The Diversity of Alien Plant Species in South Africa’s National Botanical and Zoological Gardens
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Area
2.2. Data Collection
2.3. Data Analyses
2.3.1. Species and Families
2.3.2. Species Classification: Continental Origin, Life Forms, and NEM:BA-A&IS Categories
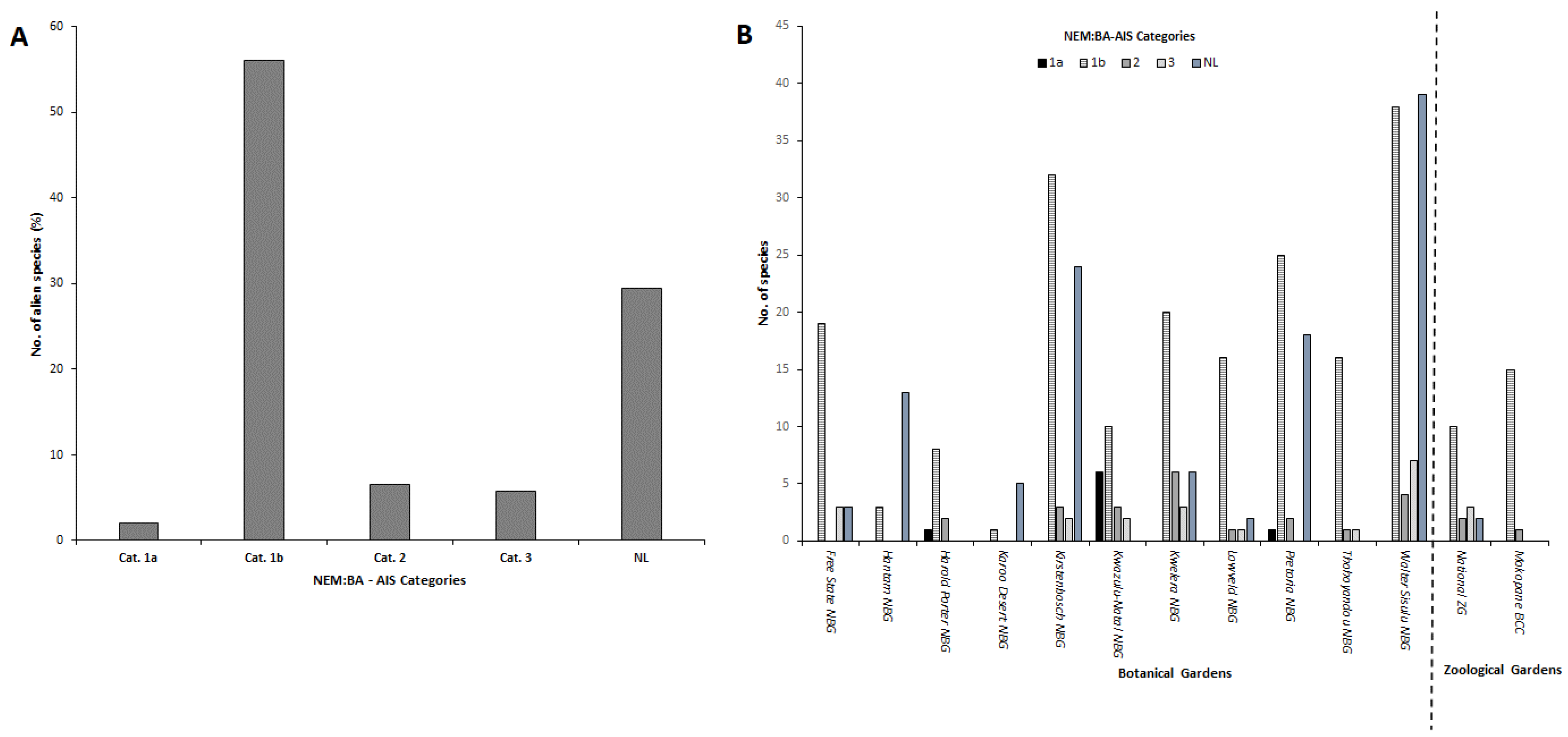
3. Results
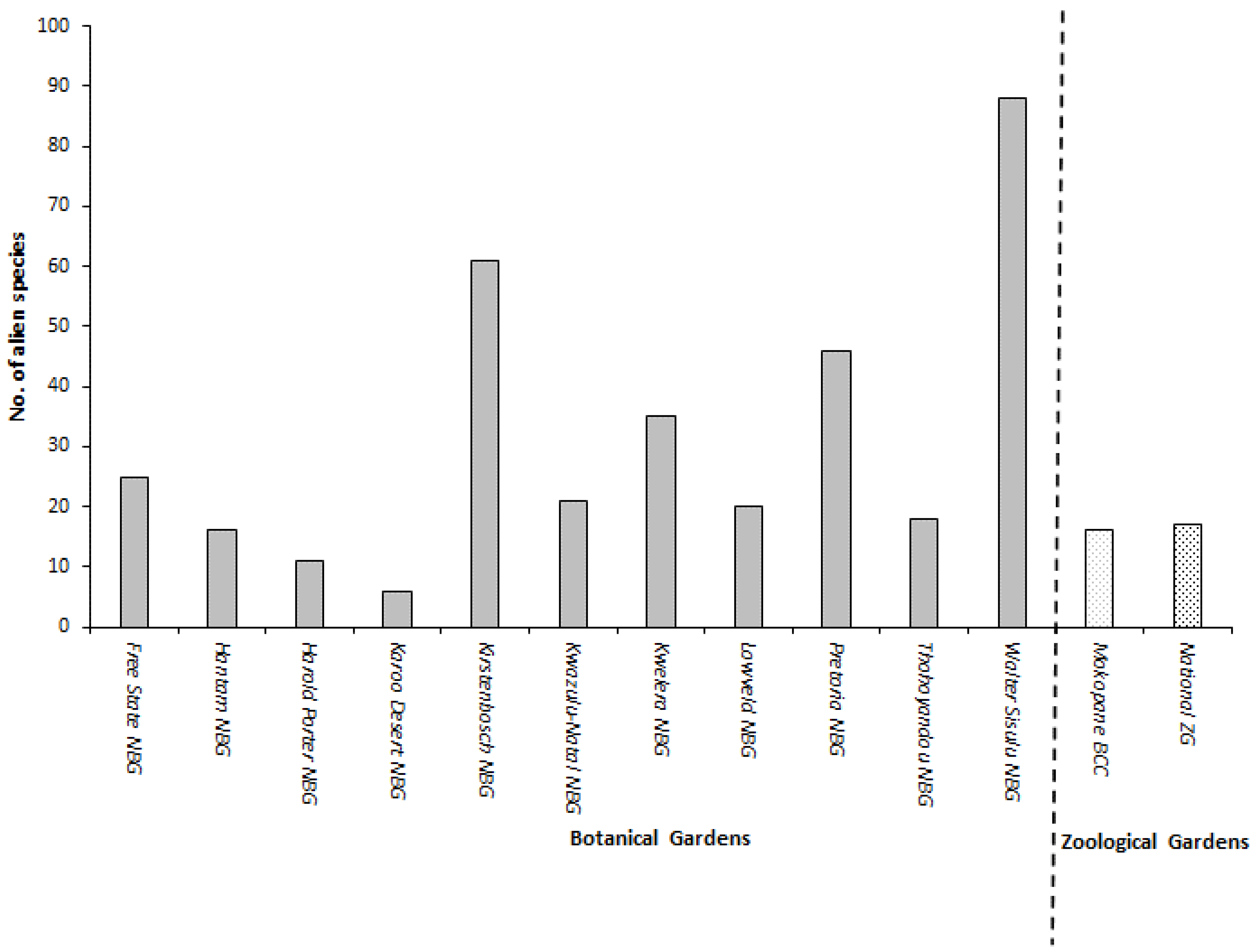
3.1. Identification of Alien Plant Species and Families
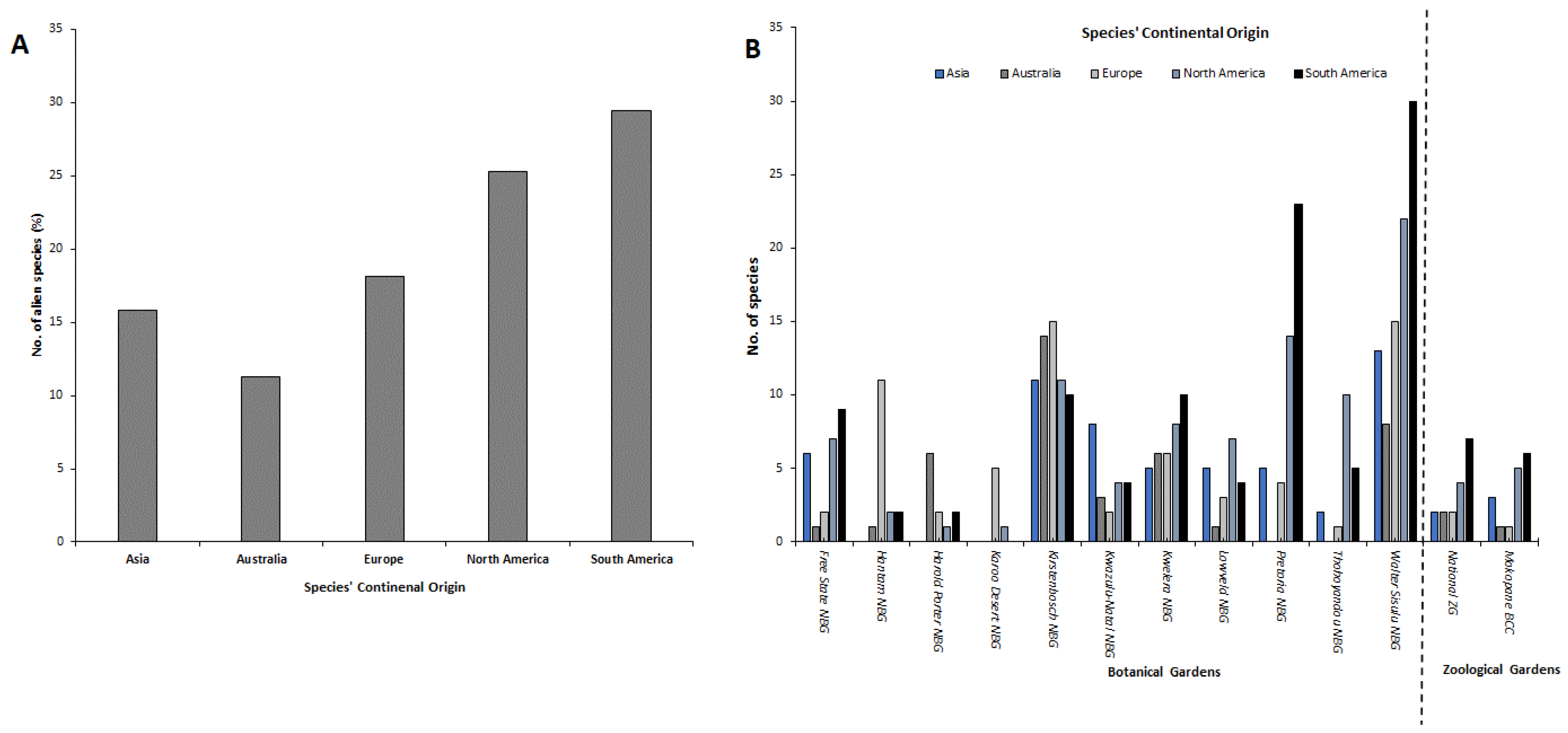
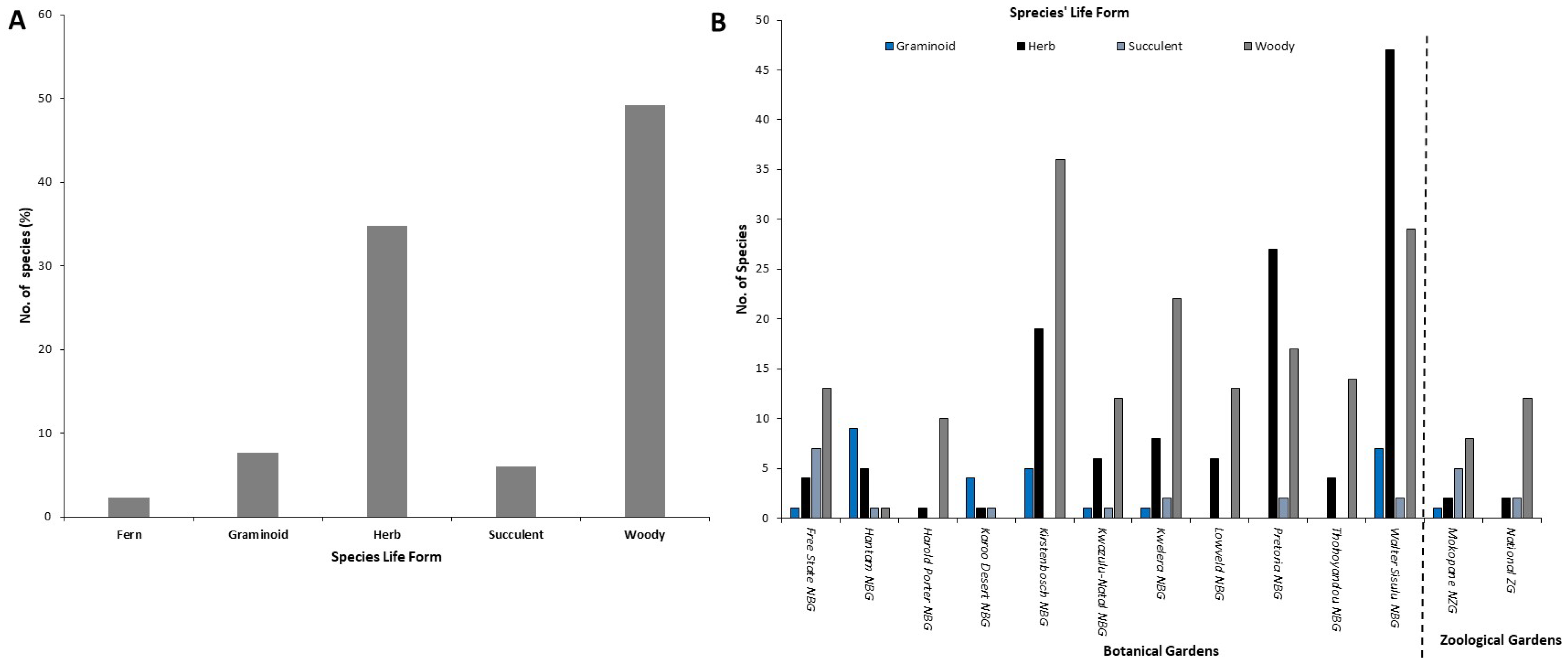
3.2. Comparing Continental Origin of Different Alien Species, Life Forms and NEM:BA-A&IS Regulations’ Categories
4. Discussions
5. Concluding Remarks
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Genus, Species and Lower Taxa | Life Form | NEM:BA-AIS Regs. Cat | Alien Status 73 |
|---|---|---|---|---|
| Adoxaceae | Viburnum tinus L. | Woody | NL | - |
| Alismataceae | Sagittaria latifolia Willd. | Herb | NL | Invasive |
| Amaranthaceae | Amaranthus hybridus L. | Herb | NL | Invasive |
| Atriplex inflata F.Muell. | Herb | 1b | Invasive | |
| Chenopodium album L. | Herb | NL | Invasive | |
| Dysphania sect. botryoides (L.) Mosyakin & Clemants | Herb | NL | - | |
| Salsola kali L. | Herb | 1b | Invasive | |
| Anacardiaceae | Schinus molle L. | Woody | NL | Invasive |
| Apiaceae | Foeniculum vulgare A.W.Hill | Herb | NL | Invasive |
| Cyclospermum leptophyllum (Pers.) | Herb | NL | - | |
| Apocynaceae | Araujia sericifera Brot. | Herb | 1b | Invasive |
| Thevetia peruviana (Pers.) K. Schum. | Woody | 1b | Invasive | |
| Vinca major L. | Herb | 1b | Invasive | |
| Aristolochiaceae | Aristolochia elegans Mast. | Woody | 1b | Invasive |
| Asparagaceae | Agave americana L. var. americana | Succulent | NL | Invasive |
| Agave sisalana Perrine | Succulent | 2 | Invasive | |
| Furcraea foetida L. | Succulent | 1a | Invasive | |
| Yucca sp. | Succulent | NL | - | |
| Asteraceae | Ageratum conyzoides (Mill.) M.Sharma | Herb | 1b | Invasive |
| Ageratina adenophora (Spreng.) R.M.King & H.Rob. | Herb | 1b | Invasive | |
| Bidens bipinnata L. | Herb | NL | Invasive | |
| Bidens pilosa L. | Herb | NL | Invasive | |
| Campuloclinium macrocephalum (Less.) DC. | Herb | 1b | Invasive | |
| Chromolaena odorata (L.) R.M.King & H.Rob. | Herb | 1b | Invasive | |
| Cirsium vulgare (Savi) Ten. | Herb | 1b | Invasive | |
| Conyza sumatrensis (Retz.) E.Walker | Herb | NL | Invasive | |
| Cosmos bipinnatus Cav. | Herb | NL | Invasive | |
| Flaveria bidentis (L) Kuntze | Herb | 1b | Invasive | |
| Galinsoga ciliata (Raf.) Blake | Herb | NL | - | |
| Hypochaeris microcephala (Sch.Bip.) Cabrera | Herb | NL | - | |
| Hypochaeris radicata L. | Herb | NL | Invasive | |
| Lactuca indica L. | Herb | NL | - | |
| Schkuhria pinnata (Lam.) Kuntze ex Thell. | Herb | NL | Invasive | |
| Sonchus oleraceus L. | Herb | NL | Invasive | |
| Sphagneticola trilobata (L.) Pruski | Herb | 1b | Invasive | |
| Tagetes minuta L. | Herb | NL | Invasive | |
| Tithonia diversifolia (Hemsl.) A.Gray | Herb | 1b | Invasive | |
| Tithonia rotundifolia S.F.Blake (Mill.) | Herb | 1b | Invasive | |
| Zinnia peruviana L. | Herb | NL | Invasive | |
| Basellaceae | Anredera cordifolia (Ten.) Steenis | Herb | 1b | Invasive |
| Bignoniaceae | Dolichandra unguis-cati L. (A.Gentry) | Woody | 1b | Invasive |
| Jacaranda mimosifolia D.Don | Woody | 1b | Invasive | |
| Tecoma stans (L.) Juss. ex Kunth | Woody | 1b | Invasive | |
| Boraginaceae | Amsinckia menziesii var. retrorsa (Lehm.) A.Nelson & J.F.Macbr. | Herb | NL | Invasive |
| Echium plantagineum L. | Herb | 1b | Invasive | |
| Heliotropium amplexicaule Vahl | Herb | NL | Invasive | |
