Abstract
The success of plant reproduction is highly dependent on effective seed dispersal. This study aimed to evaluate the potential seed dispersal effectiveness of cattle for Malus sieversii. The impact of cattle on the dispersal quantity and dispersal quality of M. sieversii seeds was explored based on camera trapping, GPS tracking, and germination trials. The results showed that, on average, cattle visited M. sieversii trees 477.33 times during a two-month observation period. Out of these visits, 315 were specifically for fruit removal. The fruit removal rate per cattle visit was as high as 96.67%. Additionally, cattle were able to disperse M. sieversii seeds up to a maximum distance of 533.67 m, with an average dispersal distance of 134.62 m. The average distance of cattle movement was recorded as 176.95 m/h, with peak activity observed during 11:00–13:00 and 19:00–21:00. The germination rate of M. sieversii seeds that passed through the digestive tract of cattle was significantly higher than that of control seeds. Finally, the emergence rate and survival rate of seeds dispersed by cattle to forest edges and gaps were significantly higher than those dispersed to understory. These findings suggest that cattle can serve as effective long-distance dispersers of M. sieversii seeds and may play a crucial role in the regeneration and expansion of M. sieversii populations in the Ili Botanical Garden.
1. Introduction
Seed dispersal is an important ecological process that involves the movement of plant seeds away from the parent plant and toward suitable germination habitats [1]. This process plays a crucial role in the persistence of plant populations and the maintenance of species diversity in communities [2]. Seed dispersal can occur through various means, including wind, water, and animals [3]. Among these, animal dispersal is particularly significant as it supports the natural regeneration cycles of approximately 60–80% of plant species [4]. Animal dispersal involves three main mechanisms: epizoochory [5], synzoochory [6], and endozoochory [7]. However, endozoochory is widely recognized as the predominant animal dispersal mechanism [8]. Endozoochory is a common seed dispersal strategy in which animals consume fruits and subsequently disperse the seeds through processes like ruminating, chewing, or excretion [9]. Animals are able to detect important information, such as smell or color, and identify fruits as a valuable food resource [10,11]. As animals consume the fruits, the seeds pass through their digestive tracts and are dispersed, allowing plants to colonize new environments [12,13]. This leads to the formation of a distinct mutualistic network between animals and plants [3]. Within a mutualistic network, plants often interact with various types of dispersal animals [14]. However, the contributions of different animals to plant regeneration vary depending on the seed dispersal effectiveness [15,16,17].
Seed dispersal effectiveness serves as an indicator of the mutualistic relationship between plants and animals and is utilized to assess the role of animals in plant regeneration [18,19]. Over the last 15 years, seed dispersal by frugivores has been widely studied in primates [10,20], frugivorous birds [21,22], tortoises [23], and ungulates [24]. At the same time, our knowledge of seed dispersal effectiveness by frugivores has also greatly increased. Large frugivores have a high level of seed dispersal because they can consume more fruits, disperse more seeds, and move the seeds further away than smaller species [25,26]. For example, the larger Onychognathus tristramii provides a longer dispersal distance for Ochradenus baccatus seeds, while the smaller Pycnonotus xanthopygos has a shorter seed dispersal distance [16]. However, the renewal of plant populations depends not only on how many seeds are consumed and dispersed, and how far they are dispersed, but also on whether the seeds can be dispersed by frugivores to suitable habitats for seedling growth, and whether the digestive processes are beneficial for seed germination [27]. Therefore, evaluating the seed dispersal effectiveness of frugivores requires comprehensive quantitative and qualitative studies on all the impacts caused by endozoochory.
Quantifying seed dispersal by frugivores is crucial for expanding our understanding of the complex dispersal systems of plants [28]. Seed dispersal effectiveness by frugivores depends on both the “quantity” and “quality” of dispersal [29]. The quantity focuses on the frequency and intensity of animals’ visits to the mother plant, which is quantified by the volume of visits and the amount of fruit or seeds removed within a specific time [18,30]. On the other hand, the quality revolves around the conditions that enable seed germination and seedling establishment throughout the animal dispersal process [30]. These conditions are evaluated through factors such as the distance of seed dispersal, the location of seeds after passing through the digestive tract, and the germination rate [27].
