DNA Barcoding of Fish Species Diversity in Guizhou, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Morphological Identification
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Data Analysis
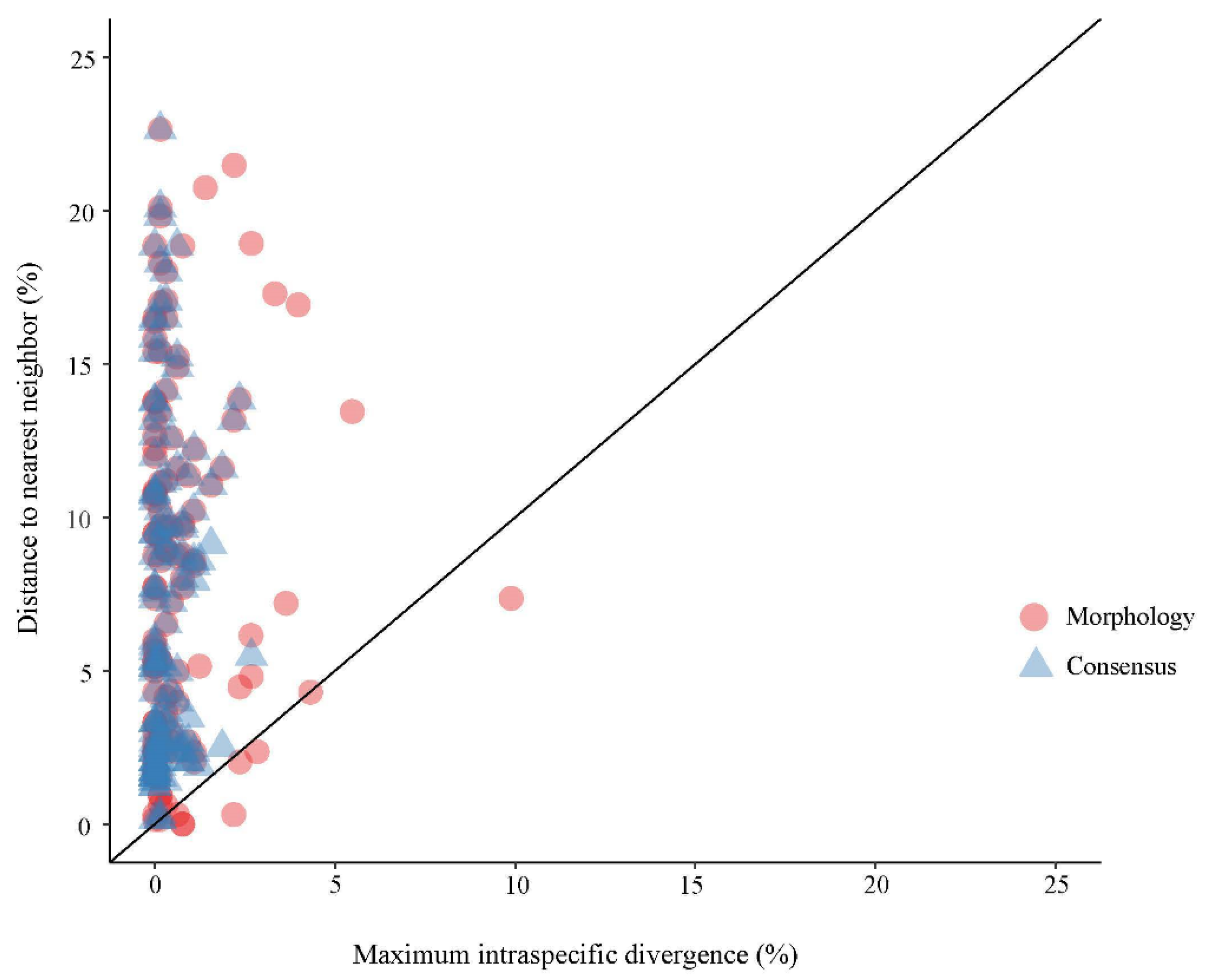
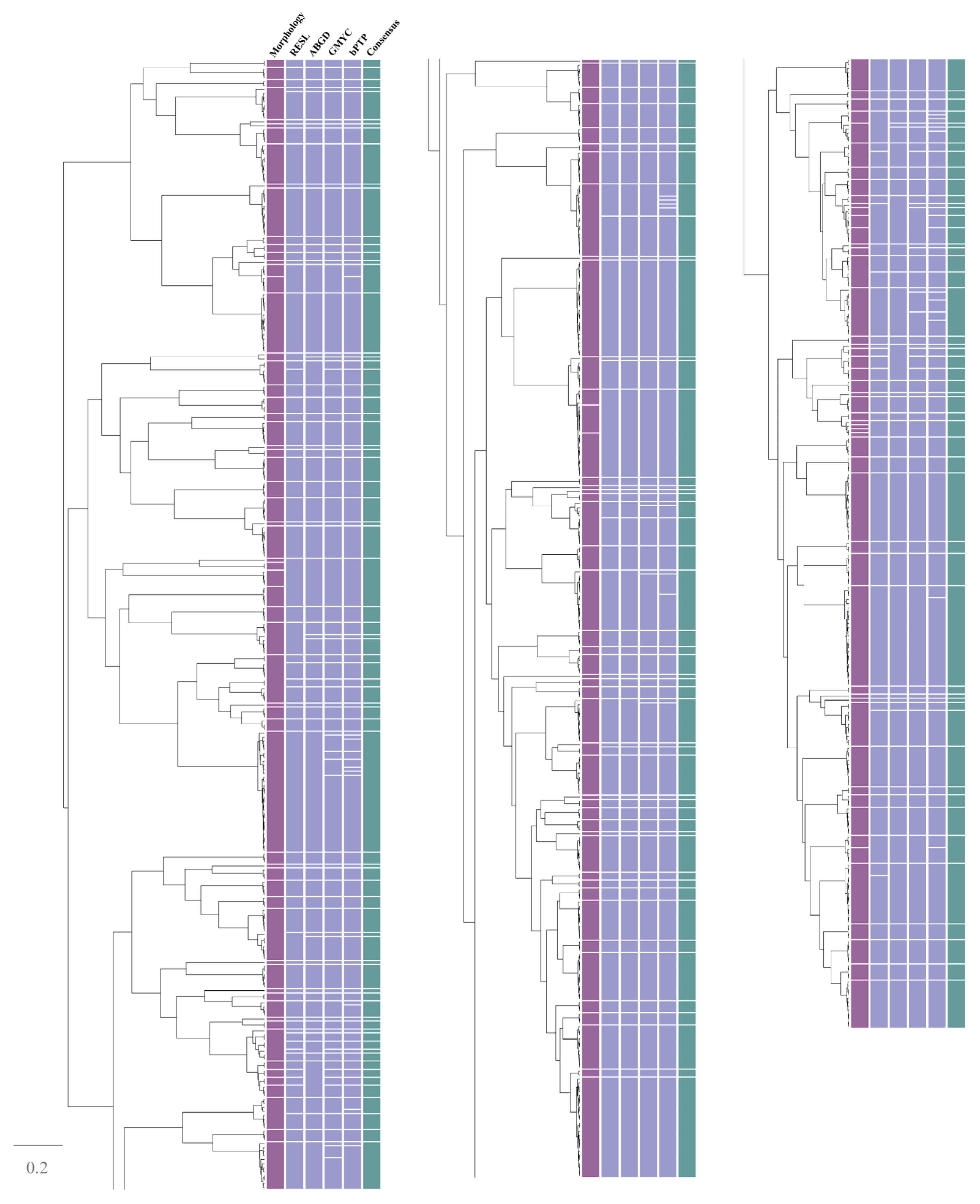
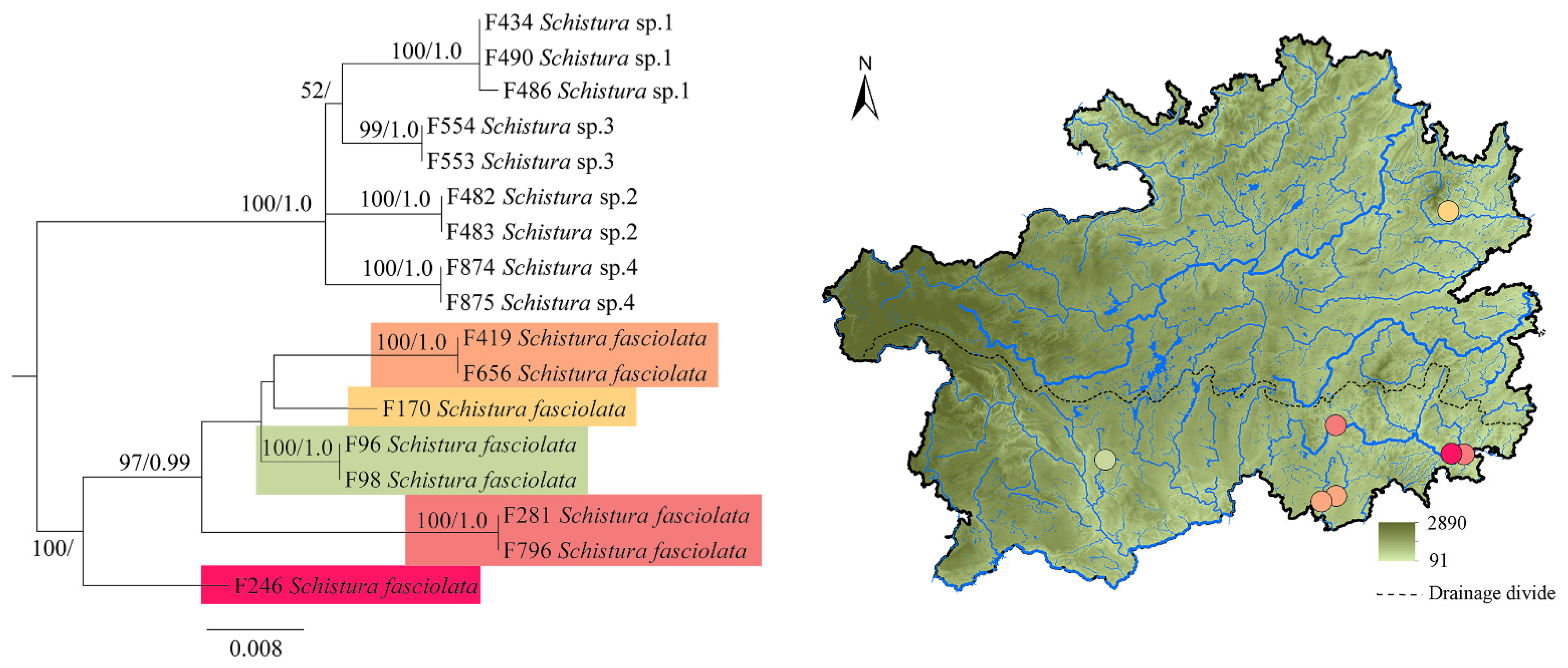
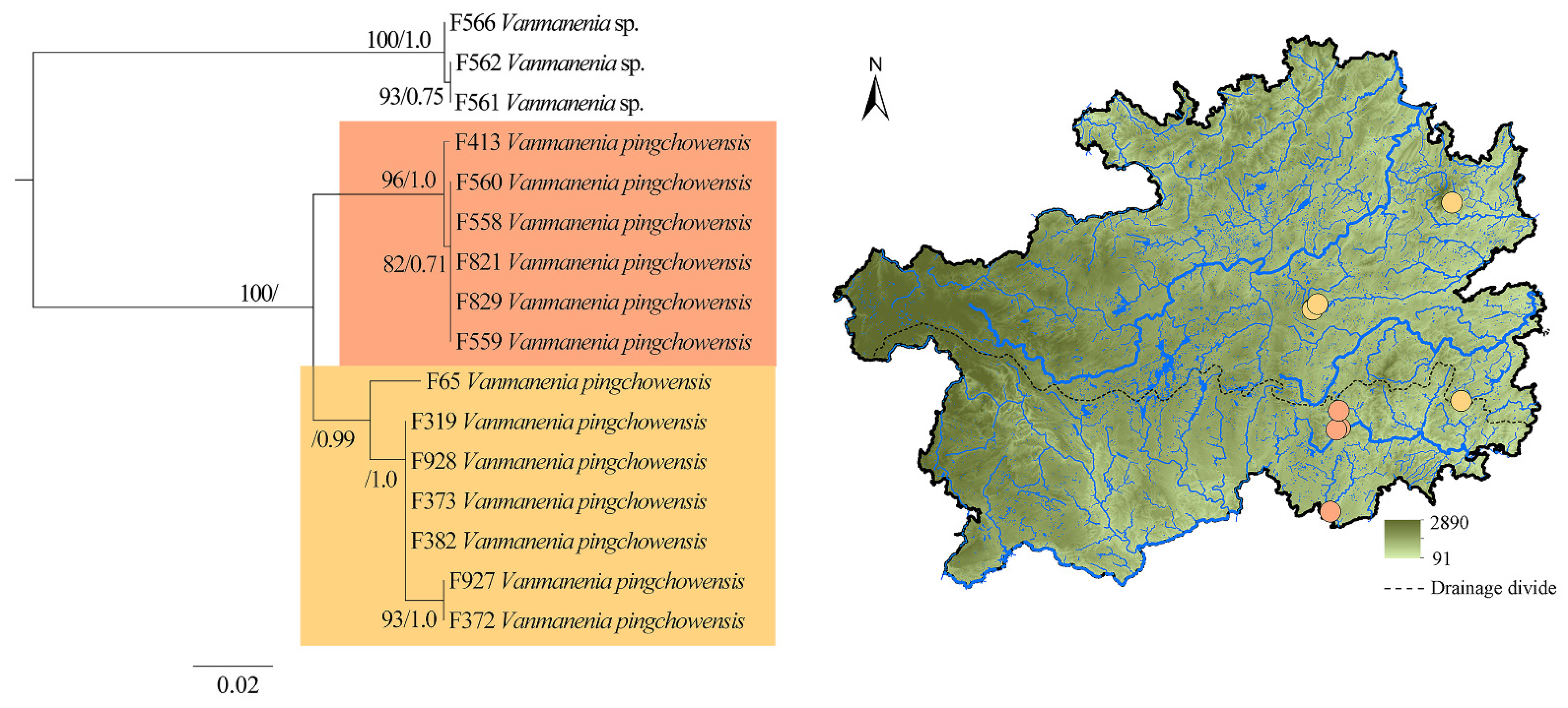
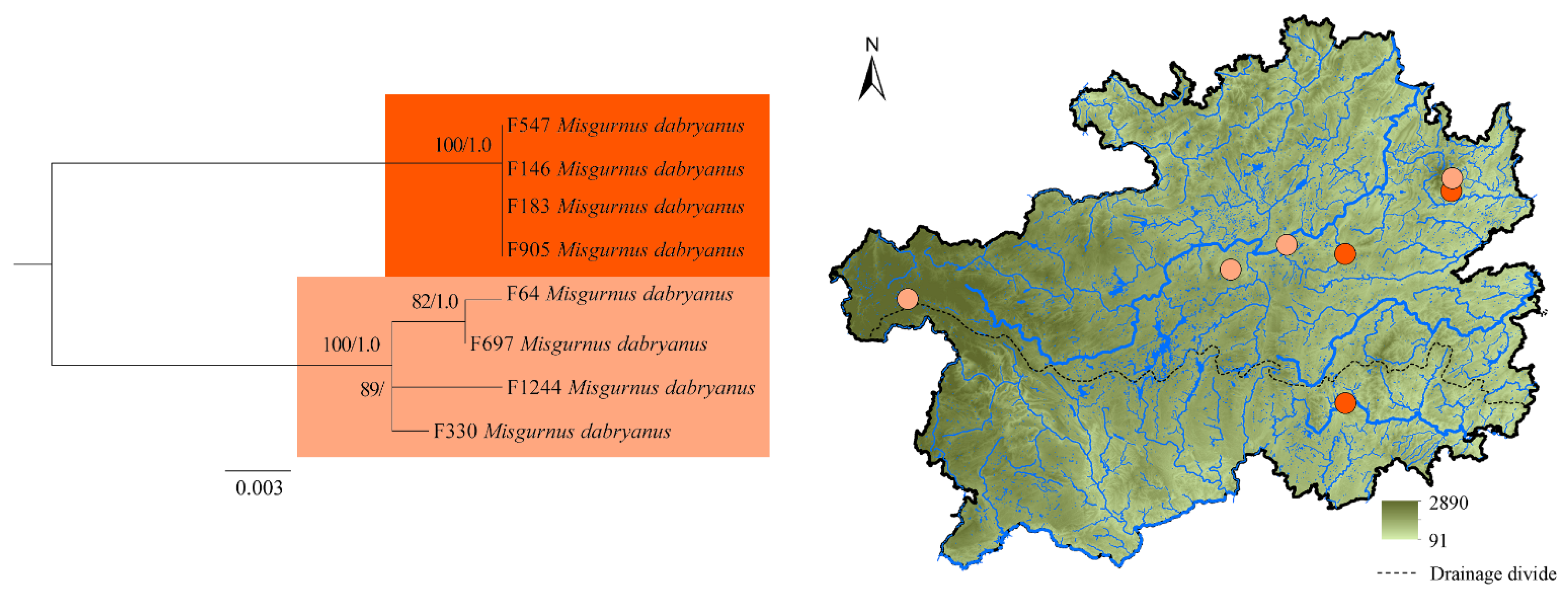
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheraliev, B.; Peng, Z.G. Molecular diversity of Uzbekistan’s fishes assessed with DNA barcoding. Sci. Rep. 2021, 11, 16894. [Google Scholar] [CrossRef]
- Oishi, T. Ethnoecology and ethnomedicinal use of fish among the Bakwele of southeastern Cameroon. Revue d’ethnoécologie 2016, 10, 2267–2419. [Google Scholar] [CrossRef]
- Barman, A.S.; Singh, M.; Singh, S.K.; Saha, H.; Singh, Y.J.; Laishram, M.; Pandey, P.K. DNA barcoding of freshwater fishes of Indo-Myanmar biodiversity hotspot. Sci. Rep. 2018, 8, 8579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hanner, R. Molecular approach to the identification of fish in the South China Sea. PLoS ONE 2012, 7, e30621. [Google Scholar] [CrossRef] [PubMed]
- Keskn, E.; Atar, H.H. DNA barcoding commercially important fish species of Turkey. Mol. Ecol. Resour. 2013, 13, 788–797. [Google Scholar] [CrossRef] [PubMed]
- DeSalle, R.; Goldstein, P. Review and interpretation of trends in DNA barcoding. Front. Ecol. Evol. 2019, 7, 302. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Galal-Khallaf, A.; Ardura, A.; Mohammed-Geba, K.; Borrell, Y.J.; Garcia-Vazquez, E. DNA barcoding reveals a high level of mislabeling in Egyptian fish fillets. Food Control 2014, 46, 441–445. [Google Scholar] [CrossRef]
