High-Latitude Dinosaur Nesting Strategies during the Latest Cretaceous in North-Eastern Russia
Abstract
1. Introduction
2. Material and Method
3. Results
4. Discussion
4.1. Primary Preservation of the Carbon and Oxygen Isotope Compositions of Tooth Apatite and Eggshell Calcite
- Oxygen isotope compositions of tooth phosphate show a large range of variation from 9.6‰ to 22.6‰, precluding any re-equilibration with diagenetic fluids that would tend to homogenize the oxygen isotope compositions of phosphate towards a possible diagenetic end member [47].
- The relative amount of carbonate in dinosaur apatites estimated from the measured CO2 peak intensity of the mass spectrometer ranges from 4.4 Wt% (weight percent) to 8.6 Wt% (Table 1), within the expected range of 2–13 Wt% measured in extant vertebrates [51,52,53]. A higher amount would have indicated a contamination of the apatite carbonate stable isotope compositions with diagenetic calcite that would not have been removed during the chemical cleaning.
- No correlation is observed between the carbon and oxygen isotope compositions of eggshell calcite that would hint to a partial re-equilibration with environmental diagenetic fluids [54].
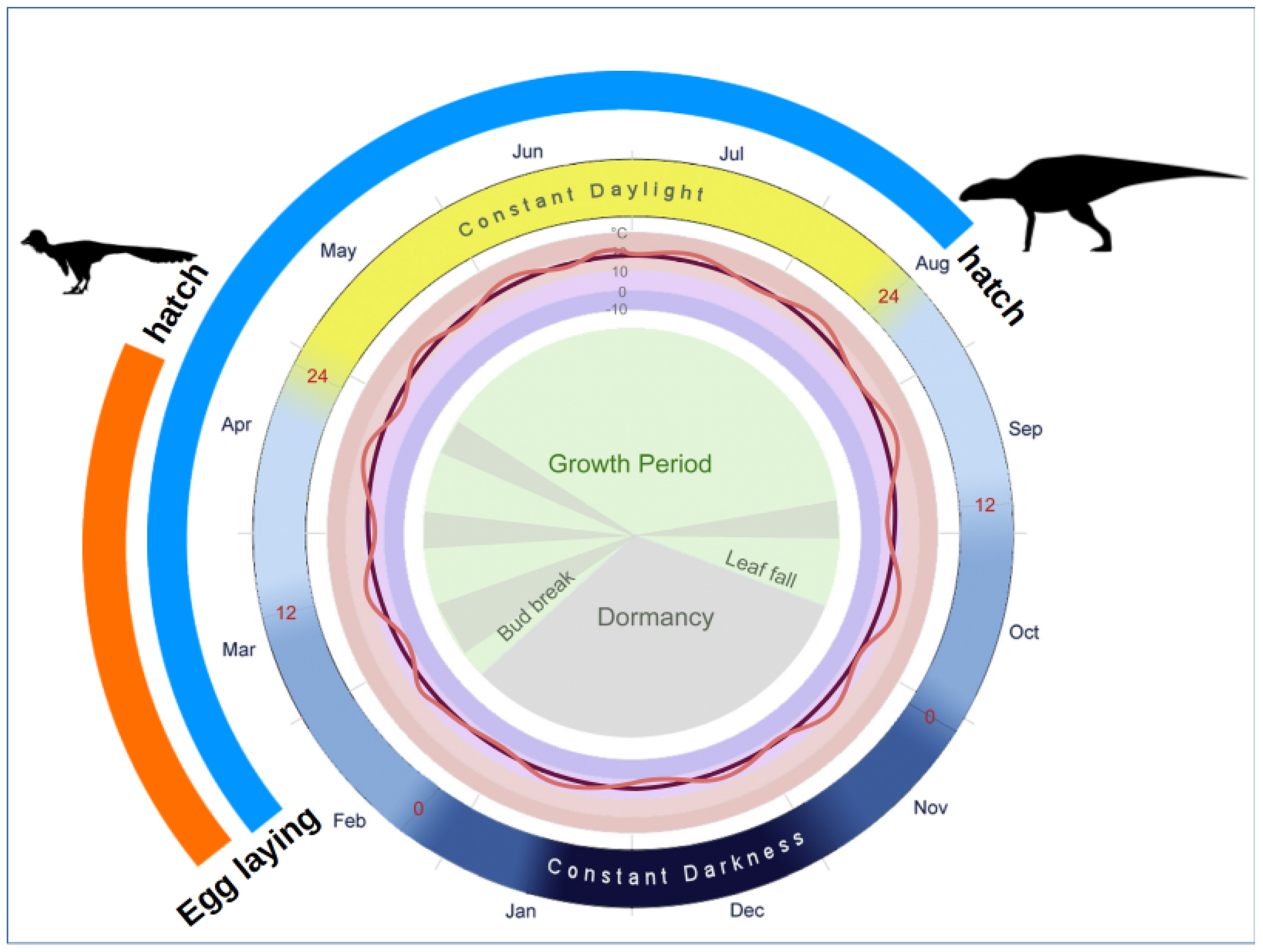
4.2. Environmental Conditions in the Kakanaut Area during the Latest Cretaceous
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, J.R. The Relation between Latitude and Breeding Seasons in Birds. Proc. Zool. Soc. Lond. 1939, 108, 557–582. [Google Scholar] [CrossRef]
- Tanaka, K.; Zelenitsky, D.K.; Therrien, F.; Kobayashi, Y. Nest Substrate Reflects Incubation Style in Extant Archosaurs with Implications for Dinosaur Nesting Habits. Sci. Rep. 2018, 8, 3170. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Bauer, A.M.; Meiri, S.; Uetz, P. Global Taxonomic Diversity of Living Reptiles. PLoS ONE 2013, 8, e59741. [Google Scholar] [CrossRef] [PubMed]
- Artuso, C.; Houston, C.S.; Smith, D.G.; Rohner, C. Great Horned Owl (Bubo virginianus), version 1.1. In Birds of the World; Sly, N.D., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2022. [Google Scholar] [CrossRef]
- Benkman, C.W. White-winged Crossbill (Loxia leucoptera), version 1.0. In Birds of the World; Sly, N.D., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Buehler, D.A. Bald Eagle (Haliaeetus leucocephalus), version 2.0. In Birds of the World; Sly, N.D., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2022. [Google Scholar] [CrossRef]
- Carpenter, K. Eggs, Nests, and Baby Dinosaurs: A Look at Dinosaur Reproduction; Indiana University Press: Bloomington, IN, USA; Indianapolis, IN, USA, 1999. [Google Scholar]
- Spicer, R.A.; Herman, A.B.; Amiot, R.; Spicer, T.E.V. Environmental Adaptations and Constraints on Latest Cretaceous Arctic Dinosaurs. Glob. Geol. 2016, 19, 241–254. [Google Scholar] [CrossRef]
