First Record of Corallivorous Nudibranch Pinufius (Gastropoda: Nudibranchia) in the South China Sea: A Suspected New Species of Pinufius †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Analysis
2.2.1. External Morphology
2.2.2. Internal Morphology
2.3. Molecular Analysis
2.3.1. DNA Extraction and Sequencing
2.3.2. Phylogenetic Analysis
3. Results
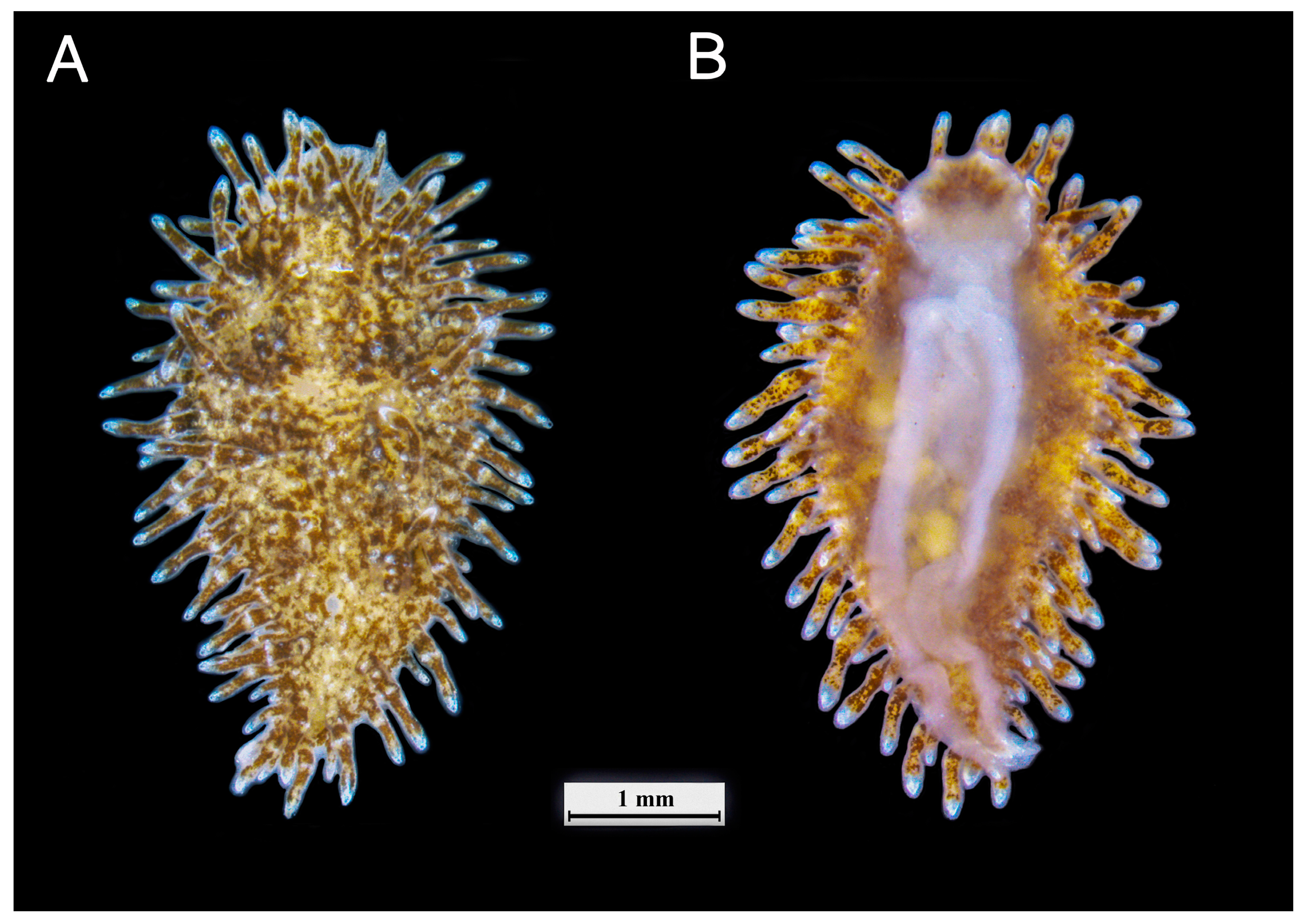
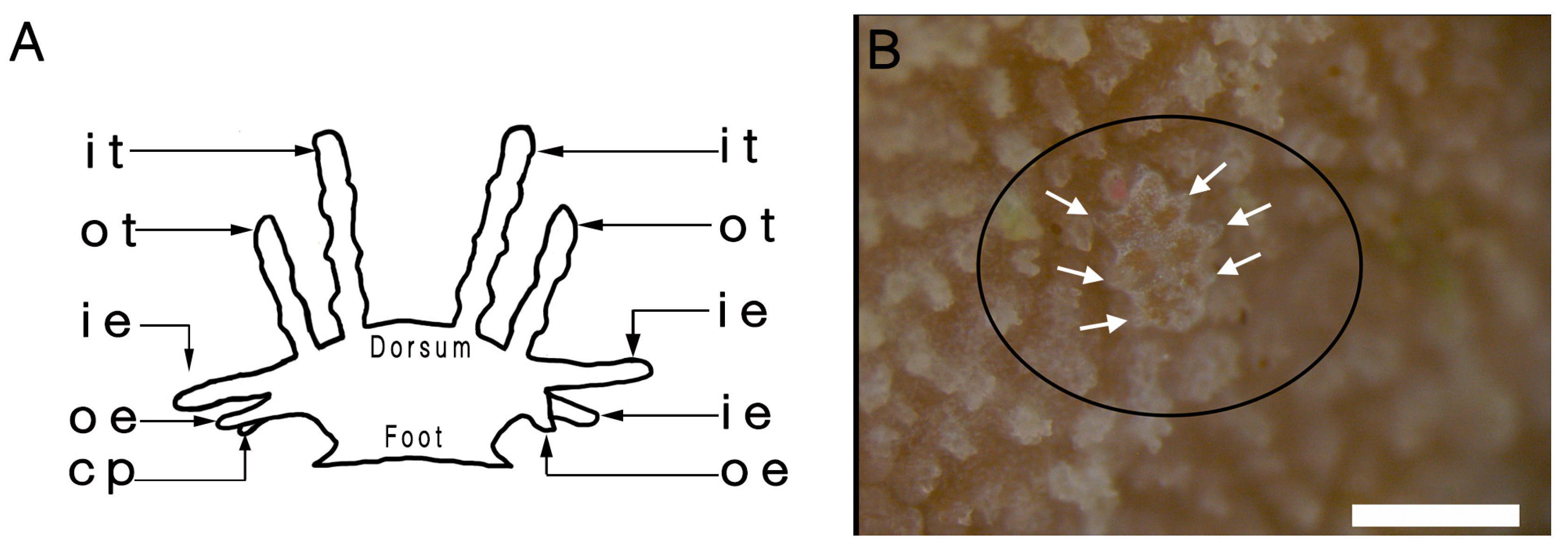
3.1. External Morphology
3.2. Internal Morphology
3.2.1. Radula and Jaw
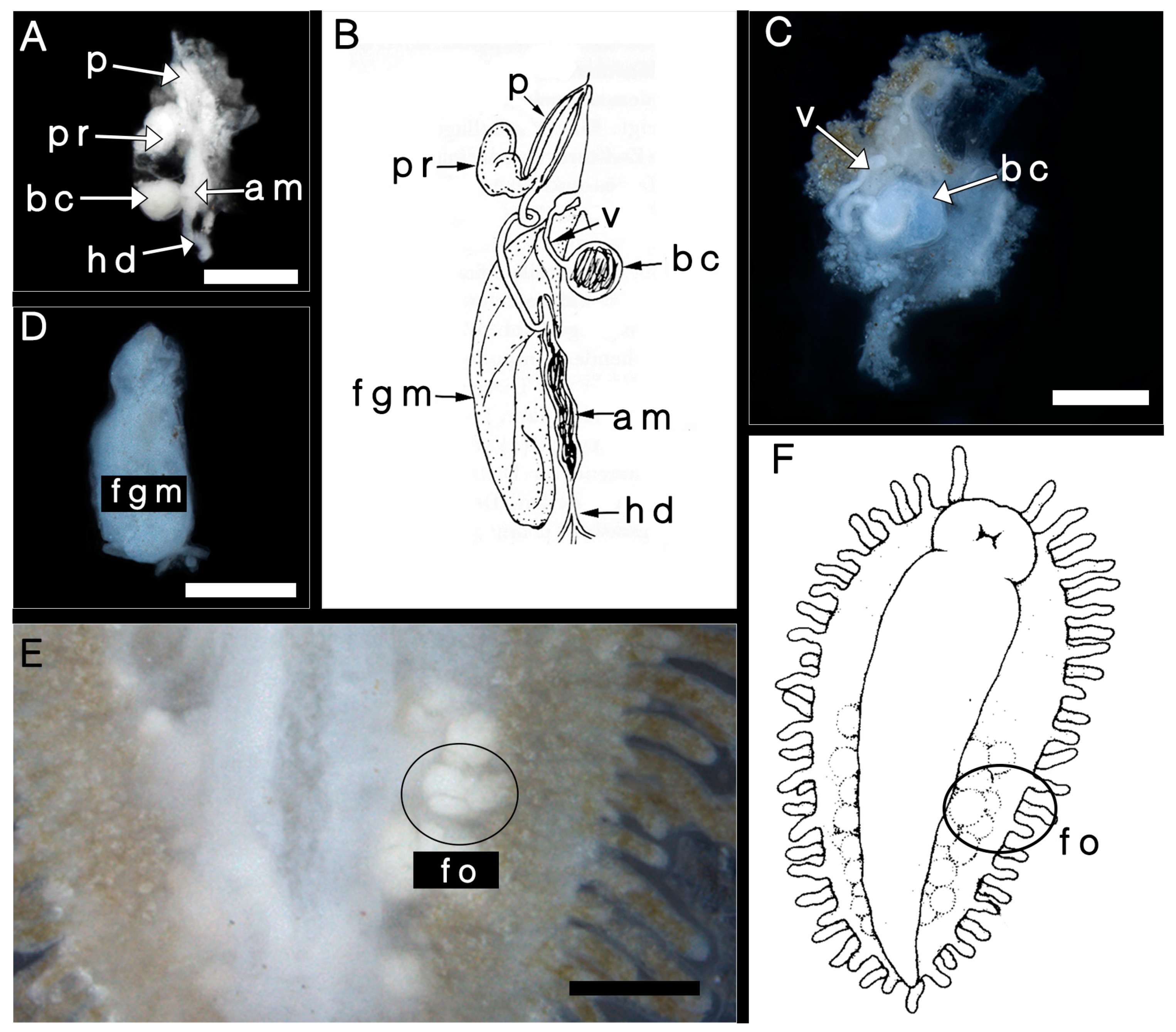
3.2.2. Reproductive System
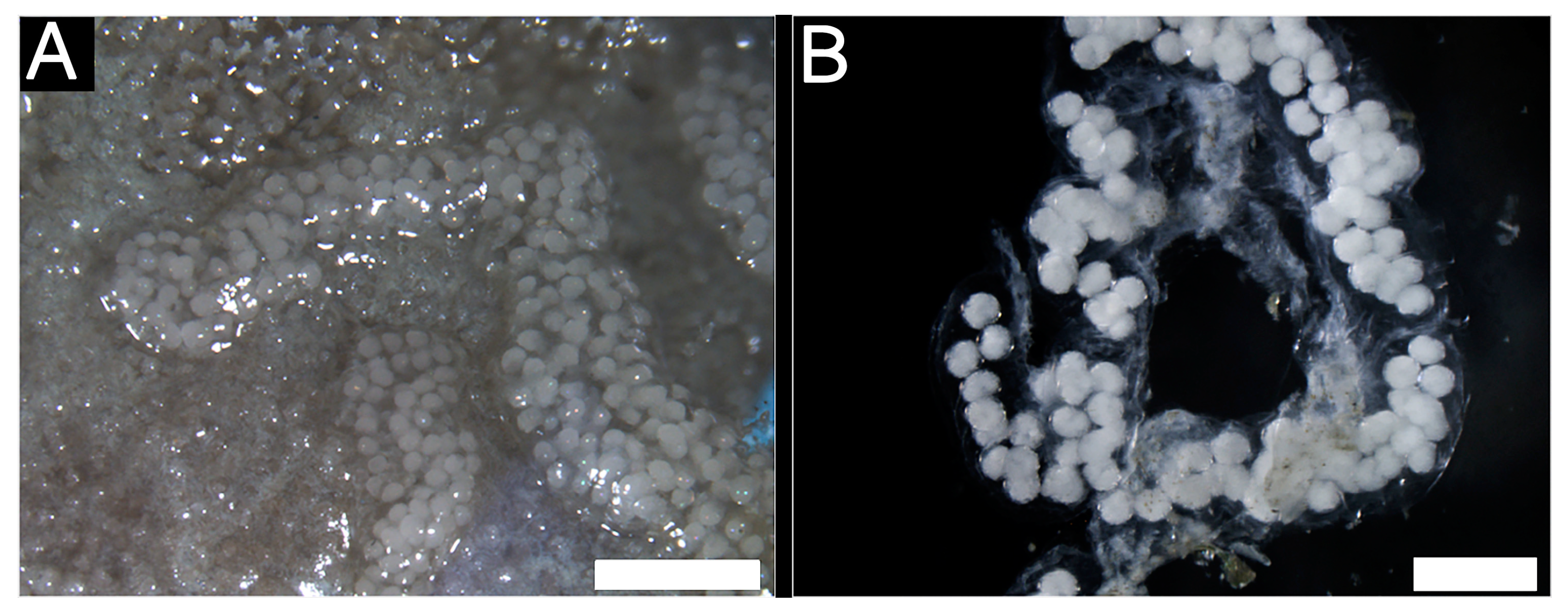
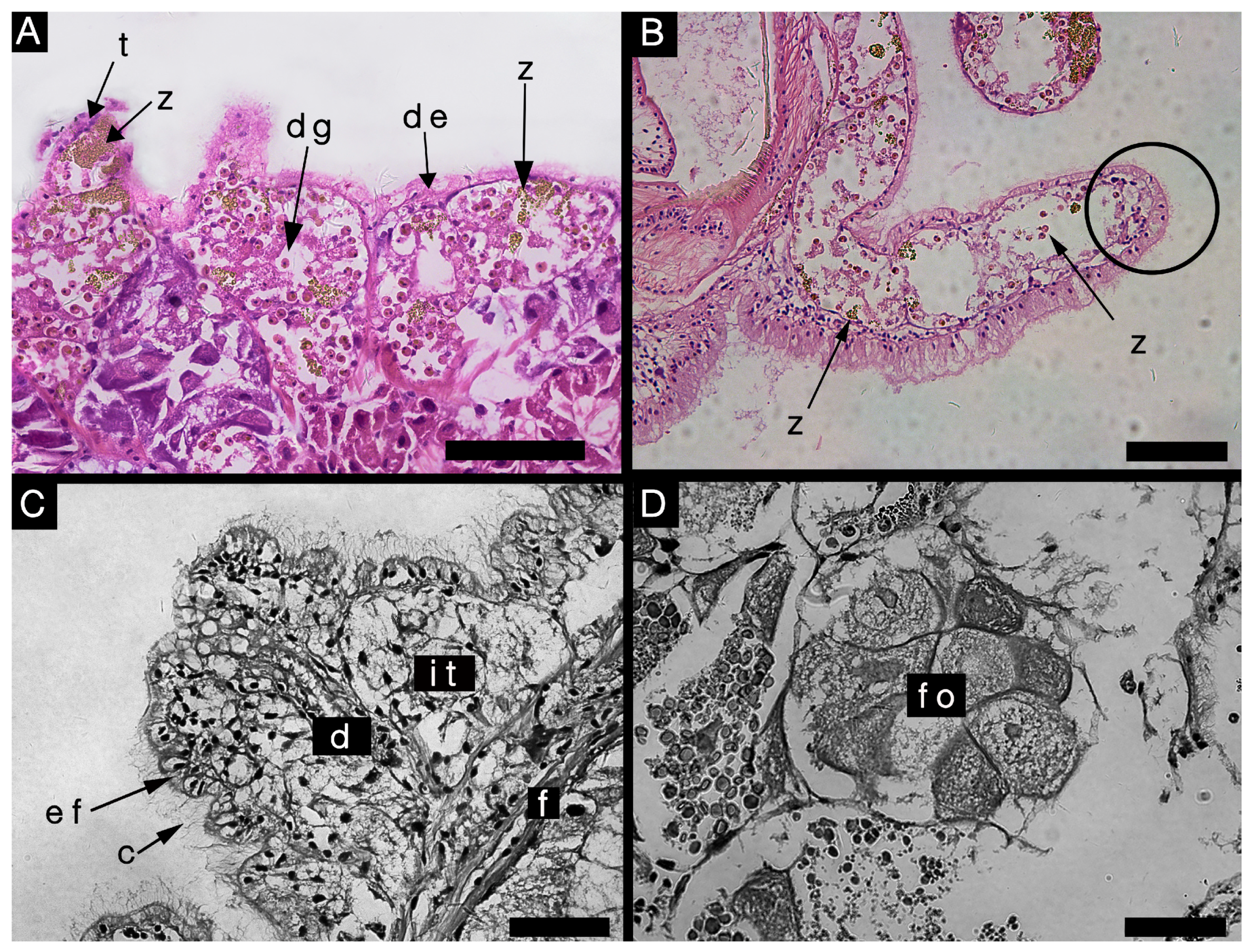
3.2.3. Histological Characteristics
3.3. Phylogenetic Relationships