- Raharinaivo, L.R.; Jaonalison, H.; Mahafina, J.; Ponton, D. How to efficiently determine the size at maturity of small-sized tropical fishes: A case study based on 144 species identified via DNA barcoding from southwestern Madagascar. J. Appl. Ichthyol. 2020, 36, 402–413. [Google Scholar] [CrossRef]
- Wang, Y.H.; Duan, J.N.; Shi, H.L.; Guo, J.X.; Wang, X.Y.; Gao, T.X.; Ping, H.L.; Li, Z.L. Species identification of small fish in Xixuan Island coastal waters of Zhoushan using DNA barcoding. J. Appl. Ichthyol. 2019, 36, 75–84. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Proc. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Zemlak, T.S.; Ward, R.D.; Connell, A.D.; Holmes, B.H.; Hebert, P.D. DNA barcoding reveals overlooked marine fishes. Mol. Ecol. Resour. 2009, 9, 237–242. [Google Scholar] [CrossRef]
- Lara, A.; Ponce de Leon, J.L.; Rodriguez, R.; Casane, D.; Cote, G.; Bernatchez, L.; Garcia-Machado, E. DNA barcoding of Cuban freshwater fishes: Evidence for cryptic species and taxonomic conflicts. Mol. Ecol. Resour. 2010, 10, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Dahruddin, H.; Hutama, A.; Busson, F.; Sauri, S.; Hanner, R.; Keith, P.; Hadiaty, R.; Hubert, N. Revisiting the ichthyodiversity of Java and Bali through DNA barcodes: Taxonomic coverage, identification accuracy, cryptic diversity and identification of exotic species. Mol. Ecol. Resour. 2016, 17, 288–299. [Google Scholar] [CrossRef]