| Heliotropium europaeum L. | Herb | NL | Invasive | |
| Brassicaceae | Brassica juncea (L.) Czern. | Herb | NL | - |
| Capsella bursa-pastoris (L.) Medik. | Herb | NL | Introduced but not naturalized | |
| Nasturtium officinale R.Br. | Herb | 2 | Invasive | |
| Raphanus raphanistrum L. | Herb | NL | Invasive | |
| Cactaceae | Cereus jamacaru DC. | Succulent | 1b | Invasive |
| Cylindropuntia imbricata (Haw.) F.M.Knuth | Succulent | 1b | Invasive | |
| Trichocereus spachianus (Lem.) Riccob | Succulent | 1b | Invasive | |
| Opuntia aurantiaca Lindl. | Succulent | 1b | Invasive | |
| Opuntia engelmannii Salm-Dyck ex Engelm. | Succulent | 1b | Invasive | |
| Opuntia ficus-indica (L.) Mill. | Succulent | 1b | Invasive | |
| Opuntia leucotricha DC. | Succulent | 1b | Invasive | |
| Opuntia microdasys (Lehm.) Pfeiff. | Succulent | 1b | Invasive | |
| Opuntia pubescens J.C.Wendl. ex Pfeiff. | Succulent | 1a | Introduced but not naturalized | |
| Opuntia stricta (Haw.) Haw. | Succulent | 1b | Invasive | |
| Cannabaceae | Celtis australis L. | Woody | 3 | Introduced but not naturalized |
| Cannaceae | Canna indica L. | Herb | 1b | Invasive |
| Caprifoliaceae | Centranthus ruber (L.) DC. | Herb | 1b | Invasive |
| Lonicera japonica Thunb.’Halliana’ | Woody | 3 | Invasive | |
| Caryophyllaceae | Silene gallica L. | Herb | NL | - |
| Cistaceae | Cistus ladanifer L. | Woody | NL | Invasive |
| Commelinaceae | Tradescantia fluminensis Vell. | Herb | 1b | Invasive |
| Convolvulaceae | Convolvulus arvensis L. | Herb | 1b | Introduced but not naturalized |
| Cuscuta campestris Yunck. | Herb | 1b | Invasive | |
| Ipomoea purpurea (L.) Roth | Herb | 1b | Invasive | |
| Crassulaceae | Bryophyllum delagoense (Eckl. & Zeyh.) Schinz | Succulent | 1b | Invasive |
| Cucurbitaceae | Diplocyclos palmatus L. | Woody | 1a | Invasive |
| Cyatheaceae | Sphaeropteris excelsa (Endl.) R.M.Tryon | Fern | NL | Invasive |
| Cyperaceae | Cyperus eragrostis Lam. | Graminoids | NL | - |
| Euphorbiaceae | Euphorbia heterophylla L. | Herb | NL | - |
| Euphorbia peplus L. | Herb | NL | - | |
| Homalanthus populifolius Graham. | Woody | 1b | Invasive | |
| Mercurialis annua L. | Herb | NL | - | |
| Ricinus communis L. | Woody | 2 | Invasive | |
| Fabaceae | Acacia cyclops A.Cunn. ex G.Don | Woody | 1b | Invasive |
| Acacia dealbata Link | Woody | 2 | Invasive | |
| Acacia elata A.Cunn. ex Benth. | Woody | 1b | Invasive | |
| Acacia longifolia (Andrews) Willd. | Woody | 1b | Invasive | |
| Acacia mearnsii De Wild. | Woody | 2 | Invasive | |
| Acacia melanoxylon R.Br. | Woody | 2 | Invasive | |
| Acacia podalyriifolia A.Cunn. ex G.Don | Woody | 1b | Invasive | |
| Acacia saligna (Labill.) H.L.Wendl. | Woody | 1b | Invasive | |
| Caesalpinia decapetala (Roth) Alston | Woody | 1b | Invasive | |
| Caesalpinia gilliesii Wall. ex. Hook. | Woody | 1b | Invasive | |
| Crotalaria agatiflora Schweinf. | Woody | 1b | Invasive | |
| Cytisus palmensis (Christ) Hutch. | Woody | NL | - | |
| Desmodium sp. | Woody | NL | - | |
| Gleditsia triacanthos L. | Woody | 1b | Invasive | |
| Medicago lupulina L. | Herb | NL | - | |
| Medicago polymorpha L. var. brevispina (Benth.) Heyn | Herb | NL | - | |
| Paraserianthes lophantha (Willd.) I.C.Nielsen | Woody | 1b | Invasive | |
| Prosopis glandulosa var. torreyana (L.D.Benson) M.C.Johnst. | Woody | 1b | Invasive | |
| Robinia pseudoacacia L. | Woody | 1b | Invasive | |
| Senna bicapsularis (L.) Roxb. | Woody | 1b | Invasive | |
| Senna septemtrionalis (Viv.) H.S.Irwin & Barneby | Woody | 1b | Invasive | |
| Senna sp. | Woody | 1b | - | |
| Sesbania bispinosa (Jacq.) W.Wight | Woody | NL | - | |
