Abstract
Animals capable of moving in different environments might face conflicting selection on morphology, thus posing trade-offs on the relationships between morphology and locomotor performance in each of these environments. Moreover, given the distinct ecological roles of the sexes, these relationships can be sexually dimorphic. In this article, I studied the relationships between morphological traits and locomotor performance in male and female semiaquatic Pleurodeles waltl newts in their aquatic and their terrestrial stages. Morphology was sexually dimorphic: males have proportionally longer limbs and tails, as well as a better body condition (only in the aquatic phase), whereas females were larger and had greater body mass in both phases. Nonetheless, these morphological differences did not translate into sexual divergence in locomotor performance in either stage. This finding suggests other functions for the morphological traits measured, among which only SVL showed a positive relationship with locomotor performance in both stages, whereas the effect of SMI was negative only in the terrestrial stage, and that of tail length was positive only in the aquatic stage. In any case, the morphological correlates of terrestrial and aquatic locomotion did not conflict, which suggests no trade-off between both locomotory modes in the newts studied.
Keywords:
amphibian; biphasic animal; Iberian ribbed newt; locomotor performance; trade-off; urodele 1. Introduction
Purposeful locomotion is among the fundamental capabilities of most metazoans [1,2]. For instance, animals frequently rely on their locomotory function to succeed in social interactions [3] and take over larger territories [4]. Likewise, faster males have access to more females, probably because they outcompete other males [5], and have improved reproductive success [6]. Moreover, locomotion is in many cases essential for animals to reach their food [7], or to flee from predators [8]. In fact, the locomotory skills of the contenders play a central role in the outcome of predator–prey interactions [9]. As a consequence, locomotor performance is known to enhance fitness [10,11], and to be subjected to selection [12].
Selection on locomotion is primarily exerted through morphology [13,14]. This is due to the strong link between the different locomotory modes and the anatomy of the body structures responsible for them. For instance, leg length in jumping insects [15], wing size in flying insects [16], fin size in fish [17,18], limb length in terrestrial amphibians [19,20], reptiles [21,22], and mammals [23,24], and wing size in flying birds [25,26] are positively correlated with the locomotor performance of their bearers. Therefore, sexual dimorphism in traits involved in locomotion, related or not to reproduction itself, may drive divergent relationships between morphology and locomotor performance between males and females [27,28].
However, the effects of morphology on locomotion are not necessarily straightforward. Body mass represents an example of such complexity. A larger body size could, on the one hand, involve a burden that hinders locomotion [29]. On the other hand, it could favor locomotion by increasing stability and inertia [30]. How locomotor performance is affected by body mass might depend on the taxon and the locomotory mode. For example, body mass is positively correlated with speed in terrestrial Porcellio laevis isopods [31] and, albeit weakly, in terrestrial mammals [32]. However, in the latter example, when speed is expressed in relation to body size, its relationship with body mass becomes negative, especially in large species [33]. The relationship between terrestrial locomotion and body mass is negative in Psammodromus algirus lizards [21] and Pelophylax perezi frogs [34]. Likewise, body mass impairs flying speed in birds, but this effect can be compensated behaviorally [35]. A larger body size can also hinder agility, as observed in the flight performance of male Phyllium philippinicum leaf insects [36]. In turn, body mass appears positively related with swimming performance in fish [37] and Ambystoma tigrinum salamanders [38]. Swimming speed of Crocodylus porosus juvenile crocodiles also increases with body length, but body length-specific swimming speed decreases with increasing body length [39]. However, swimming speed of marine animals such as seabirds, seals, and whales, appears unrelated to body size [40].
The optimal development (both in evolutionary and ontogenetic terms) of a given character involved in locomotion may be tuned to the characteristics of different habitats and substrates [41]. A particularly interesting scenario is posed by animals capable of displaying various locomotory modes and/or exploiting dissimilar habitats. On the one hand, distinct locomotory modes may involve different body features [42,43]. As a consequence, separate locomotor modules might appear, for instance, for terrestrial and aerial locomotion in birds [44] or for terrestrial and aquatic locomotion in amphibians [45], which could prevent or soften evolutionary conflicts between locomotory modes. However, in other cases, a given state for a character might be beneficial or detrimental in some contexts, but not in others. Accordingly, humerus retractor muscles represent an advantage for burrowing anurans but not for nonburrowing species [46]. Divergent selective pressures on various locomotory modes exerted on diverse substrates may even drive intraspecific differences in performance between habitats according to the proportions of such substrates [47]. Moreover, adaptations of a given body structure to a particular locomotory mode may be detrimental to others. For instance, reduced limb length improves hydrodynamism in river otters (Lontra canadensis), while increasing the energetic costs of terrestrial locomotion [48]. The general rule in mustelids, however, is that locomotor ecology drives covariation between morphological traits, but this does not lead to reduced integration originated by functional partitioning [49]. On a different note, limb diversification is limited in terrestrial urodeles as compared with the fully aquatic, probably because limb morphology is more relevant to cursorial locomotion than it is to swimming performance [50].
In this article, I explore whether aquatic and terrestrial locomotion are associated statistically with morphological features in semiaquatic Pleurodeles waltl newts. In addition, I test whether the potential relationships between morphology and locomotion conflict between the terrestrial and the aquatic stages. For instance, based on the aforementioned rationales, larger tails could improve aquatic locomotion, while being a burden in the terrestrial phase. Conversely, larger limbs could improve terrestrial locomotion, while hindering hydrodynamism. As for body mass, it could reduce locomotor performance in the terrestrial stage, while improving inertia in the aquatic phase. In order to assess potential sexual differences in morphology and locomotor performance, these and the relationships among them were also compared between females and males.
2. Materials and Methods
2.1. Study Species
The species studied was the Iberian ribbed newt (Pleurodeles waltl), a large salamandrid (252 mm of maximum total length in this sample; n = 113) distributed throughout northwestern Africa and most of the Iberian Peninsula [51]. It can be found in a variety of habitats, usually linked to waterbodies such as ponds, pools, dams, or creeks, which can be permanent or, most frequently, temporary [51]. Although some individuals may remain aquatic year-round, these newts typically display a biphasic circa-annual cycle, with an aquatic phase that encompasses the reproductive period from autumn to spring, and a terrestrial stage largely coincident with the summer drought and the concomitant aestivation [51]. Hibernation only happens in the coldest areas of its range [51]. While they can be active day and night in the aquatic stage, their terrestrial activity is typically restricted to rainy or very humid nights [51]. In both stages, these newts are subjected to a strong pressure by a wide array of predators (birds such as Ardea purpurea, Athene noctua, Ciconia ciconia, Milvus migrans, Neophron percnopterus, or Nycticorax nycticorax, mammals such as Lutra lutra, Rattus norvegicus, or Sus scrofa; or snakes such as Natrix maura; [51]). Despite being equipped with diverse antipredator strategies [52], its primary defense in both stages is an attempt to escape the threat by means of a quick flight, either by crawling or swimming [51].
2.2. Study Area
This work was performed in Pinares de Cartaya (Huelva; SW Spain: 37°20′ N, 7°09′ W), a Pinus pinea grove of 11,000 hectares whose understory is dominated by Cistus ladanifer, Pistacia lentiscus and Rosmarinus officinalis. In the rainy season, from October to May approximately, this forest abounds in waterbodies inhabitable by this species. Areas of agricultural land divide this forest into three separate patches. Fieldwork was conducted in two of these patches.
2.3. Animal Capture and Management
In October-November 2020, I captured a subsample of newts (32 females and 31 males) in their terrestrial stage in one of these forest patches, of approximately 5000 hectares. These newts were caught while active along random transects in rainy nights. In February 2021, I captured a different subsample of newts (27 females and 23 males) in their aquatic stage in the other forest patch. These newts were caught with a dip net in six different ponds (hereafter nominated as “location”). In all cases, newts were released at their capture sites a few days after capture. Thus, this design prevented long-term captivity, which would have been needed so as to test the same individuals at both stages. Long-term captivity has shown negative effects on this species’ locomotion [53]. The two forest patches sampled were roughly 2 km away from each other at its narrowest stretch. Such distance surpasses the dispersal rate of this species: according to a study in the central Iberian Peninsula, 99.49% of the individuals studied dispersed less than 250 m over 8 years [54]. Therefore, newts released at the first patch (see below) were unlikely to reach the other patch during this study, particularly because croplands, such as those that separate both forest patches, are negatively selected by this species [55]. Nonetheless, the short distance between both forest patches makes it unlikely for these patches to harbor genetically distinct populations.