- Spicer, R.A.; Herman, A.B. The Late Cretaceous Environment of the Arctic: A Quantitative Reassessment Based on Plant Fossils. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 295, 423–442. [Google Scholar] [CrossRef]
- Zolina, A.A.; Golovneva, L.B.; Spicer, R.A. Latest Cretaceous (Maastrichtian) Climate of the Koryak Upland of North-East Russia Based on a Quantitative Analysis of a Palaeo-Polar Flora. Palaeogeogr. Palaeoclim. Palaeoecol. 2020, 560, 109997. [Google Scholar] [CrossRef]
- Godefroit, P.; Golovneva, L.; Shchepetov, S.; Garcia, G.; Alekseev, P. The Last Polar Dinosaurs: High Diversity of Latest Cretaceous Arctic Dinosaurs in Russia. Naturwissenschaften 2009, 96, 495–501. [Google Scholar] [CrossRef]
- Fiorillo, A.R. Alaska Dinosaurs: An Ancient Arctic World, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-138-06087-6. [Google Scholar]
- Rich, T.H.; Vickers-Rich, P. Dinosaurs of Darkness: In Search of the Lost Polar World, 2nd ed.; Indiana University Press: Bloomington, IN, USA, 2020; ISBN 978-0-253-04739-7. [Google Scholar]
- Druckenmiller, P.S.; Erickson, G.M.; Brinkman, D.; Brown, C.M.; Eberle, J.J. Nesting at Extreme Polar Latitudes by Non-Avian Dinosaurs. Curr. Biol. 2021, 31, 3469–3478.e5. [Google Scholar] [CrossRef]
- Chiarenza, A.A.; Fiorillo, A.R.; Tykoski, R.S.; McCarthy, P.J.; Flaig, P.P.; Contreras, D.L. The First Juvenile Dromaeosaurid (Dinosauria: Theropoda) from Arctic Alaska. PLoS ONE 2020, 15, e0235078. [Google Scholar] [CrossRef]
- Amiot, R.; Wang, X.; Wang, S.; Lécuyer, C.; Mazin, J.-M.; MO, J.; Flandrois, J.-P.; Fourel, F.; Wang, X.; Xu, X.; et al. δ18O-Derived Incubation Temperatures of Oviraptorosaur Eggs. Palaeontology 2017, 60, 633–647. [Google Scholar] [CrossRef]
- Bi, S.; Amiot, R.; Peyre de Fabrègues, C.; Pittman, M.; Lamanna, M.C.; Yu, Y.; Yu, C.; Yang, T.; Zhang, S.; Zhao, Q.; et al. An Oviraptorid Preserved atop an Embryo-Bearing Egg Clutch Sheds Light on the Reproductive Biology of Non-Avialan Theropod Dinosaurs. Sci. Bull. 2021, 66, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.M.; Zelenitsky, D.K.; Kay, D.I.; Norell, M.A. Dinosaur Incubation Periods Directly Determined from Growth-Line Counts in Embryonic Teeth Show Reptilian-Grade Development. Proc. Natl. Acad. Sci. USA 2017, 114, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A. Incubation Times of Dinosaur Eggs via Embryonic Metabolism. Phys. Rev. E 2016, 94, 022402. [Google Scholar] [CrossRef] [PubMed]
- Longinelli, A. Oxygen Isotopes in Mammal Bone Phosphate: A New Tool for Paleohydrological and Paleoclimatological Research? Geochim. Cosmochim. Acta 1984, 48, 385–390. [Google Scholar] [CrossRef]
