Resistance of Lichens and Mosses of Regenerated Alpine Communities to Repeated Experimental Trampling in the Belianske Tatras, Northern Slovakia
Abstract
1. Introduction
2. Materials and Methods
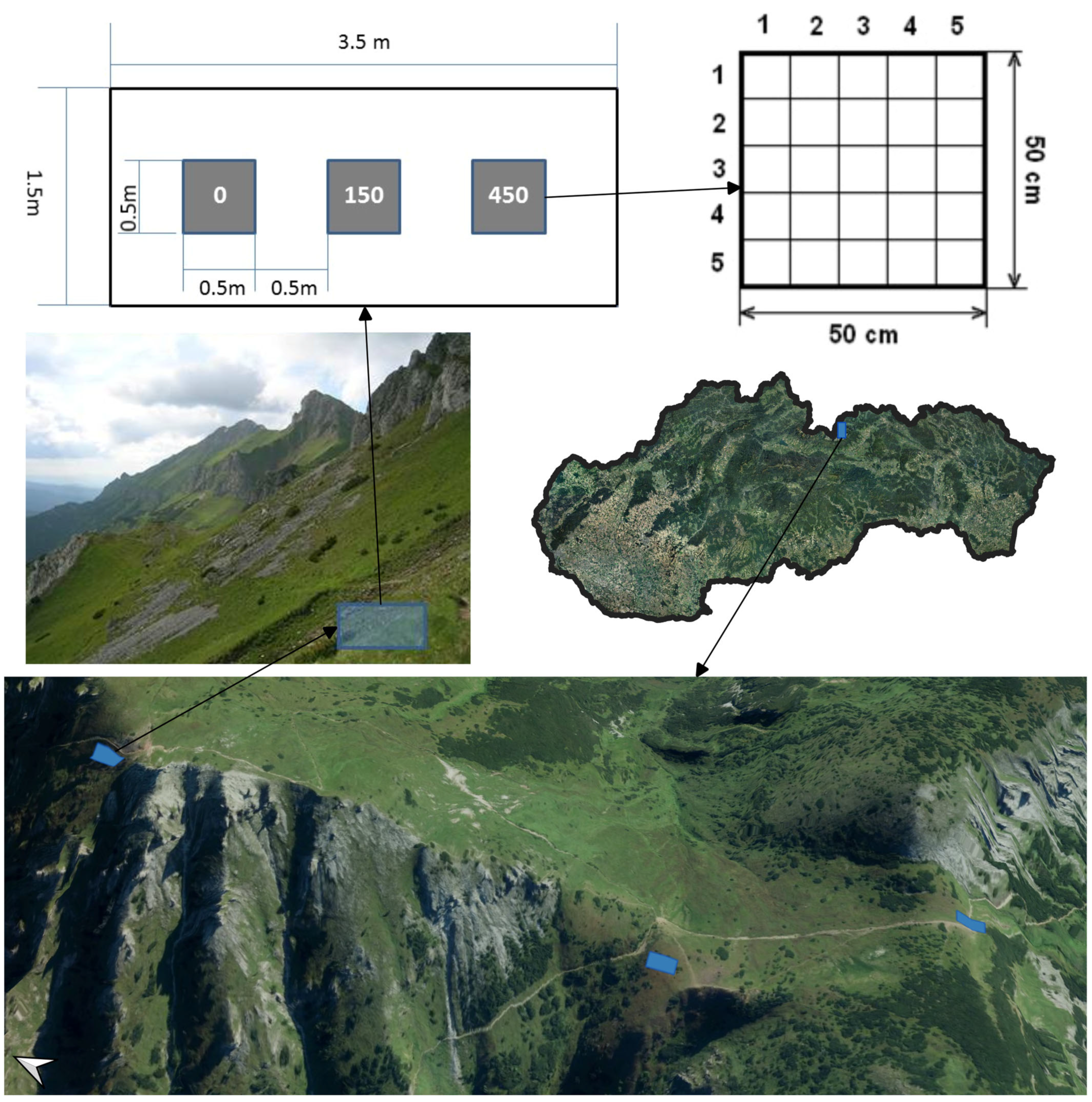
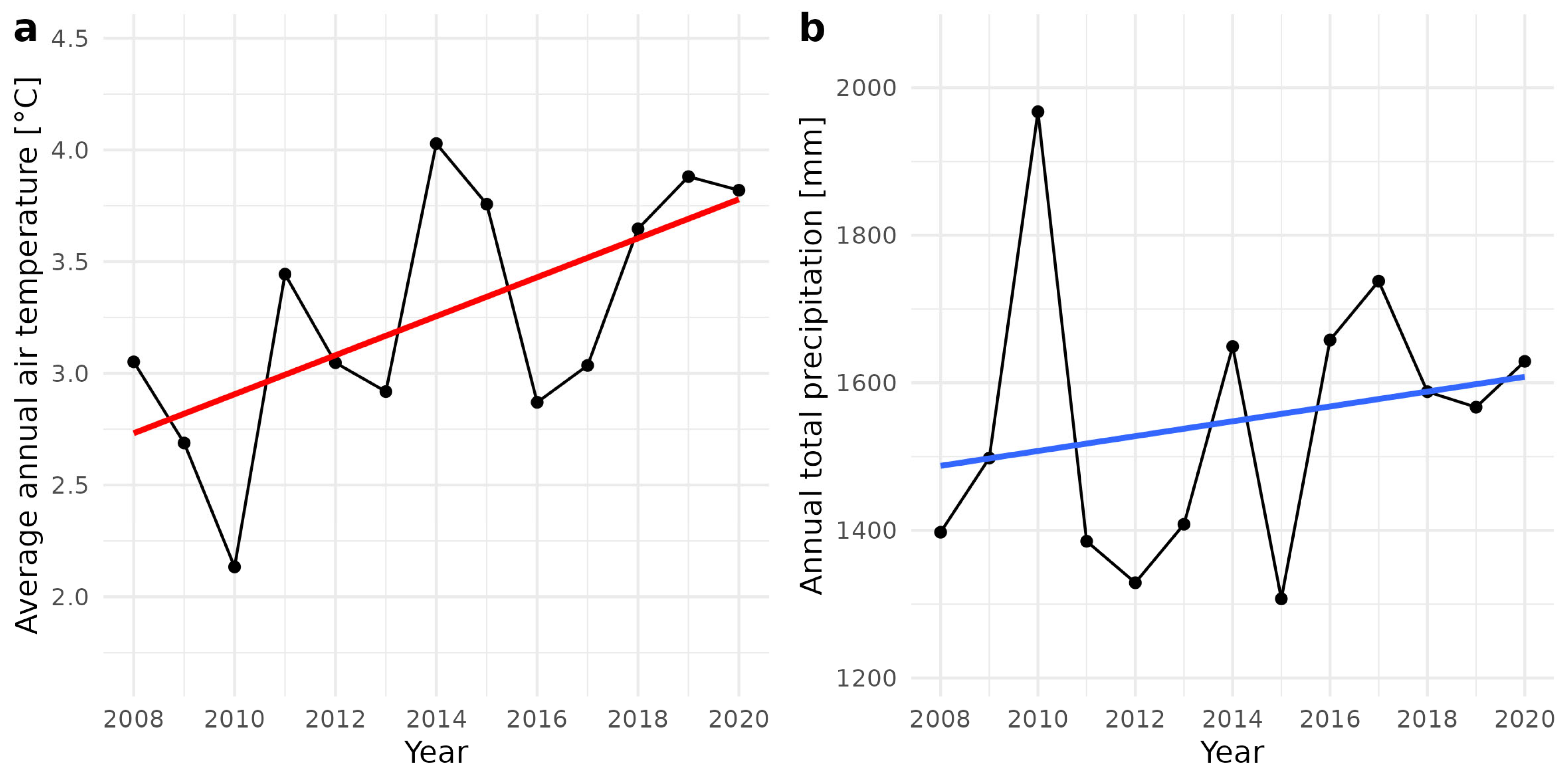
2.1. Study Area
2.2. Experimental Design
2.3. Trampling Treatment and Timing
- Coverage (%) of the vascular plant species (E1 layer), mosses and lichens (E0 layer; the lichens and mosses were determined by a specialist). Only green photosynthetic material should be included in the cover estimates. It is inappropriate to include the cover of the surviving stems that have been defoliated by the trampling. The cover values were round integral numbers, and if the cover was less than 1%, a value of 0.5% or 0% was used, indicating a complete lack of cover.
- (a)
- Visual estimates of the top coverage perpendicular to each subplot;
- (b)
- Visual estimates of the coverage of each vascular plant species, mosses and lichens per subplot.
- Coverage (%) of the bare ground (i.e., ground not covered by live vegetation). Bare ground can be either mineral or soil.
- (c)
- Visual estimates of the top coverage of the bare ground perpendicular to each subplot;
- (d)
- Visual estimates of the coverage of the bare ground per subplot.
- Coverage (%) of the litter (including the litter of the recently trampled plants).
- (e)
- Visual estimates of the top coverage of the litter perpendicular to each subplot;
- (f)
- Visual estimates of the coverage of the litter per subplot.
2.4. Data Analysis
2.4.1. Relative Cover
2.4.2. Statistical Processing
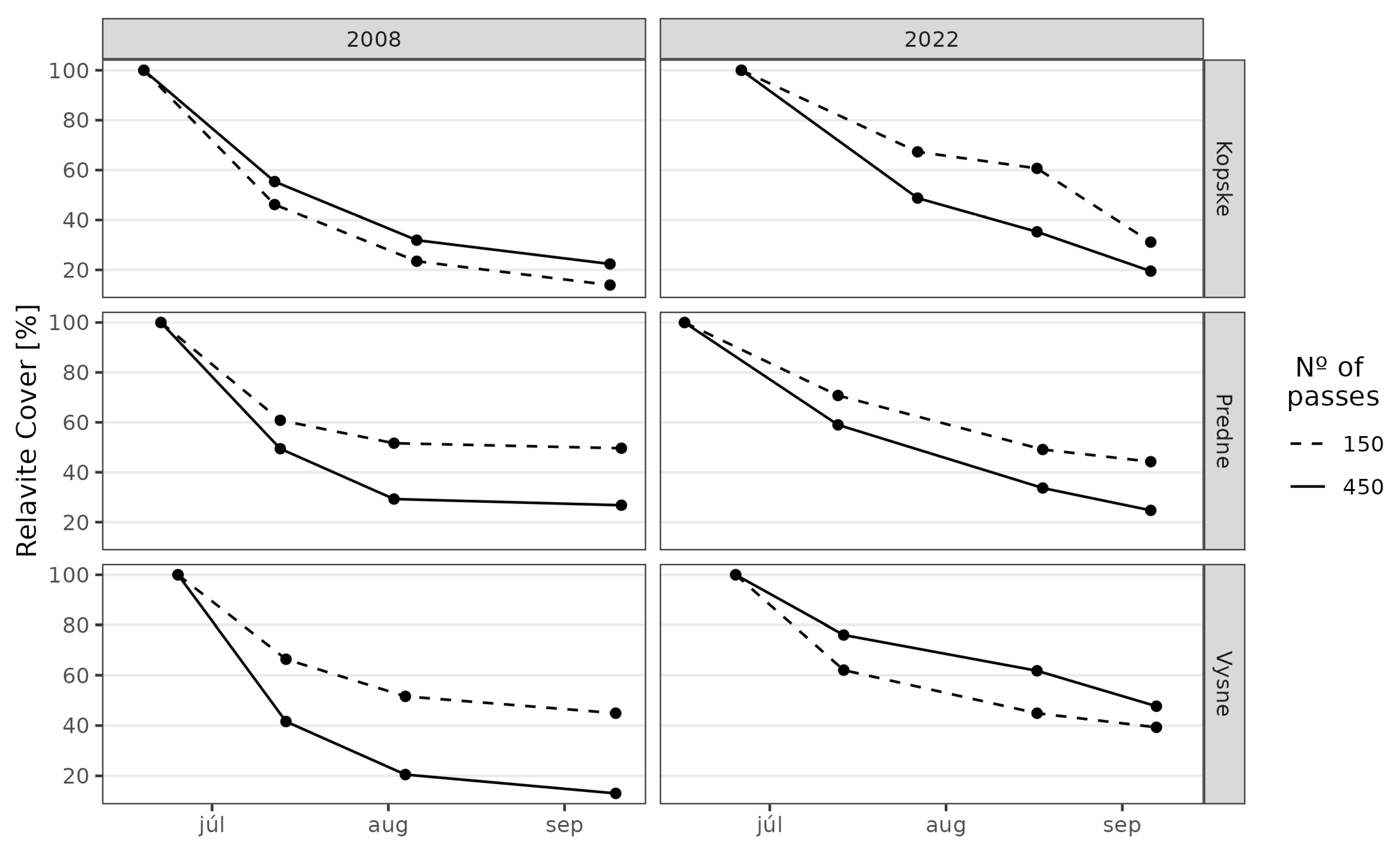
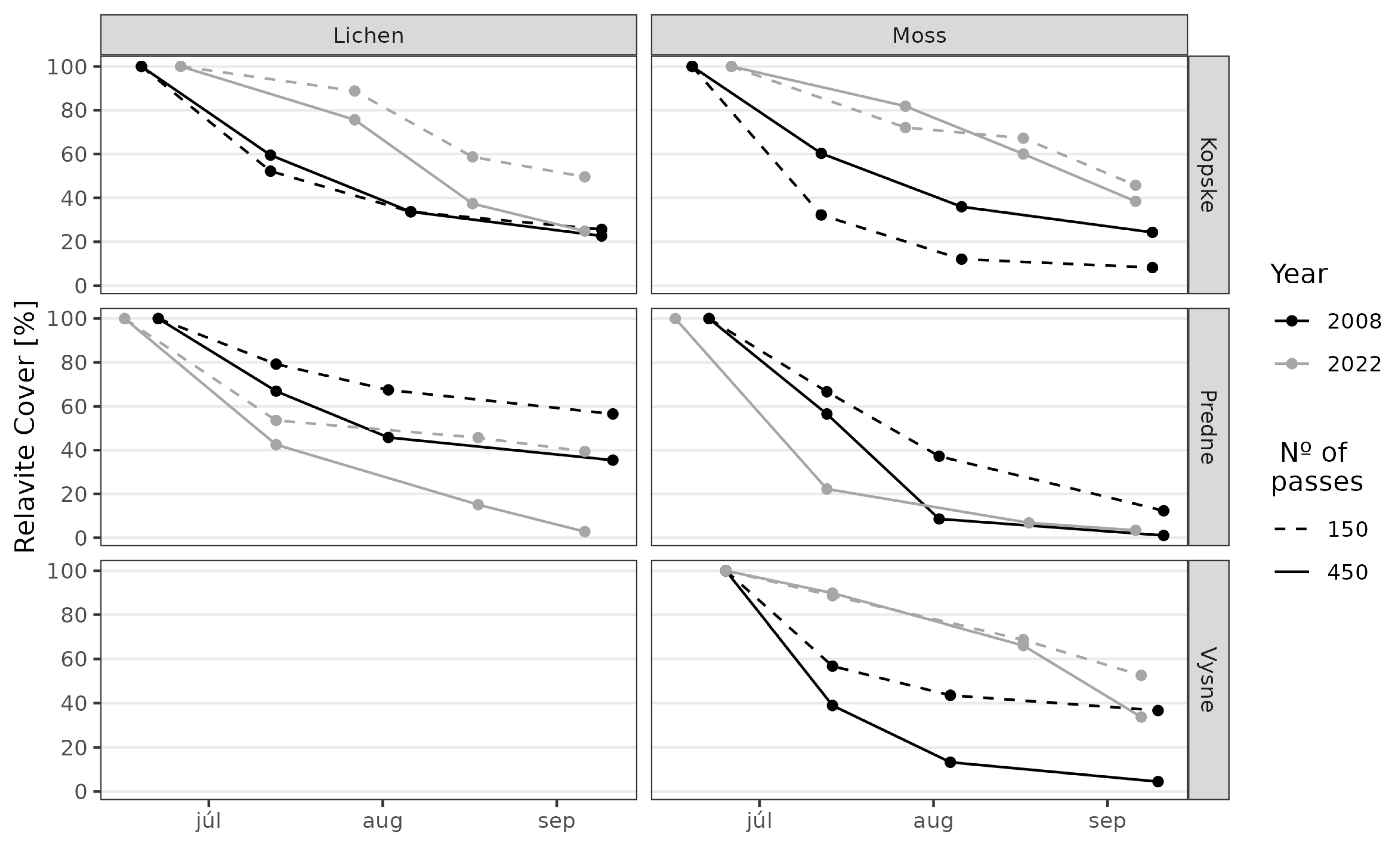
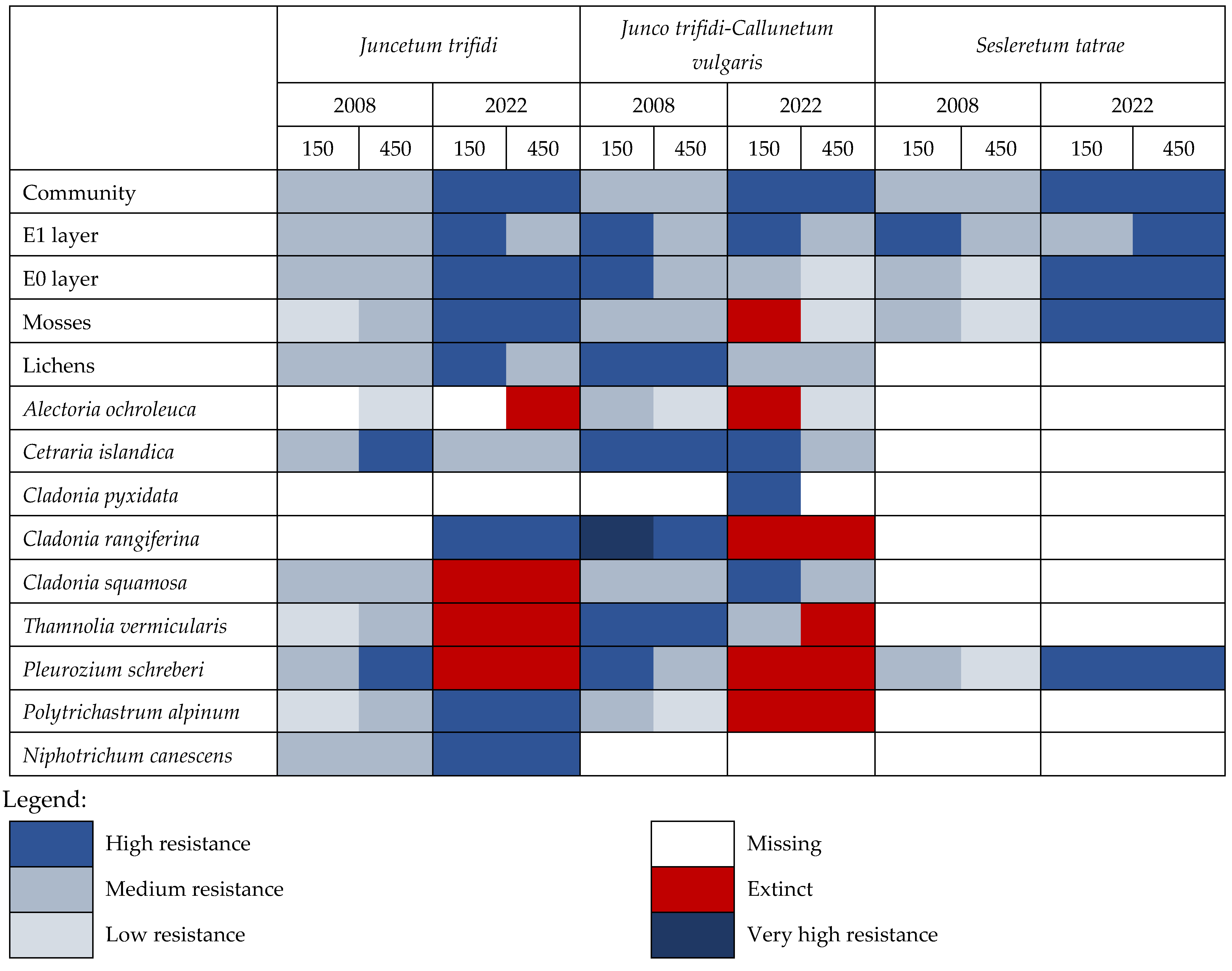
3. Results
3.1. Juncetum trifidi Community
3.2. Junco trifidi-Callunetum vulgaris Community
3.3. Seslerietum tatrae Community
3.4. Community Resistance Scheme
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Buckley, R. Tourism in the most fragile environments. Tour. Recreat. Res. 2000, 25, 31–40. [Google Scholar] [CrossRef]
- Roovers, P.; Verheyen, K.; Hermy, M.; Gulinck, H. Experimental trampling and vegetation recovery in some forest and heathland communities. Appl. Veg. Sci. 2004, 7, 111–118. [Google Scholar] [CrossRef]
- Atik, M.; Sayan, S.; Karaguzel, O. Impact of recreational trampling on the natural vegetation in Termessos National Park, Antalya-Turkey. Tarim Bilim. Derg. 2009, 15, 249–258. [Google Scholar]
- McDougall, K.; Wright, G. The impact of trampling on feldmark vegetation in Kosciuszko National Park, New South Wales. Aust. J. Bot. 2004, 52, 315–320. [Google Scholar] [CrossRef]
- Perevoznikova, V.D.; Zubareva, O.N. Geobotanical Indication of the State of Suburban Forests (an Example of Birch Grove in Akademgorodok, Krasnoyarsk. Russ. J. Ecol. 2002, 33, 1–6. [Google Scholar] [CrossRef]
- Whinam, J.; Cannell, E.J.; Kirkpatrick, J.B.; Comfort, M. Studies on the potential impact of recreational horseriding on some Alpine Environments of the Central Plateau Tasmania. J. Environ. Manag. 1994, 40, 103–117. [Google Scholar] [CrossRef]