I housed the newts in the terrestrial phase individually in plastic terraria (19 × 38 × 27 cm) with a substrate of wet peat and a shelter in the form of a concave piece of opaque plastic. In turn, newts in the aquatic stage were also set individually, in aquaria of the same size, with 6 L of untreated stream water, plus a shelter in the form of a concave piece of opaque plastic. In all cases, circadian rhythms could be adjusted thanks to the natural daylight that a window let in. Room temperature was in the range of 16–18 °C.
2.4. Locomotor Performance Measures
Locomotor performance trials were performed by each individual 48 h after capture. In all cases, a plastic linear runway (100 × 20 × 20 cm) was utilized. Each newt was individually released at one end of the runway, and gently but constantly chased by a simulated predator (my finger; humans are perceived as predators by numerous animals [56]), which would prod the individual’s tail every time it stopped moving, until the length of the runway was covered three times (forth, back, and forth, to avoid manipulating the newt during the trial other than for the stimulating prodding). A white-light bulb of 80 W pended 2 m above the center of the runway, thus homogeneously illuminating the area. Room temperature was 16–18 °C in all cases, thus avoiding any potential effect of temperature on locomotion [57]. All footages included a ruler. In the case of newts in the terrestrial stage, the runway was sprayed with water, so as to imitate the conditions that favor newt activity, but this slight humidity never involved an actual accumulation of water on the bottom of the runway. In the case of newts in the aquatic stage, the runway was filled with 15 L of untreated stream water, so the depth of the water column was about 7.5 cm. In all cases, I thoroughly rinsed the runway with untreated stream water between trials.
Afterwards, I analyzed these videos with the software Tracker v.4.92, which pinpoints the position of the newt in each frame of the footage, thus calculating the shift in position between consecutive frames. As the ruler included allows distance calibration, and the time between frames is known, the speed of the newt between each pair of frames was calculated. I made use of the fastest speed of each newt in each footage. Then, I obtained relative speed as the residuals of regression of maximum speed against SVL. In this way, the potential effects of SVL on maximum speed were controlled for.
2.5. Morphological Measures
Twenty-four hours after these measures, newts were weighed in a digital scale (model CDS-100; accuracy 0.01 g), and I measured their snout–vent length (hereafter, SVL), forelimb length, hindlimb length (always the right limb in both cases), and tail length with a ruler, to the nearest mm. Then, I obtained relative forelimb length, relative hindlimb length, and relative tail length as the residuals of regression of forelimb length, hindlimb length, and tail length, respectively, on SVL. Moreover, for each individual, I calculated body condition of each individual as the scaled mass index (hereafter, SMI) pursuant to the formula by Peig and Green [58]:
where Mi represents the body mass of a given individual, L0 represents the average SVL of the sample, Li represents the SVL of a given individual, and bSMA is the quotient between the slope resulting from the ordinary least squares regression of body mass on SVL (both ln-transformed) and the Pearson’s correlation coefficient, r [58].
2.6. Statistics
The data met the criteria of homoscedasticity and residual normality (Table S1 in Supplementary Material), as required for parametric statistics [59]. Firstly, I conducted a series of mixed models in which location was included as a random factor, phase, sex, and their interaction were included as factors, and either relative speed, relative forelimb length, relative hindlimb length, relative tail length, SVL, body mass, or SMI were included as response variables. Then, I conducted a mixed model analysis where location was a random factor, relative speed was the response variable, sex and phase were included as factors, relative forelimb length, relative hindlimb length, relative tail length, SVL, body mass, and SMI were included as covariates, and all twofold interactions including at least one of the factors were also implemented. For simplicity’s sake, all other interactions were avoided, to prevent the model from excessive complexity. In all cases, backward stepwise selection was applied to all full models, by which method non-significant interactions first, and factors then, were removed one-by-one, starting with those with the greater p-value [59]. The final models after backward stepwise selection are below, but I presented the full model as Supplementary Material. I used the package nlme [60] in the R environment to conduct these analyses.
3. Results
3.1. Effects of Sex and Phase on Morphology and Locomotor Performance
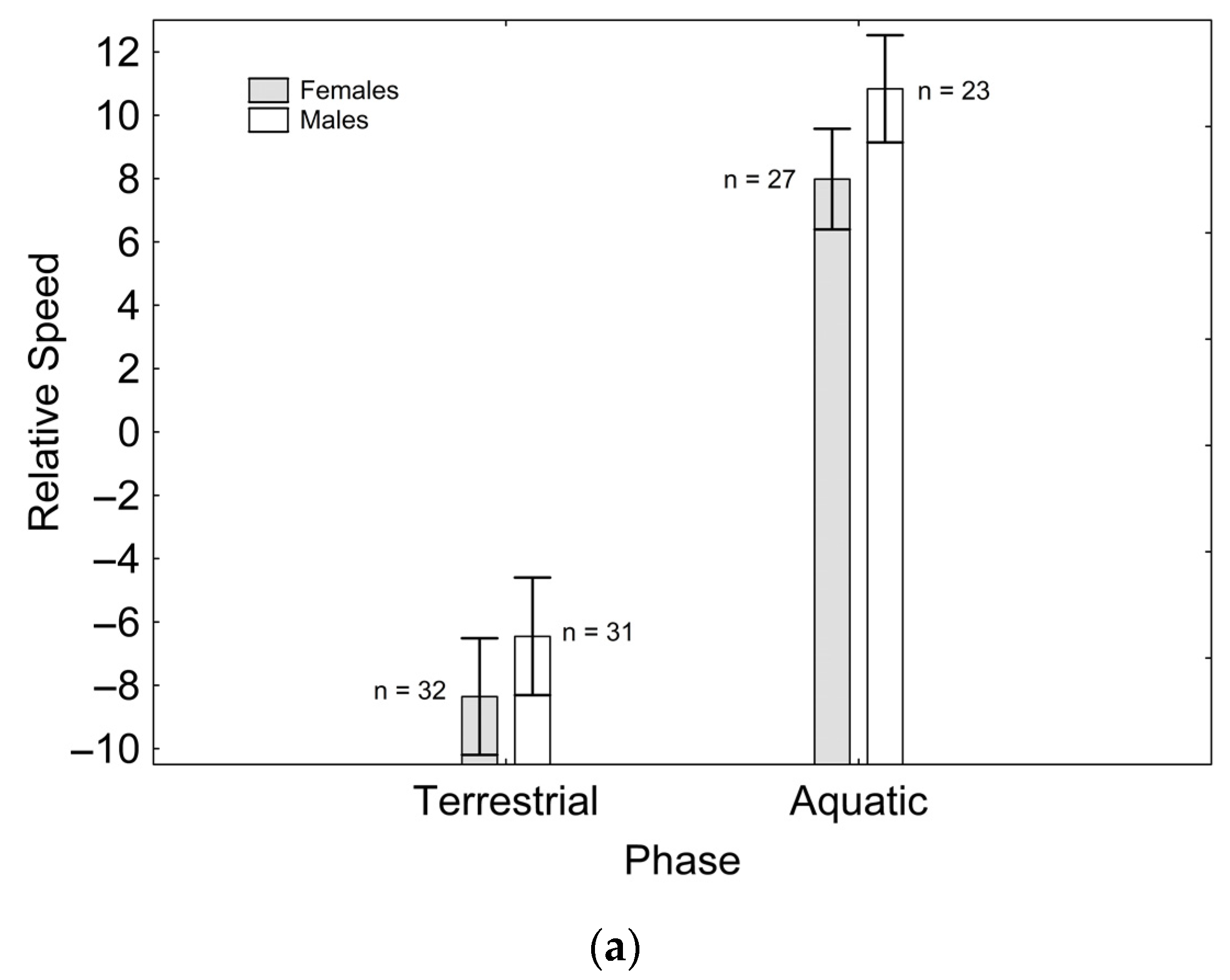
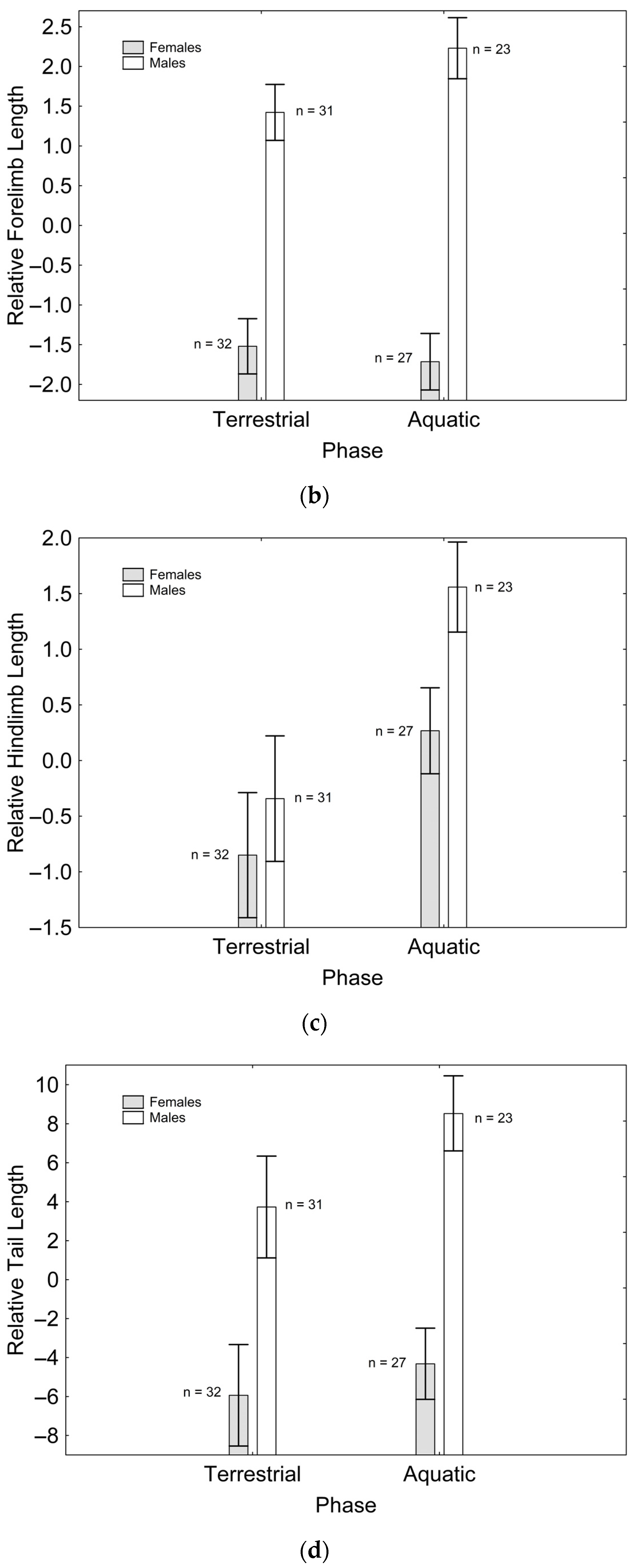
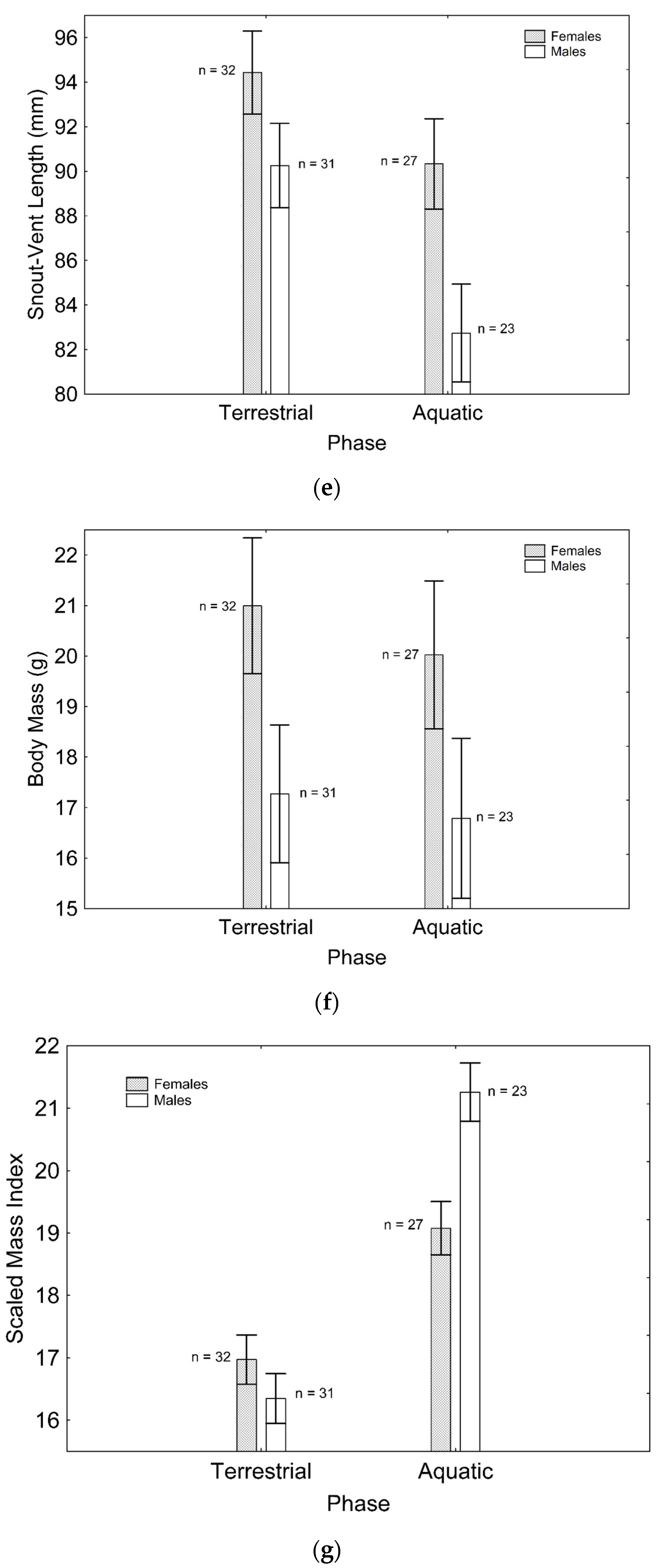
After backward stepwise selection was applied to the full model testing the effects of the factors sex, phase, and their interaction on relative speed and the morphological features measured, relative speed was greater in the aquatic than in the terrestrial phase (Χ21, 111 = 59.260; p < 0.001; Figure 1a). Relative fore (Χ21, 111 = 97.163; p < 0.001; Figure 1b) and hindlimb length (Χ21. 110 = 7.906; p = 0.005; Figure 1c) were greater in males than in females, while only the latter was greater in the aquatic than in the terrestrial phase (Χ21, 110 = 5.953; p = 0.015; Figure 1c). Relative tail length was greater in males than in females (Χ21, 111 = 57.848; p < 0.001; Figure 1d), whereas SVL (Χ21, 110 = 8.230; p = 0.004; Figure 1e) and body mass (Χ21, 111 = 6.012; p = 0.014; Figure 1f) were greater in females than in males, but only SVL was greater in the terrestrial than in the aquatic phase (Χ21, 111 = 8.247; p = 0.004; Figure 1e). SMI was greater in males than in females (Χ21, 109 = 11.938; p = 0.001; Figure 1g), and in the aquatic than in the terrestrial phase (Χ21, 109 = 13.145; p < 0.001; Figure 1g), although sexual differences were only present in the aquatic phase, according to the significant phase*sex interaction (Χ21, 109 = 11.059; p = 0.001; Figure 1g). These results correspond to the final models after backward stepwise selection. Full models are presented as Supplementary Material (Table S2).