| Sesbania punicea (Cav.) Benth. | Woody | 1b | Invasive | |
| Spartium junceum L. | Woody | 1b | Invasive | |
| Tipuana tipu (Benth.) Kuntze | Woody | 3 | Invasive | |
| Vicia atropurpurea L. | Herb | NL | - | |
| Vicia sativa L. | Herb | NL | - | |
| Fagaceae | Quercus robur L. | Woody | NL | Invasive |
| Hypericaceae | Hypericum canariense L. | Woody | NL | - |
| Iridaceae | Iris pseudacorus L. | Herb | 1a | Invasive |
| Sisyrynchium sp. | Herb | NL | - | |
| Juncaceae | Juncus bufonius L. aggregate | Herb | NL | - |
| Lamiaceae | Salvia tiliifolia Vahl. | Herb | 1b | Invasive |
| Lauraceae | Cinnamomum camphora (L.) J.Presl | Woody | 1b | Invasive |
| Liliaceae | Lilium formosanum Wallace | Herb | 1a | Invasive |
| Malvaceae | Hibiscus trionum L. | Herb | NL | Invasive |
| Meliaceae | Melia azedarach L. | Woody | 1b | Invasive |
| Moraceae | Morus alba L. | Woody | 3 | Invasive |
| Morus nigra L. | Woody | NL | - | |
| Myrtaceae | Eucalyptus camaldulensis Dehnh. | Woody | 1b | Invasive |
| Eucalyptus grandis W.Hill ex Maiden | Woody | 1b | Invasive | |
| Eucalyptus paniculata Sm. | Woody | NL | Introduced but not naturalized | |
| Eucalyptus saligna Sm. | Woody | NL | - | |
| Leptospermum laevigatum (Gaertn.) F.Muell. | Woody | 1b | Invasive | |
| Callistemon rigidus R.Br.. | Woody | 1b | Invasive | |
| Metrosideros excelsa Sol. ex Gaertn. | Woody | 1a | Invasive | |
| Myrtus communis L. | Woody | NL | - | |
| Psidium guajava L. | Woody | 2 | Invasive | |
| Syzygium paniculatum Gaertn. | Woody | NL | Invasive | |
| Nyctaginaceae | Mirabilis jalapa L. | Herb | 1b | Invasive |
| Nymphaeaceae | Nymphaea mexicana Zucc | Herb | 1b | Invasive |
| Oleaceae | Ligustrum japonicum Thun. | Woody | 1b | Invasive |
| Ligustrum lucidum W.T. Aiton | Woody | 1b | Invasive | |
| Ligustrum vulgare L. | Woody | 1b | Invasive | |
| Syringa vulgaris L. | Woody | NL | - | |
| Onagraceae | Oenothera rosea L’Herit. ex Aiton | Herb | NL | Invasive |
| Oenothera stricta Ledeb. ex Link | Herb | NL | Invasive | |
| Oenothera tetraptera Cav. | Herb | NL | Introduced but not naturalized | |
| Oxalidaceae | Oxalis corniculata L. | Herb | NL | Invasive |
| Oxalis latifolia Kunth | Herb | NL | Invasive | |
| Papaveraceae | Argemone ochroleuca Sweet | Herb | 1b | Invasive |
| Fumaria muralis Sond. ex Koch | Herb | NL | Introduced but not naturalized | |
| Papaver rhoeas L. | Herb | NL | Invasive | |
| Passifloraceae | Passiflora caerulea L. | Herb | 1b | Invasive |
| Passiflora edulis Sims. | Herb | 2 | Invasive | |
| Passiflora ligularis Juss. | Herb | NL | - | |
| Passiflora subpeltata Ortega. | Herb | 1b | Invasive | |
| Phytolaccaceae | Phytolacca americana L. | Herb | 1b | Invasive |
| Phytolacca dioica L. | Woody | 3 | Invasive | |
| Phytolacca octandra L. | Herb | 1b | Invasive | |
| Pinaceae | Pinus patula Schiede ex Schltdl. & Cham. | Woody | 2 | Invasive |
| Pinus pinaster Aiton | Woody | 1b | Invasive | |
| Pittosporaceae | Pittosporum undulatum Vent. | Woody | 1b | Invasive |
| Plantaginaceae | Plantago lanceolata L. | Herb | NL | Invasive |
| Plantago major L. | Herb | NL | Invasive | |
| Poaceae | Arundo donax L. 1753 | Graminoids | 1b | Invasive |
| Avena barbata Pott ex Link | Graminoids | NL | Introduced but not naturalized | |
| Avena fatua L. | Graminoids | NL | Invasive | |
| Brachypodium distachyon (L.) P.Beauv. | Graminoids | NL | - | |
| Briza maxima L. | Graminoids | NL | Introduced but not naturalized | |
| Bromus diandrus Roth | Graminoids | NL | Introduced but not naturalized | |
| Bromus pectinatus Thunb. | Graminoids | NL | Introduced but not naturalized | |
| Bromus rigidus Roth. | Graminoids | NL | - | |