- Monz, C.A. The response of two arctic tundra plant communities to human trampling disturbance. J. Environ. Manag. 2002, 64, 207–217. [Google Scholar] [CrossRef]
- Pickering, C.M.; Hill, W. Impacts of recreation and tourism on plant biodiversity and vegetation in protected areas in Australia. J. Environ. Manag. 2007, 85, 791–800. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Xiao, J.; Li, L. Variation of plant functional diversity along a disturbance gradient in mountain meadows of the Donglingshan reserve, Beijing, China. Russ. J. Ecol. 2015, 46, 157–166. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 1st ed.; Springer: Berlin Germany, 2003; p. 343. [Google Scholar]
- Körner, C.; Spehn, E.M. Mountain Biodiversity: A Global Assessment, 1st ed.; Routledge: London, UK, 2002; p. 350. [Google Scholar]
- Willard, B.E.; Cooper, D.J.; Forbes, B.C. Natural Regeneration of Alpine Tundra Vegetation after Human Trampling: A 42-yearData Set from Rocky Mountain National Park, Colorado, U.S.A. Arct. Antarct. Alp. Res. 2007, 39, 177–183. [Google Scholar] [CrossRef]
- Barros, A.; Pickering, C.M. Impacts of experimental trampling by hikers and pack animals on a high-altitude alpine sedge meadow in the Andes. Plant. Ecol. Divers. 2015, 8, 265–276. [Google Scholar] [CrossRef]
- Pescott, O.L.; Stewart, G.B. Assessing the impact of human trampling on vegetation: A systematic review and meta-analysis of experimental evidence. PeerJ 2014, 2, e360. [Google Scholar] [CrossRef]
- Barros, A.; Aschero, V.; Mazzolari, A.; Cavieres, L.A.; Pickering, C.M. Going off trails: How dispersed visitor use affects alpine vegetation. J. Environ. Manag. 2020, 267, 110546. [Google Scholar] [CrossRef]
- Goh, E. Walking off-trail in national parks: Monkey see monkey do. Leis. Sci. 2020, 45, 1–23. [Google Scholar] [CrossRef]
- Park, L.O.; Manning, R.E.; Marion, J.L.; Lawson, S.R.; Jacobi, C. Managing visitor impacts in parks: A multi-method study of the effectiveness of alternative management practices. J. Park Recreat. Adm. 2008, 26, 97–121. [Google Scholar]
- Gheza, G.; Assini, S.; Marini, L.; Nascimbene, J. Impact of an invasive herbivore and human trampling on lichen-rich dry grasslands: Soil-dependent response of multiple taxa. Sci. Total Environ. 2018, 639, 633–639. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Alatalo, J.M. Effects of human trampling on abundance and diversity of vascular plants, bryophytes and lichens in alpine heath vegetation. Northern Sweden. SpringerPlus 2015, 4, 95. [Google Scholar] [CrossRef]
- Törn, A.; Rautio, J.; Norokorpi, Y.; Tolvanen, A. Revegetation after short-term trampling at subalpine heath vegetation. Ann. Bot. Fenn. 2006, 43, 129–138. [Google Scholar]
- Barros, A.; Gonnet, J.; Pickering, C. Impacts of informal trails on vegetation and soils in the highest protected area in the Southern Hemisphere. J. Environ. Manag. 2013, 127, 50–60. [Google Scholar] [CrossRef]
- Scott, J.J.; Kirkpatrick, J.B. Effects of human trampling on the sub-Antarctic vegetation of Macquarie Island. Polar Rec. 1994, 30, 207–220. [Google Scholar] [CrossRef]