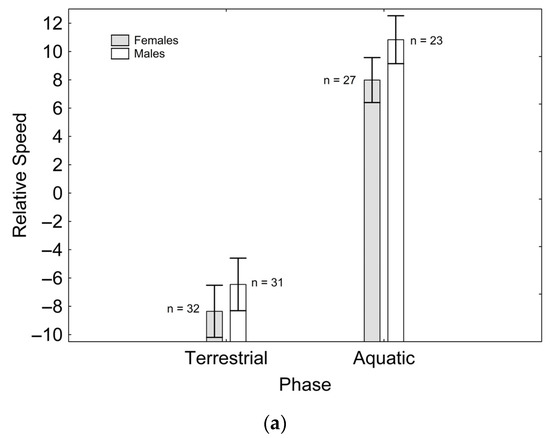
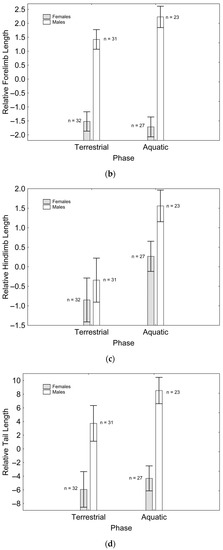
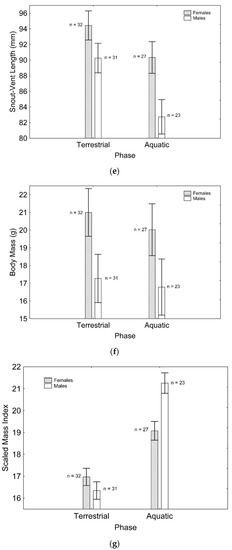
Figure 1.
Effects of phase and sex on relative speed (a), relative forelimb length (b), relative hindlimb length (c), relative tail length (d), SVL (e), body mass (f), and SMI (g). Sample sizes are indicated. Vertical whiskers represent standard errors.
3.2. Effects of Morphology on Locomotor Performance
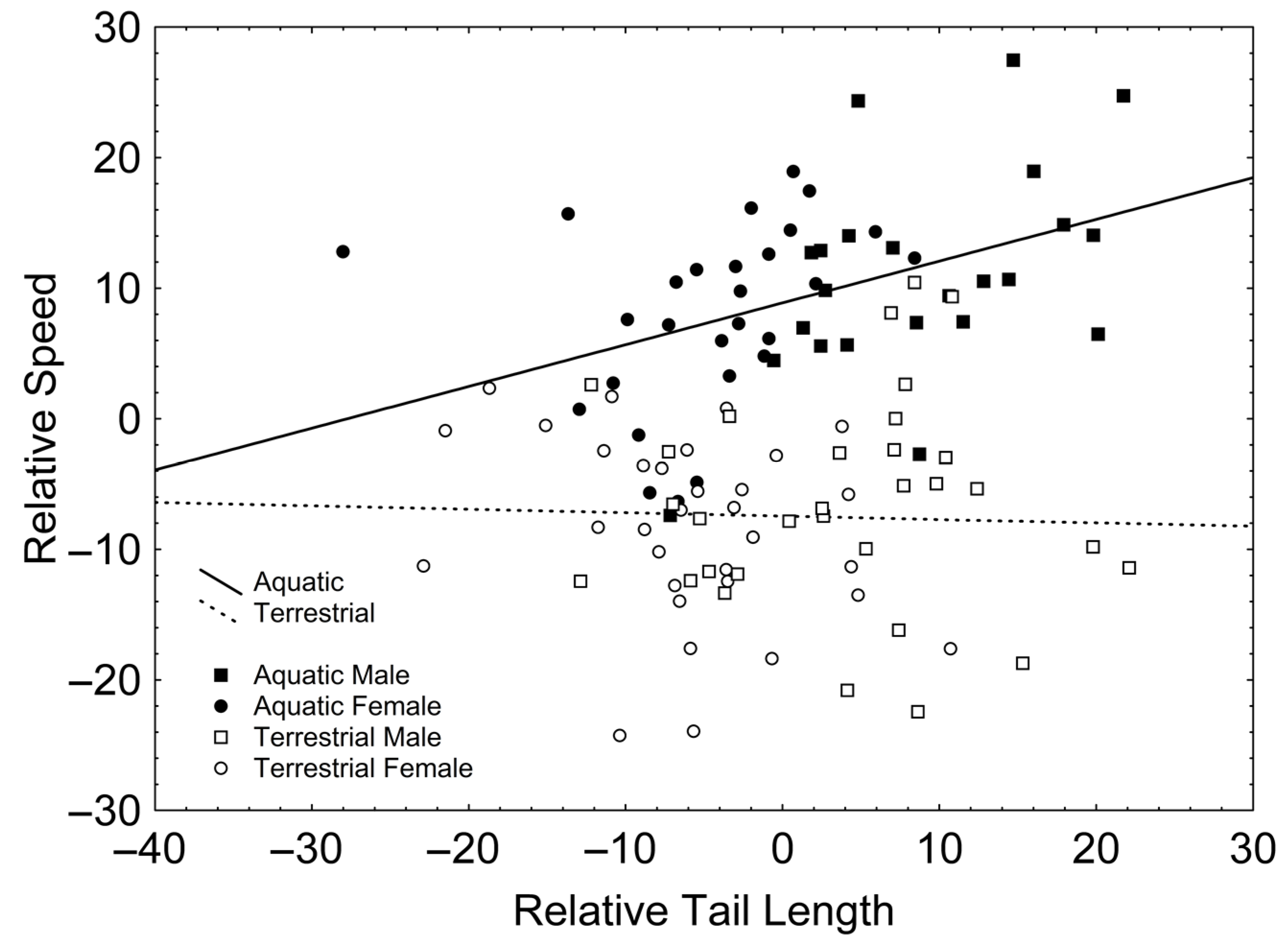
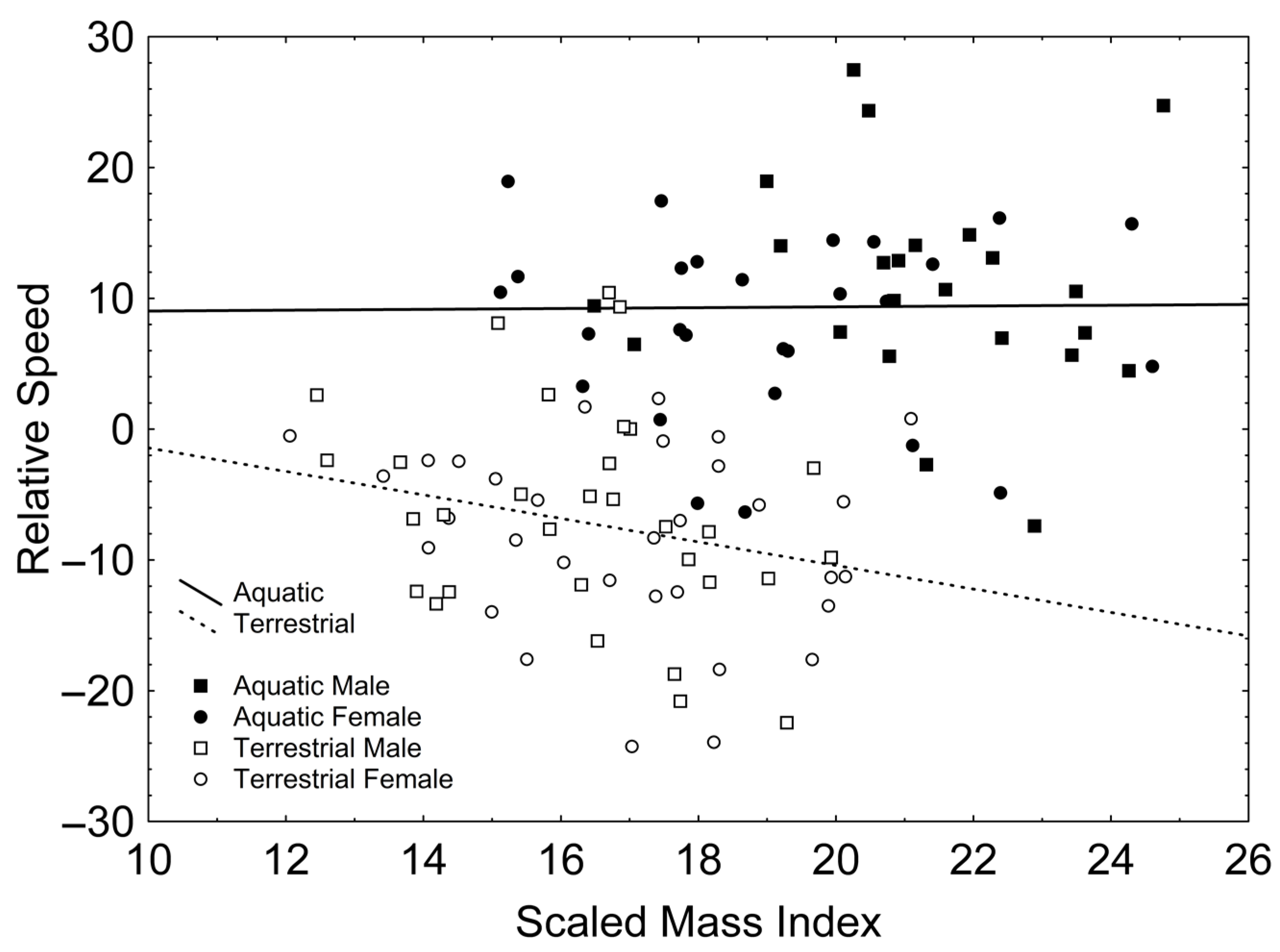
After backward stepwise selection was applied to the full model (available in Table S3, Supplementary Material) testing the effects of the factors sex and phase, the covariates relative forelimb length, relative hindlimb length, relative tail length, SMI, and the interactions involving at least one of the factors, on relative speed, only a positive effect of SVL (Χ21, 106 = 16.954; β = 0.265; p < 0.001) and relative tail length (Χ21, 106 = 11.313; β = 0.340; p = 0.001) appeared. Additionally, there were significant interactions between phase and relative tail length (Χ21, 106 = 5.503; p = 0.019; Figure 2), according to which relative tail length had a positive effect on relative speed in the aquatic stage (r = 0.416; p = 0.003; Figure 2) but not in the terrestrial stage (r = −0.032; p = 0.803; Figure 2); and between phase and SMI (Χ21, 106 = 6.204; p = 0.013; Figure 3), according to which SMI had a negative effect on relative speed only in the terrestrial stage (r = −0.251; p = 0.047; Figure 3) but not in the aquatic stage (r = 0.011; p = 0.941; Figure 3).
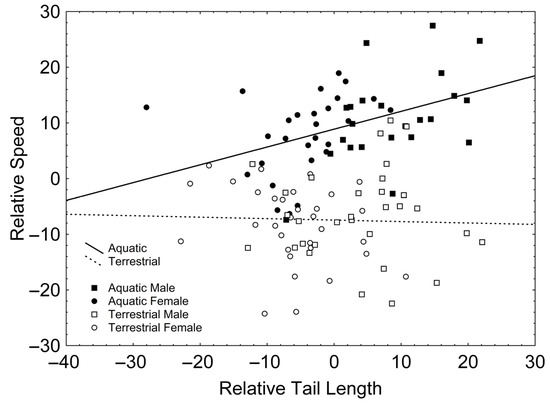
Figure 2.
Correlations between relative tail length and relative speed in the aquatic (solid line) and the terrestrial phases (dashed line). Squares represent males whereas circles represent females. Solid symbols represent newts in the aquatic phase whereas empty symbols represent newts in the terrestrial phase.
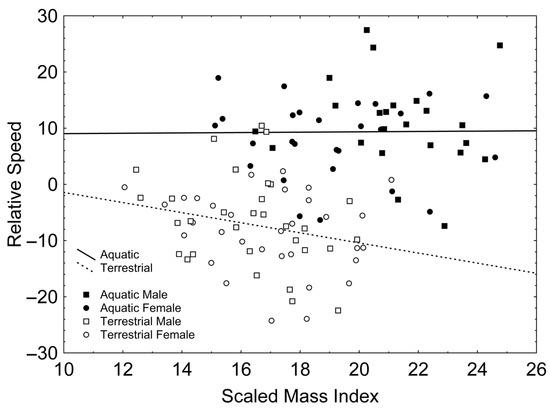
Figure 3.
Correlations between the scaled mass index and relative speed in the aquatic (solid line) and the terrestrial phases (dashed line). Squares represent males whereas circles represent females. Solid symbols represent newts in the aquatic phase whereas empty symbols represent newts in the terrestrial phase.
4. Discussion
Sexual dimorphism was detected in all the morphological variables measured: males had proportionally longer limbs and tails, and higher SMI, whereas females were larger, with greater SVL and body mass. Similar patterns of sexual dimorphism have been reported in other populations of P. waltl [61] and in some salamandridae [62,63,64]. However, these morphological differences between the sexes did not translate into disparate locomotor performance, which could suggest a different role for them. For instance, longer bodies in females, perhaps to the detriment of tail development, could increase their reproductive success by accommodating more eggs, as detected in other female urodeles [65,66]. Similarly, longer bodies and ova could lay behind the greater body mass of females as compared with males. However, SMI was greater in males (albeit only in the aquatic stage), which indicates that males were proportionally more massive per