| Calamagrostis acutiflora (Schrad.) Rchb. | Graminoids | NL | NA | |
| Cortaderia jubata (Lemoine) Stapf. | Graminoids | 1b | - | |
| Digitaria debilis (Desf.) Willd. | Graminoids | NL | - | |
| Eragrostis mexicana (Hornem.) Link | Graminoids | NL | - | |
| Hordeum murinum L. | Graminoids | NL | Invasive | |
| Imperata cylindrica (L.) Raeusch | Graminoids | NL | - | |
| Lolium rigidum Gaudin | Graminoids | NL | Introduced | |
| Nassella trichotoma (Nees) Hack. ex Arechav. | Graminoids | 1b | invasive | |
| Paspalum dilatatum Poir. | Graminoids | NL | Invasive | |
| Paspalum urvillei Steud. | Graminoids | NL | Invasive | |
| Pennisetum clandestinum Hochst. ex Chiov. | Graminoids | 1b | Invasive | |
| Pennisetum setaceum (Forssk.) Chiov. | Graminoids | 1b | Invasive | |
| Pennisetum villosum R.Br. ex Fresen. | Graminoids | 1b | - | |
| Phalaris minor Retz. (1783) | Graminoids | NL | - | |
| Stipa capensis Thunb. | Graminoids | NL | Introduced | |
| Vulpia myuros (L.) C.C. Gmel. | Graminoids | NL | - | |
| Pontederiaceae | Pontederia crassipes (Mart.) Solms | Herb | 1b | Invasive |
| Polypodiaceae | Nephrolepis cordifolia L. | Fern | 1b | Invasive |
| Nephrolepis exaltata (L.) Schott | Fern | 1b | Invasive | |
| Primulaceae | Ardisia crenata Sims | Woody | 1b | Invasive |
| Lysimachia arvensis L. | Herb | NL | Introduced but not naturalized | |
| Pteridaceae | Adiantum raddianum Presl | Fern | NL | Introduced but not naturalized |
| Rosaceae | Cotoneaster franchetii Bois | Woody | 1b | Invasive |
| Cotoneaster pannosus Franch. | Woody | 1b | Invasive | |
| Potentilla indica (Jacks.) Focke | Herb | NL | Invasive | |
| Prunus persica (L.) Batsch | Woody | NL | Invasive | |
| Pyracantha angustifolia (Franch.) C.K.Schneid. | Woody | 1b | Invasive | |
| Pyracantha coccinea M.Roem. | Woody | 1b | Invasive | |
| Rosa rubiginosa L. | Woody | 1b | Invasive | |
| Rubus cuneifolius Pursh. | Woody | 1b | Invasive | |
| Rubus fruticosus Lour. | Woody | 2 | Invasive | |
| Rubus odoratus L. | Woody | NL | - | |
| Rubiaceae | Richardia brasiliensis Gomes | Herb | NL | Invasive |
| Salicaceae | Populus canescens (Aiton) Sm. | Woody | 2 | Invasive |
| Salviniaceae | Azolla filiculoides Lam. | Fern | 1b | Invasive |
| Sapindaceae | Cardiospermum grandiflorum Swartz | Woody | 1b | Invasive |
| Scrophulariaceae | Verbascum chaixii Vill. | Herb | NL | - |
| Simaroubaceae | Ailanthus altissima (Mill.) Swingle | Woody | 1b | Invasive |
| Solanaceae | Cestrum aurantiacum Lindl. | Woody | 1b | Invasive |
| Cestrum laevigatum Schltdl. | Woody | 1b | Invasive | |
| Cestrum parqui L’Her. | Woody | 1b | Invasive | |
| Datura ferox L. | Herb | 1b | Invasive | |
| Datura innoxia Mill. | Herb | 1b | Invasive | |
| Datura stramonium L. | Herb | 1b | Invasive | |
| Physalis angulata L. | Herb | NL | Introduced but not naturalized | |
| Physalis peruviana L. | Herb | NL | Invasive | |
| Physalis viscosa L. | Herb | NL | Invasive | |
| Solanum elaeagnifolium Cav. | Woody | 1b | Invasive | |
| Solanum mauritianum Scop. | Woody | 1b | Invasive | |
| Solanum nigrum L. | Herb | NL | - | |
| Solanum pseudocapsicum L. | Herb | 1b | Invasive | |
| Solanum seaforthianum Andrews | Woody | 1b | Invasive | |
| Solanum sisymbriifolium Lam. | Herb | 1b | Invasive | |
| Tropaeolaceae | Tropaeolum majus L. | Herb | NL | Invasive |
| Tropaeolum speciosum Poepp. & Endl. | Herb | 3 | NA | |
| Verbenaceae | Lantana camara L. | Woody | 1b | Invasive |
| Phyla nodiflora (L.) Greene | Herb | NL | - | |
| Verbena aristigera S.Moore | Herb | NL | - | |
| Verbena bonariensis L. | Herb | 1b | Invasive | |
| Zingiberaceae | Hedychium coronarium J.Koenig. | Herb | 1b | Invasive |