- Longinelli, A.; Nuti, S. Revised Phosphate-Water Isotopic Temperature Scale. Earth Planet. Sci. Lett. 1973, 19, 373–376. [Google Scholar] [CrossRef]
- Kohn, M.J. Predicting Animal δ18O: Accounting for Diet and Physiological Adaptation. Geochim. Cosmochim. Acta 1996, 60, 4811–4829. [Google Scholar] [CrossRef]
- Langlois, C.; Simon, L.; Lécuyer, C. Box-Modeling of Bone and Tooth Phosphate Oxygen Isotope Compositions as a Function of Environmental and Physiological Parameters. Isot. Environ. Health Stud. 2003, 39, 259–272. [Google Scholar] [CrossRef]
- Amiot, R.; Angst, D.; Legendre, S.; Buffetaut, E.; Fourel, F.; Adolfssen, J.; André, A.; Bojar, A.V.; Canoville, A.; Barral, A.; et al. Oxygen Isotope Fractionation between Bird Bone Phosphate and Drinking Water. Sci. Nat. 2017, 104, 47. [Google Scholar] [CrossRef]
- Lazzerini, N.; Lécuyer, C.; Amiot, R.; Angst, D.; Buffetaut, E.; Fourel, F.; Daux, V.; Betancort, J.F.; Sánchez-Marco, A.; Lomoschitz, A. Oxygen Isotope Fractionation between Bird Eggshell Calcite and Body Water: Application to Fossil Eggs from Lanzarote (Canary Islands). Sci. Nat. 2016, 103, 81. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable Isotopes in Precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Goedert, J.; Amiot, R.; Boudad, L.; Buffetaut, E.; Fourel, F.; Godefroit, P.; Kusuhashi, N.; Suteethom, V.; Tong, H.; Watanabe, M.E.; et al. Preliminary Investigation of Seasonal Patterns Recorded in the Oxygen Isotope Composition of Theropod Dinosaur Tooth Enamel. Palaios 2016, 31, 10–19. [Google Scholar] [CrossRef]
- Passey, B.H.; Robinson, T.F.; Ayliffe, L.K.; Cerling, T.E.; Sponheimer, M.; Dearing, M.D.; Roeder, B.L.; Ehleringer, J.R. Carbon Isotope Fractionation between Diet, Breath CO2, and Bioapatite in Different Mammals. J. Archaeol. Sci. 2005, 32, 1459–1470. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Monson, R.K. Evolutionary and Ecological Aspects of Photosynthetic Pathway Variation. Annu. Rev. Ecol. Syst. 1993, 24, 411–439. [Google Scholar] [CrossRef]
- Tipple, B.J.; Pagani, M. The Early Origins of Terrestrial C4 Photosynthesis. Annu. Rev. Earth Planet. Sci. 2007, 35, 435–461. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Angst, D.; Lécuyer, C.; Amiot, R.; Buffetaut, E.; Fourel, F.; Martineau, F.; Legendre, S.; Abourachid, A.; Herrel, A. Isotopic and Anatomical Evidence of an Herbivorous Diet in the Early Tertiary Giant Bird Gastornis. Implications for the Structure of Paleocene Terrestrial Ecosystems. Naturwissenschaften 2014, 101, 313–322. [Google Scholar] [CrossRef]
- Fricke, H.C.; Rogers, R.R.; Backlund, R.; Dwyer, C.N.; Echt, S. Preservation of Primary Stable Isotope Signals in Dinosaur Remains, and Environmental Gradients of the Late Cretaceous of Montana and Alberta. Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 266, 13–27. [Google Scholar] [CrossRef]
