Phenotypic Plasticity Drives the Successful Expansion of the Invasive Plant Pedicularis kansuensis in Bayanbulak, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Site
2.2. Field Sample Collection
2.3. Determination of Morphological Indicators
2.4. Biomass Determination
2.5. Determination of Nutrient Characteristics
2.6. Statistical Analyses
3. Results
3.1. Morphological Characteristics of P. kansuensis
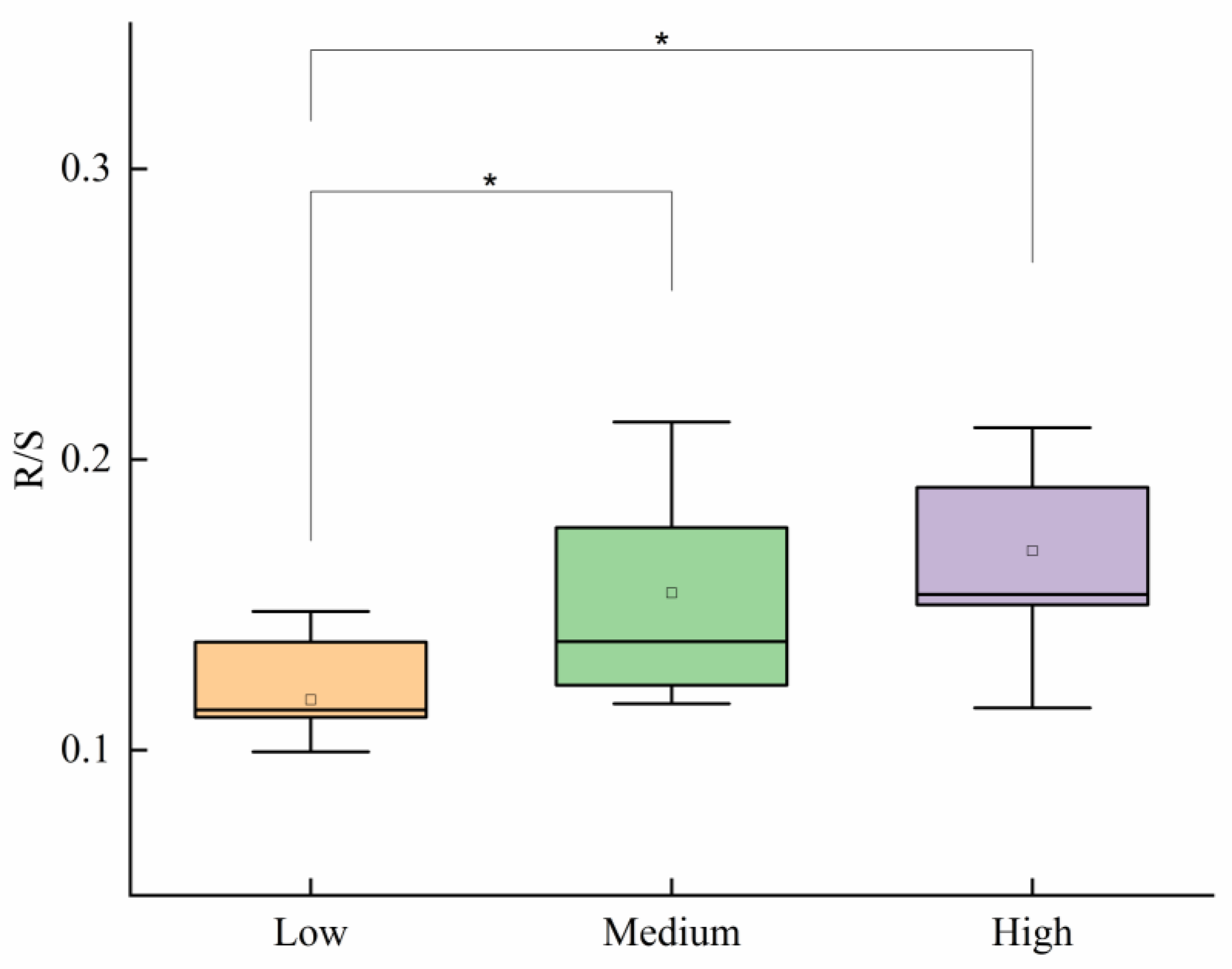
3.2. Biomass Accumulation and Allocation of P. kansuensis
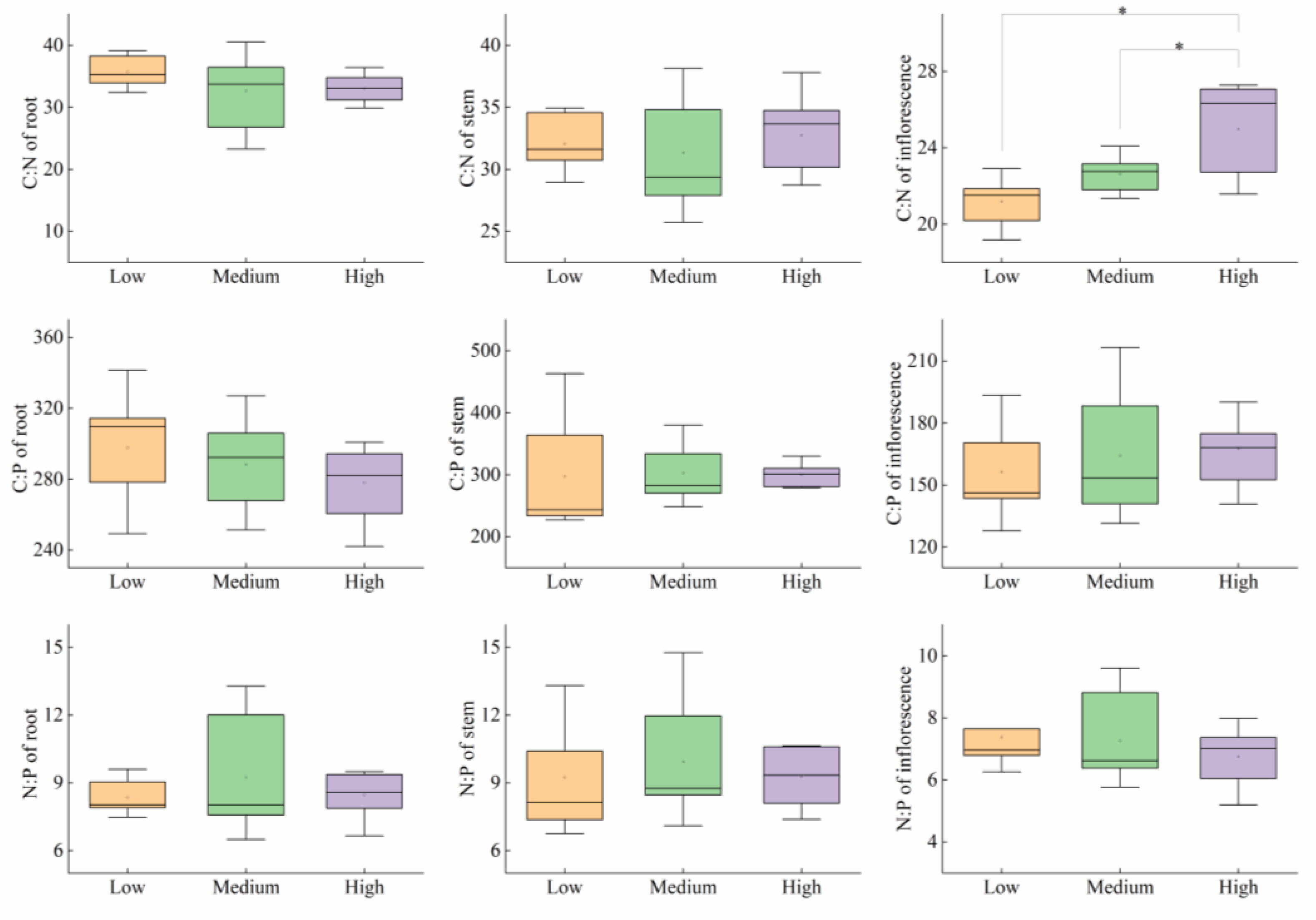
3.3. Nutrient Characteristics of P. kansuensis
3.3.1. Nutrient Characteristics of Aboveground Parts among the Three Invasion Levels
3.3.2. Nutrient Characteristics of Each Structure in the Three Invasion Levels
4. Discussion
4.1. Pedicularis kansuensis Has Strong Phenotypic Plasticity
4.2. Morphological Characteristics of P. kansuensis in Different Invasion Levels
4.3. Biomass Allocation of P. kansuensis in Different Invasion Levels
4.4. Nutrient Characteristics of P. kansuensis Components under Different Invasion Levels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drenovsky, R.E.; Grewell, B.J.; D’antonio, C.M.; Funk, J.L.; James, J.J.; Molinari, N.; Psker, I.M.; Richards, C.L. A functional trait perspective on plant invasion. Ann. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef]
- Warren, R.J.; Candeias, M.; Lafferty, A.; Chick, L.D. Regional-scale environmental resistance to non-native ant invasion. Biol. Invasions 2020, 22, 813–825. [Google Scholar] [CrossRef]
- Aldorfová, A.; Knobová, P.; Münzbergová, Z. Plant–soil feedback contributes to predicting plant invasiveness of 68 alien plant species differing in invasive status. Oikos 2020, 129, 1257–1270. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Ren, L.; Guo, X.; Liu, S.; Yu, T.; Guo, W.; Wang, R.; Ye, S.; Lambertini, C.; Brix, H.; Eller, F. Intraspecific variation in Phragmites australis: Clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. J. Ecol. 2020, 108, 2531–2543. [Google Scholar] [CrossRef]