Malus sieversii (Lebed.) M. Roem., belonging to the genus Malus (Rosaceae), is a woody plant commonly found on sunny or semi-shady slopes of mountainous areas at altitudes of 900–1700 m [31]. It holds significant importance as a component of the global apple gene bank and is considered one of the ancestors of many modern cultivated apple varieties [32]. The natural distribution range of this species includes Kazakhstan, Kyrgyzstan, Tajikistan, and China [33]. In China, it is primarily found in the Ili River Valley and the Tacheng area of Xinjiang [34]. The population of M. sieversii exhibits a rich genetic diversity, which plays a significant role in maintaining the stability of regional ecosystems, soil and water conservation, and biodiversity [35]. Over a long process of evolution, it has acquired excellent traits such as cold resistance, drought tolerance, and disease resistance [36,37]. Unfortunately, in the past three decades, the natural regeneration of M. sieversii has faced obstacles due to climate change, disease outbreaks, pests, and human disturbances [31,38,39].
In consideration of the significant value of M. sieversii, various studies have been conducted to safeguard its biological resources [36,37,40]. Presently, research on M. sieversii primarily focuses on resource surveys, disease and pest status, and genetic characteristics of the population, with limited attention given to animal-mediated seed dispersal [38,41,42]. Based on our field observations at the Ili Botanical Garden, we found that various grazing livestock and wild animals consume the ground fruits of M. sieversii during its fruiting period (July–September). Additionally, we discovered that the distribution area of M. sieversii is abundant with cattle feces containing its seeds and seedlings (Figure S1). Xu et al. (2022) found that recovery rate of intact seeds of M. sieversii seeds after passing through the digestive tract of cattle was as high as 54.05%, and the recovered seed vitality was 100% [43]. However, comprehensive studies on the seed dispersal effectiveness of M. sieversii by cattle are still lacking.
In this study, we aimed to evaluate the potential seed dispersal effectiveness of cattle for M. sieversii by surveying cattle-visit frequency and intensity to M. sieversii, quantifying seed dispersal distances, and testing seed germination, emergence, and survival rates after passing through the cattle digestive tract. The results of this study will not only offer theoretical support for the recovery of M. sieversii populations but also provide practical grazing recommendations for local forestry management departments.
2. Materials and Methods
2.1. Study Site
The study was conducted at the Ili Botanical Garden (83°34′25″–83°37′39″ E, 43°20′14″–43°27′00″ N), located in Xinyuan County, Xinjiang, China (Figure 1). The site has a temperate continental climate [44], characterized by an average elevation of 1360 m, an annual average temperature of 7.7 °C, and an average annual precipitation of 479 mm. The soil in the study area is classified as mountainous black-brown soil, known for its high content of carbonates and basic substances, which contribute to its exceptional fertility [44]. The botanical garden, which covers over 3000 acres in the wild fruit forest, is mainly for the in-site conservation of wild fruit trees. The study area was located around the conservation district separated by fences and was frequently utilized as a summer and autumn pasture. This area shares the same native vegetation as the conservation district. The dominant plant species of this native vegetation is M. sieversii, which is accompanied by woody plants including Prunus cerasifera, Juglans regia, and Crataegus cuneata [45,46]. Additionally, dominant herbaceous plants mainly include Urtica fissa, Arctium lappa, and Dactylis glomerata [42,46].
Figure 1.
(A) Map of China highlighting the geopolitical division of Xinjiang (green); (B) Ili Ka-zakh autonomous prefecture (yellow) showing Xinyuan County (red).
