Day-Time Roost Patterns of New and Previously Translocated North Island Brown Kiwi (Apteryx mantelli)
Abstract
1. Introduction
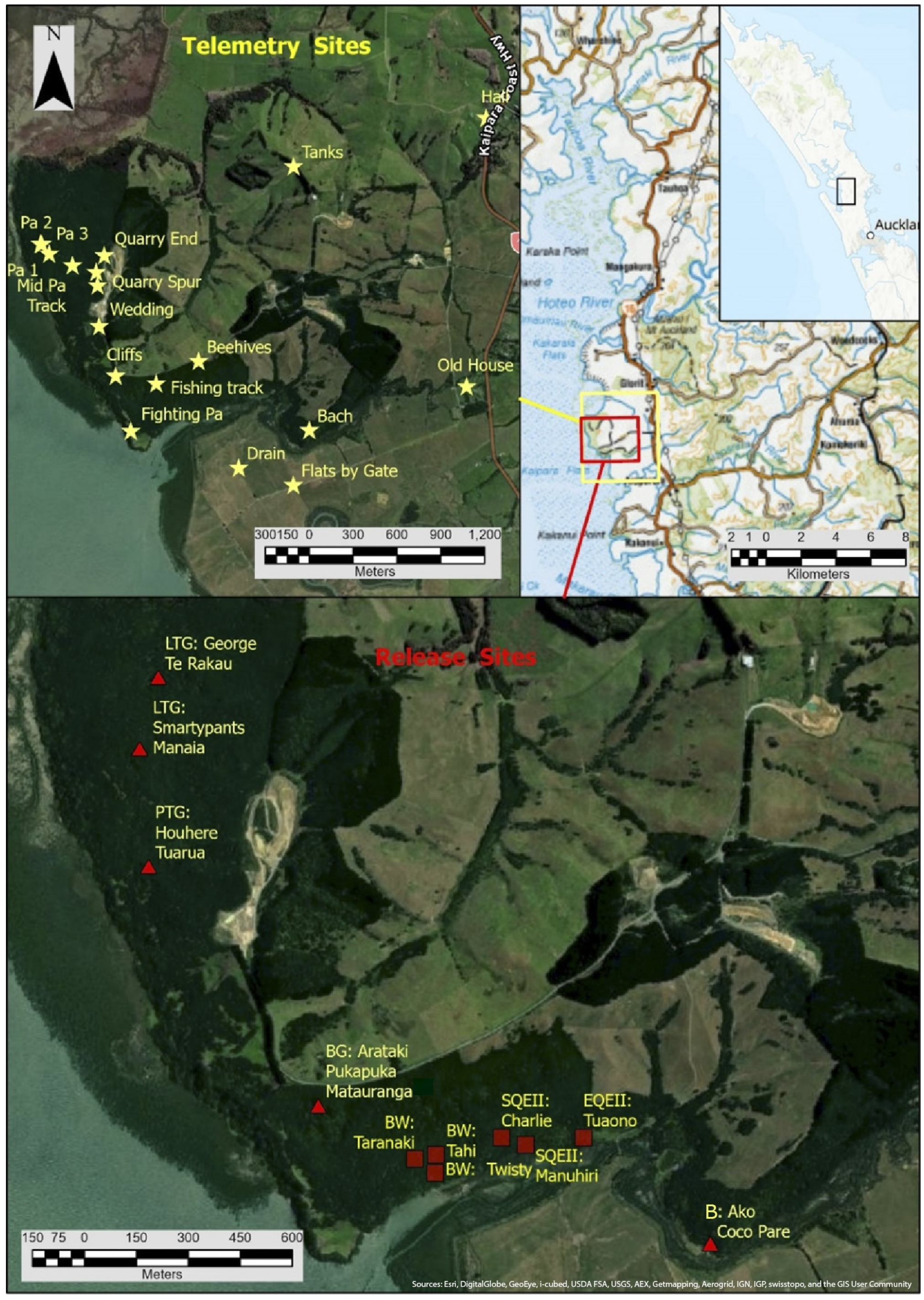
2. Materials and Methods
3. Results
3.1. Bird Data used in the Analysis