| Hedychium flavescens Carey ex Roscoe. | Herb | 1b | Invasive |
References
- Blackwell-Hackney, A. Botanical Gardens: Driving Plant Conservation Law. Ky. J. Equine Agric. Nat. Resour. Law 2012, 5, 2. Available online: https://uknowledge.uky.edu/kjeanrl/vol5/iss1/2 (accessed on 15 February 2020).
- Wyse Jackson, P.S.; Sutherland, L.A. International Agenda for Botanic Gardens in Conservation; Botanic Gardens Conservation International: San Marino, CA, USA, 2000; p. 56. [Google Scholar]
- Wyse Jackson, P.S. The development of feasibility studies for the creation of new botanic gardens. Bot. Gard. Conserv. News 2003, 3, 46–48. [Google Scholar]
- Krishnan, S.; Novy, A. The Role of Botanic Gardens in the Twenty-First Century. CAB Rev. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Powledge, F. The evolving role of botanical gardens. BioScience 2011, 61, 743–749. [Google Scholar] [CrossRef]
- Botanic Gardens Conservation International (BGCI). Available online: http://www.bgci.org/garden_search.php (accessed on 3 December 2021).
- Barham, E.; Sharrock, S.; Lane, C.; Baker, R. The International Plant Sentinel Network: A tool for Regional and National Plant Protection Organizations. Bull. OEPP/EPPO Bull. 2016, 46, 156–162. [Google Scholar] [CrossRef]
- Paap, T.; de Beer, Z.W.; Migliorini, D.; Nel, W.; Wingfield, M.J. The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: A new invasion in South Africa. Australas. Plant Pathol. 2018, 47, 231–237. [Google Scholar] [CrossRef]
- Kenis, M.; Hurley, B.P.; Colombari, F.; Lawson, S.; Sun, J.; Wilcken, C.; Weeks, R.; Sathyapala, S. Guide to the Classical Biological Control of Insect Pests in Planted and Natural Forests. FAO Forestry Paper; FAO: Rome, Italy, 2019. [Google Scholar]
- Wondafrash, M.; Wingfeld, M.J.; Wilson, J.R.U.; Hurley, B.P.; Slippers, B.; Paap, T. Botanical gardens as key resources and hazards for biosecurity. Biodivers. Conserv. 2021, 30, 1929–1946. [Google Scholar] [CrossRef]
- Brockway, L.H. Science and Colonial Expansion: The Role of the British Royal Botanic Gardens; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; González-Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. 2018, 93, 1421–1437. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database. Published by The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), 12p. First Published as Special Lift-Out in Aliens 12, December 2000. Updated and Reprinted Version: November 2004. Available online: https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf) (accessed on 14 July 2022).
- Hulme, P.E. Addressing the threat to biodiversity from botanic gardens. Trends Ecol. Evol. 2011, 26, 168–174. [Google Scholar] [CrossRef]
- Ni, M.; Hulme, P.E. Botanic gardens play key roles in the regional distribution of first records of alien plants in China. Glob. Ecol. Biogeogr. 2021, 30, 1572–1582. [Google Scholar] [CrossRef]
- Malcom, S.M. Education and Biodiversity; Levin, S.A., Ed.; Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2001; pp. 383–394. [Google Scholar]
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, D.; Nentwig, W.; Olenin, S.; Panov, V.; Pergl, J.; et al. Grasping at the routes of biological invasions: A framework for integrating pathways into policy. J. Appl. Ecol. 2008, 45, 403–414. [Google Scholar] [CrossRef]
- Willis, K.C. State of Research in South Africa’s National Botanical Gardens; South African National Biodiversity Institute: Pretoria, South Africa, 2018. [Google Scholar]
- South African National Biodiversity Institute (SANBI). Vision, Mission and Values. 2020. Available online: https://www.sanbi.org.za (accessed on 21 April 2020).