- Cole, D.N. Impacts of hiking and camping on soils and vegetation: A review. In Environmental Impact of Ecotourism, 1st ed.; Buckley, R., Ed.; CABI Publishing: Oxfordshire, UK, 2004; pp. 41–60. [Google Scholar]
- Pickering, C.M.; Growcock, A.J. Impacts of experimental trampling on tall alpine herbfields and subalpine grasslands in the Australian Alps. J. Environ. Manag. 2009, 91, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Crisfield, V.E.; Macdonald, S.E.; Gould, A.J. Effects of Recreational Traffic on Alpine Plant Communities in the Northern Cana-dian Rockies. Arct. Antarct. Alp. Res. 2012, 44, 277–287. [Google Scholar] [CrossRef]
- Farrell, T.A.; Marion, J.L. Trail impacts and trail impact management related to visitation at Torres del Paine National Park Chile. Leis. Loisir. 2001, 26, 31–59. [Google Scholar] [CrossRef]
- Pickering, C.M.; Hill, W.; Newsome, D.; Leung, Y.-F. Comparing hiking, mountain biking and horse riding impacts on vegetation and soils in Australia and the United States of America. J. Environ. Manag. 2010, 91, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.N. Experimental Trampling of Vegetation. I. Relationship between Trampling Intensity and Vegetation Response. J. Appl. Ecol. 1995, 32, 203. [Google Scholar] [CrossRef]
- Xu, L.; Freitas, S.M.A.; Yu, F.-H.; Dong, M.; Anten, N.P.R.; Werger, M.J.A. Effects of Trampling on Morphological and Mechan-ical Traits of Dryland Shrub Species Do Not Depend on Water Availability. PLoS ONE 2013, 8, e53021. [Google Scholar] [CrossRef]
- Niu, L.; Cheng, Z. Impact of tourism disturbance on forest vegetation in Wutai Mountain China. Environ. Monit. Assess. 2019, 191, 81. [Google Scholar] [CrossRef]
- Liddle, M. A selective review of the ecological effects of human trampling on natural ecosystems. Biol. Conserv. 1975, 7, 17–36. [Google Scholar] [CrossRef]
- Dunne, T.; Dietrich, W.E. Effects of cattle trampling on vegetation, infiltration, and erosion in a tropical rangeland. J. Arid Environ. 2011, 75, 58–69. [Google Scholar] [CrossRef]
- Czortek, P.; Eycott, A.E.; Grytnes, J.-A.; Delimat, A.; Kapfer, J.; Jaroszewicz, B. Effects of grazing abandonment and climate change on mountain summits flora: A case study in the Tatra Mts. Plant Ecol. 2018, 219, 261–276. [Google Scholar] [CrossRef]
- Pauler, C.M.; Isselstein, J.; Braunbeck, J.T.; Schneider, M.K. Influence of Highland and production-oriented cattle breeds on pasture vegetation: A pairwise assessment across broad environmental gradients. Agric. Ecosyst. Environ. 2019, 284, 106585. [Google Scholar] [CrossRef]
- Kuba, K.; Monz, C.; Bårdsen, B.-J.; Hausner, V.H. Role of site management in influencing visitor use along trails in multiple alpine protected areas in Norway. J. Outdoor Recreat. Tour. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Li, W.; He, S.; Cheng, X.; Zhang, G. Short-term effects of experimental trampling on alpine grasslands in Shangri-la, China. Glob. Ecol. Conserv. 2020, 23, e01161. [Google Scholar] [CrossRef]