- Ali, F.S.; Ismail, M.; Aly, W. DNA barcoding to characterize biodiversity of freshwater fishes of Egypt. Mol. Biol. Rep. 2020, 47, 5865–5877. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Dhar, B.; Ghosh, S.K. Design of character-based DNA barcode motif for species identification: A computational approach and its validation in fishes. Mol. Ecol. Resour. 2017, 17, 1359–1370. [Google Scholar] [CrossRef]
- Ward, R.D. FISH-BOL, A Case Study for DNA Barcodes. In DNA Barcodes. Methods in Molecular Biology; Kress, W., Erickson, D., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 858, pp. 423–439. [Google Scholar] [CrossRef]
- Eberle, J.; Ahrens, D.; Mayer, C.; Niehuis, O.; Misof, B. A plea for standardized nuclear markers in metazoan DNA taxonomy. Trends Ecol. Evol. 2020, 35, 336–345. [Google Scholar] [CrossRef]
- Galimberti, A.; Assandri, G.; Maggioni, D.; Ramazzotti, F.; Baroni, D.; Bazzi, G.; Chiandetti, I.; Corso, A.; Ferri, V.; Galuppi, M.; et al. Italian odonates in the Pandora’s box: A comprehensive DNA barcoding inventory shows taxonomic warnings at the Holarctic scale. Mol. Ecol. Resour. 2020, 21, 183–200. [Google Scholar] [CrossRef]
- Breman, F.C.; Loix, S.; Jordaens, K.; Snoeks, J.; Van Steenberge, M. Testing the potential of DNA barcoding in vertebrate radiations: The case of the littoral cichlids (Pisces, Perciformes, Cichlidae) from Lake Tanganyika. Mol. Ecol. Resour. 2016, 16, 1455–1464. [Google Scholar] [CrossRef]