- Vukeya, L.R.; Mokotjomela, T.M.; Malebo, N.J.; Smith, D.A.E.; Oke, S. The vegetation cover dynamics and potential drivers of habitat change over 30 years in the Free State National Botanical Garden, South Africa. Reg. Environ. Chang. 2023, 23, 1–16. [Google Scholar] [CrossRef]
- Willis, C.K.; Mutsinyalo, T.M. National Gardens Expansion Strategy 2016–2030; South African National Biodiversity Institute: Pretoria, South Africa, 2019. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Skowno, A.L.; Poole, C.J.; Raimondo, D.C.; Sink, K.J.; van Deventer, H.; van Niekerk, L.; Harris, L.R.; Smith-Adao, L.; Tolley, K.; Zengya, T.; et al. National Biodiversity Assessment 2018: The Status of South Africa’s Ecosystems and Biodiversity. Synthesis Report; South African National Biodiversity Institute, an entity of the Department of Environment, Forestry and Fisheries: Pretoria, South Africa, 2019; pp. 1–214. [Google Scholar]
- Zengeya, T.A.; Wilson, J.R. The Status of Biological Invasions and Their Management in South Africa in 2019; South African National Biodiversity Institute, Kirstenbosch and DSI-NRF Centre of Excellence for Invasion Biology: Stellenbosch, South Africa, 2020; p. 71. [Google Scholar]
- Van Wilgen, B.W.; Richardson, D.M.; Wilson, J.R.; Zengeya, T.A. Biological invasions in South Africa. 2020. Available online: https://link.springer.com/book/10.1007/978-3-030-32394-3> (accessed on 12 January 2021).
- Van Wilgen, B.W.; Wilson, J.R. The Status of Biological Invasions and Their Management in South Africa in 2017; South African National Biodiversity Institute, Kirstenbosch and DST-NRF Centre of Excellence for Invasion Biology: Stellenbosch, South Africa, 2018. [Google Scholar]
- Reynolds, C.; Venter, N.; Cowie, B.W.; Marlin, D.; Mayonde, S.; Tocco, C.; Byrne, M.J. Mapping the socio-ecological impacts of invasive plants in South Africa: Are poorer households with high ecosystem service use most at risk? Ecosyst. Serv. 2020, 42, 101075. [Google Scholar] [CrossRef]
- Department of Environmental Affairs. National Environmental Management: Biodiversity Act 2004 (Act No 10 of 2004) Alien and Invasive Species Regulations, 2014 Government Gazette 590(37885). 2014. Available online: https://www.dffe.gov.za/sites/default/files/legislations/nema_amendment_act10_0.pdf (accessed on 14 July 2022).
- McGeoch, M.A.; Spear, D.; Kleynhans, E.J.; Marais, E. Uncertainty in invasive alien species listing. Ecol. Appl. 2012, 22, 959–971. [Google Scholar] [CrossRef]
- Wilson, J.R.; Faulkner, K.T.; Rahlao, S.J.; Richardson, D.M.; Zengeya, T.A.; van Wilgen, B.W. Indicators for monitoring biological invasions at a national level. J. Appl. Ecol. 2018, 55, 2612–2620. [Google Scholar] [CrossRef]
- Zengeya, T.A.; Kumschick, S.; Weyl, O.L.F.; van Wilgen, B.W. An Evaluation of the Impacts of Alien Species on Biodiversity in South Africa using Different Assessment Methods; Van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 487–512. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; van Wilgen, B.W.; Abrahams, B.; Esler, K.J.; Wannenburgh, A. Knowing-Doing Continuum or Knowing Doing Gap? Information Flow between Researchers and Managers of Biological Invasions in South Africa; Van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 827–850. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Nemurangoni, T.; Mundalamo, T.; Jaca, T.; Kuhudzai, A.G. The value of dump sites in monitoring biological invasions in South Africa. Biol. Invasions 2022, 24, 971–986. [Google Scholar] [CrossRef]
- Bromilow, C. Problem Plants and Alien Weeds of Southern Africa, 4th ed.; Briza Publications: Pretoria, South Africa, 2018. [Google Scholar]
- Bromilow, C. Problem Plants of South Africa: A Guide to the Identification and Control of More Than 300 Invasive Plants and Other Weeds; Briza Publications: Pretoria, South Africa, 2001. [Google Scholar]
- Henderson, L. Invasive Alien Plants in South Africa; Agriculture Research Council: Pretoria, South Africa, 2020; ISBN 978-0-620-86146-5. [Google Scholar]
- The Invasive Species South African Database. 2023. Available online: https://www.invasives.org.za (accessed on 22 May 2022).
- South African National Biodiversity Institute. Botanical Database of Southern Africa (BODATSA). 2016. Available online: http://posa.sanbi.org/ (accessed on 14 October 2022).
- Integrated Taxonomic Information System (ITIS). 2023. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt#null (accessed on 29 January 2023).
- Pyšek, P.; Jarosik, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vila, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Change Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Faulkner, K.T.; Robertson, M.P.H.; Rouget, M.; Wilson, J.R.U. Understanding and managing the introduction pathways of alien taxa: South Africa as a case study. Biol. Invasions 2016, 18, 73–87. [Google Scholar] [CrossRef]
- Kumschick, S.; Foxcroft, L.C.; Wilson, J.R. Analysing the Risks Posed by Biological Invasions to South Africa; Van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 569–592. [Google Scholar] [CrossRef]
- Wilson, J.R.U.; Ivey, P.; Manyama, P.; Nanni, I. A new national unit for invasive species detection, assessment and eradication planning. S. Afr. J. Sci 2013, 109, 1–13. [Google Scholar] [CrossRef]
- Department of Forestry, Fisheries and the Environmental Affairs. National Environmental Management: Biodiversity Act (NEM: BA) (Act No. 10 of 2004), Pretoria, South Africa. 2021. Available online: https://www.dffe.gov.za/national_environmental_management_biodiversity_act_2004_act_no_10_2004_alien_and_invasive_species_regulations_g_43735_%E2%80%93_gon_1020_1 (accessed on 14 July 2022).