2.2. Survey of Animals Visiting Ground Fruits
For the survey on the species of animals visiting M. sieversii ground fruits and the role of cattle among these species, we conducted a camera trapping experiment during the fruiting period of M. sieversii in 2022. Three productive mother trees were selected in the study area and located at the following coordinates: 83°36′14″ E, 43°22′41″ N; 83°36′13″ E, 43°23′37″ N; and 83°36′16″ E, 43°23′33″ N. These trees were separated by more than 100 m. Infrared cameras (HC900Pro, Shenzhen Zhen Shijie Technology Co., Ltd., Shenzhen, China) were placed on the trees and adjusted to monitor fallen fruit on the ground (Figure 2). Continuous filming was conducted for a duration of 2 months, covering the entire fruiting period of the fruit tree. Photos and videos captured by the cameras were used to record information about the species of visiting animals, visit frequency, visit time, and visit behaviors. Animal species were identified by examining characteristics such as fur color, tail length, and body length, as well as animal expert identification [47].
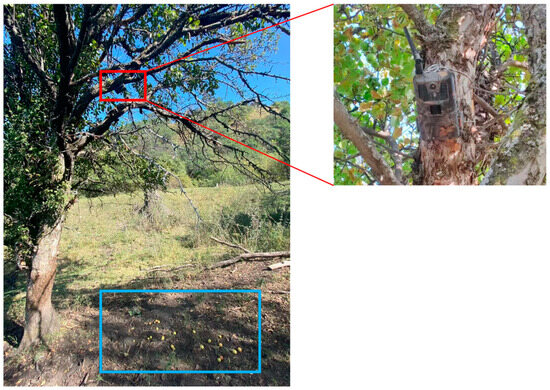
Figure 2.
Sampling station using infrared camera trapping. Infrared cameras placed at 2.50 m height focused on 20 Malus sieversii fruits. The red box highlights where a camera was placed on a tree to observe visiting animals, while the blue box highlights the ground fruits.
To evaluate the fruit removal rate of visiting animals and the role of cattle among these species, we conducted a ground-fruit placement experiment during the late fruiting period of M. sieversii in 2022. In order to minimize bias resulting from fruit drop from the mother trees, we used M. sieversii fruits collected from the study site. We placed 20 fruits on the ground where cameras could monitor them every day (Figure 2), and this process was repeated for seven consecutive days. By recording the number of fruits supplied and the duration of the survey, we were able to quantify the removed fruits and identify the animal species visiting the ground fruits [48,49]. The fruit removal rate of the visiting animals could be determined by inferring the number of fruits removed based on the recording time.
To minimize the chances of the infrared cameras being falsely triggered, we cleared the vegetation within a 1 square meter area around the mother tree. Before conducting the experiment, we tested and determined the optimal settings. Photography was initiated within 0.5 s of detecting movement. The captured content included three consecutive photos and a 10 s video, with a 30 s interval between each capture. This setting shares similarities with the approach proposed by Prasad et al. (2010) [50]. The following situations were considered independent visit events: consecutive records of individuals of different species or consecutive records of individuals of the same species with a time interval exceeding 10 min.
2.3. Estimation of Seed Dispersal Distance by Cattle
The seed dispersion pattern was estimated by integrating the movement trajectory distribution of cattle during seed retention in the digestive tract after the cattle had consumed fruits [51]. Xu et al. (2022) observed that the seeds were no longer detectable in the cattle’s feces on the sixth day following the ingestion of M. sieversii seeds [43]. To estimate the dispersal distance of seeds by cattle, we conducted GPS-tracking experiments during the fruiting period of M. sieversii in 2022. We fitted three adult cattle (weights ranging from 450 to 500 kg) with global positioning system (GPS) electronic collars (ZG001, Hongwei Xintong Technology Co., Ltd., Shenzhen, China). The cattle were released from a common starting point (representing the position of the mother trees) and allowed to move freely without supplementary feed for six consecutive days. All experimental procedures involving animals were approved by the Animal Welfare and Ethics Committee of Xinjiang Agricultural University, Urumqi, Xinjiang, China; animal protocol number: 2021081.