4. Discussion
4.1. A New Distribution Record for Pinufius in the South China Sea
4.2. The Phylogeny of the Genus Pinufius
4.3. A Suspected New Species of the Genus Pinufius
4.4. Potential Threats to Local Coral Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.V. Coral-reef area and the contributions of reefs to processes and resources of the world’s oceans. Nature 1978, 273, 225–226. [Google Scholar] [CrossRef]
- Halpern, B.; Walbridge, S.; Selkoe, K.; Kappel, C.; Micheli, F.; D’Agrosa, C.; Bruno, J.; Casey, K.; Ebert, C.; Fox, H.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Rotjan, R.D.; Lewis, S.M. Impact of coral predators on tropical reefs. Mar. Ecol. Prog. Ser. 2008, 367, 73–91. [Google Scholar] [CrossRef]
- Selig, E.R.; Casey, K.S.; Bruno, J.F. New insights into global patterns of ocean temperature anomalies: Implications for coral reef health and management. Glob. Ecol. Biogeogr. 2010, 19, 397–411. [Google Scholar] [CrossRef]
- Morton, B.; Blackmore, G.; Kwok, C.T. Corallivory and prey choice by Drupella rugosa (Gastropoda: Muricidae) in Hong Kong. J. Molluscan Stud. 2002, 68, 217–223. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Cvitanovic, C.; Raymundo, M.L.; Babcock, R.C.; Bonin, M.C.; Bozec, Y.M.; Burn, D.; Byrne, M.; Castro-Sanguino, C.; et al. Knowledge gaps in the biology, ecology, and management of the Pacific crown-of-thorns sea star Acanthaster sp. on Australia’s Great Barrier Reef. Biol. Bull. 2021, 241, 330–346. [Google Scholar] [CrossRef]