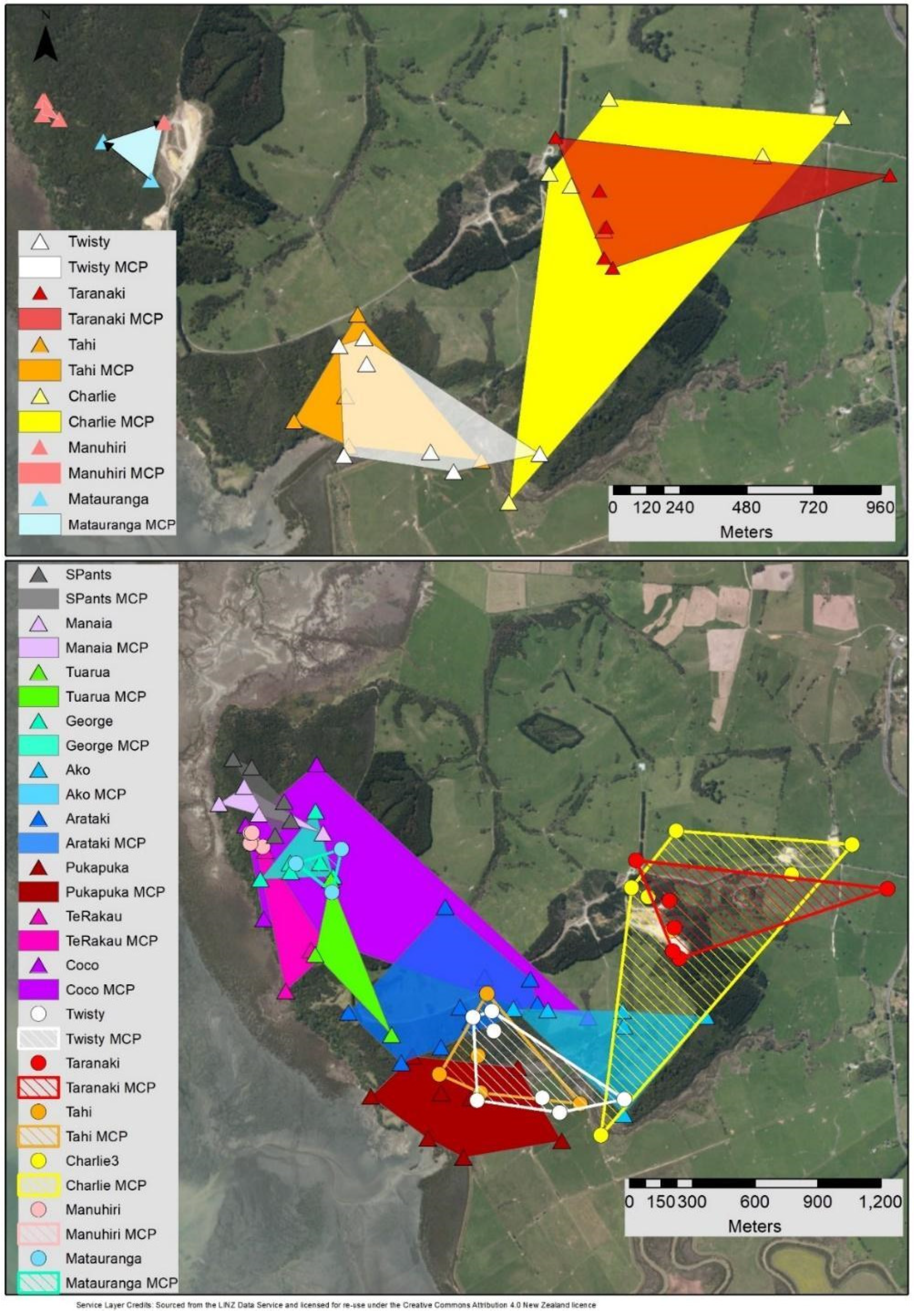
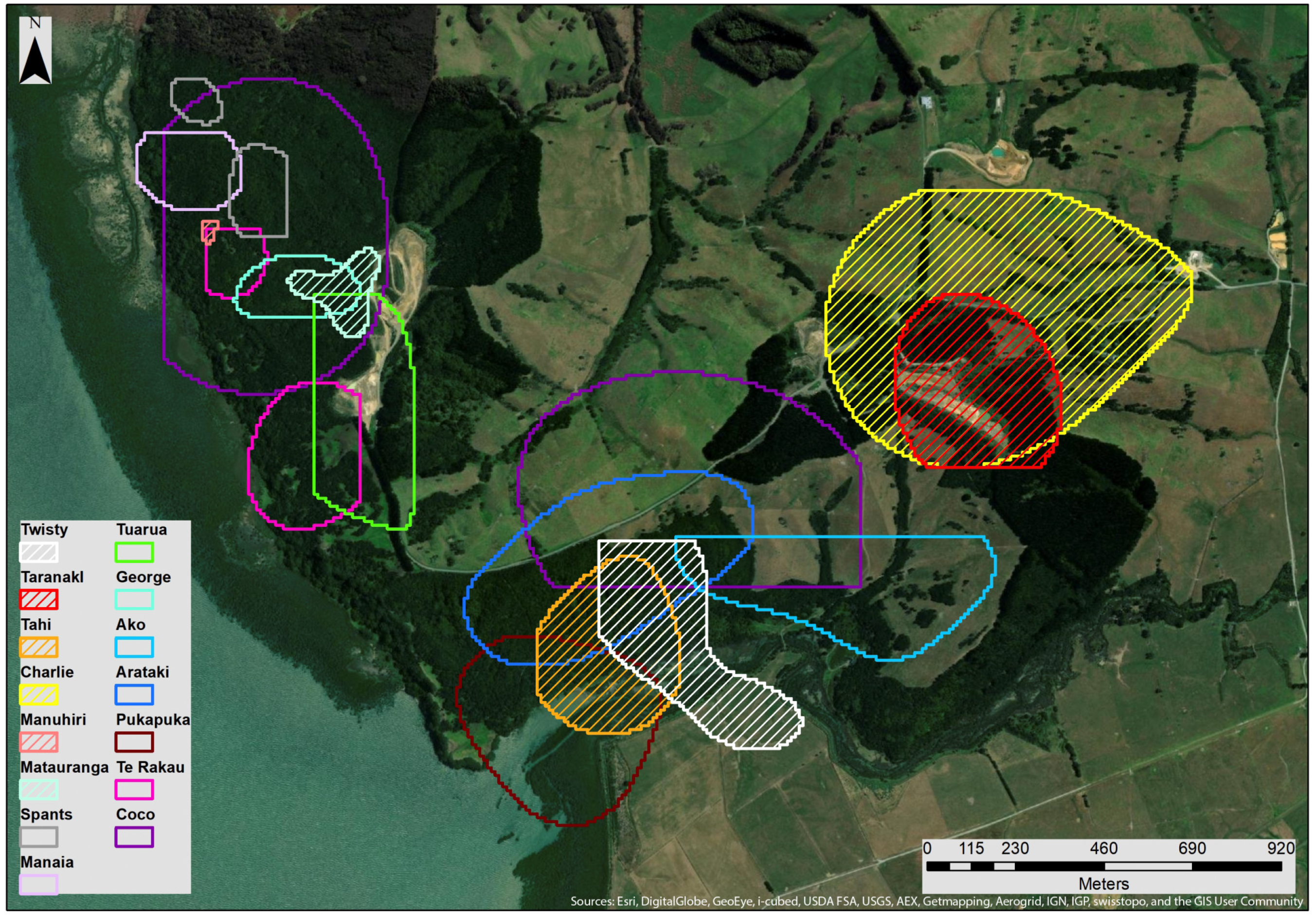
3.2. MCP & Core KDE50 Roost Area
4. Discussion
4.1. Roost Area
4.2. Overlap, Roost Locations and Characteristics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN The IUCN Red List of Threatened Species. Version 2022-2. 2022. Available online: https://www.iucnredlist.org/search?query=north%20island%20brown%20kiwi&searchType=species (accessed on 12 December 2022).