- Etges, W.J.; Trotter, M.V.; de Oliveira, C.C.; Rajpurohit, S.; Gibbs, A.G.; Tuljapurkar, S. Deciphering life history transcriptomes in different environments. Mol. Ecol. 2015, 24, 151–179. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.Y.; Scheepens, J.F.; Li, W.T.; Wang, R.F.; Zheng, Y.L.; Feng, Y.L. Biomass reallocation and increased plasticity might contribute to successful invasion of Chromolaena odorata. Flora 2019, 256, 79–84. [Google Scholar] [CrossRef]
- Rathee, S.; Ahmad, M.; Sharma, P.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kaur, A.; Yadav, S.; Kohli, R.K. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot. 2021, 184, 104392. [Google Scholar] [CrossRef]
- Wang, J.H.; Chen, W.; Zhu, H. Ecological stoichiometry and invasive strategies of two alien species (Bidens pilosa and Mikania micrantha) in subtropical China. Ecol. Res. 2019, 34, 612–623. [Google Scholar] [CrossRef]
- Postma, J.A.; Hecht, V.L.; Hikosaka, K.; Nord, E.A.; Pons, T.L.; Poorter, H. Dividing the pie: A quantitative review on plant density responses. Plant Cell Environ. 2021, 44, 1072–1094. [Google Scholar] [CrossRef]
- Rehling, F.; Sandner, T.M.; Matthies, D. Biomass partitioning in response to intraspecific competition depends on nutrients and species characteristics: A study of 43 plant species. J. Ecol. 2021, 109, 2219–2233. [Google Scholar] [CrossRef]
- Ren, G.; He, F.; Sun, J.; Hu, W.; Azeem, A.; Qi, S.; Yang, B.; Cui, M.; Jiang, K.; Du, D. Resource conservation strategy helps explain patterns of biological invasion in a low-N environment. Biochem. Syst. Ecol. 2021, 94, 104205. [Google Scholar] [CrossRef]
- Bloom, R.G.; Mallik, A.U. Indirect effects of black spruce (Picea mariana) cover on community structure and function in sheep laurel (Kalmia angustifolia) dominated heath of eastern Canada. Plant Soil 2004, 265, 279–293. [Google Scholar] [CrossRef]
- Elser, J. Biological stoichiometry: A chemical bridge between ecosystem ecology and evolutionary biology. Am. Nat. 2006, 168, S25–S35. [Google Scholar] [CrossRef] [PubMed]
- Dybzinski, R.; Kelvakis, A.; McCabe, J.; Panock, S.; Anuchitlertchon, K.; Vasarhelyi, L.; McCormack, M.; McNickle, G.; Poorter, H.; Trinder, C.; et al. How are nitrogen availability, fine-root mass, and nitrogen uptake related empirically? Implications for models and theory. Glob. Change Biol. 2019, 25, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef]
