; Li, G.; Uchida, Y.; Nakamura, M.; Ikeda, T.; Liu, H. Measurement of Time-Varying Kinematics of a Dolphin in Burst Accelerating Swimming. PLoS ONE 2019, 14, e0210860. [Google Scholar] [CrossRef]
- Butti, C.; Corain, L.; Cozzi, B.; Podestà, M.; Pirone, A.; Affronte, M.; Zotti, A. Age Estimation in the Mediterranean Bottlenose Dolphin Tursiops truncatus (Montagu 1821) by Bone Density of the Thoracic Limb. J. Anat. 2007, 211, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Smuts, B.B. Natal Attraction: Allomaternal Care and Mother–Infant Separations in Wild Bottlenose Dolphins. Anim. Behav. 1998, 55, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Connor, R.C.; Barre, L.M.; Heithaus, M.R. Female Reproductive Success in Bottlenose Dolphins (Tursiops Spp.): Life History, Habitat, Provisioning, and Group-Size Effects. Behav. Ecol. 2000, 11, 210–219. [Google Scholar] [CrossRef]
- Díaz López, B. Interactions between Mediterranean Bottlenose Dolphins (Tursiops truncatus) and Gillnets off Sardinia, Italy. ICES J. Mar. Sci. 2006, 63, 946–951. [Google Scholar] [CrossRef]
- Perrin, W.F.; Reilly, S.B. Reproductive Parameters of Dolphins and Small Whales of the Family Deiphinidae. Rep. Int. Whal. Comm. 1984, 6, 97–133. [Google Scholar]
- Cozzi, B.; Huggenberger, S.; Oelschläger, H. Urinary System, Genital Systems, and Reproduction. In Anatomy of Dolphins; Cozzi, B., Huggenberger, S., Oelschläger, H., Eds.; Academic Press: San Diego, CA, USA, 2017; Chapter 9; pp. 369–409. ISBN 978-0-12-407229-9. [Google Scholar]
- Anderson, R.C.; Chabaud, A.G.; Willmott, S. Keys to Genera of the Superfamily Metastrongyloidea. In C.I.H. Keys to the Nematode Parasites of Vertebrates; Commonwealth Agricultural Bureau: Farnham Royal, UK, 1978; Volume 5, pp. 1–40. [Google Scholar]
- Khalil, L.F.; Jones, A.; Bray, R.A. Keys to the Cestode Parasites of Vertebrates; CAB International: Wallingford, UK, 1994; p. 752. [Google Scholar]
- Giorda, F.; Crociara, P.; Iulini, B.; Gazzuola, P.; Favole, A.; Goria, M.; Serracca, L.; Dondo, A.; Crescio, M.I.; Audino, T.; et al. Neuropathological Characterization of Dolphin Morbillivirus Infection in Cetaceans Stranded in Italy. Animals 2022, 12, 452. [Google Scholar] [CrossRef]
- Di Guardo, G.; Proietto, U.; Di Francesco, C.E.; Marsilio, F.; Zaccaroni, A.; Scaravelli, D.; Mignone, W.; Garibaldi, F.; Kennedy, S.; Forster, F.; et al. Cerebral Toxoplasmosis in Striped Dolphins (Stenella coeruleoalba) Stranded Along the Ligurian Sea Coast of Italy. Vet. Pathol. 2010, 47, 245–253. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Terrestrial Manual), 8th ed.; World Organisation for Animal Health (OIE): Paris, France, 2018; ISBN 978-92-95108-18-9. [Google Scholar]
- Verna, F.; Giorda, F.; Miceli, I.; Rizzo, G.; Pautasso, A.; Romano, A.; Iulini, B.; Pintore, M.D.; Mignone, W.; Grattarola, C.; et al. Detection of Morbillivirus Infection by RT-PCR RFLP Analysis in Cetaceans and Carnivores. J. Virol. Methods 2017, 247, 22–27. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and Analysis of Diverse Herpesviral Species by Consensus Primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef]
- Vitale, M. A High Sensitive Nested PCR for Toxoplasma Gondii Detection in Animal and Food Samples. J. Microb. Biochem. Technol. 2013, 5, 39–41. [Google Scholar] [CrossRef]
- Bounaadja, L.; Albert, D.; Chénais, B.; Hénault, S.; Zygmunt, M.S.; Poliak, S.; Garin-Bastuji, B. Real-Time PCR for Identification of Brucella Spp.: A Comparative Study of IS711, Bcsp31 and per Target Genes. Vet. Microbiol. 2009, 137, 156–164. [Google Scholar] [CrossRef]
- Osorio, C.R.; Romalde, J.L.; Barja, J.L.; Toranzo, A.E. Presence of Phospholipase-D (Dly) Gene Coding for Damselysin Production Is Not a Pre-Requisite for Pathogenicity in Photobacterium damselae Subsp. damselae. Microb. Pathog. 2000, 28, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.; Dastjerdi, A.; Davison, N.; Deaville, R.; Everest, D.; Peake, J.; Finnegan, C.; Jepson, P.; Steinbach, F. Identification of Novel Cetacean Poxviruses in Cetaceans Stranded in South West England. PLoS ONE 2015, 10, e01243. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mora, G.; González-Barrientos, R.; Morales, J.-A.; Chaves-Olarte, E.; Guzmán-Verri, C.; Baquero-Calvo, E.; De-Miguel, M.-J.; Marín, C.-M.; Blasco, J.-M.; Moreno, E. Neurobrucellosis in Stranded Dolphins, Costa Rica. Emerg. Infect. Dis. 2008, 14, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, M.; de los Monteros, A.E.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; Jepson, P.D.; Fernández, A. Pathology and Causes of Death of Stranded Cetaceans in the Canary Islands (1999−2005). Dis. Aquat. Org. 2013, 103, 87–99. [Google Scholar] [CrossRef] [PubMed]
- ASCOBANS/MOP9/Inf.6.2.3a. 2020. Available online: https://www.ascobans.org/sites/default/files/document/ascobans_mop9_inf6.2.3a_report-iwc-workshop-marine-debris.pdf (accessed on 19 January 2023).