- Cole, D.N. Experimental Trampling of Vegetation. II. Predictors of Resistance and Resilience. J. Appl. Ecol. 1995, 32, 215. [Google Scholar] [CrossRef]
- Whinam, J.; Chilcott, N. Impacts of trampling on alpine environments in central Tasmania. J. Environ. Manag. 1999, 57, 205–220. [Google Scholar] [CrossRef]
- Cole, D.N.; Monz, C.A. Trampling Disturbance of High-Elevation Vegetation, Wind River Mountains, Wyoming, U.S.A. Arct. Antarct. Alp. Res. 2002, 34, 365. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Gray, A.; Vanbergen, A.J.; Bergès, L.; Bohner, A.; Brooker, R.W.; De Bruyn, L.; De Cinti, B.; Dirn-böck, T.; Grandin, U.; et al. Functional traits and local environment predict vegetation responses to disturbance: A pan-Euro-pean multi-site experiment. J. Ecol. 2011, 99, 777–787. [Google Scholar] [CrossRef]
- Pertierra, L.R.; Lara, F.; Tejedo, P.; Quesada, A.; Benayas, J. Rapid denudation processes in cryptogamic communities fromMaritime Antarctica subjected to human trampling. Antarct. Sci. 2013, 25, 318–328. [Google Scholar] [CrossRef]
- Gremmen, N.J.M.; Smith, V.R.; Van Tongeren, O.F.R. Impact of Trampling on the Vegetation of Subantarctic Marion Island. Arct. Antarct. Alp. Res. 2003, 35, 442–446. [Google Scholar] [CrossRef]
- Longton, R.E. Bryophyte vegetation in polar regions. In Bryophyte Ecology, 1st ed.; Smith, A.J.E., Ed.; Springer: Amsterdam, The Netherlands, 1982; pp. 123–165. [Google Scholar]
- Matveyeva, N.; Chernov, Y. Biodiversity of terrestrial ecosystems. In The Arctic: Environment, People, Policy; Nuttall, M., Calla-ghan, T.V., Eds.; Harwood Academic Publishers: Groningen, The Netherlands, 2000; pp. 233–273. [Google Scholar]
- Longton, R.E. The role of bryophytes in terrestrial ecosystems. J. Hattori Bot. Lab. 1984, 55, 147–163. [Google Scholar]
- Nash, T.H., III. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Lange, O.L.; Hahn, S.C.; Meyer, A.; Tenhunen, J.D. Upland tundra in the foothills of the Brooks range, Alaska, USA: Lichen long-term photosynthetic CO2 uptake and net carbon gain. Arct. Antarct. Alp. Res. 1998, 30, 252–261. [Google Scholar] [CrossRef]
- Crittenden, P.D. The role of lichens in the nitrogen economy of subarctic woodlands: Nitrogen loss from the nitrogen-fixing lichen Stereocaulon paschale during rainfall. In Nitrogen as an Ecological Factor, 1st ed.; Lee, J.A., McNeill, S., Rorison, I.H., Eds.; Blackwell Science: Oxford, UK, 1983; pp. 43–68. [Google Scholar]
- Kielland, K. Role of free amino acids in the nitrogen economy of arctic cryptogams. Ecoscience 1997, 4, 75–79. [Google Scholar] [CrossRef]
- Longton, R.E. The role of bryophytes and lichens in polar ecosystems. In Ecology of Arctic Environments, 1st ed.; Woodin, S.J., Marquiss, M., Eds.; Blackwell Science: Oxford, UK, 1997; pp. 69–96. [Google Scholar]
- Longton, R.E. British Bryological Society. Biology of Polar Bryophytes and Lichens, 1st ed.; Cambridge University Press: Cambridge, UK, 1988; p. 391. ISBN 0-521-250153. [Google Scholar]