unit of length. This could be a consequence of a potential difference in body composition: males could be more muscular, which is a denser tissue than the fat stored and the ova produced by females in the aquatic phase, when reproduction takes place [67]. In turn, greater forelimb length could aid males in grasping the females during the mating display [68]. However, that does not explain the proportionally larger hindlimbs of males, especially considering that these are used by females to wrap the eggs among submerged plants during oviposition [68], as most aquatic urodeles do [69,70]. The fact that hindlimbs were proportionally longer in males could be a mere byproduct of a correlation among the length of different extremities, as observed in other taxa between fore and hindlimb length [71,72], or even between limbs and other appendages [73,74].
In parallel with the absence of sexual differences in locomotion despite sexual dimorphism in morphology, limb length was unrelated to locomotor performance. That is hardly surprising in the aquatic phase, when the limbs are not involved in locomotion. However, the limbs play a fundamental role in the terrestrial locomotion of urodeles [75,76], including P. waltl [77]. In other legged amphibians (i.e., anurans), a positive relationship between hindlimb length and locomotion has been detected in terrestrial leaping [78,79] and cursorial locomotion [19]. However, in P. waltl, as well as in other urodeles [38], locomotor performance in terrestrial environments appears unrelated to limb length. This mismatch between morphology and performance might reveal the limitations of terrestrial locomotion in these newts. Recent postural analyses have revealed that the medial displacement of ground reaction forces in P. waltl newts as compared with terrestrial salamanders could limit their locomotor performance on land [80]. In fact, locomotor performance was poorer in the terrestrial than in the aquatic stage. This finding could also reflect the little relevance of terrestrial locomotion in these newts. The terrestrial stage of these animals largely consists of an extended period of inactivity (the aestivation that comes with the summer drought) only interrupted in rainy or exceedingly humid nights, oftentimes in the process of relocation towards the water bodies where most of their activity takes place [51]. Therefore, selection on terrestrial locomotion could be mild in these animals.
In both phases, longer individuals were proportionally faster, which supports findings on other urodeles [81]. This fact might be representative of the role of epaxial undulations on both terrestrial and aquatic locomotion in this [82,83] and other urodeles [84]. However, the negative relationship between SMI and locomotion in the terrestrial stage involves that proportionally more massive individuals had hindered terrestrial locomotion, likely due to the burden of extra body mass. This is in contrast with findings that swimming speed of larvae and crawling speed of metamorphs of Ambystoma californiense salamanders are independent of body mass [85], whereas terrestrial and aquatic locomotor performance of Ambystoma tigrinum salamanders are positively related to body mass [38].