- Bonsembiante, F.; Centelleghe, C.; Rossi, G.; Giglio, S.; Madeo, E.; Gelain, M.E.; Mazzariol, S. Clinico-Pathological Findings in a Striped Dolphin (Stenella Coeruleoalba) Affected by Rhabdomyolysis and Myoglobinuric Nephrosis (Capture Myopathy). J. Vet. Med. Sci. 2017, 79, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Herráez, P.; Espinosa de los Monteros, A.; Fernández, A.; Edwards, J.F.; Sacchini, S.; Sierra, E. Capture Myopathy in Live-Stranded Cetaceans. Vet. J. 2013, 196, 181–188. [Google Scholar] [CrossRef]
- Marsili, L.; Focardi, S. Organochlorine Levels in Subcutaneous Blubber Biopsies of Fin Whales (Balaenoptera physalus) and Striped Dolphins (Stenella coeruleoalba) from the Mediterranean Sea. Environ. Pollut. 1996, 91, 1–9. [Google Scholar] [CrossRef]
- Ballschmiter, K.; Zell, M. Analysis of Polychlorinated Biphenyls (PCB) by Glass Capillary Gas Chromatography. Z. Anal. Chem. 1980, 302, 20–31. [Google Scholar] [CrossRef]
- Fishbein, L. Identification of Carcinogenic, Mutagenic and Teratogenic Substances in the Environment. Environ. Qual Saf. 1975, 4, 200–225. [Google Scholar]
- De Voogt, P.; Wells, D.E.; Reutergårdh, L.; Brinkman, U.A.T. Biological Activity, Determination and Occurence of Planar, Mono- and Di-Ortho PCBs. Int. J. Environ. Anal. Chem 1990, 40, 1–46. [Google Scholar] [CrossRef]
- Høyer, A.P.; Grandjean, P.; Jørgensen, T.; Brock, J.W.; Hartvig, H.B. Organochlorine Exposure and Risk of Breast Cancer. Lancet 1998, 352, 1816–1820. [Google Scholar] [CrossRef] [PubMed]
- Dorgan, J.F.; Brock, J.W.; Rothman, N.; Needham, L.L.; Miller, R.; Stephenson, H.E.; Schussler, N.; Taylor, P.R. Serum Organochlorine Pesticides and PCBs and Breast Cancer Risk: Results from a Prospective Analysis (USA). Cancer Causes Control 1999, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Malats, N.; Jariod, M.; Grimalt, J.O.; Rifà, J.; Carrato, A.; Guarner, L.; Salas, A.; Santiago-Silva, M.; Corominas, J.M.; et al. Serum Concentrations of Organochlorine Compounds and K-Ras Mutations in Exocrine Pancreatic Cancer. Lancet 1999, 354, 2125–2129. [Google Scholar] [CrossRef]
- Aronson, K.J.; Miller, A.B.; Woolcott, C.G.; Sterns, E.E.; McCready, D.R.; Lickley, L.A.; Fish, E.B.; Hiraki, G.Y.; Holloway, C.; Ross, T.; et al. Breast Adipose Tissue Concentrations of Polychlorinated Biphenyls and Other Organochlorines and Breast Cancer Risk1. Cancer Epidemiol. Biomark. Prev. 2000, 9, 55–63. [Google Scholar]
- Holford, T.R.; Zheng, T.; Mayne, S.T.; Zahm, S.H.; Tessari, J.D.; Boyle, P. Joint Effects of Nine Polychlorinated Biphenyl (PCB) Congeners on Breast Cancer Risk. Int. J. Epidemiol. 2000, 29, 975–982. [Google Scholar] [CrossRef]
- Demers, A.; Ayotte, P.; Brisson, J.; Dodin, S.; Robert, J.; Dewailly, É. Plasma Concentrations of Polychlorinated Biphenyls and the Risk of Breast Cancer: A Congener-Specific Analysis. Am. J. Epidemiol. 2002, 155, 629–635. [Google Scholar] [CrossRef]
- Ferrante, M.C.; Mattace Raso, G.; Esposito, E.; Bianco, G.; Iacono, A.; Clausi, M.T.; Amero, P.; Santoro, A.; Simeoli, R.; Autore, G.; et al. Effects of Non-Dioxin-like Polychlorinated Biphenyl Congeners (PCB 101, PCB 153 and PCB 180) Alone or Mixed on J774A.1 Macrophage Cell Line: Modification of Apoptotic Pathway. Toxicol. Lett. 2011, 202, 61–68. [Google Scholar] [CrossRef]
- Adami, H.-O.; Lipworth, L.; Titus-Ernstoff, L.; Hsieh, C.; Hanberg, A.; Ahlborg, U.; Baron, J.; Trichopoulos, D. Organochlorine Compounds and Estrogen-Related Cancers in Women. Cancer Causes Control 1995, 6, 551–566. [Google Scholar] [CrossRef]
- Sohoni, P.; Sumpter, J. Several Environmental Oestrogens Are Also Anti-Androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef]
- Hilscherova, K.; Machala, M.; Kannan, K.; Blankenship, A.L.; Giesy, J.P. Cell Bioassays for Detection of Aryl Hydrocarbon (AhR) and Estrogen Receptor (ER) Mediated Activity in Environmental Samples. Environ. Sci. Pollut. Res. 2000, 7, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Morsey, B.; Mori, C.; Guise, S.D. Specific Non-Coplanar Pcb-Mediated Modulation of Bottlenose Dolphin and Beluga Whale Phagocytosis Upon in Vitro Exposure. J. Toxicol. Environ. Health Part A 2004, 67, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Morsey, B.; Mori, C.; Nambiar, P.R.; De Guise, S. Non-Coplanar PCB-Mediated Modulation of Human Leukocyte Phagocytosis: A New Mechanism for Immunotoxicity. J. Toxicol. Environ. Health Part A 2005, 68, 1977–1993. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Morsey, B.; Mori, C.; Nambiar, P.R.; De Guise, S. PCBs and TCDD, Alone And In Mixtures, Modulate Marine Mammal But Not B6C3F1 Mouse Leukocyte Phagocytosis. J. Toxicol. Environ. Health Part A 2005, 68, 635–656. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Corcoran, D.; Dils, R.R.; Darbre, P. 3,4,3′,4′-Tetrachlorobiphenyl Acts as an Estrogen in Vitro and in Vivo. Mol. Endocrinol. 1996, 10, 923–936. [Google Scholar] [CrossRef]
- Wong, W.; Pessah, N. Structural Specificity toward Skeletal- and Cardiac-Type Microsomal Calcium Release Channels. Mol. Pharmacol. 1996, 49, 740–751. [Google Scholar]
- Safe, S.; Astroff, B.; Harris, M.; Zacharewski, T.; Dickerson, R.; Romkes, M.; Biegel, L. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds as Antioestrogens: Characterization and Mechanism of Action. Pharmacol. Toxicol. 1991, 69, 400–409. [Google Scholar] [CrossRef]
- Guise, S.D. Effects of in Vitro Exposure of Beluga Whale Leukocytes to Selected Organochlorines. J. Toxicol. Environ. Health Part A 1998, 55, 479–493. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of a Binary Mixture of 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) (Cas No. 57465-28-8) and 2,2′,4,4′,5,5′-Hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in Female Harlan Sprague-Dawley Rats (Gavage Studies). 2006; pp. 1–258. Available online: https://pubmed.ncbi.nlm.nih.gov/17160104/ (accessed on 20 January 2023).
- National Toxicology Program. Toxicology and Carcinogenesis Studies of 2,3′,4,4′,5-Pentachlorobiphenyl (PCB 118) (CAS No. 31508-00-6) in Female Harlan Sprague-Dawley Rats (Gavage Studies). 2010; pp. 1–174. Available online: https://pubmed.ncbi.nlm.nih.gov/21383778/ (accessed on 20 January 2023).
- Stata Corp Stata Treatment Effects Reference Manual. 202. Available online: https://www.stata.com/manuals/te.pdf (accessed on 20 January 2023).