- Zhao, W.X.; Liu, T.; Liu, Y.; Wang, H.Y.; Wang, R.L.; Ma, Q.Q.; Dong, H.G.; Bi, X. The significance of biomass allocation to population growth of the invasive species Ambrosia artemisiifolia and Ambrosia trifida with different densities. BMC Ecol. Evol. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Swart, E.M.; Cornelissen, J.H. Integrated plant phenotypic responses to contrasting above-and below-ground resources: Key roles of specific leaf area and root mass fraction. New Phytol. 2015, 206, 1247–1260. [Google Scholar] [CrossRef]
- Alpert, P.; Bone, E.; Holzapfel, C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 52–66. [Google Scholar] [CrossRef]
- Olmstead, R.G.; de Pamphilis, C.W.; Wolfe, A.D.; Young, N.D.; Elisons, W.J.; Reeves, P.A. Disintegration of the Scrophulariaceae. Am. J. Bot. 2001, 88, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.B.; Holmgren, N.H.; Mill, R.R.; Pedicularis, L. Flora of China; Wu, Z.Y., Peter, H.R., Hong, D.Y., Eds.; Missouri Botanical Garden Press: St. Louis, MO, USA; Science Press: Beijing, China, 1998; Volume 18, pp. 97–209. [Google Scholar]
- Sui, X.; Kuss, P.; Li, W.; Yang, M.; Guan, K.; Li, A. Identity and distribution of weedy Pedicularis kansuensis Maxim.(Orobanchaceae) in Tianshan Mountains of Xinjiang: Morphological, anatomical and molecular evidence. J. Arid Land 2016, 8, 453–461. [Google Scholar] [CrossRef]
- Bao, G.S.; Suetsugu, K.j.; Wang, H.S.; Yao, X.; Liu, L.; Ou, J.; LI, C.J. Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol. Res. 2015, 30, 507–515. [Google Scholar] [CrossRef]
- LI, X.P.; Zhao, C.Z.; REN, Y.; Zhang, j.; Lei, L. Relationship between root forks and link number, branch angle of Pedicularis kansuensis under different density conditions in Gahai Wetland. Acta Ecol. Sin. 2019, 39, 3670–3676. [Google Scholar]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. The Agro-Chemical Analysis of Soil; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Song, S.; Leng, H.; Feng, S.; Meng, C.; Luo, B.; Zhao, L.; Zhang, C. Biomass allocation pattern of urban shrubs in the Yangtze River Delta region, China–A field observation of 13 shrub species. Urban For. Urban Green. 2021, 63, 127228. [Google Scholar] [CrossRef]