- Hou, G.; Chen, W.T.; Lu, H.S.; Cheng, F.; Xie, S.G. Developing a DNA barcode library for perciform fishes in the South China Sea: Species identification, accuracy and cryptic diversity. Mol. Ecol. Resour. 2018, 18, 137–146. [Google Scholar] [CrossRef]
- Nneji, L.M.; Adeola, A.C.; Ayoola, A.O.; Oladipo, S.O.; Wang, Y.Y.; Malann, Y.D.; Anyaele, O.; Nneji, I.C.; Rahman, M.M.; Olory, C.S. DNA barcoding and species delimitation of butterflies (Lepidoptera) from Nigeria. Mol. Biol. Rep. 2020, 47, 9441–9457. [Google Scholar] [CrossRef] [PubMed]
- Papa, Y.; Le Bail, P.Y.; Covain, R. Genetic landscape clustering of a large DNA barcoding data set reveals shared patterns of genetic divergence among freshwater fishes of the Maroni Basin. Mol. Ecol. Resour. 2021, 21, 2109–2124. [Google Scholar] [CrossRef] [PubMed]
- Arida, E.; Ashari, H.; Dahruddin, H.; Fitriana, Y.S.; Hamidy, A.; Irham, M.; Kadarusman; Riyanto, A.; Wiantoro, S.; Zein, M.S.A.; et al. Exploring the vertebrate fauna of the Bird’s Head Peninsula (Indonesia, West Papua) through DNA barcodes. Mol. Ecol. Resour. 2021, 21, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Rabaoui, L.; Yacoubi, L.; Sanna, D.; Casu, M.; Scarpa, F.; Lin, Y.J.; Shen, K.N.; Clardy, T.R.; Arculeo, M.; Qurban, M.A. DNA barcoding of marine fishes from Saudi Arabian waters of the Gulf. J. Fish Biol. 2019, 95, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, C.Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem. Geol. 2004, 204, 1–21. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Wu, M.J.; Yin, Z.L.; Tan, J.B. General situation and basic characteristics of river system in Guizhou Province. Jilin Water Resour. 2017, 12, 29–32. [Google Scholar] [CrossRef]