- Peat, J.; Barton, B. Medical Statistics, a Guide to Data Analysis and Critical Appraisal. Annals 2005, 88, 603. [Google Scholar] [CrossRef]
- LifeDELFI. 2020. Available online: https://lifedelfi.eu/wp-content/uploads/2022/02/LifeDELFI.Deliverable_D2_First-annual-report-on-bycatch-of-stranded-dolphins_Part1.pdf (accessed on 8 February 2023).
- Wells, R.S.; Scott, M.D. The Bottlenose Dolphin. In Handbook of Marine Mammals: Small Cetaceans; Ridgway, S.H., Harrison, R.J., Eds.; Academic Press: London, UK, 1999; pp. 1–55. [Google Scholar]
- WHO. Environmental Health Criteria 9, DDT and Its Derivatives; Environmental health criteria; World Health Organization: Gland, Switzerland; Albany, NY, USA, 1979; ISBN 978-92-4-154069-8.
- Smith, B.D.; Crespo, E.A.; Notarbartolo di Sciara, G. Dolphins, Whales and Porpoises: 2002–2010 Conservation Action Plan for the World’s Cetaceans; Reeves, R.R., Ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2003; ISBN 978-2-8317-0656-6. [Google Scholar]
- Derraik, J.G.B. The Pollution of the Marine Environment by Plastic Debris: A Review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Read, A.J.; Drinker, P.; Northridge, S. Bycatch of Marine Mammals in U.S. and Global Fisheries. Conserv. Biol. 2006, 20, 163–169. [Google Scholar] [CrossRef] [PubMed]
- López, B.D. Bottlenose Dolphins and Aquaculture: Interaction and Site Fidelity on the North-Eastern Coast of Sardinia (Italy). Mar. Biol. 2012, 159, 2161–2172. [Google Scholar] [CrossRef]
- Methion, S.; López, B.D. Abundance and Demographic Parameters of Bottlenose Dolphins in a Highly Affected Coastal Ecosystem. Mar. Freshw. Res. 2018, 69, 1355–1364. [Google Scholar] [CrossRef]
- Natoli, A.; Genov, T.; Kerem, D.; Gonzalvo, J.; Lauriano, G.; Holcer, D.; Labach, H.; Marsili, L.; Mazzariol, S.; Moura, A.E.; et al. The IUCN Red List of Threatened Species 2021: E.T16369383A215248781; International Union for Conservation of Nature (IUCN): Gland, Switzerland, 2021. [Google Scholar]
- Notarbartolo di Sciara, G.; Tonay, A.M. Conserving Whales, Dolphins and Porpoises in the Mediterranean Sea, Black Sea and Adjacent Areas; ACCOBAMS: Monaco City, Monaco, 2021; 160p, ISBN 978-2-9579273-1-9. [Google Scholar]
- Ascheri, D.; Fontanesi, E.; Ballardini, M.; Nani, B.; Alessi, J. Occurrence, Site Fidelity, and Abundance of Bottlenose Dolphins (Tursiops truncatus) in the Western Ligurian Sea. J. Cetacean Res. Manag. 2022, 23, 191–204. [Google Scholar] [CrossRef]
- De Santis, V.; Azzellino, A.; Lanfredi, C.; Airoldi, S. Are Bottlenose Dolphins (Tursiops truncatus) Increasing in the North–Westernpart of the Pelagos Sanctuary (Mediterranean Sea)? 2018. Available online: https://www.researchgate.net/publication/330937157_Are_bottlenose_dolphins_Tursiops_truncatus_increasing_in_the_North-Western_part_of_the_Pelagos_Sanctuary_Mediterranean_Sea (accessed on 24 January 2023).
- Gnone, G.; Bellingeri, M.; Dhermain, F.; Dupraz, F.; Nuti, S.; Bedocchi, D.; Moulins, A.; Rosso, M.; Alessi, J.; McCrea, R.S.; et al. Distribution, Abundance, and Movements of the Bottlenose Dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (North-West Mediterranean Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 372–388. [Google Scholar] [CrossRef]
- Corazzola, G.; Centelleghe, C.; Gallo, E.; Mazzariol, S. Anatomo- Pathological Findings on Encephalic Tissue of Stranded Cetaceans Affected by Toxoplasma gondii. 2017, p. 69. Available online: https://sief.it/data/documents/ATTI-IV-CONGRESSO-SIEF-2017.pdf (accessed on 24 January 2023).
- Di Guardo, G.; Falconi, A.; Di Francesco, A.; Mazzariol, S.; Centelleghe, C.; Casalone, C.; Pautasso, A.; Cocumelli, C.; Eleni, C.; Petrella, A.; et al. Western Blot Expression of 5-Lipoxygenase in the Brain from Striped Dolphins (Stenella coeruleoalba) and Bottlenose Dolphins (Tursiops truncatus) with or without Encephalitis/Meningo-Encephalitis of Infectious Nature. J. Biol. Regul. Homeost. Agents. 2015, 29, 245–250. [Google Scholar]
- Fernández-Escobar, M.; Giorda, F.; Mattioda, V.; Audino, T.; Di Nocera, F.; Lucifora, G.; Varello, K.; Grattarola, C.; Ortega-Mora, L.M.; Casalone, C.; et al. Toxoplasma gondii Genetic Diversity in Mediterranean Dolphins. Pathogens 2022, 11, 909. [Google Scholar] [CrossRef]
- Di Guardo, G.; Mazzariol, S. Toxoplasma gondii: Clues From Stranded Dolphins. Vet. Pathol. 2013, 5, 737. [Google Scholar] [CrossRef]
- Van Bressem, M.-F.; Duignan, P.J.; Banyard, A.; Barbieri, M.; Colegrove, K.M.; De Guise, S.; Di Guardo, G.; Dobson, A.; Domingo, M.; Fauquier, D.; et al. Cetacean Morbillivirus: Current Knowledge and Future Directions. Viruses 2014, 6, 5145–5181. [Google Scholar] [CrossRef]
- Pintore, M.D.; Mignone, W.; Di Guardo, G.; Mazzariol, S.; Ballardini, M.; Florio, C.L.; Goria, M.; Romano, A.; Caracappa, S.; Giorda, F.; et al. Neuropathologic findings in cetaceans stranded in Italy (2002–14). J. Wildl. Dis. 2018, 54, 295–303. [Google Scholar] [CrossRef]
- Minoia, L.; Consales, G.; Mazzariol, S.; Mancusi, C.; Terracciano, G.; Ceciarini, I.; Capanni, F.; Neri, A.; D’Agostino, A.; Marsili, L. Preliminary Assessment of Persistent Organic Pollutants (POPs) in Tissues of Risso’s Dolphin (Grampus griseus) Specimens Stranded along the Italian Coasts. Mar. Pollut. Bull. 2023, 186, 114470. [Google Scholar] [CrossRef] [PubMed]
- López-Berenguer, G.; Acosta-Dacal, A.; Luzardo, O.P.; Peñalver, J.; Martínez-López, E. POPs Concentrations in Cetaceans Stranded along the Agricultural Coastline of SE Spain Show Lower Burdens of Industrial Pollutants in Comparison to Other Mediterranean Cetaceans. Sci. Total Environ. 2023, 858, 159743. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Blankenship, A.L.; Jones, P.D.; Giesy, J.P. Toxicity Reference Values for the Toxic Effects of Polychlorinated Biphenyls to Aquatic Mammals. Hum. Ecol. Risk Assess. Int. J. 2000, 6, 181–201. [Google Scholar] [CrossRef]
- Helle, E.; Olsson, M.; Jensen, S. PCB Levels Correlated with Pathological Changes in Seal Uteri. Ambio 1976, 5, 261–262. [Google Scholar]
- Tanabe, S.; Tatsukawa, R.; Maruyama, K.; Miyazaki, N. Transplacental Transfer of PCBs and Chlorinated Hydrocarbon Pesticides from the Pregnant Striped Dolphin (Stenella coeruleoalba) to Her Fetus. Agric. Biol. Chem. 1982, 46, 1249–1254. [Google Scholar] [CrossRef]