- Miller, P.J.; Pierce, R.J. Distribution and decline of the North Island brown kiwi (Apteryx australis mantelli) in Northland. Notornis 1995, 42, 203–211. [Google Scholar]
- New Zealand Birds Online. North Island Brown Kiwi, Kiwi-Nui. 2013. Available online: https://www.nzbirdsonline.org.nz/species/north-island-brown-kiwi (accessed on 12 December 2022).
- Dixon, T. What They Do in the Shadows: Habitat Utilisation and Diet of Brown Kiwi (Apteryx mantelli) Adults within High-Density Island Populations. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2015. [Google Scholar]
- Jamieson, S.E.; Castro, I.; Jensen, T.; Morrison, K.W.; Durrant, B. Roosting Preferences of North Island Brown Kiwis (Apteryx mantelli). Wilson J. Ornithol. 2016, 128, 857–866. [Google Scholar] [CrossRef]
- Germano, J.; Barlow, S.; Castro, I.; Colbourne, R.; Cox, M.; Gillies, C.; Hackwell, K.; Harawira, J.; Impey, M.; Reuben, A.; et al. Kiwi Recovery Plan 2018–2028; Threatened Species Recovery Plan 64; Terrestrial Ecosystems Unit, Department of Conservation: Wellington, New Zealand, 2018. Available online: https://www.doc.govt.nz/globalassets/documents/science-and-technical/tsrp64entire.pdf (accessed on 24 August 2021).
- Miskelly, C.; Powelesland, R. Conservation translocations of New Zealand birds, 1863–2012. Notornis 2013, 60, 3–28. [Google Scholar]
- Parker, K.A.; Ewen, J.G.; Seddon, P.J.; Armstrong, D.P. Post-release monitoring of bird translocations: Why is it important and how do we do it? Notornis 2013, 60, 85–92. [Google Scholar]
- Innes, J.; Fitzgerald, N.; Binny, R.; Byrom, A.; Pech, R.; Watts, C.; Gillies, C.; Maitland, M.; Campbell-Hunt, C.; Burns, B. New Zealand ecosanctuaries: Types, attributes and outcomes. J. R. Soc. N. Z. 2019, 49, 370–393. [Google Scholar] [CrossRef]
- Toy, R.; Toy, S. Post-translocation dispersal and home range establishment of roroa (great spotted kiwi, Apteryx haastii): Need for long-term monitoring and a flexible management strategy. Notornis 2020, 67, 511–525. [Google Scholar]
- Richardson, K.M.; Doerr, V.; Ebrahimi, M.; Lovegrove, T.G.; Parker, K.A. Considering dispersal in reintroduction and restoration planning. In Advances in Reintroduction Biology of Australian and New Zealand Fauna; Armstrong, D.P., Hayward, M.W., Moro, D., Seddon, P.J., Eds.; CSIRO Publications Clayton South: Melbourne, Australia, 2015; pp. 59–72. [Google Scholar]
- Zang, J.; Pannell, J.L.; Case, B.S.; Hinchliffe, G.; Stanley, M.C.; Buckley, H.L. Interactions between landscape structure and bird morbidity traits affect the connectivity of agroecosystems networks. Ecol. Indic. 2021, 129, 107962. [Google Scholar] [CrossRef]
- Ziesemann, B. The Social Organisation and Mating System of Brown Kiwi (Aptyrex mantelli). Ph.D. Thesis, Massey University, Auckland, New Zealand, 2011. [Google Scholar]
- McLennan, J.A.; Rudge, M.R.; Potter, M.A. Range size and denning behaviour of brown kiwi, Apteryx australis mantelli, in Hawke’s Bay, New Zealand. N. Z. J. Ecol. 1987, 10, 97–107. [Google Scholar]
- Jahn, P.; Cagua, E.F.; Molles, L.E.; Ross, J.G.; German, J.M. Kiwi translocation review: Are we releasing enough birds and to the right places? N. Z. J. Ecol. 2022, 46, 3454. [Google Scholar] [CrossRef]
- Bradley, D.W.; Ninnes, C.E.; Valderrama, S.V.; Waas, J.R. And Does ‘acoustic anchoring’ reduce post-translocation dispersal of North Island robins? Wildl. Res. 2011, 38, 69–76. [Google Scholar] [CrossRef]