The electronic collar used in this study has a positioning accuracy of 2–3 m and weighs 350 g, which is approximately 0.08% of the cattle’s body weight. The collar records the location information of the cattle every 10 min. In this experiment, the start time was 15:00. Therefore, the first recorded data after 15:00 each day were considered as the starting point of the cattle’s movement. Duplicate data records from the collar were removed. In theory, the collar can record 144 data points within 24 h. After filtering, a minimum of 130 data points per day (approximately 90%) were considered valid. This is because signal issues can cause significant displacement in the recorded location information, which can affect the accuracy of the data. To calculate the movement distance based on the coordinate positions, the following formula is used:
In the equation, Δlat and Δlon correspond to the variations in latitude and longitude, respectively, R symbolizes the Earth’s radius, and d denotes the distance between the two coordinates.
2.4. Germination Trials
Before commencing the experiment, we conducted a seed recovery experiment to collect seeds that had passed through the digestive tract. To minimize experimental errors resulting from different seed sources [52], we collected seeds from a single mother tree and stored them in envelopes for further testing. We selected one adult cattle and isolated it in a dedicated room with sufficient water and forage in the study area. When the cattle had adapted to the environment, we incorporated a portion of the collected seeds into its feed and ensured that all feed was consumed. The cattle’s feces were collected daily and washed in an 8-mesh wire sieve to recover M. sieversii seeds. The recovered seeds were subsequently washed, dried, and stored in envelopes until the germination experiment began.
2.4.1. Seed Germination Experiment in Laboratory
To investigate the impact of cattle on the germination of M. sieversii seeds that have passed through the digestive tract, we conducted seed germination experiments in a laboratory. Based on Baskin’s (1998) classification criteria of seed dormancy, M. sieversii seeds need to undergo a period of cold stratification before germination [53]. Liu et al. (2021) found that M. sieversii seeds need 60–70 d of cold stratification to overcome dormancy and achieve peak germination rates at 80 d of cold stratification [54]. We obtained collected seeds (control seeds) and recovered seeds (post-digestion seeds), which were then transported to the laboratory. After 80 d of cold stratification, the seeds were extracted, washed with distilled water, and 25 seeds from each treatment group were placed in petri dishes with two filter papers to initiate germination. The germination experiment was conducted in a controlled laboratory with 12 h of light at 25 °C and 12 h of darkness at 15 °C, with three replicates for each treatment. Seed germination was determined when the embryonic root penetrated the seed coat. The experiment was terminated if no seeds germinated within 1 week, and the number of germinated seeds was recorded.
2.4.2. Seed Germination Experiment in Wild
To investigate the seedling emergence and survival of M. sieversii seeds deposited in the wild after passing through the digestive tract of cattle, we conducted seed germination experiments in the wild. Zhang et al. (2022) found that the dispersal range of M. sieversii seed rain without seed dispersers was mainly concentrated under the crown of the mother tree [55]. Our field surveys found that M. sieversii seeds can be excreted by cattle into three habitats: forest edges, gaps, and understory (Figure S2). In addition, our field survey of 30 cattle feces found that fresh feces with seeds contained an average of 6.5 seeds per 100 g of feces.
During the late fruiting period of M. sieversii in 2022, we selected a 1 × 1 m sample area with relatively flat terrain in different habitats for germination experiments. Treatment W: 30 recovered seeds (post-digestion seeds) were inserted into 500 g of cattle feces and placed in the center of soil-filled flowerpots; the feces were collected when there were no M. sieversii seeds in the digestive tract of the cattle. Treatment W was placed in three habitats (forest edges, gaps, and understory) and replicated three times in each habitat. Control treatment C: 30 collected seeds (control seeds) were sown in soil-filled flowerpots to a depth of 0.02 m; the soil was in situ soil. Treatment C was placed in the forest understory and replicated three times. The plastic flowerpot was 0.16 m in diameter and 0.15 m in depth. The placement method was to bury it in the soil so that the upper edge was flush with the ground. To avoid disturbances, such as human activities and animal trampling, closed cages with dimensions of 0.7 × 0.3 × 0.3 m made of wire sieve (8 mesh) were used to cover the three replicates of each treatment.