- Robeck, T.R.; O’Brien, J.K.; Atkinson, S. Reproduction. In CRC Handbook of Marine Mammal Medicine; CRC Press: Boca Raton, FL, USA, 2018; pp. 169–207. [Google Scholar]
- Blasi, M.F.; Bruno, C.; Boitani, L. Female Reproductive Output in a Mediterranean Bottlenose Dolphin Tursiops truncatus Population. Aquat. Biol. 2020, 29, 123–136. [Google Scholar] [CrossRef]
- Martini, M.; Salari, F.; Altomonte, I. The Macrostructure of Milk Lipids: The Fat Globules. Crit. Rev. Food Sci. Nutr. 2016, 56, 1209–1221. [Google Scholar] [CrossRef]
- Martini, M.; Altomonte, I.; Sommer, M.F.; Gili, C.; Biancani, B.; Licitra, R.; Salari, F. Milk Composition, Fatty Acids Profile and Fat Globule Size of Bottlenose Dolphin (Tursiops truncatus, Montagu 1821) Milk at Early Lactation. Vet. Res. Commun. 2022, 46, 577–583. [Google Scholar] [CrossRef]
- Koopman, H.N. Phylogenetic, Ecological, and Ontogenetic Factors Influencing the Biochemical Structure of the Blubber of Odontocetes. Mar. Biol. 2007, 151, 277–291. [Google Scholar] [CrossRef]
- Schwacke, L.H.; Voit, E.O.; Hansen, L.J.; Wells, R.S.; Mitchum, G.B.; Hohn, A.A.; Fair, P.A. Probabilistic Risk Assessment of Reproductive Effects of Polychlorinated Biphenyls on Bottlenose Dolphins (Tursiops truncatus) from the Southeast United States Coast. Environ. Toxicol. Chem. 2002, 21, 2752–2764. [Google Scholar] [CrossRef]
- Wells, R.S.; Tornero, V.; Borrell, A.; Aguilar, A.; Rowles, T.K.; Rhinehart, H.L.; Hofmann, S.; Jarman, W.M.; Hohn, A.A.; Sweeney, J.C. Integrating Life-History and Reproductive Success Data to Examine Potential Relationships with Organochlorine Compounds for Bottlenose Dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Sci. Total Environ. 2005, 349, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Focardi, S. Chlorinated hydrocarbon (HCB, DDTs and PCBs levels in cetaceans stranded along the italian coasts: An overview. Environ. Monit Assess 1997, 45, 129–180. [Google Scholar] [CrossRef]
- Marsili, L.; Maltese, S.; Coppola, D.; Carletti, L.; Mazzariol, S.; Fossi, M.C. Ecotoxicological Status of Seven Sperm Whales (Physeter macrocephalus) Stranded along the Adriatic Coast of Southern Italy. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 103–118. [Google Scholar] [CrossRef]
- Fair, P.A.; Adams, J.; Mitchum, G.; Hulsey, T.C.; Reif, J.S.; Houde, M.; Muir, D.; Wirth, E.; Wetzel, D.; Zolman, E.; et al. Contaminant Blubber Burdens in Atlantic Bottlenose Dolphins (Tursiops truncatus) from Two Southeastern US Estuarine Areas: Concentrations and Patterns of PCBs, Pesticides, PBDEs, PFCs, and PAHs. Sci. Total Environ. 2010, 408, 1577–1597. [Google Scholar] [CrossRef]
- Safe, S.; Bandiera, S.; Sawyer, T.; Zmudzka, B.; Mason, G.; Romkes, M.; Denomme, M.A.; Sparling, J.; Okey, A.B.; Fujita, T. Effects of Structure on Binding to the 2,3,7,8-TCDD Receptor Protein and AHH Induction--Halogenated Biphenyls. Environ. Health Perspect. 1985, 61, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Thornton, J.; Fischbein, A.; Lilis, R.; Selikoff, I.J. Disposition of Polychlorinated Biphenyl Congeners in Occupationally Exposed Persons. Toxicol. Appl. Pharmacol. 1982, 62, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Bush, B.; Snow, J.; Koblintz, R. Polychlorobiphenyl (PCB) Congeners,p,p′-DDE, and Hexachlorobenzene in Maternal and Fetal Cord Blood from Mothers in Upstate New York. Arch. Environ. Contam. Toxicol. 1984, 13, 517–527. [Google Scholar] [CrossRef]
- Clarke, J.U. Structure-Activity Relationships in PCBs: Use of Principal Components Analysis to Predict Inducers of Mixed-Function Oxidase Activity. Chemosphere 1986, 15, 275–287. [Google Scholar] [CrossRef]
- Boon, J.P.; Van arnhem, E.; Jansen, S.; Kannan, N.; Petrick, G.; Schulz, D.; Duinker, J.C.; Reijnders, P.J.H.; Goksøyr, A. The Toxicokinetics of PCBs in Marine Mammals with Special Reference to Possible Interactions of Individual Congeners with the Cytochrome P450-Dependent Monooxygenase System: An Overview. In Persistent Pollutants in Marine Ecosystems; Walker, C.H., Livingstone, D.R., Eds.; Society of Environment Toxicology and Chemistry; Pergamon: Amsterdam, The Netherlands, 1992; pp. 119–159. ISBN 978-0-08-041874-2. [Google Scholar]
- Stenberg, M.; Andersson, P.L. Selection of Non-Dioxin-like PCBs for in Vitro Testing on the Basis of Environmental Abundance and Molecular Structure. Chemosphere 2008, 71, 1909–1915. [Google Scholar] [CrossRef]
- (EFSA) European Food Safety. Opinion of the Scientific Committee on a Request from EFSA Related to A Harmonised Approach for Risk Assessment of Substances Which Are Both Genotoxic and Carcinogenic. EFSA J. 2005, 3, 282. [CrossRef]
- (EFSA) European Food Safety. Results of the Monitoring of Non Dioxin-like PCBs in Food and Feed. EFSA J. 2010, 8, 1701. [CrossRef]
- Elnar, A.A.; Diesel, B.; Desor, F.; Feidt, C.; Bouayed, J.; Kiemer, A.K.; Soulimani, R. Neurodevelopmental and Behavioral Toxicity via Lactational Exposure to the Sum of Six Indicator Non-Dioxin-like-Polychlorinated Biphenyls (∑6 NDL-PCBs) in Mice. Toxicology 2012, 299, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Van Larebeke, N.; Hens, L.; Schepens, P.; Covaci, A.; Baeyens, J.; Everaert, K.; Bernheim, J.L.; Vlietinck, R.; De, P.G. The Belgian PCB and Dioxin Incident of January-June 1999: Exposure Data and Potential Impact on Health. Environ. Health Perspect. 2001, 109, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rokoff, L.B.; Shoaff, J.R.; Coull, B.A.; Enlow, M.B.; Bellinger, D.C.; Korrick, S.A. Prenatal Exposure to a Mixture of Organochlorines and Metals and Internalizing Symptoms in Childhood and Adolescence. Environ. Res. 2022, 208, 112701. [Google Scholar] [CrossRef]
- Nowell, L.H.; Capel, P.D.; Dileanis, P.D. Pesticides in Stream Sediment and Aquatic Biota; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-429-10443-5. [Google Scholar]
- Viselli, R. La Contaminazione Da DDT e Dai Suoi Derivati Nei Comparti Abiotici Del Bacino Del Basso Toce e Del Lago Maggiore; ANPA Dipartimento Prevenzione e Risanamento Ambientali, Settore Applicazione Direttiva IPPC: Roma, Italy, 1999.