As opposed to terrestrial locomotion, however, aquatic locomotion was unrelated to SMI in these newts, probably because the water column alleviates the mass burden. Conversely, locomotor performance in the aquatic stage was positively correlated with tail length. This result is expectable, as the tail is the main responsible for swimming propulsion in newts. Indeed, tails have played a significant role in the evolution of tetrapod locomotion in the water [86]. Similarly, tail size is positively correlated with swimming speed in Lissotriton helveticus newts [87]. Remarkably, I detected no evidence of tail length impairing terrestrial locomotion, which contradicts my predictions. However, a study on Notophthalmus viridescens newts revealed equivalent results concerning the role of tail length in aquatic and terrestrial locomotion [88]. In any case, the morphology of these newts does not seem to be under conflicting selective forces between aquatic and terrestrial locomotion, as no interference between these two locomotory modes and morphology was detected. This finding is supported by a previous study in a group of smaller semiaquatic salamandridae, where no evidence of a trade-off between terrestrial and aquatic locomotion was found [89]. The generic body plan of urodeles, along with their scarcely specialized locomotory abilities in both environments, could lie behind the absence of any potential conflict between locomotion in the aquatic and the terrestrial stages [45,90]. However, the present study was conducted in a limited area and with only one species, so these conclusions must be taken cautiously.
One unexpected result of this study involved the morphological differences between newts in the aquatic and the terrestrial stages. The fact that SMI was greater in the aquatic than in the terrestrial stage could simply mirror a greater degree of hydration in the former, probably facilitated by simple osmosis [91]. However, the reasons why SVL was greater in newts in the terrestrial stage whereas hindlimb length was greater in newts in the aquatic stage remain obscure. Newts in each stage were captured in two different forest patches. Therefore, newts sampled in each stage might represent distinct populations. Nonetheless, given the geographical proximity between those patches (around 2 km away), these newts might not be genetically isolated. However, this possibility was not explored in this work.
5. Conclusions
To conclude, morphology was sexually dimorphic in P. waltl newts, with males having proportionally longer limbs and tails, as well as greater SMI (only in the aquatic phase), whereas females were neatly larger and more massive. Nonetheless, these morphological differences did not translate into sexual divergence in locomotor performance in either the terrestrial or the aquatic stage. This finding suggests other functions for the morphological traits measured, probably related to reproduction. Indeed, among the morphological traits measured, only SVL showed a positive relationship with locomotor performance in both stages, whereas the effect of SMI was negative only in the terrestrial stage, and that of tail length was positive only in the aquatic stage. In any case, the morphological correlates of terrestrial and aquatic locomotion did not conflict, which suggests no trade-off between both locomotory modes in this newt. Locomotor performance was greater in the aquatic stage, which is when activity peaks in this species, whereas the terrestrial phase mostly consists in a period of inactivity coincident with the seasonal desiccation of the water bodies where these animals reproduce. In any case, it should be noted that this study was performed at a local scale, so its conclusions must be taken cautiously.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15020188/s1, Table S1: Levene’s and Shapiro-Wilk’s tests to test respectively homoscedasticity and residual normality of the tests were either relative speed, relative forelimb length, relative hindlimb length, relative tail length, and SMI were the response variables; Table S2: Full models testing the effects of sex, phase and their interaction on either relative speed, relative forelimb length, relative hindlimb length, relative tail length, and SMI, prior to backward stepwise selection. Χ2-values are indicated. Degrees of freedom were 1 and 109. Symbols indicate: ns = non-significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001. Significant results are in boldface; Table S3: Full models testing the effects of the factors sex and phase, the covariates relative forelimb length, relative hindlimb length, relative tail length, SMI, and the interactions involving at least one of the factors, on relative speed. Χ2- and p-values are indicated. Degrees of freedom were 1 and 91.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Junta de Andalucía government (protocol code AWG/mgd/GB-370-20, approved on 23 November 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The author was partly supported by a Juan de la Cierva-Incorporación fellowship by the Spanish Ministerio de Economía, Industria y Competitividad. Mar Comas and Gregorio Moreno-Rueda kindly gave logistic support. Comments by three anonymous reviewers improved the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- McMahon, T.A. Muscles, Reflexes, and Locomotion; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Schaeffer, P.J.; Lindstedt, S.L. How animals move: Comparative lessons on animal locomotion. Compr. Physiol. 2013, 3, 289–314. [Google Scholar] [PubMed]
- Perry, G.; Levering, K.; Girard, I.; Garland, T. Locomotor performance and social dominance in male Anolis cristatellus. Anim. Behav. 2004, 67, 37–47. [Google Scholar] [CrossRef]
- Peterson, C.C.; Husak, J.F. Locomotor performance and sexual selection: Individual variation in sprint speed of collared lizards (Crotaphytus collaris). Copeia 2006, 2006, 216–224. [Google Scholar] [CrossRef]
- Husak, J.F.; Fox, S.F.; Van Den Bussche, R.A. Faster male lizards are better defenders not sneakers. Anim. Behav. 2008, 75, 1725–1730. [Google Scholar] [CrossRef]
- Husak, J.F.; Fox, S.F.; Lovern, M.B.; Van Den Bussche, R.A. Faster lizards sire more offspring: Sexual selection on whole-animal performance. Evolution 2006, 60, 2122–2130. [Google Scholar] [PubMed]
- Higham, T.E. The integration of locomotion and prey capture in vertebrates: Morphology, behavior and performance. Integr. Comp. Biol. 2007, 47, 82–95. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.R.; Julius, M.L.; Vajda, A.M.; Norris, D.O.; Barber, L.B.; Schoenfuss, H.L. Predator avoidance performance of larval fathead minnows (Pimephales promelas) following short-term exposure to estrogen mixtures. Aquat. Toxicol. 2009, 91, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.Y.; Biewener, A.A. Outrun or outmaneuver: Predator-prey interactions as a model system for integrating biomechanical studies in a broader ecological and evolutionary context. Integr. Comp. Biol. 2015, 55, 1188–1197. [Google Scholar] [CrossRef]
- Lailvaux, S.P.; Husak, J.F. Predicting life-history trade-offs with whole-organism performance. Integr. Comp. Biol. 2017, 57, 325–332. [Google Scholar] [CrossRef]
- Kraskura, K.; Nelson, J.A. Hypoxia and sprint swimming performance of juvenile striped bass Morone saxatilis. Physiol. Biochem. Zool. 2018, 91, 682–690. [Google Scholar] [CrossRef]
- Gordon, M.S.; Blickhan, R.; Dabiri, J.O.; Videler, J.J. Animal Locomotion: Physical Principles and Adaptations; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Calsbeek, R. An ecological twist on the morphology–performance–fitness axis. Evol. Ecol. Res. 2008, 10, 197–212. [Google Scholar]