- Dorrepaal, E.; Aerts, R.; Cornelissen, J.H.C.; Van Logtestijn, R.S.P.; Callaghan, T.V. Sphagnum modifies climate-change im-pacts on subarctic vascular bog plants. Funct. Ecol. 2006, 20, 31–41. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Van Bodegom, P.M.; Aerts, R.; Callaghan, T.V.; Van Logtestijn, R.S.P.; Alatalo, J.; Stuart Chapin, F.; Gerdol, R.; Gud-mundsson, J.; Gwynn-Jones, D.; et al. Global negativevegetation feedback to climate warming responses of leaf litterdecomposition rates in cold biomes. Ecol. Lett. 2007, 10, 619–627. [Google Scholar] [CrossRef]
- Casanova-Katny, A.; Palfner, G.; Torres-Mellado, G.A.; Cavieres, L.A. Do Antarctic lichens modify microclimate and facilitatevascular plants in the maritime Antarctic? A comment to Molina-Montenegro et al. J. Veg. Sci. 2014, 25, 601–605. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Callaghan, T.V.; Alatalo, J.M.; Michelsen, A.; Graglia, E.; Hartley, A.E.; Hik, D.S.; Hobbie, S.E.; Press, M.C.; Robinson, C.H.; et al. Global change and arctic ecosystems:is lichen decline a function of increases in vascular plant biomass? J. Ecol. 2001, 89, 984–994. [Google Scholar] [CrossRef]
- Jägerbrand, A.K.; Kudo, G.; Alatalo, J.M.; Molau, U. Effects of neighboring vascular plants on the abundance of bryophytes indifferent vegetation types. Polar Sci. 2012, 6, 200–208. [Google Scholar] [CrossRef]
- Sedia, E.G.; Ehrenfeld, J.G. Lichens and mosses promotealternate stableplant communities in the New Jersey Pinelands. Oikos J. 2003, 100, 447–458. [Google Scholar] [CrossRef]
- Bayfield, N.G.; Urquhart, U.H.; Cooper, S.M. Susceptibility of Four Species of Cladonia to Disturbance by Trampling in the Cairngorm Mountains, Scotland. J. Appl. Ecol. 1981, 18, 303–310. [Google Scholar] [CrossRef]
- Heinken, T. Dispersal Patterns of Terricolous Lichens by Thallus Fragments. Lichenologist 1999, 31, 603–612. [Google Scholar] [CrossRef]
- Heggenes, J.; Odland, A.; Chevalier, T.; Ahlberg, J.; Berg, A.; Larsson, H.; Bjerketvedt, D.K. Herbivore Grazing—Or Trampling? Trampling Effects by a Large Ungulate in Cold High-Latitude Ecosystems. Ecol. Evol. 2017, 7, 6423–6431. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, T.; Michelsen, O.; Graae, B.J.; Kyrkjeeide, M.O.; Holien, H.; Hassel, K.; Lindmo, S.; Kapás, R.E.; De Frenne, P. Impact of climate change on alpine vegetation of mountain summits in Norway. Ecol. Res. 2017, 32, 579–593. [Google Scholar] [CrossRef]
- Gignac, L.D. Bryophytes as Indicators of Climate Change. Bryologist 2001, 104, 410–420. [Google Scholar] [CrossRef]
- Lenoir, J.J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2014, 37, 15–28. [Google Scholar] [CrossRef]
- Evangelista, A.; Frate, L.; Carranza, M.L.; Attorre, F.; Pelino, G.; Stanisci, A. Changes in composition, ecology and structure of high-mountain vegetation: A re-visitation study over 42 years. AoB Plants 2016, 8, plw004. [Google Scholar] [CrossRef] [PubMed]
- Tuba, Z.; Slack, N.G.; Stark, L.R. Bryophyte Ecology and Climate Change; Cambridge University Press: Cambridge, UK, 2011; 114p. [Google Scholar]
- Ferrenberg, S.; Sasha, C.R.; Jayne, B. Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc. Natl. Acad. Sci. USA 2015, 112, 12116–12121. [Google Scholar] [CrossRef]