- Lamarque, L.J.; Porte, A.J.; Eymeric, C.; Lasnier, J.B.; Lortie, C.J.; Delzon, S. A test for pre-adapted phenotypic plasticity in the invasive tree Acer negundo L. PLoS ONE 2013, 8, e74239. [Google Scholar] [CrossRef]
- Huey, R.B.; Gilchrist, G.W.; Carlson, M.L.; Berrigan, D.; Serra, L. Rapid evolution of a geographic cline in size in an introduced fly. Science 2000, 287, 308–309. [Google Scholar] [CrossRef]
- Li, W.J. Taxonomy of Pedicularis in Xinjiang and Genetic Diversity and Distribution Model of P. kansuensis Maxim. Doctoral Dissertation, University of Chinese Academy of Sciences:, Beijing, China, 2016. (In Chinese). [Google Scholar]
- Hou, Z.J.; Zhao, C.Z.; Li, Y.; Zhang, Q.; Ma, X.L. Trade-off between height and branch numbers in Stellera chamaejasme on slopes of different aspects in a degraded alpine grassland. Chin. J. Plant Ecol. 2014, 38, 281–288. (In Chinese) [Google Scholar]
- Doust, J.L.; Silvertown, J.W. Introduction to plant population ecology. J. Ecol. 1988, 76, 912. [Google Scholar] [CrossRef]
- Fan, G.H.; Zhang, J.W.; Huang, Y.X.; Shen, X.J.; Yu, P.J.; Zhao, X.Y. Influence of population density on morphological traits and allometric growth of Corispermum macrocarpum. Acta Ecol. Sin. 2018, 38, 3931–3942. (In Chinese) [Google Scholar]
- Zheng, W.; Fan, G.H.; Huang, Y.X.; Wang, T.; Yu, P.J.; Wang, H.Q. Allometric relationships between the morphological traits and biomass allocation strategies of Salsola collina under different population density. Acta Ecol. Sin. 2021, 41, 2845–2854. (In Chinese) [Google Scholar]
- Burns, J.H.; Strauss, S.Y. Effects of competition on phylogenetic signal and phenotypic plasticity in plant functional traits. Ecology 2012, 93, S126–S137. [Google Scholar] [CrossRef]
- Heger, T.; Jacobs, B.S.; Latimer, A.M.; Kollmann, J.; Rice, K.J. Does experience with competition matter? Effects of source competitive environment on mean and plastic trait expression in Erodium cicutarium. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 236–246. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. N. Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Belter, P.R.; Cahill, J.F. Disentangling root system responses to neighbours: Identification of novel root behavioural strategies. Aob Plants 2015, 7, plv059. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Cuassolo, F.; Balseiro, E.; Modenutti, B. Alien vs. native plants in a Patagonian wetland: Elemental ratios and ecosystem stoichiometric impacts. Biol. Invasions 2012, 14, 179–189. [Google Scholar] [CrossRef]
- Kleyer, M.; Trinogga, J.; Cebrián-Piqueras, M.A.; Trenkamp, A.; Fløjgaard, C.; Ejrnæs, R.; Bouma, T.J.; Minden, V.; Maier, M.; Mantilla-Contreras, J.; et al. Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. J. Ecol. 2019, 107, 829–842. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, X.; Korpelainen, H.; Li, C. Stronger intra-specific competition aggravates negative effects of drought on the growth of Cunninghamia lanceolata. Environ. Exp. Bot. 2020, 175, 104042. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W.; Verhoeven, J.T. Biomass N: P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol. Appl. 2003, 13, 372–384. [Google Scholar] [CrossRef]
- Elser, J.J.; Peace, A.L.; Kyle, M.; Wojewodzic, M.; McCrackin, M.L.; Andersen, T.; Hessen, D.O. Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol. Lett. 2010, 13, 1256–1261. [Google Scholar] [CrossRef]
- Penuelas, J.; Sardans, J.; Llusià, J.; Owen, S.M.; Carnicer, J.; Giambelluca, T.W.; Glambelluca, E.L.; Rezende, M.W.; ÜLO, N. Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob. Change Biol. 2010, 16, 2171–2185. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef]
- Naddafi, R.; Eklöv, P.; Pettersson, K. Stoichiometric constraints do not limit successful invaders: Zebra mussels in Swedish lakes. PLoS ONE 2009, 4, e5345. [Google Scholar] [CrossRef]
- Makino, W.; Cotner, J.B.; Sterner, R.W.; Elser, J.J. Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C: N: P stoichiometry. Funct. Ecol. 2003, 17, 121–130. [Google Scholar] [CrossRef]
- González, A.L.; Kominoski, J.S.; Danger, M.; Ishida, S.; Iwai, N.; Rubach, A. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 2010, 119, 779–790. [Google Scholar] [CrossRef]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; et al. Growth rate–stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef]