- Barton, J.A.; Bourne, D.G.; Humphrey, C.; Hutson, K.S. Parasites and coral associated invertebrates that impact coral health. Rev. Aquac. 2020, 12, 2284–2303. [Google Scholar] [CrossRef]
- Hume, B.C.C.; D’Angelo, C.; Cunnington, A.; Smith, E.G.; Wiedenmann, J. The corallivorous flatworm Amakusaplana acroporae: An invasive species threat to coral reefs? Coral Reefs 2014, 33, 267–272. [Google Scholar] [CrossRef]
- Rawlinson, K.A.; Stella, J.S. Discovery of the corallivorous polyclad flatworm, Amakusaplana acroporae, on the Great Barrier Reef, Australia—The first report from the wild. PLoS ONE 2012, 7, e42240. [Google Scholar] [CrossRef]
- Gosliner, T.M.; Fahey, S.J. Previously undocumented diversity and abundance of cryptic species: A phylogenetic analysis of Indo-Pacific Arminidae Rafinesque, 1814 (Mollusca: Nudibranchia) with descriptions of 20 new species of Dermatobranchus. Zool. J. Linn. Soc. 2011, 161, 245–356. [Google Scholar] [CrossRef]
- Odhner, N.H. Opisthobranchiate Mollusca from the western and northern coasts of Norway. K. Nor. Vidensk. Selsk. Skr. 1939, 1, 1–93. [Google Scholar]
- Wägele, H.; Willan, R.C. Phylogeny of the Nudibranchia. Zool. J. Linn. Soc. 2000, 130, 83–181. [Google Scholar] [CrossRef]
- MolluscaBase eds. Cladobranchia. Available online: http://www.molluscabase.org/aphia.php?p=taxdetails&id=827889 (accessed on 27 January 2023).