- Pang, F. Study on Guizhou Electric Power Development. Master’s Thesis, Wuhan University, Wuhan, China, 2004. [Google Scholar]
- Ning, M.Q.; Zhao, J.; Xiong, K.N.; Lan, A.J. Analysis on spatial and temporal pattern of karst rocky desertification of the Yangtze river basin and Pearl river basin in Guizhou. Guizhou Agric. Sci. 2014, 42, 39–42. [Google Scholar] [CrossRef]
- Wu, L. The Fishes of Guizhou; Guizhou People’s Publishing House: Guiyang, China, 1989. [Google Scholar]
- Yao, J.J.; Li, C.; Yang, X.; Li, Z.Y. Present situation and protection countermeasures of fish resources in Guizhou Province. Mod. Fish. Inform. 2009, 24, 12–14. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.G.; Wang, Y.Y. The Fishes of Guizhou; Science Press: Beijing, China, 2022. [Google Scholar]
- Wu, S. Analysis of river pollution characteristics and influencing factors in Guizhou Province. Earth Environ. 2010, 28, 230–234. [Google Scholar] [CrossRef]
- Sambrook, J. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2001. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1981, 16, 111–120. [Google Scholar] [CrossRef]
- Brown, S.D.J.; Collins, R.A.; Boyer, S.; Lefort, M.C.; Malumbres-Olarte, J.; Vink, C.J.; Cruickshank, R.H. Spider: An R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Mol. Ecol. Resour. 2012, 12, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Reid, N.M.; Carstens, B.C. Phylogenetic estimation error can decrease the accuracy of species delimitation: A Bayesian implementation of the general mixed Yule-coalescent model. BMC Evol. Biol. 2012, 12, 196. [Google Scholar] [CrossRef]