- Larson, D.W. Patterns of lichen photosynthesis and respiration following prolonged frozen storage. Can. J. Bot. 1978, 56, 2119–2123. [Google Scholar] [CrossRef]
- Váczi, P. Physiological Properties of Lichen Photobionts of the Genus Trebouxia. Ph.D. Thesis, Department of Plant Physiology and Anatomy, Faculty of Natural Sciences, Masaryk University, Brno, Czech Republic, 2005. [Google Scholar]
- Hale, M.E. Growth. In The Lichens, 1st ed.; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: London, UK, 1973; pp. 473–492. [Google Scholar]
- Rhoades, F.M. Growth rates of Lobaria oregana as determined from sequential photographs. Can. J. Bot. 1977, 55, 2226–2233. [Google Scholar] [CrossRef]
- Kahan, P. Lichens, 1st ed.; Strom Života: Bratislava, Slovakia, 2013; p. 17. [Google Scholar]
- Šimonovičová, A.; Nosalj, S.; Machariková, M.; Pelechová Drongová, Z.; Takáčová, A.; Mišíková, K.; Guttová, A. Soil Microscopic Filamentous Fungi, Cyanobacteria, Algae, Bryophytes, Lichenized Fungi and Their Biodiversity, 1st ed.; Comenius University in Bratislava: Bratislava, Slovakia, 2021; p. 264. [Google Scholar]
- Andersen, U.V. Resistance of Danish coastal vegetation types to human trampling. Biol. Conserv. 1995, 71, 223–230. [Google Scholar] [CrossRef]
- Arnesen, T. Vegetation dynamics following trampling in grassland and heathland in Sølendet Nature Reserve, a boreal uplandarea in Central Norway. Nord. J. Bot. 1999, 19, 47–69. [Google Scholar] [CrossRef]
- Grabherr, G. The impact of trampling by tourists on a high altitudinal grassland in the Tyrolean Alps, Austria. Vegetation 1982, 48, 209–217. [Google Scholar] [CrossRef]
- Kuss, F.R.; Hall, C.N. Ground flora trampling studies: Five years after closure. Environ. Manag. 1991, 15, 715–727. [Google Scholar] [CrossRef]
- Cole, D.N.; Bayfield, N.G. Recreational trampling of vegetation: Standard experimental procedures. Biol. Conserv. 1993, 63, 209–215. [Google Scholar] [CrossRef]
- Gallet, S.; Rozé, F. Long-term effects of trampling on Atlantic Heathland in Brittany (France): Resilience and tolerance in relationto season and meteorological conditions. Biol. Conserv. 2002, 103, 267–275. [Google Scholar] [CrossRef]
- Šťastný, P.; Nieplová, E.; Melo, M. Average air temperature in January 1:2,000,000. In Landscape Atlas of the Slovak Republic, 1st ed.; Ministry of the Environment of the Slovak Republic: Bratislava, Slovakia, 2002; p. 99. [Google Scholar]
- Faško, P.; Handžák, Š.; Šrámková, N. Number of days with snow cover and its average height 1:2,000,000. In Landscape Atlas of the Slovak Republic, 1st ed.; Ministry of the Environment of the Slovak Republic: Bratislava, Slovakia, 2002; p. 99. [Google Scholar]
- Piscová, V.; Ševčík, M.; Hreško, J.; Petrovič, F. Effects of a Short-Term Trampling Experiment on Alpine Vegetation in the Tatras, Slovakia. Sustainability 2021, 13, 2750. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 17 July 2022).
- Apollo, M.; Andreychouk, V. Trampling Intensity and Vegetation Response and Recovery according to Altitude: An Experi-mental Study from the Himalayan Miyar Valley. Resources 2020, 9, 98. [Google Scholar] [CrossRef]