- Bouchet, P.; Rocroi, J.P.; Hausdorf, B.; Kaim, A.; Kano, Y.; Nützel, A.; Parkhaev, P.; Schrödl, M.; Strong, E.E. Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 2017, 61, 1–526. [Google Scholar] [CrossRef]
- Cella, K.; Carmona, L.; Ekimova, I.; Chichvarkhin, A.; Schepetov, D.; Gosliner, T.M. A radical solution: The phylogeny of the nudibranch family Fionidae. PLoS ONE 2016, 11, e0167800. [Google Scholar] [CrossRef]
- Korshunova, T.; Martynov, A.; Picton, B. Ontogeny as an important part of integrative taxonomy in tergipedid aeolidaceans (Gastropoda: Nudibranchia) with a description of a new genus and species from the Barents Sea. Zootaxa 2017, 4324, 1–22. [Google Scholar] [CrossRef]
- Marcus, E.; Marcus, E. Opisthobranchia aus dem Roten Meer und von den Malediven. Akad. Wiss. Lit. Abh. Math. Nat. Kl. 1960, 12, 873–933. [Google Scholar]
- Rudman, W.B. Further-studies on the anatomy and ecology of opisthobranch mollusks feeding on the scleractinian coral Porites. Zool. J. Linn. Soc. 1981, 71, 373–412. [Google Scholar] [CrossRef]
- Fritts-Penniman, A.L.; Gosliner, T.M.; Mahardika, G.N.; Barber, P.H. Cryptic ecological and geographic diversification in coral-associated nudibranchs. Mol. Phylogenetics Evol. 2020, 144, 106698. [Google Scholar] [CrossRef]
- Pola, M.; Gosliner, T.M. The first molecular phylogeny of cladobranchian opisthobranchs (Mollusca, Gastropoda, Nudibranchia). Mol. Phylogenetics Evol. 2010, 56, 931–941. [Google Scholar] [CrossRef]
- Rudman, W.B. The taxonomy and biology of further aeolidacean and arminacean nudibranch molluscs with symbiotic zooxanthellae. Zool. J. Linn. Soc. 1982, 74, 147–196. [Google Scholar] [CrossRef]
- Beesley, P.L.; Ross, G.J.B.; Wells, A. Mollusca: The Southern Synthesis. Fauna of Australia; CSIRO Publishing: Melbourne, Australia, 1998; Volume 5, pp. 1007–1011. ISBN 0643057560. [Google Scholar]
- Jensen, K.R. An outline of the systematics and classification of Nudibranchia (Gastropoda, Opisthobranchia). Phuket Mar. Biol. Cent. Spec. Publ. 2000, 21, 431–446. [Google Scholar]
- Mahguib, J.; Valdés, Á. Molecular investigation of the phylogenetic position of the polar nudibranch Doridoxa (Mollusca, Gastropoda, Heterobranchia). Polar Biol. 2015, 38, 1369–1377. [Google Scholar] [CrossRef]
- Liu, R. Checklist of Marine Biota of China Seas; China Science Publishing & Media Ltd. (CSPM): China, Beijing, 2008; pp. 536–545. ISBN 978-7-03-023722-4. [Google Scholar]
- Hu, J.; Zhang, Y.; Yiu, S.K.F.; Xie, J.Y.; Qiu, J.W. A new species of predatory nudibranch (Gastropoda: Trinchesiidae) of the scleractinian coral Goniopora. Zool. Stud. 2020, 59, 62. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Xie, J.Y.; Qiu, J.W. A new species of predatory nudibranch (Gastropoda: Trinchesiidae) of the Coral Pavona decussata. Zool. Stud. 2020, 59, 30. [Google Scholar] [CrossRef]
- Yiu, S.K.F.; Chung, S.S.W.; Qiu, J.W. New observations on the corallivorous nudibranch Phestilla melanobrachia: Morphology, dietary spectrum and early development. J. Molluscan Stud. 2021, 87, yab034. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Wr, H.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial Cytochrome Coxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Puslednik, L.; Serb, J.M. Molecular phylogenetics of the Pectinidae (Mollusca: Bivalvia) and effect of increased taxon sampling and outgroup selection on tree topology. Mol. Phylogenetics Evol. 2008, 48, 1178–1188. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Kessing, B.; Martin, A. The Simple Fool’s Guide to PCR; Department of Zoology, University of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