- Biewener, A.A.; Patek, S.N. Animal Locomotion, 2nd ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Burrows, M.; Sutton, G.P. The effect of leg length on jumping performance of short- and long-legged leafhopper insects. J. Exp. Biol. 2008, 211, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Jahant-Miller, C.; Miller, R.; Parry, D. Size-dependent flight capacity and propensity in a range-expanding invasive insect. Insect Sci. 2022, 29, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Plaut, I. Effects of fin size on swimming performance, swimming behaviour and routine activity of zebrafish Danio rerio. J. Exp. Biol. 2000, 203, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, Y.; Ogino, K.; Hirata, H. Swimming capability of zebrafish is governed by water temperature, caudal fin length and genetic background. Sci. Rep. 2019, 9, 16307. [Google Scholar] [CrossRef]
- Zamora-Camacho, F.J. Locomotor performance in a running toad: Roles of morphology, sex, and agrosystem versus natural habitat. Biol. J. Linn. Soc. 2018, 123, 411–421. [Google Scholar] [CrossRef]
- Mariotto, L.R.; Bars-Closel, M.; Kohlsdorf, T. Limb length and poison glands size as predictors of anti-predatory performance in South American true toads. Zool. Anz. 2022, 296, 50–57. [Google Scholar] [CrossRef]
- Zamora-Camacho, F.J.; Reguera, S.; Rubino-Hispan, M.V.; Moreno-Rueda, G. Effects of limb length, body mass, gender, gravidity, and elevation on escape speed in the lizard Psammodromus algirus. Evol. Biol. 2014, 61, 509–517. [Google Scholar] [CrossRef]
- González-Morales, J.C.; Rivera-Rea, J.; Moreno-Rueda, G.; Bastiaans, E.; Castro-López, M.; Fajardo, V. Fast and dark: The case of Mezquite lizards at extreme altitudes. J. Therm. Biol. 2021, 102, 103115. [Google Scholar] [CrossRef]
- Garland, T.; Janis, C.M. Does metatarsal/femur ratio predict maximal running speed in cursorial mammals? J. Zool. 1993, 229, 133–151. [Google Scholar] [CrossRef]
- Day, L.M.; Jayne, B.C. Interspecific scaling of the morphology and posture of the limbs during locomotion in cats (Felidae). J. Exp. Biol. 2007, 210, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rueda, G. The capacity to escape from predators in Passer domesticus: An experimental study. J. Ornithol. 2003, 144, 438–444. [Google Scholar] [CrossRef]
- Sullivan, T.N.; Meyers, M.A.; Arzt, E. Scaling of bird wings and feathers for efficient flight. Sci. Adv. 2019, 5, eaat4269. [Google Scholar] [CrossRef] [PubMed]
- Plaut, I. Does pregnancy affect swimming performance of female mosquitofish, Gambusia affinis? Funct. Ecol. 2002, 16, 290–295. [Google Scholar] [CrossRef]
- Lowie, A.; Gillet, E.; Vanhooydonck, B.; Irschick, D.J.; Losos, J.B.; Herrel, A. Do the relationships between hindlimb anatomy and sprint speed variation differ between sexes in Anolis lizards? J. Exp. Biol. 2021, 222, jeb188805. [Google Scholar]
- Jagnandan, K.; Higham, T.E. How rapid changes in body mass affect the locomotion of terrestrial vertebrates: Ecology, evolution and biomechanics of a natural perturbation. Biol. J. Linn. Soc. 2018, 124, 279–293. [Google Scholar] [CrossRef]
- Kilbourne, B.M.; Hoffman, L.C. Scale effects between body size and limb design in quadrupedal mammals. PLoS ONE 2013, 8, e78392. [Google Scholar] [CrossRef]
- Dailey, T.M.; Claussen, D.L.; Ladd, G.B.; Buckner, S.T. The effects of temperature, desiccation, and body mass on the locomotion of the terrestrial isopod Porcellio laevis. Comp. Biochem. Physiol. A 2009, 153, 162–166. [Google Scholar] [CrossRef]
- Christiansen, P. Locomotion in terrestrial mammals: The influence of body mass, limb length and bone proportions on speed. Zool. J. Linn. Soc. 2002, 136, 685–714. [Google Scholar] [CrossRef]
- Iriarte-Díaz, J. Differential scaling of locomotor performance in small and large terrestrial mammals. J. Exp. Biol. 2002, 205, 2897–2908. [Google Scholar] [CrossRef]
- Moreno-Rueda, G.; Requena-Blanco, A.; Zamora-Camacho, F.J.; Comas, M.; Pascual, G. Morphological determinants of jumping performance in the Iberian green frog. Curr. Zool. 2020, 66, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.S.; Metcalfe, N.B.; Houston, D.C. A reassessment of the effect of body mass upon flight speed and predation risk in birds. Anim. Behav. 1998, 56, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, R.P.; Büscher, T.H.; Klawitter, L.J.; Gorb, S.N.; Emlen, D.J.; Tobalske, B.W. Multi-modal locomotor costs favor smaller males in a sexually dimorphic leaf-mimicking insect. BMC Ecol. Evol. 2022, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gracia, F.; García-Berthou, E.; Guasch, H.; Zamora, L.; Vila-Gispert, A. Size-related effects and the influence of metabolic traits and morphology on swimming performance in fish. Curr. Zool. 2020, 66, 493–503. [Google Scholar] [CrossRef]
- Bennett, A.F.; Garland, T.; Else, P.L. Individual correlation of morphology, muscle mechanics, and locomotion in a salamander. Regul. Integr. Comp. Physiol. 1989, 256, R1200–R1208. [Google Scholar] [CrossRef]
- Elsworth, P.G.; Seebacher, F.; Franklin, C.E. Sustained swimming performance in crocodiles (Crocodylus porosus): Effects of body size and temperature. J. Herpetol. 2003, 37, 363–368. [Google Scholar] [CrossRef]
- Sato, K.; Watanuki, Y.; Takahashi, A.; O’Miller, P.J.; Tanaka, H.; Kawabe, R.; Ponganis, P.J.; Handrich, Y.; Akamatsu, T.; Watanabe, Y.; et al. Stroke frequency, but not swimming speed, is related to body size in free-ranging seabirds, pinnipeds and cetaceans. Proc. R. Soc. B 2007, 274, 471–477. [Google Scholar] [CrossRef]
- Citadini, J.M.; Brandt, R.; Williams, C.R.; Gomes, F.R. Evolution of morphology and locomotor performance in anurans: Relationships with microhabitat diversification. J. Evol. Biol. 2018, 31, 371–381. [Google Scholar] [CrossRef]
- Heers, A.M.; Dial, K.P. Wings versus legs in the avian bauplan: Development and evolution of alternative locomotor strategies. Evolution 2014, 69, 305–320. [Google Scholar] [CrossRef]
- Burrows, M.; Ghosh, A.; Yeshwanth, H.M.; Dorosenko, M.; Sane, S.P. Effectiveness and efficiency of two distinct mechanisms for take-off in a derbid planthopper insect. J. Exp. Biol. 2019, 222, jeb191494. [Google Scholar] [CrossRef]
- Gatesy, S.M.; Dial, K.P. Locomotor modules and the evolution of avian flight. Evolution 1996, 50, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Cabelguen, J.M.; Charrier, V.; Mathou, A. Modular functional organisation of the axial locomotor system in salamanders. Zoology 2014, 117, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Engelkes, K.; Kath, L.; Kleinteich, T.; Hammel, J.U.; Beerlink, A.; Haas, A. Ecomorphology of the pectoral girdle in anurans (Amphibia, Anura): Shape diversity and biomechanical considerations. Ecol. Evol. 2020, 10, 11467–11487. [Google Scholar] [CrossRef] [PubMed]
- Winchell, K.M.; Maayan, I.; Fredette, J.R.; Revell, L.J. Linking locomotor performance to morphological shifts in urban lizards. Proc. R. Soc. B 2018, 285, 20180229. [Google Scholar] [CrossRef]
- Williams, T.M.; Ben-David, M.; Noren, S.; Rutishauser, M.; McDonald, K.; Heyward, W. Running energetics of the North American river otter: Do short legs necessarily reduce efficiency on land? Comp. Biochem. Physiol. A 2002, 133, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Botton-Divet, L.; Houssaye, A.; Herrel, A.; Fabre, A.C.; Cornette, R. Swimmers, diggers, climbers and more, a study of integration across the mustelids’ locomotor apparatus (Carnivora: Mustelidae). Evol. Biol. 2018, 45, 182–195. [Google Scholar] [CrossRef]
- Ledbetter, N.M.; Bonett, R.M. Terrestriality constrains salamander limb diversification: Implications for the evolution of pentadactyly. J. Evol. Biol. 2019, 32, 642–652. [Google Scholar] [CrossRef]
- Salvador, A. Gallipato—Pleurodeles waltl Michahelles, 1830. In Enciclopedia Virtual de Los Vertebrados Españoles; Salvador, A., Martínez-Solano, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, España, 2014; Available online: http://vertebradosibericos.org (accessed on 29 January 2023).