- Qiu, X.; Zhu, T.; Yao, B.; Hu, J.; Hu, S. Contribution of Dicofol to the Current DDT Pollution in China. Environ. Sci. Technol. 2005, 39, 4385–4390. [Google Scholar] [CrossRef]
- Sereda, B.; Bouwman, H.; Kylin, H. Comparing Water, Bovine Milk, and Indoor Residual Spraying as Possible Sources of DDTand Pyrethroid Residues in Breast Milk. J. Toxicol. Environ. Health Part A 2009, 72, 842–851. [Google Scholar] [CrossRef]
- Wong, M.H.; Leung, A.O.W.; Chan, J.K.Y.; Choi, M.P.K. A Review on the Usage of POP Pesticides in China, with Emphasis on DDT Loadings in Human Milk. Chemosphere 2005, 60, 740–752. [Google Scholar] [CrossRef]
- Aguilar, A. Relationship of DDE/ΣDDT in Marine Mammals to the Chronology of DDT Input into the Ecosystem. Can. J. Fish. Aquat. Sci. 1984, 41, 840–844. [Google Scholar] [CrossRef]
- Borrell, A.; Aguilar, A. Variations in DDE Percentage Correlated with Total DDT Burden in the Blubber of Fin and Sei Whales. Mar. Pollut. Bull. 1987, 18, 70–74. [Google Scholar] [CrossRef]
- Tsydenova, O.; Minh, T.B.; Kajiwara, N.; Batoev, V.; Tanabe, S. Recent Contamination by Persistent Organochlorines in Baikal Seal (Phoca sibirica) from Lake Baikal, Russia. Mar. Pollut. Bull. 2004, 48, 749–758. [Google Scholar] [CrossRef]
- Calamari, D.; Bacci, E.; Focardi, S.; Gaggi, C.; Morosini, M.; Vighi, M. Role of Plant Biomass in the Global Environmental Partitioning of Chlorinated Hydrocarbons. Environ. Sci. Technol. 1991, 25, 1489–1495. [Google Scholar] [CrossRef]
- Bosch, C.; Grimalt, J.O.; Fernández, P. Enantiomeric Fraction and Isomeric Composition to Assess Sources of DDT Residues in Soils. Chemosphere 2015, 138, 40–46. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, T.; Li, J.; Pan, H.; Li, Q.; Miao, G.; Gong, J. Organochlorine Pesticides in the Air around the Taihu Lake, China. Environ. Sci. Technol. 2004, 38, 1368–1374. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, T. Using the o,p′-DDT/p,p′-DDT Ratio to Identify DDT Sources in China. Chemosphere 2010, 81, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- European Union Commission Delegated Regulation (EU) 2022/643. 2022. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32022R0643&from=EN (accessed on 26 January 2023).
- Beasley, V.R. Direct and Indirect Effects of Environmental Contaminants on Amphibians. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-409548-9. [Google Scholar]
- Turgut, C.; Gokbulut, C.; Cutright, T.J. Contents and Sources of DDT Impurities in Dicofol Formulations in Turkey. Environ. Sci Pollut. Res. 2009, 16, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Alegria, H.A.; Bidleman, T.F.; Alvarado, V.; Angeles, F.; Galarza, A.Á.; Bandala, E.R.; de la Cerda Hinojosa, I.; Estrada, I.G.; Reyes, G.G.; et al. Passive Air Sampling of Organochlorine Pesticides in Mexico. Environ. Sci. Technol. 2009, 43, 704–710. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Qi, S.; Li, X.; Peng, X. Concentrations, Enantiomeric Compositions, and Sources of HCH, DDT and Chlordane in Soils from the Pearl River Delta, South China. Sci. Total Environ. 2006, 372, 215–224. [Google Scholar] [CrossRef]
- Marsili, L. Lipophilic Contaminants in Marine Mammals: Review of the Results of Ten Years’ Work at the Department of Environmental Biology, Siena University (Italy). Int. J. Environ. Pollut. 2000, 13, 416–452. [Google Scholar] [CrossRef]
- Fossi, M.C.; Marsili, L.; Lauriano, G.; Fortuna, C.; Canese, S.; Ancora, S.; Leonzio, C.; Romeo, T.; Merino, R.; Abad, E.; et al. Assessment of Toxicological Status of a SW Mediterranean Segment Population of Striped Dolphin (Stenella coeruleoalba) Using Skin Biopsy. Mar. Environ. Res. 2004, 58, 269–274. [Google Scholar] [CrossRef] [PubMed]


| Case ID | IZS Code | Histological | Immuno-Histochemical | Bacteriological | Biomolecular | Serological |
|---|---|---|---|---|---|---|
| 1 | 18013 | X | X (Morbillivirus; Toxoplasma gondii) | X | X | X |
| 2 | 42472 | X * | X (Morbillivirus) | X | X | X |
| 3 | 44599 | X | X (Morbillivirus; Toxoplasma gondii) | X | X | X |
| 4 | 59260 | X | X (Morbillivirus; Toxoplasma gondii) | X | X | X |
| 5 | 51352 | X | X (Morbillivirus; Toxoplasma gondii) | X | X | X |
| 6 | 41716 | X | X (Morbillivirus; Toxoplasma gondii) | X | X | |
| 7 | 50946 | X | X | |||
| 8 | 53638 | X | X (Morbillivirus; Toxoplasma gondii) | X | X | |
| 9 | 61838 | X | ||||