- Fujisawa, T.; Barraclough, T.G. Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent (GMYC) approach: A revised method and evaluation on simulated datasets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.J.; Hubert, N.; Huang, Y.; Wang, X.Z.; Gan, X.N.; Peng, Z.G.; He, S.P. DNA barcoding the ichthyofauna of the Yangtze River: Insights from the molecular inventory of a mega-diverse temperate fauna. Mol. Ecol. Resour. 2019, 19, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.C.; Aquino, P.D.P.U.; Breitman, M.F.; Langeani, F.; Colli, G.R. DNA barcode and minibarcode identification of freshwater fishes from Cerrado headwater streams in Central Brazil. J. Fish Biol. 2019, 95, 1046–1060. [Google Scholar] [CrossRef]
- Phillips, J.D.; Gillis, D.J.; Hanner, R.H. Incomplete estimates of genetic diversity within species: Implications for DNA barcoding. Ecol. Evol. 2019, 9, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Ran, K.; Li, Q.; Qi, L.; Li, W.D.; Kong, L.F. DNA barcoding for identification of marine gastropod species from Hainan island, China. Fish. Res. 2020, 225, 105504. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Guan, L.; Du, Y.; Lou, Z.; Jiao, W. Current freshwater fish resources and the application of DNA barcoding in species identification in Gansu Province. Biodivers. Sci. 2015, 23, 306–313. [Google Scholar] [CrossRef]