- Bates, G.H. The Vegetation of Footpaths, Sidewalks, Cart-Tracks and Gateways. J. Ecol. 1935, 23, 470. [Google Scholar] [CrossRef]
- Speight, M.C. Outdoor Recreation and Its Ecological Effects: A Bibliography and Review; University College: London, UK, 1973; Volume 4. [Google Scholar]
- Dale, D.; Weaver, T. Trampling Effects on Vegetation of the Trail Corridors of North Rocky Mountain Forests. J. Appl. Ecol. 1974, 11, 767. [Google Scholar] [CrossRef]
- Nepal, S.K. Tourism in protected areas: The Nepalese Himalaya. Ann. Tour. Res. 2000, 27, 661–681. [Google Scholar] [CrossRef]
- Marek, A.; Wieczorek, M. Tourist Traffic in the Aconcagua Massif Area. Quaest. Geogr. 2015, 34, 65–76. [Google Scholar] [CrossRef][Green Version]
- Barros, A.; Monz, C.; Pickering, C. Is tourism damaging ecosystems in the Andes? Current knowledge and an agenda for futureresearch. Ambio 2014, 44, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Hertlová, B.; Popelka, O.; Zeidler, M.; Banaš, M. Alpine plant communities responses to simulated mechanical disturbances of tourism, case study from the High Sudetes Mts. J. Landsc. Manag. 2016, 7, 16–21. [Google Scholar]
- Bayfield, N.G. Recovery of four montane heath communities on Cairngorm, Scotland, from disturbance by trampling. Biol. Conserv. 1979, 15, 165–179. [Google Scholar] [CrossRef]
- Gargas, A.; DePriest, P.T.; Grube, M.; Tehler, A. Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny. Science 1995, 268, 1492–1495, Erratum in Science 1995, 268, 1833. [Google Scholar] [CrossRef] [PubMed]
- Lutzoni, F.; Pagel, M.; Reeb, V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature 2001, 411, 937–940. [Google Scholar] [CrossRef]


| 2008 | 2022 | |||||||
|---|---|---|---|---|---|---|---|---|
| Area | Trampling | Species | Formula | R2 | Mean Change 1 | Formula | R2 | Mean Change 1 |
| Kopske | 450 | Alectoria ochroleuca | 96.31 − 3.42x + 0.03x2 | 0.91 | 32.92 | |||
| Kopske | 150 | Cetraria islandica | 98.84 − 1.9x + 0.01x2 | 0.98 | 23.3 | 103.4 − 0.76x | 0.87 | 16.64 |
| Kopske | 450 | Cetraria islandica | 99.92 − 1.89x + 0.01x2 | 0.99 | 25.44 | 101.99 − 1.12x | 0.95 | 25.39 |
| Kopske | 150 | Cladonia rangiferina | 100.8 + 0.41x − 0.02x2 | 0.96 | 19.84 | |||
| Kopske | 450 | Cladonia rangiferina | 104.94 − 0.86x | 0.92 | 20.37 | |||
| Kopske | 150 | Cladonia squamosa | 93.87 − 1.33x | 0.76 | 33.33 | |||
| Kopske | 450 | Cladonia squamosa | 100.32 − 2.63x + 0.02x2 | 0.99 | 23.23 | |||
| Kopske | 150 | Pleurozium schreberi | 100.63 − 2.6x + 0.02x2 | 0.99 | 28.37 | |||
| Kopske | 450 | Pleurozium schreberi | 96.39 − 0.81x | 0.92 | 21.61 | |||
| Kopske | 150 | Polytrichastrum alpinum | 96.32 − 3.14x + 0.03x2 | 0.9 | 30.48 | 101.35 − 0.64x | 0.93 | 16.3 |
| Kopske | 450 | Polytrichastrum alpinum | 98.85 − 2.2x + 0.01x2 | 0.99 | 29.94 | 101.6 − 0.73x | 0.99 | 17.73 |
| Kopske | 150 | Niphotrichum canescens | 97.19 − 3.13x + 0.03x2 | 0.94 | 30.71 | 96.7 − 0.79x | 0.92 | 19.98 |