During the seed germination period of M. sieversii in April 2023, we observed seedling emergence in different habitats. The seedling emergence criterion was that the cotyledons broke through the surface of the substrate. Seedling emergence was recorded every 48 h, and seedling survival was counted when the seedlings grew to 30 d. The emergence rate and survival rate were calculated by the following formulas:
Seedling emergence rate = (number of emergence/total number of seeds) × 100%
Seedling survival rate = (number of survival/total number of emergence) × 100%
2.5. Statistical Analyses
Before conducting the statistical tests, the data were evaluated for normality and the homogeneity of variances. Outliers were identified and removed using box plots. One-way analysis of variance (ANOVA) was utilized to evaluate the significance of differences among the various groups based on animal visitation data. T-tests were conducted to examine the significance of differences in seed germination rate, seedling emergence rate, and seedling survival rate data between groups. Non-normally distributed data were analyzed using a nonparametric test (Kruskal–Wallis test). The data were analyzed using SPSS 23.0 software (SPSS Inc., Chicago, IL, USA) for statistical analysis, and graphs were created using Origin 2021 and ArcGIS (ArcMap 10.8 Esri., Redlands, CA, USA).
3. Results
3.1. Animals Visiting Ground Fruits
During the period from the end of July to the end of September, infrared cameras were utilized for continuous shooting, resulting in a collection of 8894 photos and videos. Out of these, 3590 photos and videos had unambiguous records of the animals’ visiting behavior. By analyzing the characteristics of these animals, the animal species that visited the ground fruits of M. sieversii are shown in Figure 3. These animal species include cattle (Bos taurus), horses (Equus caballus caballus), wild boars (Sus scrofa), roe deer (Capreolus pygargus), badgers (Meles meles), and red foxes (Vulpes vulpes). It was observed that five of these species removed ground fruits and exhibited predatory behavior by swallowing the fruits whole.

Figure 3.
Visiting animal species of Malus sieversii ground fruits (photos taken by camera). (A) Bos taurus; (B) Equus caballus caballus; (C) Sus scrofa; (D) Capreolus pygargus; (E) Meles meles; (F) Vulpes vulpes.
The visit records of various animals to the ground fruits of M. sieversii within two months and the removal rate of ground fruits within seven days are shown in Table 1; the corresponding raw data are shown in Tables S1 and S2. Significant differences were observed in the total number of visits, visits with fruit removal, and fruit removal rates among the various animals (Kruskal–Wallis test, p = 0.013, p = 0.015, p = 0.012, respectively). Among them, cattle showed the highest seed dispersal effectiveness, as indicated by the combination of frequency (number of visits) and intensity (fruit removal rate) of cattle visits to the ground fruits. Cattle searched for and consumed almost all the fruits on the ground throughout the entire 24 h period. However, no observed behavior of red foxes removing ground fruits was documented.

Table 1.
Visiting animal species of Malus sieversii ground fruits (data obtained by camera trapping). Data include the total number of visits (frequency of visits), visits with fruit removal, visiting behaviors, consumption time by animals within a two-month period, and the rate of fruits removed (intensity of visits) by animals within a seven-day period. Values are mean ± SE (n = 3).
3.2. Seed Dispersal Distance by Cattle
A total of 2794 GPS location records were collected during the retention period of M. sieversii seeds in the cattle’s digestive tract. After filtering, 2586 coordinates were identified as valid data points. The activity range and trajectory distribution of three cattle in the study area are shown in Figure 4; the corresponding raw data are shown in Table S3. The figure shows that the starting point represents the simulated position of the mother tree, while the blue, yellow, and red dots represent the movement trajectories of Cattle 1, Cattle 2, and Cattle 3, respectively. The farthest trajectory point from the starting point is recognized as the farthest distance for seed dispersal through the cattle’s digestive tract. According to the statistical analysis, cattle attained a farthest dispersal distance of 533.67 m for M. sieversii seeds, with an average dispersal distance of 134.62 m. Field surveys based on the distribution of cattle trajectories indicated that they could disperse M. sieversii seeds to three habitats: forest edges, gaps, and understory (Figure S2).