- Zamora-Camacho, F.J. Contextualizing the bizarre: The integrated functioning of rib puncture as an antipredator defense in the Iberian ribbed newt (Pleurodeles waltl). Freshw. Biol. 2022, in press. [Google Scholar] [CrossRef]
- Zamora-Camacho, F.J.; Comas, M.; Moreno-Rueda, G. Immune challenge does not impair short-distance escape speed in a newt. Anim. Behav. 2020, 167, 101–109. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, J.; Sánchez-Montes, G.; Martínez-Solano, I. Effective to census population size ratio in two near Threatened Mediterranean amphibians: Pleurodeles waltl and Pelobates cultripes. Conserv. Genet. 2017, 18, 1201–1211. [Google Scholar] [CrossRef]
- Albero, L.; Martínez-Solano, I.; Arias, A.; Lizana, M.; Bécares, E. Amphibian metacommunity responses to agricultural intensification in a Mediterranean landscape. Land 2021, 10, 924. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L.M. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Gvoždík, L. Mismatch between ectotherm thermal preferenda and optima for swimming: A test of the evolutionary pace hypothesis. Evol. Biol. 2015, 42, 137–145. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883e1891. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambrigde University Press: Cambridge, UK, 2002. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R. Development Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-103; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Fontanet, X.; Horta, N. Biometría y dimorfismo sexual en Pleurodeles waltli Michahelles, 1830 (Amphibia: Salamandridae) de una población del NE de la península ibérica. Misc. Zool. 1989, 13, 202–206. [Google Scholar]
- Raxworthy, C.J. Courtship, fighting and sexual dimorphism of the banded newt, Triturus vittatus ophryticus. Ethology 1989, 81, 148–170. [Google Scholar] [CrossRef]
- Malmgren, J.C.; Thollesson, M. Sexual size and shape dimorphism in two species of newts, Triturus cristatus and T. vulgaris (Caudata: Salamandridae). J. Zool. 1999, 249, 127–136. [Google Scholar] [CrossRef]
- Reinhard, S.; Kupfer, A. Sexual dimorphism in a French population of the marbled newt, Triturus marmoratus (Urodela: Salamandridae). Salamandra 2015, 51, 121–128. [Google Scholar]
- Salthe, S.N. Reproductive modes and the number and sizes of ova in the urodeles. Am. Midl. Nat. 1969, 81, 467–490. [Google Scholar] [CrossRef]
- Verrell, P.A.; Francillon, H. Body size, age, and reproduction in the smooth newt, Triturus vulgaris. J. Zool. 1986, 210, 89–100. [Google Scholar] [CrossRef]
- Luu, Y.K.; Lublinsky, S.; Ozcivici, E.; Capilla, E.; Pessin, J.E.; Rubin, C.T.; Judex, S. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Med. Eng. Phys. 2009, 31, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Joven, A.; Kirkham, M.; Simon, A. Husbandry of Spanish ribbed newts (Pleurodeles waltl). Methods Mol. Biol. 2015, 1290, 269–277. [Google Scholar]
- Díaz-Paniagua, C. Oviposition behavior of Triturus marmoratus pygmaeus. J. Herpetol. 1989, 23, 159–163. [Google Scholar] [CrossRef]
- Orizaola, G.; Braña, F. Oviposition behaviour and vulnerability of eggs to predation in four newt species (genus Triturus). Herpetol. J. 2003, 13, 121–124. [Google Scholar]
- Tallman, M. Forelimb to hindlimb shape covariance in extant hominoids and fossil hominins. Anat. Rec. 2012, 296, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.L.; Garland, T.; Schmitz, L.; Higham, T.E. Skink ecomorphology: Forelimb and hindlimb lengths, but not static stability, correlate with habitat use and demonstrate multiple solutions. Biol. J. Linn. Soc. 2018, 125, 673–692. [Google Scholar] [CrossRef]
- Losos, J.B. The evolution of form and function: Morphology and locomotor performance in west Indian Anolis lizards. Evolution 1990, 44, 1189–1203. [Google Scholar] [CrossRef]
- Böhmer, C.; Plateau, O.; Cornette, R.; Abourachid, A. Correlated evolution of neck length and leg length in birds. R. Soc. Open Sci. 2019, 6, 181588. [Google Scholar] [CrossRef]
- Ashley-Ross, M.A. Metamorphic and speed effects on hindlimb kinematics during terrestrial locomotion in the salamander Dicamptodon tenebrosus. J. Exp. Biol. 1994, 193, 285–305. [Google Scholar] [CrossRef]
- Pierce, S.E.; Lamas, L.P.; Pelligand, L.; Schilling, N.; Hutchinson, J.R. Patterns of limb and epaxial muscle activity during walking in the fire salamander, Salamandra salamandra. Integr. Org. Biol. 2020, 2, obaa015. [Google Scholar] [CrossRef]
- Devolvé, I.; Bem, T.; Cabelguen, J.M. Epaxial and limb muscle activity during swimming and terrestrial stepping in the adult newt, Pleurodeles waltl. J. Neurophysiol. 1997, 78, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Camacho, F.J.; Aragón, P. Antipredator responses of the morphs of an amphibian species match their differential predation pressures. Behav. Ecol. Sociobiol. 2022, 76, 26. [Google Scholar] [CrossRef]
- Zamora-Camacho, F.J.; Zambrano-Fernández, S.; Aragón, P. Carryover effects of chronic exposure to ammonium during the larval stage on post-metamorphic frogs. Aquat. Toxicol. 2022, 248, 106196. [Google Scholar] [CrossRef]
- Kawano, S.M.; Blob, R.W. Terrestrial force production by the limbs of a semi-aquatic salamander provides insight into the evolution of terrestrial locomotion mechanics. J. Exp. Biol. 2022, 225, jeb242795. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.L.; Wu, Q.; Geng, J.; Dang, W. Swimming performance and thermal resistance of juvenile and adult newts acclimated to different temperatures. Acta Herpetol. 2016, 11, 189–195. [Google Scholar]
- Bem, T.; Cabelguen, J.M.; Ekeberg, Ö.; Grillner, S. From swimming to walking: A single basic network for two different behaviors. Biol. Cybern. 2003, 88, 79–90. [Google Scholar] [CrossRef]
- Ryczko, D.; Knüsel, J.; Crespi, A.; Lamarque, S.; Mathou, A.; Ijspeert, I.J.; Cabelguen, J.M. Flexibility of the axial central pattern generator network for locomotion in the salamander. J. Neurophysiol. 2015, 113, 1921–1940. [Google Scholar] [CrossRef]
- Omura, A.; Anzai, W.; Koyabu, D.; Endo, H. Positional strategy on trunk muscles among aquatic, semiaquatic and terrestrial species in Urodela. J. Vet. Med. Sci. 2015, 77, 1043–1048. [Google Scholar] [CrossRef]
- Shaffer, H.B.; Austin, C.C.; Huey, R.B. The consequences of metamorphosis on salamander (Ambystoma) locomotor performance. Physiol. Zool. 1991, 64, 212–231. [Google Scholar] [CrossRef]
- Fish, F.E.; Rybczynski, N.; Lauder, G.V.; Duff, C.M. The role of the tail or the lack thereof in the evolution of tetrapod aquatic propulsion. Integr. Comp. Biol. 2021, 61, 398–413. [Google Scholar] [CrossRef]
- Secondi, J.; Lepetz, V.; Cossard, G.; Sourice, S. Nitrate affects courting and breathing but not escape performance in adult newts. Behav. Ecol. Sociobiol. 2013, 67, 1757–1765. [Google Scholar] [CrossRef]
- Brossman, K.H.; Carlson, B.E.; Swierk, L.; Langkilde, T. Aquatic tail size carries over to the terrestrial phase without impairing locomotion in adult Eastern red-spotted newts (Notophthalmus viridescens viridescens). Can. J. Zool. 2013, 91, 7–12. [Google Scholar] [CrossRef]
- Gvoždík, L.; Van Damme, R. Triturus newt defy the running-swimming dilemma. Evolution 2006, 60, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Omura, A.; Ejima, K.I.; Honda, K.; Anzai, W.; Taguchi, Y.; Koyabu, D.; Endo, H. Locomotion pattern and trunk musculoskeletal architecture among Urodela. Acta Zool. 2015, 96, 225–235. [Google Scholar] [CrossRef]
- Brown, S.C.; Brown, P.S. Water balance in the California newt Taricha torosa. Regul. Integr. Comp. Physiol. 1980, 238, R113–R118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).