- Colgan, D.J.; McLauchlan, A.; Wilson, G.D.F.; Livingston, S.P.; Edgecombe, G.D.; Macaranas, J.; Cassis, G.; Gray, M.R. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ekimova, I.; Deart, Y.; Schepetov, D. Living with a giant parchment tube worm: A description of a new nudibranch species (Gastropoda: Heterobranchia) associated with the annelid Chaetopterus. Mar. Biodivers. 2017, 49, 289–300. [Google Scholar] [CrossRef]
- Kitano, Y.F.; Benzoni, F.; Arrigoni, R.; Shirayama, Y.; Wallace, C.C.; Fukami, H. A phylogeny of the family Poritidae (Cnidaria, Scleractinia) based on molecular and morphological analyses. PLoS ONE 2014, 9, e98406. [Google Scholar] [CrossRef]
- Mcdonald, G.; Nybakken, J. A worldwide review of the food of nudibranch mollusks. I. Introduction and the suborder Arminacea. Veliger 1997, 40, 157–159. [Google Scholar]
- Pola, M.; Hallas, J.M.; Gosliner, T.M. Welcome back Janolidae and Antiopella: Improving the understanding of Janolidae and Madrellidae (Cladobranchia, Heterobranchia) with description of four new species. J. Zool. Syst. Evol. Res. 2019, 57, 345–368. [Google Scholar] [CrossRef]
- Thompson, T.E.; Cattaneo, R.; Wong, Y.M. Eastern Mediterranean Opisthobranchia: Dotidae (Dendronotoidea), Arminidae and Madrellidae (Arminoidea). J. Molluscan Stud. 1990, 56, 393–413. [Google Scholar] [CrossRef]
- Burn, R.; Miller, M.C. A new genus, Caldukia and an extended description of the type species, Proctonotus? Affinis Burn, 1958 (Mollusca Gastropoda: Arminacea, Antiopellidae). J. Malacol. Soc. Aust. 1969, 1, 23–31. [Google Scholar] [CrossRef]
- Goodheart, J.A.; Bazinet, A.L.; Valdes, A.; Collins, A.G.; Cummings, M.P. Prey preference follows phylogeny: Evolutionary dietary patterns within the marine gastropod group Cladobranchia (Gastropoda: Heterobranchia: Nudibranchia). BMC Evol. Biol. 2017, 17, 221. [Google Scholar] [CrossRef]
- Faucci, A.; Toonen, R.J.; Hadfield, M.G. Host shift and speciation in a coral-feeding nudibranch. Proc. R. Soc. Biol. Sci. 2007, 274, 111–119. [Google Scholar] [CrossRef]
- Mehrotra, R.; Arnold, S.; Wang, A.; Chavanich, S.; Hoeksema, B.W.; Caballer, M. A new species of coral-feeding nudibranch (Mollusca: Gastropoda) from the Gulf of Thailand. Mar. Biodivers. 2020, 50, 36. [Google Scholar] [CrossRef]
- Miller, M.C. Aeolid nudibranchs (Gastropoda: Opisthobranchia) of the family Tergipedidae from New Zealand waters. Zool. J. Linn. Soc. 1977, 60, 197–222. [Google Scholar] [CrossRef]
- Gochfeld, D.J.; Aeby, G.S. Control of populations of the coral-feeding nudibranch Phestilla sibogae by fish and crustacean predators. Mar. Biol. 1997, 130, 63–69. [Google Scholar] [CrossRef]
- Cowan, Z.L.; Dworjanyn, S.A.; Caballe, C.F.; Pratchett, M. Benthic predators influence microhabitat preferences and settlement success of crown-of-thorns starfish (Acanthaster cf. solaris). Diversity 2016, 8, 27. [Google Scholar] [CrossRef]
- Cowan, Z.L.; Pratchett, M.; Messmer, V.; Ling, S. Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in Mitigating, if not preventing, population outbreaks. Diversity 2017, 9, 7. [Google Scholar] [CrossRef]