- Fricke, H.C.; Pearson, D.A. Stable Isotope Evidence for Changes in Dietary Niche Partitioning among Hadrosaurian and Ceratopsian Dinosaurs of the Hell Creek Formation, North Dakota. Paleobiology 2008, 34, 534–552. [Google Scholar] [CrossRef]
- Domingo, L.; Barroso-Barcenilla, F.; Cambra-Moo, O. Seasonality and Paleoecology of the Late Cretaceous Multi-Taxa Vertebrate Assemblage of “Lo Hueco” (Central Eastern Spain). PLoS ONE 2015, 10, e0119968. [Google Scholar] [CrossRef] [PubMed]
- Tütken, T. The Diet of Sauropod Dinosaurs: Implications from Carbon Isotope Analysis of Teeth, Bones, and Plants. In Biology of the Sauropod Dinosaurs: Understanding the Life of Giants; Klein, N., Remes, K., Sander, M., Eds.; Indiana University Press: Bloomington, IN, USA, 2011; pp. 57–79. [Google Scholar]
- Golovneva, L.B.; Gnilovskaya, A.A. Fossil Plants from the Vysokorechenskaya Formation (the Upper Cretaceous, Koryak Upland). Palaeobotany 2015, 6, 36–47. [Google Scholar] [CrossRef]
- Golovneva, L.B.; Shczepetov, S.V. Stratigraphy of the Maastrichtian Deposits of the Kakanaut River Basin (Eastern Part or the Koryak Upland). Palaeobotany 2010, 1, 96–119. [Google Scholar] [CrossRef]
- Shczepetov, S.V.; Herman, A.B. The Formation Conditions of the Burial Site of Late Cretaceous Dinosaurs and Plants in the Kakanaut River Basin (Koryak Highlands, Northeastern Asia). Strat. Geol. Correl. 2017, 25, 400–418. [Google Scholar] [CrossRef]
- Torsvik, T.H.; Van der Voo, R.; Preeden, U.; Mac Niocaill, C.; Steinberger, B.; Doubrovine, P.V.; van Hinsbergen, D.J.J.; Domeier, M.; Gaina, C.; Tohver, E.; et al. Phanerozoic Polar Wander, Palaeogeography and Dynamics. Earth Sci. Rev. 2012, 114, 325–368. [Google Scholar] [CrossRef]
- Scotese, C.R. PALEOMAP PaleoAtlas for Gplates and the PaleoData Plotter Program, PALEOMAP Project. 2016. Available online: https://www.earthbyte.org/paleomap-paleoatlas-for-gplates/ (accessed on 22 April 2022).
- Lécuyer, C. Oxygen Isotope Analysis of Phosphate. In Handbook of Stable Isotope Analytical Techniques; Elsevier: Amsterdam, The Netherlands, 2004; pp. 482–496. [Google Scholar]
- Lécuyer, C.; Grandjean, P.; O’Neil, J.R.; Cappetta, H.; Martineau, F. Thermal Excursions in the Ocean at the Cretaceous-Tertiary Boundary (Northern Morocco): δ18O Record of Phosphatic Fish Debris. Palaeogeogr. Palaeoclim. Palaeoecol. 1993, 105, 235–243. [Google Scholar] [CrossRef]
- Hut, G. Consultants’ Group Meeting on Stable Isotope Reference Samples for Geochemical and Hydrological Investigations. 1987. Available online: http://www.Iaea.org/inis/collection/NCLCollectionStore/_Public/18/075/18075746.Pdf (accessed on 22 April 2022).