- Richardson, D.M.; Foxcroft, L.C.; Latombe, G.; Le Maitre, D.C.; Rouget, M.; Wilson, J.R. The Biogeography of South African Terrestrial Plant Invasions; Van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 65–94. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Naturalization of introduced plants: Ecological drivers of biogeographical patterns. New Phytol. 2012, 196, 383–396. [Google Scholar] [CrossRef]
- Faulkner, K.T.; Burness, A.; Byrne, M.J.; Kumschick, S.; Peters, K.; Robertson, M.P.; Saccaggi, D.L.; Weyl, O.L.; Wiliams, V.L. South Africa’s Pathways of Introduction and Dispersal and How They Have Changed Over Time; Van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 311–352. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Hoffmann, J.H.; Downs, C.T. The potential for birds to disperse the seeds of Acacia cyclops, an invasive alien plant in South Africa. Ibis 2015, 157, 449–458. [Google Scholar] [CrossRef]
- Mokotjomela, T.M.; Musil, C.F.; Esler, K.J. Potential seed dispersal distances of native and non-native fleshy fruiting shrubs in the South African Mediterranean climate region. Plant Ecol. 2013, 214, 1127–1137. [Google Scholar] [CrossRef]
- Vukeya, L.R.; Mokotjomela, T.M.; Malebo, N.J.; Oke, S. Seed dispersal phenology of encroaching woody species in the Free State National Botanical Garden, South Africa. Afr. J. Ecol. 2022, 60, 723–735. [Google Scholar] [CrossRef]
- Vukeya, L.R.; Mokotjomela, T.M.; Malebo, N.J.; Oke, S. Interspecific competition in germination of bird-dispersed seeds in a habitat with sparse tree vegetation in South Africa. Bot. Stud. 2021, 62, 10. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Lavergne, S.; Dutoit, T.; Bertaudiere-Montes, V. From the backyard to the backcountry: How ecological and biological traits explain the escape of garden plants into Mediterranean old fields. Biol. Invasions 2010, 12, 761–779. [Google Scholar] [CrossRef]
- McLean, P.; Wilson, J.R.U.; Gaertner, M.; Kritzinger-Klopper, S.; Richardson, D.M. The distribution and status of alien plants in a small South African town. S. Afr. J. Bot. 2018, 117, 18–71. [Google Scholar] [CrossRef]
- Vukeya, L.R.; Mokotjomela, T.M. Free State National Botanical Garden Alien and Invasive Species Management Plan 2020/21; South African National Biodiversity Institute: Pretoria, South Africa, 2020. [Google Scholar]
- Richardson, D.M.; Pyšek, P.; Rejma’nek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants—Concepts and definitions. Divers Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Blackwell Publishing Ltd.: Malden, MA, USA, 2007. [Google Scholar]
- Buckley, Y.M.; Catford, J. Does the biogeographic origin of species matter? Ecological effects of native and non-native species and the use of origin to guide management. J. Ecol. 2016, 104, 4–17. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Clusella-Trullas, S.; Mokotjomela, T.M.; Mairal, M.; Richardson, D.M.; Skein, L.; Wilson, J.R.; Weyl, O.L.F.; Geerts, S. Biotic Interactions as Mediators of Biological Invasions: Insights from South Africa; Van Wilgen, B., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Biological Invasions in South Africa; Springer: Cham, Switzerland, 2020; pp. 387–427. [Google Scholar]
- Van Wilgen, B.W.; Zengeya, T.A.; Richardson, D.M. A review of the impacts of biological invasions in South Africa. Biol. Invasions 2021, 24, 27–50. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejma’nek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.-J.; Holes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J.; et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Traveset, A.; Richardson, D.M. Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol. Evol. 2006, 21, 208–216. [Google Scholar] [CrossRef]
- Wilson, J.R.; Panetta, F.D.; Lindgren, C. Detecting and Responding to Alien Plant Incursions Ecology Biodiversity and Conservation; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Visser, V.; Maitre, D.; Wilson, J.R.U.; Nänni, I.; Canavan, K.; Canavan, S.; Fish, L.; Mashau, C.; O’Connor, T.G.; Ivey, P.; et al. Grasses as invasive plants in South Africa revisited: Patterns, pathways and management. Bothalia 2017, 47, a2169. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Van Rensburg, J.; Richardson, D.M. Reconstructing the spread of invasive alien plants on privately-owned land in the Cape Floristic Region: Vergelegen Wine Estate as a case study. S. Afr. Geogr. J.-Suid-Afr. Geogr. Tydskr. 2018, 100, 180–195. [Google Scholar]
- Simberloff, D. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar] [CrossRef]
- Mokotjomela, T.M. A Comparison of Bird Foraging Preferences for Fruits of Indigenous and Alien Shrubs and Seed Dispersal Potentials in the Cape Floristic Region. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar] [CrossRef]
- Pyšek, P.; Pergl, J.; van Kleunen, M.; Dawson, W.; Essl, F.; Kreft, H.; Weigelt, P.; Wilson, J.R.; Winter, M.; Richardson, D.M. South Africa as a Donor of Naturalized and Invasive Plants to Other Parts of the World; Van Wilgen, B.W., Measey, J., Richardson, D.M., Wilson, J.R., Zengeya, T.A., Eds.; Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 755–782. [Google Scholar]
- Matsika, R. Land-Cover Change: Threats to the Grassland Biome of South Africa. Master of Science in Resource Conservation Biology; University of the Witwatersrand: Johannesburg, South Africa, 2008. [Google Scholar]
- Richardson, D.M.; Macdonald, I.A.W.; Holmes, P.M.; Cowling, R.M. Plant and animal invasions. In The Ecology of Fynbos. Nutrients, Fire and Diversity; Cowling, R.M., Ed.; Oxford University Press: Cape Town, South Africa, 1992; pp. 271–308. ISBN 0195706617. [Google Scholar]