| 10 | 73951 | X | X | X | ||
| 11 | 177 | X | X (Morbillivirus; Toxoplasma gondii) | X | X |
| Compounds | T-OCs | EDC-OCs | IS-OCs | TEI-OCs |
|---|---|---|---|---|
| HCB | × | |||
| PCB95 | × | |||
| o,p′DDE | + | × | ||
| PCB101 | × | • | ||
| PCB99 | × | |||
| p,p′DDE | + | × | • | * |
| o,p′DDD | + | × | ||
| PCB151 | ||||
| PCB144+135 | ||||
| PCB149 | ||||
| PCB118 | + | × | • | * |
| p,p′DDD | + | × | ||
| o,p′DDT | + | × | ||
| PCB146 | ||||
| PCB153 | + | × | • | * |
| PCB141 | ||||
| p,p′DDT | + | × | • | * |
| PCB138 | + | • | ||
| PCB178 | ||||
| PCB187 | ||||
| PCB183 | ||||
| PCB128 | ||||
| PCB174 | ||||
| PCB177 | ||||
| PCB156+171+202 | + | • | ||
| PCB172 | ||||
| PCB180 | + | • | ||
| PCB199 | ||||
| PCB170 | + | |||
| PCB196 | ||||
| PCB201 | ||||
| PCB195 | ||||
| PCB194 | ||||
| PCB206 |
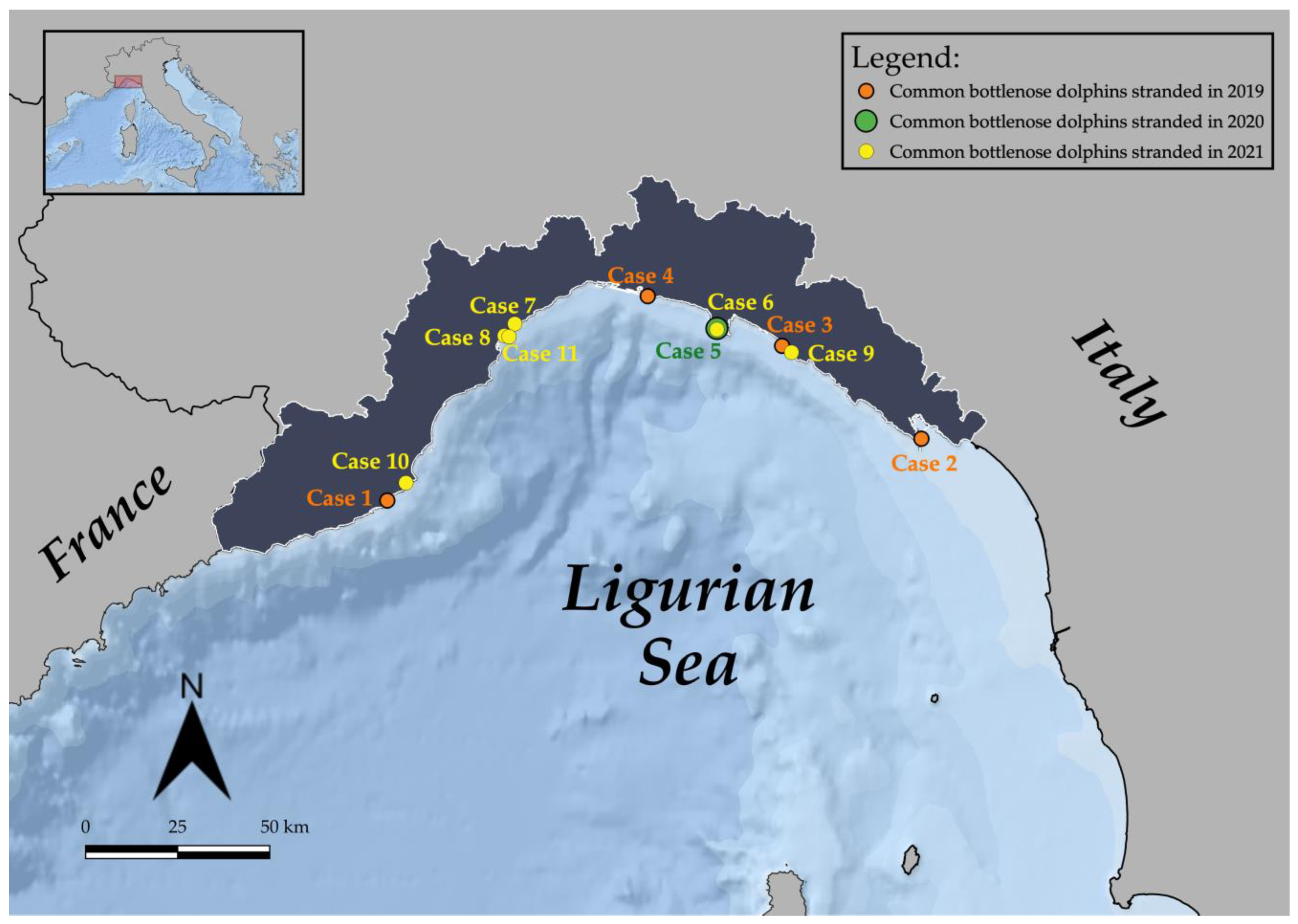
| Case ID | Stranding Date | Stranding Location | Sex | Total Body Length (TBL) | Weight (kg) | Estimated Age [43] | Estimated Age Class [44,45,46] | Sexual Maturity (Gonad Maturation) | DCC | NCC | Gastric Content | Main Lesions (Gross and Microscopic) ** | Pathogens and Helminths Detected *** | Classification of the Cause of Death | Origin | Sub-Category |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 23/02/ 2019 | Diano Marina (IM) | M | 190 | 90 | 3 | Juvenile | Immature | 3 | Go | Scarce digested milk | Two skin ulcers at the right flank; prescapular, pulmonary and rectal reactive lymphadenopathy; meningeal fibrosis. | α-HV (lung); Enterococcus faecium (lung, liver, kidney, and lns) Photobacterium damselae (lung and TB ln) | ND | ||
| 2 | 07/05/ 2019 | Isola Palmaria (SP) | M | 243 | ND | 8 | Adult | ND | 4 | Mo | Evidence of recent meal | Muscular extensive hematoma (right cervico-scapular area); severe mesenteric reactive lymphadenopathy, associated with marked lymphoid depletion. | DMV (spleen, MES ln, lung, bladder; IHC + MES ln); Photobacterium damselae (CNS). | ND | ||
| 3 | 15/05/ 2019 | Sestri levante (GE) | M | 273 | 200 | 11 | Adult | Mature (left: 18.5 cm) | 3 | Po | Scarce content (not recent meal) | Two skin ulcers at the tip of the penis; gelatinous blubber edema; mild pulmonary edema with multifocal parenchymal hemorrhages and multifocal pulmonar parasitic granulomas; lymphoplasmacytic myocarditis and moderate cardiac valvular fibrosis; mesenteric reactive lymph adenomegaly; hemorrhagic CSF. | T. gondii (heart); anti-T. gondii antibodies (1:40) (pericardial and pleuric fluid) | ND | ||
| 4 | 04/07/ 2019 | Genova (GE) | M | 284 | 232 | 12 | Adult | Mature (left: 30 cm; right: 33 cm) | 2 | Mo | Scarce content (not recent meal) | Multifocal erosive parasitic skin lesions; generalized blubber gelatinous edema; serum hemorrhagic effusion in the peritoneal cavity; moderate to severe prescapular, pulmonary and mesenteric reactive lymphadenopathy, associated with lymphoid depletion and lymphadenitis with syncytia; multifocal hemorrhages in the esophageal mucosa; severe interstitial broncho-pneumonia; hemorrhagic CSF. | DMV (lung, TB ln, spleen, kidney, CNS, laryngeal tonsil; IHC+ CNS, lung, PSC, TB, MES lns, spleen, tonsils, kidney); Photobacterium damselae (CNS, lung, liver, spleen, TB ln); T. gondii (muscle); anti-T. gondii antibodies (>1:40) (serum, HA, and pericardial fluid); Pennella sp. (skin) | natural | infectious | Coinfection (viral/bacterial/parasitic) |
| 5 | 13/07/ 2020 | Camogli (GE) | M | 310 | ND | 15 | Adult | ND | 2 | Go | Recent meal outcomes | Nets and ropes around the caudal peduncle; abrasions; protrusion and eye bleeding; hepatic congestion; severe mesenteric and pulmonary reactive lymphadenopathy; severe pulmonary edema; gas embolism in the renal capsule and mesenteric vessels; subendocardial petechiae; mononuclear-eosinophilic interstitial pneumonia; splenic hypertrophy; generalized eosinophilic lymphadenitis and lymphoid depletion; lymphoplasmacytic enteritis; NS meningoencephalitis; muscular hyaline degeneration with wavy cells and atrophy of myocytes; atrioventricular valves fibrosis; mild aortic endocardiosis. | T. gondii (PUL ln, spleen, CNS, muscle, heart); T. gondii antibodies (1:40) (serum, HA and intracardiac clot); α-HV (MES ln); Photobacterium damselae (l, lung, MES and PUL lfn, spleen, CNS); Listeria grayi (CNS); Cl. perfringens (lung, PUL ln, CNS) | anthropic | fishery interaction | bycatch (consequence of underlying pathologies) |