- Koch, P.L.; Tuross, N.; Fogel, M.L. The Effects of Sample Treatment and Diagenesis on the Isotopic Integrity of Carbonate in Biogenic Hydroxylapatite. J. Archaeol. Sci. 1997, 24, 417–429. [Google Scholar] [CrossRef]
- Fourel, F.; Martineau, F.; Tóth, E.E.; Görög, A.; Escarguel, G.; Lécuyer, C. Carbon and Oxygen Isotope Variability among Foraminifera and Ostracod Carbonated Shells. Ann. Univ. Mariae Curie Sklodowska Sect. AAA Phys. 2016, 70, 133–156. [Google Scholar]
- Lécuyer, C.; Bogey, C.; Garcia, J.-P.; Grandjean, P.; Barrat, J.A.; Floquet, M.; Bardet, N.; Pereda-Superbiola, X. Stable Isotope Composition and Rare Earth Element Content of Vertebrate Remains from the Late Cretaceous of Northern Spain (Laño): Did the Environmental Record Survive? Palaeogeogr. Palaeoclim. Palaeoecol. 2003, 193, 457–471. [Google Scholar] [CrossRef]
- Bryant, D.J.; Koch, P.L.; Froelich, P.N.; Showers, W.J.; Genna, B.J. Oxygen Isotope Partitioning between Phosphate and Carbonate in Mammalian Apatite. Geochim. Cosmochim. Acta 1996, 60, 5145–5148. [Google Scholar] [CrossRef]
- Iacumin, P.; Bocherens, H.; Mariotti, A.; Longinelli, A. Oxygen Isotope Analyses of Co-Existing Carbonate and Phosphate in Biogenic Apatite: A Way to Monitor Diagenetic Alteration of Bone Phosphate? Earth Planet. Sci. Lett. 1996, 142, 1–6. [Google Scholar] [CrossRef]
- Lécuyer, C.; Balter, V.; Martineau, F.; Fourel, F.; Bernard, A.; Amiot, R.; Gardien, V.; Otero, O.; Legendre, S.; Panczer, G. Oxygen Isotope Fractionation between Apatite-Bound Carbonate and Water Determined from Controlled Experiments with Synthetic Apatites Precipitated at 10–37 °C. Geochim. Cosmochim. Acta 2010, 74, 2072–2081. [Google Scholar] [CrossRef]
- Brudevold, F.; Soremark, R. Chemistry of the Mineral Phase of Enamel. In Structural and Chemical Organization of Teeth, Volume 2; Mills, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1967; pp. 247–277. [Google Scholar]
- Rink, W.J.; Schwarcz, H.P. Tests for Diagenesis in Tooth Enamel: ESR Dating Signals and Carbonate Contents. J. Archaeol. Sci. 1995, 22, 251–255. [Google Scholar] [CrossRef]
- Vennemann, T.W.; Hegner, E.; Cliff, G.; Benz, G.W. Isotopic Composition of Recent Shark Teeth as a Proxy for Environmental Conditions. Geochim. Cosmochim. Acta 2001, 65, 1583–1599. [Google Scholar] [CrossRef]
- Graf, J.; Tabor, N.J.; Ferguson, K.; Winkler, D.A.; Lee, Y.-N.; May, S.; Jacobs, L.L. Diagenesis of Dinosaur Eggshell from the Gobi Desert, Mongolia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 494, 65–74. [Google Scholar] [CrossRef]
- Lécuyer, C. Water on Earth: Physicochemical and Biological Properties; Wiley-ISTE: London, UK, 2014; ISBN 978-1-84821-477-4. [Google Scholar]
- Erickson, G.M. Incremental Lines of von Ebner in Dinosaurs and the Assessment of Tooth Replacement Rates Using Growth Line Counts. Proc. Natl. Acad. Sci. USA 1996, 93, 14623–14627. [Google Scholar] [CrossRef] [PubMed]
- Stanton-Thomas, K.J.; Carlson, S.J. Microscale δ18O and δ13C Isotopic Analysis of an Ontogenetic Series of the Hadrosaurid Dinosaur Edmontosaurus: Implications for Physiology and Ecology. Palaeogeogr. Palaeoclim. Palaeoecol. 2004, 206, 257–287. [Google Scholar] [CrossRef]
- Straight, W.H.; Barrick, R.E.; Eberth, D.A. Reflections of Surface Water, Seasonality and Climate in Stable Oxygen Isotopes from Tyrannosaurid Tooth Enamel. Palaeogeogr. Palaeoclim. Palaeoecol. 2004, 206, 239–256. [Google Scholar] [CrossRef]
- Lécuyer, C.; Amiot, R.; Touzeau, A.; Trotter, J. Calibration of the Phosphate δ18O Thermometer with Carbonate–Water Oxygen Isotope Fractionation Equations. Chem. Geol. 2013, 347, 217–226. [Google Scholar] [CrossRef]
- Love, J.W. Age, Growth, and Reproduction of Spotted Gar, Lepisosteus Oculatus (Lepisosteidae), from the Lake Pontchartrain Estuary, Louisiana. Southwest. Nat. 2004, 49, 18–23. [Google Scholar] [CrossRef]
- IAEA/WMO Global Network of Isotopes in Precipitation. The GNIP Database. 2022. Available online: https://nucleus.iaea.org/wiser (accessed on 22 April 2022).