- Buisson, E.; Braschi, J.; Chenot-Lescure, J.; Hess, M.C.M.; Vidaller, C.; Pavon, D.; Ramone, H.; Amy-Krebs, E.; Cottaz, C.; Passetti, A.; et al. Native plant community recovery after Carpobrotus (ice plant) removal on an island—Results of a 10-year project. Appl. Veg. Sci. 2020, 24, e12524. [Google Scholar] [CrossRef]
- Ntloko, B.R.; Siebert, S.J.; Mokotjomela, T.M. Rehabilitation of kimberlite tailings in the afro-alpine zone of Lesotho: Seed germination and plant performance of native grassland species across different topsoil mixtures. Restor. Ecol. 2021, 30, e13528. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
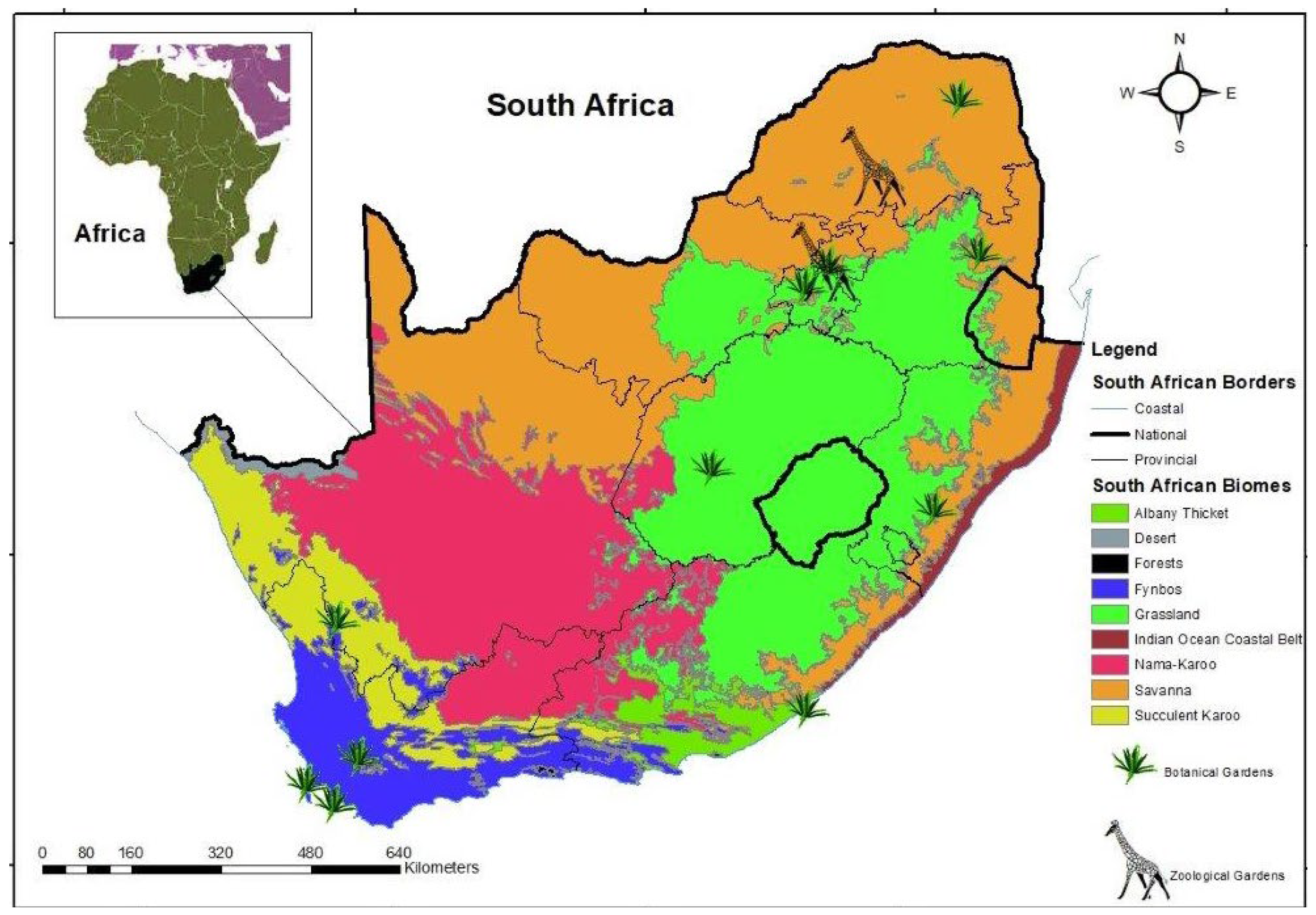
| National Botanical/Zoological Garden | Area (ha) | First Date of Current Land Use/Proclaimed | SA Province (Town) | Biome Represented (Bioregion) (Mucina & Rutherford, 2006) | Vegetation Types Represented (Mucina & Rutherford, 2006) |
|---|---|---|---|---|---|
| Free State NBG | 67 | 1967 | Free State Province (Bloemfontein) | Grassland (Dry Highveld Grassland) Azonal Vegetation (Alluvial Vegetation) | Gh 7 Winburg Grassy Shrubland Gh 8 Bloemfontein Karroid Shrubland Gh 5 Bloemfontein Dry Grassland AZa 5 Highveld Alluvial Vegetation |
| Hantam NBG | 6230 | 2008 | Northern Cape (Nieuwoudtville) | Succulent Karoo (Trans-Escarpment Succulent Karoo) Fynbos (Shale Renosterveld, and Granite and Dolerite Renosterveld) | FRd 1 Nieuwoudtville-Roggeveld Dolerite Renosterveld FRs 2 Nieuwoudtville Shale Renosterveld SKt 2 Hantam Karoo |
| Harold Porter NBG | 201 | 1959 | Western Cape (Betty’s Bay) | Fynbos (Sand Fynbos, Western Strandveld and Sandstone Fynbos) Forest (Zonal & Intrazonal) | FFd 6 Hangklip Sand Fynbos FFs 11 Kogelberg Sandstone Fynbos FOz 1 Southern Afrotemperate Forest FS 7 Overberg Dune Strandveld Freshwater (rivers) Marine biodiversity |
| Karoo Desert NBG | 154 | 1921 | Western Cape (Worcester) | Succulent Karoo (Rainshadow Valley Karoo) Fynbos (Shale Fynbos and Shale Renosterveld) | FFh 4 Breede Shale Fynbos FRs 8 Breede Shale Renosterveld SKv 7 Robertson Karoo |
| Kirstenbosch NBG | 199 | 1913 | Western Cape (Cape Town) | Fynbos (Granite Fynbos, Sandstone Fynbos, and Shale Fynbos) Forest (Zonal and Intrazonal) | FFg 3 Peninsula Granite Fynbos FFh 5 Cape Winelands Shale Fynbos FFs 9 Peninsula Sandstone Fynbos FOz 1 Southern Afrotemperate Forest Freshwater (rivers) |
| KwaZulu-Natal NBG | 48 | 1874/1969 | KwaZulu-Natal (Pietermaritzburg) | Savanna (Sub-Escarpment Savanna) | SVs 4 Ngongoni Veld Freshwater (river) |
| Kwelera NBG | 170 | 2014 | Eastern Cape (East London) | Forest (Zonal and Intrazonal) Azonal Vegetation (Eastern Strandveld) Albany Thicket | AT 9 Albany Coastal Belt FOz 6 Southern Coastal Forest AZs 2 Albany Dune Strandveld AT 12Buffels Thicket Marine biodiversity |
| Lowveld NBG | 164 | 1969 | Mpumalanga (Nelspruit) | Savanna (Lowveld) | SVl 9 Legogote Sour Bushveld SVl 10 Pretoriuskop Sour Bushveld Freshwater (river) |
| Pretoria NBG | 70 | 1958 | Gauteng (Pretoria) | Savanna (Central Bushveld) | SVcb 6 Marikana Thornveld |
| Thohoyandou NBG | 89 | 1986 | Limpopo (Thohoyandou) | Savanna (Central Bushveld) | SVcb21 Soutpansberg Mountain Bushveld |
| Pretoria NZG | 80 | 1899 | Gauteng (Pretoria) | Savanna (Central Bushveld) | SVcb 6 Marikana Thornveld |
| Mokopane Biodiversity Conservation Centre | 1398 | 1979 | Limpopo (Mokopane) | Savanna (Central Bushveld) | SVcd 20 Makhado Sweet Bushveld SVcb 23 Polokwane Plateau Bushveld |
| Walter Sisulu NBG | 276 | 1982 | Gauteng (Roodepoort/Mogale City) | Savanna (Central Bushveld) Grassland (Mesic Highveld Grassland) | SVcb 9 Gold Reef Mountain Bushveld Gm 10 Egoli Granite Grassland Freshwater (river) |
| Category | NEM:BA-A&IS Description |
|---|---|
| Category 1(a) | Species that must be combatted and are targets for eradication |
| Category 1(b) | Species that are control targets and need a national management plan |
| Category 2 | Species requiring a permit for restricted activities |
| Category 3 | Species that are subject to exemptions |
| “Not Listed” | Unlisted species: Alien species that are not listed in the NEM:BA-A&IS Regulations but have been reported as present in natural or semi-natural ecosystems in South Africa or on offshore islands |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokotjomela, T.M.; Rahlao, S.J.; Vukeya, L.R.; Baltzinger, C.; Mangane, L.V.; Willis, C.K.; Mutshinyalo, T.M. The Diversity of Alien Plant Species in South Africa’s National Botanical and Zoological Gardens. Diversity 2023, 15, 407. https://doi.org/10.3390/d15030407
Mokotjomela TM, Rahlao SJ, Vukeya LR, Baltzinger C, Mangane LV, Willis CK, Mutshinyalo TM. The Diversity of Alien Plant Species in South Africa’s National Botanical and Zoological Gardens. Diversity. 2023; 15(3):407. https://doi.org/10.3390/d15030407
Chicago/Turabian StyleMokotjomela, Thabiso M., Sebataolo J. Rahlao, Loyd R. Vukeya, Christophe Baltzinger, Lindokuhle V. Mangane, Christopher K. Willis, and Thompson M. Mutshinyalo. 2023. "The Diversity of Alien Plant Species in South Africa’s National Botanical and Zoological Gardens" Diversity 15, no. 3: 407. https://doi.org/10.3390/d15030407
APA StyleMokotjomela, T. M., Rahlao, S. J., Vukeya, L. R., Baltzinger, C., Mangane, L. V., Willis, C. K., & Mutshinyalo, T. M. (2023). The Diversity of Alien Plant Species in South Africa’s National Botanical and Zoological Gardens. Diversity, 15(3), 407. https://doi.org/10.3390/d15030407