- Clarke, M.F.; Schedvin, N. An experimental study of the translocation of noisy miners Manorina melanocephala and difficulties associated with dispersal. Biol. Conserv. 1997, 80, 161–167. [Google Scholar] [CrossRef]
- Kemink, K.M.; Kesler, D.C. Using movement ecology to inform translocation efforts: A case study with an endangered lekking bird species. Anim. Conserv. 2013, 16, 449–457. [Google Scholar] [CrossRef]
- Taborsky, B.; Taborsky, M. Habitat use and selectivity by the brown kiwi (Apteryx australis mantelli) in a patchy environment. The Auk 1995, 112, 680–689. [Google Scholar]
- Molles, L.E.; Calcott, A.; Peters, D.; Delmamare, G.; Hudson, J.; Innes, J.; Flux, I.; Waas, J. “Acoustic anchoring” and the successful translocation of North Island kokako (Callaeas cinerea wilsoni) to a New Zealand mainland management site within continuous forest. Notornis 2008, 55, 57–68. [Google Scholar]
- ESRI. Distance and Direction | ArcGIS Solutions. 2020. Available online: https://solutions.arcgis.com/defense/help/distance-direction/ (accessed on 29 May 2020).
- Hayne, D.W. Calculation of Size of Home Range. J. Mammal. 1949, 30, 1–18. [Google Scholar] [CrossRef]
- Adam, A.M.A.; Kropil, R.; Pichler, V. Differences in Evaluation of Three Different Approaches in Home Range Sizes of Red Deer Cervus Elaphus in Western Carpathians / Rozdiely vo Vyhodnotení Veľkosti Domovských Okrskov Jeleňa Lesného Cervus Elaphus v Západných Karpatoch Tromi Rôznymi Prístupmi. For. J. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Worton, B.J. Kernel methods for estimating the utilisation distribution in home-range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Fleming, C.H.; Calabrese, J.M. A new kernel density estimator for accurate home-range and species-range area estimation. Methods Ecol. Evol. 2016, 8, 571–579. [Google Scholar] [CrossRef]
- Samuel, M.D.; Pierce, D.J.; Garton, E.O. Identifying Areas of Concentrated Use within the Home Range. J. Anim. Ecol. 1985, 54, 711. [Google Scholar] [CrossRef]
- Horikoshi, C.; Battley, P.F.; Seaton, R.; Minot, E.O. Winter Habitat Use of New Zealand Falcons (Falco Novaeseelandiae Ferox) in an Intensively Managed Pine Plantation, Central North Island, New Zealand. N. Z. J. Ecol. 2017, 41, 193–206. [Google Scholar]
- Kitts-Morgan, S.E.; Caires, K.C.; Bohannon, L.A.; Parsons, E.I.; Hilburn, K.A. Free-ranging farm cats: Home range size and predation on a livestock unit in Northwest Georgia. PLoS ONE 2015, 10, e0120513. [Google Scholar] [CrossRef] [PubMed]
- Knüsel, M.A.; Lee, D.; König, B.; Bond, M.L. Correlates of home range sizes of giraffes, Giraffa camelopardalis. Anim. Behav. 2019, 149, 143–151. [Google Scholar] [CrossRef]
- Reading, R.P.; Bradley, J.; Hancock, P.; Garbett, R.; Selebatso, M.; Maude, G. Home-range size and movement patterns of Hooded Vultures Necrosyrtes monachus in southern Africa. Ostrich 2018, 90, 73–77. [Google Scholar] [CrossRef]
- Jahn, P.; Ross, J.G.; Mander, V.; Molles, L.E. Post-translocation movements and ranging behaviour of roroa (great spotted kiwi, Apteryx maxima). Notornis 2022, 69, 135–146. [Google Scholar]
- Mander, V. How translocation affects the activity, home range and pair-bonds of great spotted kiwi (Apteryx haastii). MSc Thesis, Lincoln University, Lincoln, New Zealand, 2016. [Google Scholar]
- Scrimgeour, J. Establishing a Kiwi Population at Otanewainuku. Available online: https://kiwitrust.org/wp-content/uploads/2019/03/OKT_DOC-Report.pdf (accessed on 20 July 2021).