- Spicer, R.; Valdes, P.; Hughes, A.; Yang, J.; Spicer, T.; Herman, A.; Farnsworth, A. New Insights into the Thermal Regime and Hydrodynamics of the Early Late Cretaceous Arctic. Geol. Mag. 2020, 157, 1729–1746. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, J.; Li, L.; Xin, P. Plant Water Use Strategies Indicated by Isotopic Signatures of Leaf Water: Observations in Southern and Northern China. Agric. For. Meteorol. 2019, 276–277, 107624. [Google Scholar] [CrossRef]
- Lowdon, J.A.; Dyck, W. Seasonal Variations in the Isotope Ratios of Carbon in Maple Leaves and Other Plants. Can. J. Earth Sci. 1974, 11, 79–88. [Google Scholar] [CrossRef]
- Metcalfe, J.Z. C3 Plant Isotopic Variability in a Boreal Mixed Woodland: Implications for Bison and Other Herbivores. PeerJ 2021, 9, e12167. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Diefendorf, A.F. Seasonal and Canopy Height Variation in N-Alkanes and Their Carbon Isotopes in a Temperate Forest. Org. Geochem. 2018, 116, 23–34. [Google Scholar] [CrossRef]
- Bell, P.R.; Snively, E. Polar Dinosaurs on Parade: A Review of Dinosaur Migration. Alcheringa Australas. J. Palaeontol. 2008, 32, 271–284. [Google Scholar] [CrossRef]
- Varricchio, D.J.; Kundrát, M.; Hogan, J. An Intermediate Incubation Period and Primitive Brooding in a Theropod Dinosaur. Sci. Rep. 2018, 8, 12454. [Google Scholar] [CrossRef]
- Herman, A.B.; Spicer, R.A.; Spicer, T.E.V. Environmental Constraints on Terrestrial Vertebrate Behaviour and Reproduction in the High Arctic of the Late Cretaceous. Palaeogeogr. Palaeoclim. Palaeoecol. 2016, 441, 317–338. [Google Scholar] [CrossRef]