| 6 | 25/04/ 2021 | San Fruttuoso (GE) | M | 241 | 173.5 | 8 | Adult | Mature (average length: 9 cm) | 2 | Mo | Recent meal outcomes | Five TSD, associated with proliferation of the dermal papillae and foci of hydropic degeneration of keratinocytes in the spinous layer; bilateral conjunctival hyperemia and hemorrhagic ocular discharge, blubber petechial hemorrhages associated with muscular hemorrhagic suffusions in the pre-scapular region; subcutaneous parasitization by larval cestodes; subcutaneous-muscular abscess in the right lumbar paravertebral region; multicentric reactive lymphadenopathy, associated with lymphoid depletion; pulmonary parasitic granulomas; splenomegaly; granulomatous gastritis; severe gas embolism in CNS, meningeal, mesenteric, and coronary vessels, renal capsule and lung; serum hemorrhagic effusion in the thorax cavity; abundant foamy fluids in trachea; pulmonary congestion associated with hemorrhagic lymphatic drainage to the marginal pulmonary lymph nodes; hemorrhagic CSF. | Carnobacterium spp. (abscess, MES and TB lns, kidney); Serratia spp. (abscess, MES ln, kidney); Listeria seeligeri (CNS); Cetacean poxvirus 1 (skin lesion); Pholeter gastrophilus (stomach); Phyllobothrium delphini (blubber) | anthropic | fishery interaction | bycatch (consequence of underlying pathologies) |
| 7 | 06/06/ 2021 | Albissola (SV) | F | 270 | ND | 11 | Adult | ND | 4 | ND | Absence of food | Severe dehydration; amputation of dorsal and caudal fins; fishing line around the thorax associated with a linear skin lesion; generalized advanced autolysis. | T. gondii (spleen, heart); DMV (spleen) | ND | ||
| 8 | 12/06/ 2021 | Savona (SV) | M | 140 * | 30 | <1 | Newborn /Calf | Immature | 3 | ND | Scarce digested milk | Amputation of the caudal fin; generalized blubber gelatinous edema, associated with hemorrhagic imbibition tracts; suppurative pneumonia associated with pulmonary reactive lymphadenopathy. | Enterococcus faecalis (lung, CNS, kidney, spleen, PSC and TB lns); T. gondii (liver) | natural | infectious | bacterial |
| 9 | 09/07/ 2021 | Sestri levante (GE) | M | 170 * | 31 | 2 | Juvenile | ND | 4 | ND | Absence of food | Amputation of the caudal fin; circular cut injury of the peduncle; generalized advanced autolysis. | ND | |||
| 10 | 10/09/ 2021 | Andora (SV) | F | 190 | 129.8 | 3 | Juvenile | Immature | 3 | Mo | Scarce content (not recent meal) | Multifocal parasitic skin lesions; ventral blubber gelatinous edema; subcutaneous parasitization by larval cestodes; lung parasitic nodules; foreign body in the esophagus referable to marine litter (fishing line agglomerate); generalized moderate autolyisis. | T. gondii (spleen, PSC ln, liver, CNS, muscle); DMV (lung, urinary bladder); Erysipelothrix rhusiopathiae (PSC ln, spleen, kidney); Photobacterium damselae (lung, PSC ln); Pennella sp (skin); Phyllobothrium delphini (blubber) | ND | ||
| 11 | 24/12/ 2021 | Savona (SV) | F | 280 | 211 | 12 | Adult | Mature (follicles at different development stage) | 2 | Go | Absence of food | Four sub-acute-chronic skin lesions; muscular and subcutaneous hematoma in the left prescapular region; hemothorax; moderate pulmonary oedema associated with foam in trachea; pyogranulomatous tonsillitis; granulomatous gastritis; lymphoplasmacytic endometritis; lymphoid depletion in spleen and mesenteric lymph nodes; NS meningoencephalitis. | DMV (CNS and mesenteric lymph nodes; IHC + CNS); α-HV (skin lesions); Pholeter gastrophilus (stomach) | natural | infectious | viral |
| Case ID | EOM% | HCB | DDTs | PCBs | T-OCs | EDC-OCs | IS-OCs | TEI-OCs |
|---|---|---|---|---|---|---|---|---|
| 1 | 84.78 | 280.78 | 224,46.79 | 177,609.93 | 130,334.29 (65.1%) | 90,724.62 (45.3%) | 126,420.67 (63.1%) | 74,280.54 (37.1%) |
| 2 | 83.91 | 241.21 | 39,621.54 | 262,480.14 | 200,443.90 (66.3%) | 121,294.01 (40.1%) | 178,423.27 (59.0%) | 104,702.02 (34.6%) |
| 3 | 71.50 | 158.45 | 123,374.77 | 743,861.65 | 603,010.52 (69.5%) | 347,718.54 (40.1%) | 523,707.06 (60.4%) | 313,257.21 (36.1%) |
| 4 | 24.75 | 68.76 | 94,466.53 | 1,043,474.63 | 743,699.39 (65.4%) | 358,146.57 (31.5%) | 645,605.76 (56.7%) | 336,229.43 (29.6%) |
| 5 | 70.21 | 109.94 | 73,303.76 | 575,952.02 | 443,256.75 (68.3%) | 240,098.94 (37.0%) | 388,104.78 (59.8%) | 224,646.44 (34.6%) |