- Ormond, R.; Bradbury, R.; Bainbridge, S.; Fabricius, K.; Keesing, J.; de Vantier, L.; Medlay, P.; Steven, A. Test of a model of regulation of crown-of-thorns starfish by fish predators. In Proceedings of the Acanthaster and the Coral Reef: A Theoretical Perspective, Townsville, Australia, 6–7 August 1988; pp. 189–207. [Google Scholar]
- Guo, J.; Chen, Z.; Xu, Y.; Xu, S.; Li, C. Tempo-spatial distribution characteristics of fish resources in Daya Bay. Period. Ocean Univ. China 2018, 48, 47–55. [Google Scholar] [CrossRef]
- Liu, k.; Du, F.; Li, Y.; Wang, X.; Chen, H.; Zhang, J.; Li, C. Variation characteristics of microbenthic secondary productivity in Daya Bay of South China Sea for nearly 30 years. South China Fish. Sci. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Rudman, W.B. The ecology and anatomy of a new species of aeolid opisthobranch mollusc; A predator of the scleractinian coral Porites. Zool. J. Linn. Soc. 1979, 65, 339–350. [Google Scholar] [CrossRef]
- Goodheart, J.A.; Bleidißel, S.; Schillo, D.; Strong, E.E.; Ayres, D.L.; Preisfeld, A.; Collins, A.G.; Cummings, M.P.; Wägele, H. Comparative morphology and evolution of the cnidosac in Cladobranchia (Gastropoda: Heterobranchia: Nudibranchia). Front. Zool. 2018, 15, 43. [Google Scholar] [CrossRef]
- Camacho-García, Y.E.; Gosliner, T.M. A new species of the zephyrinid nudibranch genus Janolus (Mollusca: Nudibranchia) from North America and Costa Rica. Rev. Biol. Trop. 2006, 54, 1295–1305. [Google Scholar] [CrossRef]
- Alder, J.; Hancock, A. Notice of a collection of nudibranchiate Mollusca made in India by Walter Elliot Esq. with descriptions of several new genera and species. Trans. Zool. Soc. Lond. 1864, 5, 113–147. [Google Scholar] [CrossRef]
- Baba, K. Notes on a nudibranch, Madretla sanguinea (ANGAs), with reference to its papillary glands. Jpn. J. Malacol. 1935, 5, 181–187. [Google Scholar] [CrossRef]
- Alder, J.; Hancock, A. Description of a new genus of nudibranchiate Mollusca, with some new species of Eolis. Ann. Mag. Nat. Hist. 1844, 13, 161–167. [Google Scholar] [CrossRef]
- Behrens, D.W. Nudibranch Behavior; Humann, P., DeLoach, N., Eds.; New World Publications, Inc.: Jacksonville, FL, USA, 2005; p. 43. ISBN 1-878348-41-8. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Tian, P.; Wang, W.; Cao, B.; Xu, Z.; Xiao, J.; Niu, W. First Record of Corallivorous Nudibranch Pinufius (Gastropoda: Nudibranchia) in the South China Sea: A Suspected New Species of Pinufius. Diversity 2023, 15, 226. https://doi.org/10.3390/d15020226
Jia Z, Tian P, Wang W, Cao B, Xu Z, Xiao J, Niu W. First Record of Corallivorous Nudibranch Pinufius (Gastropoda: Nudibranchia) in the South China Sea: A Suspected New Species of Pinufius. Diversity. 2023; 15(2):226. https://doi.org/10.3390/d15020226
Chicago/Turabian StyleJia, Zhiyu, Peng Tian, Wei Wang, Bingbing Cao, Ziqing Xu, Jiaguang Xiao, and Wentao Niu. 2023. "First Record of Corallivorous Nudibranch Pinufius (Gastropoda: Nudibranchia) in the South China Sea: A Suspected New Species of Pinufius" Diversity 15, no. 2: 226. https://doi.org/10.3390/d15020226
APA StyleJia, Z., Tian, P., Wang, W., Cao, B., Xu, Z., Xiao, J., & Niu, W. (2023). First Record of Corallivorous Nudibranch Pinufius (Gastropoda: Nudibranchia) in the South China Sea: A Suspected New Species of Pinufius. Diversity, 15(2), 226. https://doi.org/10.3390/d15020226