- Jing, H.; Zhou, H.; Wang, G.; Xue, S.; Liu, G.; Duan, M. Nitrogen addition changes the stoichiometry and growth rate of different organs in Pinus tabuliformis seedlings. Front. Plant Sci. 2017, 8, 1922. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yu, L.; Jiang, Y.; Lei, Y.; Korpelainen, H.; Niinemets, Ü.; Li, C. Nitrogen-controlled intra-and interspecific competition between Populus purdomii and Salix rehderiana drive primary succession in the Gongga Mountain glacier retreat area. Tree Physiol. 2017, 37, 799–814. [Google Scholar] [CrossRef] [PubMed]


| Level of Invasion | Plant Height (cm) | Inflorescence Length (cm) | Root Length (cm) | Branch Number | Single-Leaf Weight (mg) | Single-Leaf Thickness (mm) | SLA (cm2 g−1) | Single-Leaf Area (cm2) | Density (Plant·m−2) | Crown Width (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Low | 22.28 ± 0.84 a | 8.74 ± 0.34 a | 7.14 ± 0.29 a | 7.31 ± 0.64 a | 11.18 ± 1.13 a | 0.35 ± 0.02 a | 163.66 ± 3.90 a | 1.81 ± 0.17 a | 20.40 ± 3.82 c | 17.92 ± 1.19 a |
| Medium | 18.89 ± 0.65 b | 7.67 ± 0.33 ab | 6.43 ± 0.36 ab | 5.92 ± 0.41 ab | 10.00 ± 0.45 a | 0.35 ± 0.01 a | 160.86 ± 5.01 a | 1.60 ± 0.08 a | 117.07 ± 7.40 b | 13.66 ± 0.39 b |
| High | 18.05 ± 0.58 b | 6.72 ± 0.63 b | 5.81 ± 0.31 b | 4.85 ± 0.52 b | 9.17 ± 1.24 a | 0.38 ± 0.03 a | 142.89 ± 12.78 a | 1.19 ± 0.06 b | 255.91 ± 16.35 a | 13.49 ± 0.61 b |
| Level of Invasion | PPI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Height | Inflorescence Length | Root Length | Branch Number | Single-Leaf Weight | Leaf Thickness | SLA | Single-Leaf Area | Crown Width | |
| Low | 0.27 | 0.27 | 0.34 | 0.55 | 0.60 | 0.37 | 0.19 | 0.57 | 0.47 |
| Medium | 0.26 | 0.29 | 0.41 | 0.54 | 0.38 | 0.35 | 0.23 | 0.43 | 0.26 |
| High | 0.27 | 0.57 | 0.35 | 0.55 | 0.70 | 0.54 | 0.58 | 0.37 | 0.33 |
| Level of Invasion | Stem Biomass (g) | Inflorescence Biomass (g) | Root Biomass (g) | Individual Biomass (g) | Total Aboveground Biomass (g) |
|---|---|---|---|---|---|
| Low | 1.93 ± 0.24 Aa | 2.12 ± 0.3 Aa | 0.43 ± 0.04 Ba | 4.47 ± 0.55 a | 53.67 ± 3.26 c |
| Medium | 1.17 ± 0.11 Ab | 1.47 ± 0.16 Aab | 0.38 ± 0.04 Bab | 3.02 ± 0.28 b | 111.41 ± 5.96 b |
| High | 0.89 ± 0.13 Ab | 1.15 ± 0.26 Ab | 0.27 ± 0.04 Bb | 2.42 ± 0.45 b | 212.71 ± 12.76 a |