| 6 | 86.57 | 208.28 | 26,737.48 | 206,617.95 | 149,081.86 (63.8%) | 94,743.10 (40.6%) | 134,473.90 (57.6%) | 78,958.62 (33.8%) |
| 7 | 46.05 | 110.43 | 33,104.91 | 285,565.56 | 206,284.90 (64.7%) | 122,933.62 (38.6%) | 178,991.43 (56.2%) | 104,268.77 (32.7%) |
| 8 | 82.46 | 306.76 | 19,335.13 | 121,898.28 | 92,437.61 (65.3%) | 63,019.76 (44.5%) | 83,712.31 (59.1%) | 49,936.29 (35.3%) |
| 9 | 73.31 | 325.43 | 22,276.64 | 162,787.21 | 115,577.15 (62.3%) | 80,968.49 (43.7%) | 105,309.60 (56.8%) | 60,827.45 (32.8%) |
| 10 | 49.71 | 196.33 | 83,376.02 | 702,123.89 | 517,643.34 (65.9%) | 302,423.38 (38.5%) | 459,438.61 (58.5%) | 269,589.47 (34.3%) |
| 11 | 79.77 | 21.87 | 2935.19 | 36,283.59 | 20,358.92 (51.9%) | 12,018.26 (30.6%) | 18,004.87 (45.9%) | 8974.91 (22.9%) |
| Case ID | DDTs/PCBs | pp′DDE/DDTs | pp′DDE/pp′DDT | Σ2DDT/(Σ2DDE + Σ2DDD) | Σop′DDTs/DDTs | op′DDT/pp′DDT |
|---|---|---|---|---|---|---|
| 1 | 0.13 | 0.71 | 5.86 | 0.16 | 0.09 | 0.13 |
| 2 | 0.15 | 0.76 | 10.40 | 0.09 | 0.06 | 0.11 |
| 3 | 0.17 | 0.80 | 17.41 | 0.06 | 0.11 | 0.31 |
| 4 | 0.09 | 0.83 | 11.61 | 0.09 | 0.06 | 0.10 |
| 5 | 0.13 | 0.87 | 18.79 | 0.06 | 0.05 | 0.14 |
| 6 | 0.13 | 0.71 | 8.37 | 0.11 | 0.10 | 0.21 |
| 7 | 0.12 | 0.65 | 11.60 | 0.24 | 0.21 | 2.47 |
| 8 | 0.16 | 0.67 | 8.21 | 0.10 | 0.15 | 0.16 |
| 9 | 0.14 | 0.62 | 9.19 | 0.08 | 0.12 | 0.11 |
| 10 | 0.12 | 0.77 | 8.65 | 0.10 | 0.06 | 0.06 |
| 11 | 0.08 | 0.37 | 4.36 | 0.30 | 0.43 | 1.72 |
| PCBs μg/g d.w. | DDTs μg/g d.w. | IS-OCs μg/g d.w. | TEI–OCs μg/g d.w. | PCBs (101+138+153+180) μg/g d.w. | PCBs (118+138+153+180) μg/g d.w. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n° of Samples | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| Control-subgroup 1 | 6 | 331.5 | 261.3 | 47.9 | 42.2 | 227.0 | 183.3 | 133.7 | 109.0 | 171.3 | 138.7 | 178.1 | 145.2 |
| Subgroup 1 | 4 | 573.4 | 372.9 | 62.6 | 30.8 | 365.6 | 228.8 | 203.7 | 117.8 | 289.8 | 190.1 | 302.6 | 197.0 |
| Control-subgroup 2 | 6 | 365.6 | 282.3 | 50.7 | 43.0 | 246.3 | 193.7 | 145.4 | 115.4 | 186.9 | 146.5 | 195.5 | 154.2 |
| Subgroup 2 | 4 | 522.1 | 383.7 | 58.5 | 31.0 | 337.7 | 233.8 | 186.1 | 118.5 | 266.5 | 196.5 | 276.5 | 203.6 |
| Control-subgroup 3 | 4 | 301.5 | 295.8 | 46.9 | 51.0 | 209.8 | 210.0 | 124.6 | 126.2 | 156.1 | 156.7 | 162.3 | 164.1 |
| Subgroup 3 | 2 | 653.0 | 552.2 | 67.0 | 38.8 | 412.0 | 330.4 | 220.5 | 163.7 | 331.3 | 281.6 | 343.4 | 291.3 |
| Subgroup 4 | 2 | 493.8 | 294.6 | 58.2 | 35.5 | 319.2 | 198.3 | 186.9 | 116.9 | 248.4 | 149.3 | 261.8 | 157.8 |
| Subgroup 3 | 2 | 653.0 | 552.2 | 67.0 | 38.8 | 412.0 | 330.4 | 220.5 | 163.7 | 331.3 | 281.6 | 343.4 | 291.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grattarola, C.; Minoia, L.; Giorda, F.; Consales, G.; Capanni, F.; Ceciarini, I.; Franchi, E.; Ascheri, D.; Garibaldi, F.; Dondo, A.; et al. Health Status of Stranded Common Bottlenose Dolphins (Tursiops truncatus) and Contamination by Immunotoxic Pollutants: A Threat to the Pelagos Sanctuary—Western Mediterranean Sea. Diversity 2023, 15, 569. https://doi.org/10.3390/d15040569
Grattarola C, Minoia L, Giorda F, Consales G, Capanni F, Ceciarini I, Franchi E, Ascheri D, Garibaldi F, Dondo A, et al. Health Status of Stranded Common Bottlenose Dolphins (Tursiops truncatus) and Contamination by Immunotoxic Pollutants: A Threat to the Pelagos Sanctuary—Western Mediterranean Sea. Diversity. 2023; 15(4):569. https://doi.org/10.3390/d15040569
Chicago/Turabian StyleGrattarola, Carla, Lorenzo Minoia, Federica Giorda, Guia Consales, Francesca Capanni, Ilaria Ceciarini, Enrica Franchi, Davide Ascheri, Fulvio Garibaldi, Alessandro Dondo, and et al. 2023. "Health Status of Stranded Common Bottlenose Dolphins (Tursiops truncatus) and Contamination by Immunotoxic Pollutants: A Threat to the Pelagos Sanctuary—Western Mediterranean Sea" Diversity 15, no. 4: 569. https://doi.org/10.3390/d15040569
APA StyleGrattarola, C., Minoia, L., Giorda, F., Consales, G., Capanni, F., Ceciarini, I., Franchi, E., Ascheri, D., Garibaldi, F., Dondo, A., Goria, M., Serracca, L., Varello, K., Masoero, L., Di Francesco, C. E., Casalone, C., & Marsili, L. (2023). Health Status of Stranded Common Bottlenose Dolphins (Tursiops truncatus) and Contamination by Immunotoxic Pollutants: A Threat to the Pelagos Sanctuary—Western Mediterranean Sea. Diversity, 15(4), 569. https://doi.org/10.3390/d15040569