- Wang, X. Construction of Fish DNA Barcode Database and Excavation of Crypticial Species in Henan Province. Master’s Thesis, Henan Normal University, Xinxiang, China, 2017. [Google Scholar]
- van Velzen, R.; Weitschek, E.; Felici, G.; Bakker, F.T. DNA barcoding of recently diverged species: Relative performance of matching methods. PLoS ONE 2012, 7, e30490. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Mutanen, M.; Seung, J.; Lee, S. High functionality of DNA barcodes and revealed cases of cryptic diversity in Korean curved-horn moths (Lepidoptera: Gelechioidea). Sci. Rep. 2020, 10, 6208. [Google Scholar] [CrossRef]
- Sonet, G.; Snoeks, J.; Nagy, Z.T.; Vreven, E.; Boden, G.; Breman, F.C.; Decru, E.; Hanssens, M.; Ibala Zamba, A.; Jordaens, K.; et al. DNA barcoding fishes from the Congo and the Lower Guinean provinces: Assembling a reference library for poorly inventoried fauna. Mol. Ecol. Resour. 2019, 19, 728–743. [Google Scholar] [CrossRef]
- Wen, H.; Luo, T.; Wang, Y.; Wang, S.; Liu, T.; Xiao, N.; Zhou, J. Molecular phylogeny and historical biogeography of the cave fish genus Sinocyclocheilus (Cypriniformes: Cyprinidae) in southwest China. Integr. Zool. 2022, 17, 311–325. [Google Scholar] [CrossRef]
- Chen, W.T.; Ma, X.H.; Shen, Y.J.; Mao, Y.T.; He, S.P. The fish diversity in the upper reaches of the Salween River, Nujiang River, revealed by DNA barcoding. Sci. Rep. 2017, 5, 17437. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Kumar, V.; Singha, D.; Chandra, K.; Laskar, B.A.; Kundu, S.; Chakraborty, R.; Chatterjee, S. DNA Barcoding studies on Thrips in India: Cryptic species and Species complexes. Sci. Rep. 2017, 7, 4898. [Google Scholar] [CrossRef] [PubMed]