- Chiarenza, A.A.; Mannion, P.D.; Farnsworth, A.; Carrano, M.T.; Varela, S. Climatic Constraints on the Biogeographic History of Mesozoic Dinosaurs. Curr. Biol. 2022, 32, 570–585.e3. [Google Scholar] [CrossRef]
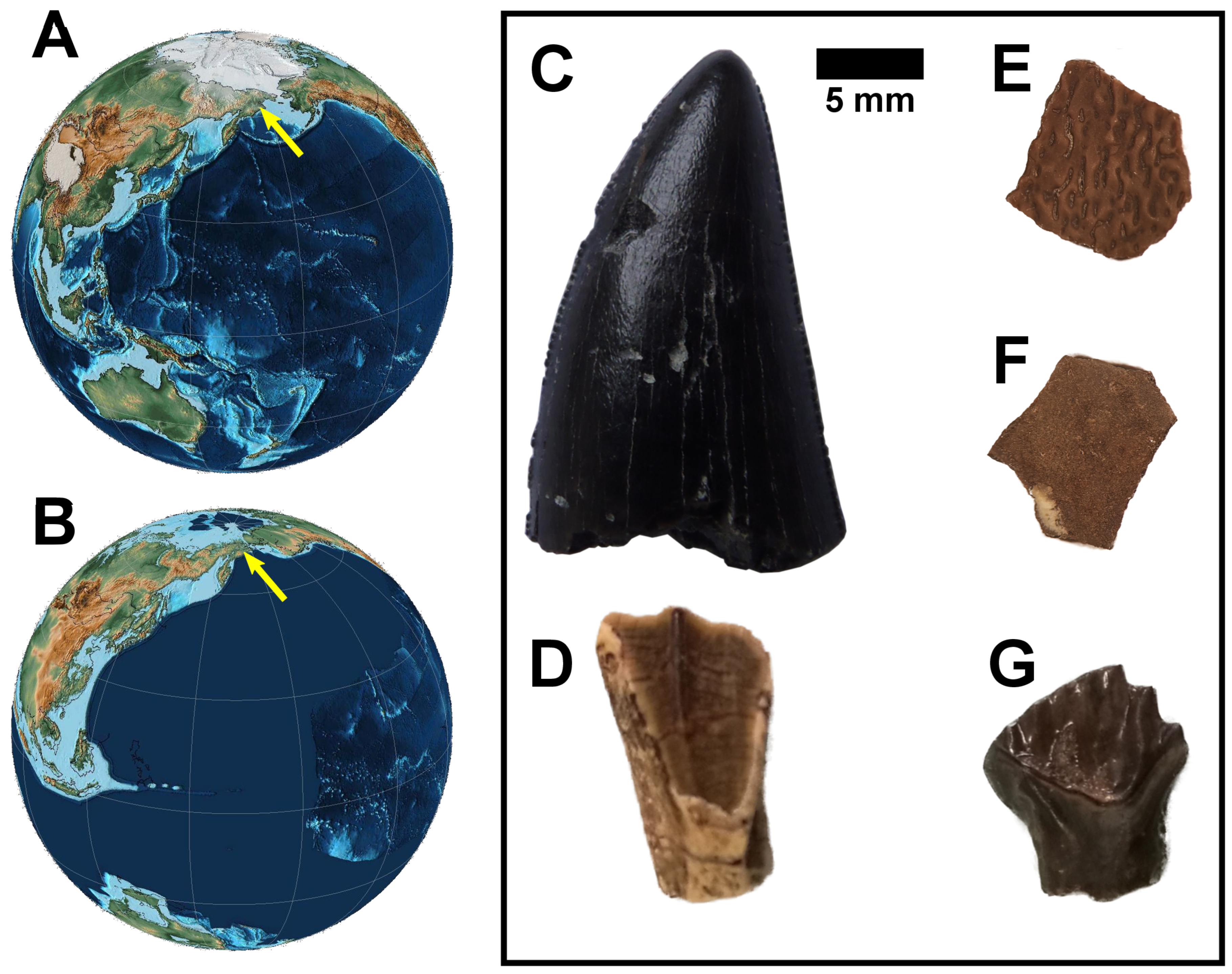

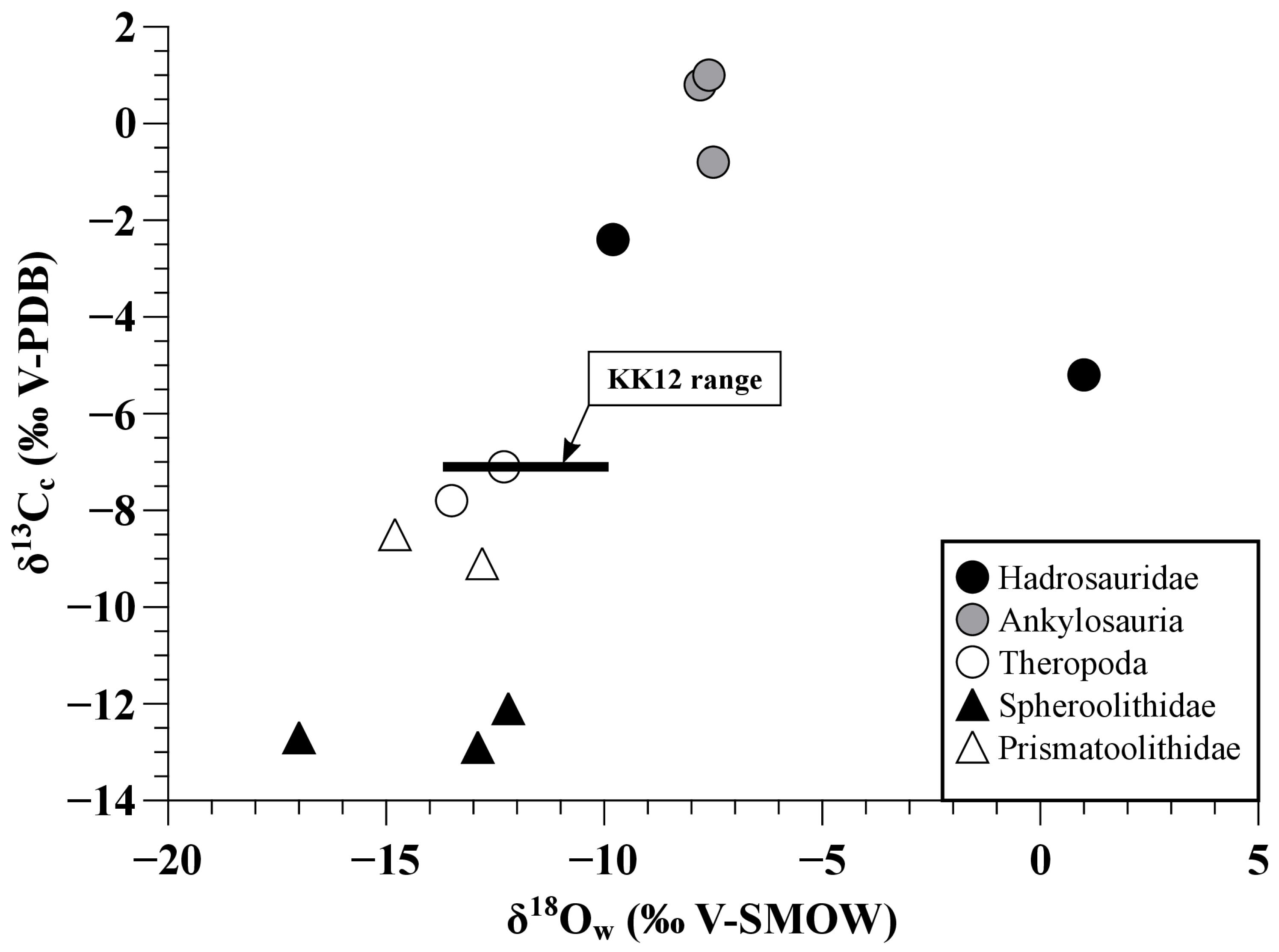
| Sample | Material | Taxon | δ18Op | δ18Oc | δ13Cc | CO3 Content | δ18Ow |
| ‰ V-SMOW | ‰ V-SMOW | ‰ V-PDB | WT% | ‰ V-SMOW | |||
| KK05 | tooth enamel | Hadrosaurid indet. | 12.9 | 21.0 | −2.4 | 6.9 | −9.8 |
| KK06 | tooth enamel | Hadrosaurid indet. | 22.6 | 28.5 | −5.2 | 5.9 | 1.0 |
| KK07 | tooth bulk | Ankylosauria indet. | 14.9 | 22.0 | −0.8 | 8.6 | −7.5 |
| KK08 | tooth bulk | Ankylosauria indet. | 14.7 | 21.3 | 0.8 | 8.2 | −7.8 |
| KK09 | tooth bulk | Ankylosauria indet. | 14.8 | 21.9 | 1.0 | 6.2 | −7.6 |
| KK10 | 3 bulk scales | lepisosteidae indet. | 14.2 | ||||
| KK11 | tooth enamel | Theropoda indet. | 9.6 | 18.8 | −7.8 | 4.4 | −13.5 |
| KK12 | tooth enamel | Tyrannosauridae indet. | 10.6 | 18.7 | −7.1 | 5.0 | −12.3 |
| KK19 | eggshell calcite | Spheroolithidae | 16.4 | −12.7 | −17.0 | ||
| KK20 | eggshell calcite | Spheroolithidae | 20.2 | −12.9 | −12.9 | ||
| KK21 | eggshell calcite | Spheroolithidae | 20.8 | −12.1 | −12.2 | ||
| KK22 | eggshell calcite | Prismatoolithidae | 18.4 | −8.5 | −14.8 | ||
| KK23 | eggshell calcite | Prismatoolithidae | 20.2 | −9.2 | −12.8 | ||
| Serial sampling of tooth KK12 | |||||||
| (Goedert et al. [22]) | |||||||
| Sample | Material | Taxon | δ18Op | Dist. from Apex | δ18Ow | ||
| ‰ V-SMOW | (mm) | ‰ V-SMOW | |||||
| R0 | tooth enamel | Tyrannosauridae indet. | 10.7 | 21 | −12.2 | ||
| R1 | tooth enamel | Tyrannosauridae indet. | 11.0 | 19 | −11.9 | ||
| R1a | tooth enamel | Tyrannosauridae indet. | 10.7 | 17 | −12.3 | ||