| Level of Invasion | C (g/kg) | N (g/kg) | P (g/kg) | C:N | C:P | N:P |
|---|---|---|---|---|---|---|
| Low | 426.39 ± 2.37 a | 18.74 ± 0.58 a | 2.51 ± 0.17 a | 22.97 ± 0.66 b | 178.35 ± 14.15 b | 7.93 ± 0.83 a |
| Medium | 426.74 ± 2.35 a | 17.79 ± 0.41 a | 2.32 ± 0.14 ab | 24.11 ± 0.46 ab | 191.86 ± 14.34 ab | 8.02 ± 0.69 a |
| High | 435.19 ± 4.29 a | 17.42 ± 0.33 a | 1.95 ± 0.13 b | 25.11 ± 0.53 a | 233.09 ± 15.33 a | 9.26 ± 0.50 a |
| Structure | Level of Invasion | C (g/kg) | N (g/kg) | P (g/kg) |
|---|---|---|---|---|
| Root | Low | 391.48 ± 4.68 Cab | 11.00 ± 0.16 Ca | 1.33 ± 0.04 Ba |
| Medium | 399.86 ± 3.35 Ba | 12.68 ± 0.85 Ba | 1.40 ± 0.04 Ba | |
| High | 379.96 ± 4.86 Bb | 11.54 ± 0.20 Ca | 1.37 ± 0.05 Ba | |
| Stem | Low | 427.48 ± 3.23 Ba | 13.38 ± 0.27 Ba | 1.55 ± 0.13 Ba |
| Medium | 435.91 ± 2.88 Aa | 14.16 ± 0.66 Ba | 1.46 ± 0.06 Ba | |
| High | 431.18 ± 3.59 Aa | 13.28 ± 0.43 Ba | 1.44 ± 0.03 Ba | |
| Inflorescence | Low | 443.53 ± 2.50 Aa | 20.98 ± 0.36 Aa | 2.88 ± 0.12 Aa |
| Medium | 442.62 ± 3.88 Aa | 19.58 ± 0.25 Ab | 2.78 ± 0.16 Aa | |
| High | 440.35 ± 2.96 Aa | 17.74 ± 0.46 Ac | 2.66 ± 0.11 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Huang, L.; Yang, L.; Liu, Y.; Chen, H.; Li, W. Phenotypic Plasticity Drives the Successful Expansion of the Invasive Plant Pedicularis kansuensis in Bayanbulak, China. Diversity 2023, 15, 313. https://doi.org/10.3390/d15030313
Li W, Huang L, Yang L, Liu Y, Chen H, Li W. Phenotypic Plasticity Drives the Successful Expansion of the Invasive Plant Pedicularis kansuensis in Bayanbulak, China. Diversity. 2023; 15(3):313. https://doi.org/10.3390/d15030313
Chicago/Turabian StyleLi, Wenchao, Liju Huang, Lei Yang, Yanyan Liu, Huimei Chen, and Wenjun Li. 2023. "Phenotypic Plasticity Drives the Successful Expansion of the Invasive Plant Pedicularis kansuensis in Bayanbulak, China" Diversity 15, no. 3: 313. https://doi.org/10.3390/d15030313
APA StyleLi, W., Huang, L., Yang, L., Liu, Y., Chen, H., & Li, W. (2023). Phenotypic Plasticity Drives the Successful Expansion of the Invasive Plant Pedicularis kansuensis in Bayanbulak, China. Diversity, 15(3), 313. https://doi.org/10.3390/d15030313