- Adshead, G.; (Mataia Restoration Project, Kaipara, New Zealand); Adshead, K.; (Mataia Restoration Project, Kaipara, New Zealand). Personal communication, 2020.
- Gibbs, S.J.; Clout, M.N. Behavioural Vulnerability of Juvenile Brown Kiwi: Habitat Use with Predators; Department of Conservation Science Internal Series 102; Department of Conservation: Wellington, New Zealand, 2003. Available online: https://www.doc.govt.nz/globalassets/documents/science-and-technical/dsis102.pdf (accessed on 20 July 2021).
- Cunningham, S.J.; Castro, I. The secret life of wild brown kiwi: Studying behaviour of a cryptic species by direct observation. N. Z. J. Ecol. 2011, 35, 209–219. [Google Scholar]
- Powell, R.A.; Mitchell, M.S. What is a home range? J. Mammal. 2012, 93, 948–958. [Google Scholar] [CrossRef]
| Release Date | Bird No | Name | Sex | Release Site 1 | No of Locations | MCP Area (Ha) | KDE50 Percentile Area (Ha) |
|---|---|---|---|---|---|---|---|
| 2013 | 1 | Twisty | Male | BW | 7 | 18.8 | 14.3 |
| 2 | Taranaki | Female | BW | 6 | 27.1 | 16.3 | |
| 4 | Tahi | Male | BW | 5 | 16.1 | 13.3 | |
| 9 | Manuhiri | Male | SQEII | 4 | 0.2 | 0.2 | |
| 10 | Charlie | Male | SQEII | 7 | 72.8 | 51.0 | |
| 12 | Mātauranga | Male | BG | 3 | 2.1 | 2.7 | |
| 2014 | 14 | Smartypants | Female | LTG | 5 | 3.3 | 4.5 |
| 15 | Manaia | Male | LTG | 4 | 2.9 | 4.8 | |
| 17 | Tuarua | Male | PTG | 3 | 7.9 | 14.4 | |
| 19 | George | Male | LTG | 6 | 5.5 | 4.3 | |
| 20 | Ako | Male | B | 6 | 22.8 | 18.4 | |
| 21 | Coco | Male | B | 6 | 76.4 | 84.2 | |
| 22 | Arataki | Male | BG | 8 | 35.2 | 24.6 | |
| 23 | Pukapuka | Male | BG | 8 | 28.2 | 19.2 | |
| 25 | Te rākau | Male | LTG | 6 | 9.3 | 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraser, D.L.; Alach, J.M.; Adams, N.J.; Aguilar, G.D. Day-Time Roost Patterns of New and Previously Translocated North Island Brown Kiwi (Apteryx mantelli). Diversity 2023, 15, 190. https://doi.org/10.3390/d15020190
Fraser DL, Alach JM, Adams NJ, Aguilar GD. Day-Time Roost Patterns of New and Previously Translocated North Island Brown Kiwi (Apteryx mantelli). Diversity. 2023; 15(2):190. https://doi.org/10.3390/d15020190
Chicago/Turabian StyleFraser, Diane L., Julie M. Alach, Nigel J. Adams, and Glenn D. Aguilar. 2023. "Day-Time Roost Patterns of New and Previously Translocated North Island Brown Kiwi (Apteryx mantelli)" Diversity 15, no. 2: 190. https://doi.org/10.3390/d15020190
APA StyleFraser, D. L., Alach, J. M., Adams, N. J., & Aguilar, G. D. (2023). Day-Time Roost Patterns of New and Previously Translocated North Island Brown Kiwi (Apteryx mantelli). Diversity, 15(2), 190. https://doi.org/10.3390/d15020190