| R2 | tooth enamel | Tyrannosauridae indet. | 10.3 | 15 | −12.7 | ||
| R2a | tooth enamel | Tyrannosauridae indet. | 10.3 | 13 | −12.7 | ||
| R3 | tooth enamel | Tyrannosauridae indet. | 10.7 | 11 | −12.3 | ||
| R3a | tooth enamel | Tyrannosauridae indet. | 11.1 | 9 | −11.8 | ||
| R4 | tooth enamel | Tyrannosauridae indet. | 11.9 | 7 | −10.9 | ||
| R4a | tooth enamel | Tyrannosauridae indet. | 10.7 | 5 | −12.2 | ||
| R5 | tooth enamel | Tyrannosauridae indet. | 10.4 | 2 | −12.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiot, R.; Golovneva, L.B.; Godefroit, P.; Goedert, J.; Garcia, G.; Lécuyer, C.; Fourel, F.; Herman, A.B.; Spicer, R.A. High-Latitude Dinosaur Nesting Strategies during the Latest Cretaceous in North-Eastern Russia. Diversity 2023, 15, 565. https://doi.org/10.3390/d15040565
Amiot R, Golovneva LB, Godefroit P, Goedert J, Garcia G, Lécuyer C, Fourel F, Herman AB, Spicer RA. High-Latitude Dinosaur Nesting Strategies during the Latest Cretaceous in North-Eastern Russia. Diversity. 2023; 15(4):565. https://doi.org/10.3390/d15040565
Chicago/Turabian StyleAmiot, Romain, Lina B. Golovneva, Pascal Godefroit, Jean Goedert, Géraldine Garcia, Christophe Lécuyer, François Fourel, Alexei B. Herman, and Robert A. Spicer. 2023. "High-Latitude Dinosaur Nesting Strategies during the Latest Cretaceous in North-Eastern Russia" Diversity 15, no. 4: 565. https://doi.org/10.3390/d15040565
APA StyleAmiot, R., Golovneva, L. B., Godefroit, P., Goedert, J., Garcia, G., Lécuyer, C., Fourel, F., Herman, A. B., & Spicer, R. A. (2023). High-Latitude Dinosaur Nesting Strategies during the Latest Cretaceous in North-Eastern Russia. Diversity, 15(4), 565. https://doi.org/10.3390/d15040565