| Kopske | 450 | Niphotrichum canescens | 99.36 − 2.03x + 0.01x2 | 0.99 | 24 | 106.72 − 1.1x | 0.92 | 25.98 |
| Kopske | 150 | Thamnolia vermicularis | 95.53 − 3.41x + 0.03x2 | 0.86 | 29.37 | |||
| Kopske | 450 | Thamnolia vermicularis | 100.36 − 2.04x + 0.02x2 | 0.99 | 21.43 | |||
| Predne | 150 | Alectoria ochroleuca | 105.05 − 2.9x + 0.02x2 | 0.85 | 33.33 | |||
| Predne | 450 | Alectoria ochroleuca | 99.42 − 3.72x + 0.03x2 | 0.99 | 33.33 | 99.18 − 2.9x + 0.02x2 | 0.99 | 33.01 |
| Predne | 150 | Cetraria islandica | 99.66 − 1.1x + 0.01x2 | 0.99 | 13.88 | 97.66 − 0.57x | 0.97 | 16.59 |
| Predne | 450 | Cetraria islandica | 99.99 − 1.85x + 0.01x2 | 0.99 | 21.42 | 87.32 − 1.09x | 0.84 | 32.57 |
| Predne | 150 | Cladonia pyxidata | 99.55 − 0.31x | 0.93 | 7.83 | |||
| Predne | 150 | Cladonia rangiferina | 102.74 − 0.48x | 0.96 | 12.75 | |||
| Predne | 450 | Cladonia rangiferina | 99.37 − 1.65x + 0.01x2 | 0.99 | 17.44 | |||
| Predne | 150 | Cladonia squamosa | 96.77 − 1.93x + 0.01x2 | 0.84 | 22.73 | 105.83 − 0.63x | 0.82 | 14.64 |
| Predne | 450 | Cladonia squamosa | 99.58 − 1.86x + 0.01x2 | 0.99 | 19.39 | 94.67 − 1.15x | 0.94 | 30.04 |
| Predne | 150 | Pleurozium schreberi | 101.88 − 1.15x | 0.98 | 30.06 | |||
| Predne | 450 | Pleurozium schreberi | 103.37 − 2.99x + 0.02x2 | 0.93 | 32.94 | |||
| Predne | 150 | Polytrichastrum alpinum | 99.11 − 2.94x + 0.02x2 | 0.99 | 28.94 | |||
| Predne | 450 | Polytrichastrum alpinum | 97.95 − 3.65x + 0.03x2 | 0.97 | 33.33 | |||
| Predne | 150 | Thamnolia vermicularis | 99.98 − 0.68x | 0.99 | 18.24 | 96.74 − 2.82x + 0.02x2 | 0.84 | 25.52 |
| Predne | 450 | Thamnolia vermicularis | 94.95 − 0.93x | 0.97 | 26.15 | |||
| Vyšne | 150 | Pleurozium schreberi | 97.69 − 2.2x + 0.02x2 | 0.93 | 21.11 | 100.51 − 0.63x | 0.99 | 15.81 |
| Vyšne | 450 | Pleurozium schreberi | 97.75 − 3.32x + 0.03x2 | 0.97 | 31.83 | 103.94 − 0.86x | 0.93 | 22.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscová, V.; Ševčík, M.; Sedlák, A.; Hreško, J.; Petrovič, F.; Slobodová, T. Resistance of Lichens and Mosses of Regenerated Alpine Communities to Repeated Experimental Trampling in the Belianske Tatras, Northern Slovakia. Diversity 2023, 15, 128. https://doi.org/10.3390/d15020128
Piscová V, Ševčík M, Sedlák A, Hreško J, Petrovič F, Slobodová T. Resistance of Lichens and Mosses of Regenerated Alpine Communities to Repeated Experimental Trampling in the Belianske Tatras, Northern Slovakia. Diversity. 2023; 15(2):128. https://doi.org/10.3390/d15020128
Chicago/Turabian StylePiscová, Veronika, Michal Ševčík, Andrej Sedlák, Juraj Hreško, František Petrovič, and Terézia Slobodová. 2023. "Resistance of Lichens and Mosses of Regenerated Alpine Communities to Repeated Experimental Trampling in the Belianske Tatras, Northern Slovakia" Diversity 15, no. 2: 128. https://doi.org/10.3390/d15020128
APA StylePiscová, V., Ševčík, M., Sedlák, A., Hreško, J., Petrovič, F., & Slobodová, T. (2023). Resistance of Lichens and Mosses of Regenerated Alpine Communities to Repeated Experimental Trampling in the Belianske Tatras, Northern Slovakia. Diversity, 15(2), 128. https://doi.org/10.3390/d15020128