- You, C.H.; Tong, J.G.; Yu, X.M. Microsatellite DNA analysis on genetic diversity of seven populations of Paramisgurnus dabryanus. J. Hydroecol. 2012, 33, 84–91. [Google Scholar] [CrossRef]
- Wang, P.X.; Bai, J.J.; Hu, Y.C.; Mou, X.D.; Wang, X.J.; Li, X.H.; Song, H.M.; Yang, Y.X.; Luo, J.R. Population genetic variations and phylogeography of Macropodus opercularis. Acta Ecol. Sin. 2011, 31, 441–448. [Google Scholar]

| Level | Min Distance (%) | Mean Distance (%) | Max Distance (%) | SE (%) |
|---|---|---|---|---|
| Species | 0 | 0.35 | 3.14 | 0.10 |
| Genus | 0.65 | 5.44 | 11.02 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Deng, L.; Luo, Q.; Duan, Q.; Wang, X.; Zhang, R. DNA Barcoding of Fish Species Diversity in Guizhou, China. Diversity 2023, 15, 203. https://doi.org/10.3390/d15020203
Tang Q, Deng L, Luo Q, Duan Q, Wang X, Zhang R. DNA Barcoding of Fish Species Diversity in Guizhou, China. Diversity. 2023; 15(2):203. https://doi.org/10.3390/d15020203
Chicago/Turabian StyleTang, Qian, Lei Deng, Qi Luo, Qian Duan, Xue Wang, and Renyi Zhang. 2023. "DNA Barcoding of Fish Species Diversity in Guizhou, China" Diversity 15, no. 2: 203. https://doi.org/10.3390/d15020203
APA StyleTang, Q., Deng, L., Luo, Q., Duan, Q., Wang, X., & Zhang, R. (2023). DNA Barcoding of Fish Species Diversity in Guizhou, China. Diversity, 15(2), 203. https://doi.org/10.3390/d15020203