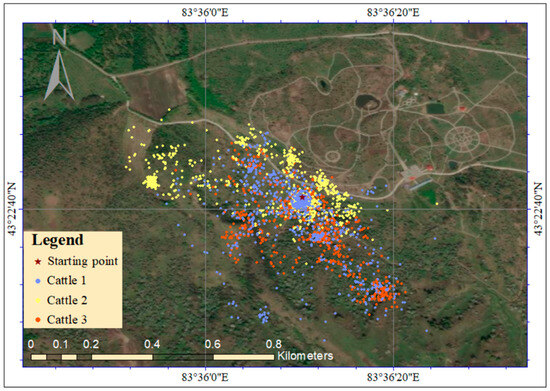
Figure 4.
The activity trajectory of cattle in the study area.
By quantitatively analyzing the movement trajectory data of three cattle during grazing, the average distance of cattle at different times are shown in Figure 5; the corresponding raw data are shown in Tables S4 and S5. The hourly movement distances of the cattle ranged from 27.00 to 795.95 m, with an average movement distance of 176.95 m. They primarily rested during 23:00 and 07:00 the following day, and became active after 07:00 in the morning. Peak activity periods were observed between 11:00 and 13:00 and between 19:00 and 21:00.
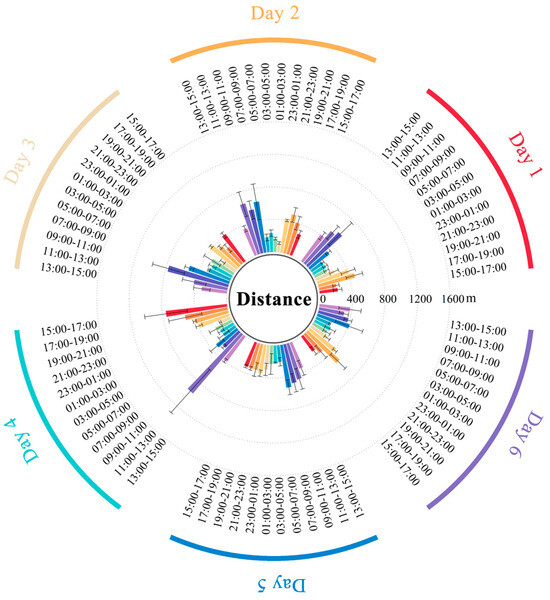
Figure 5.
The average distance of cattle at different times. Values are mean ± SE (n = 3).
3.3. Seed Germination
The results of the final germination rate (after 30 days) of M. sieversii seeds after cold stratification treatment are shown in Figure 6; the corresponding raw data are shown in Table S6. A significant difference in germination rate was observed between seeds that passed through the cattle’s digestive tract and those that did not (T-test, p = 0.008). The seeds that passed through the cattle’s digestive tract showed a faster germination speed at the initial stage, as well as a longer germination duration, compared to the control seeds. Additionally, the germination rate of seeds that passed through the cattle’s digestive tract was 72%, which was significantly higher than that of the control seeds.
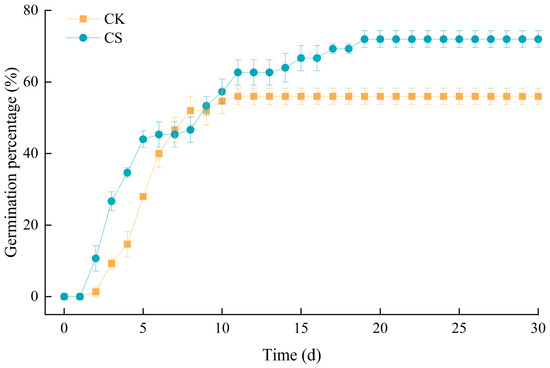
Figure 6.
Germination of Malus sieversii seeds as a function of time. CK are control seeds; CS are post-digestion seeds. Values are mean ± SE (n = 3).
3.4. Seedling Emergence and Survival
The emergence and survival rates of M. sieversii seedlings are shown in Table 2; The corresponding raw data are shown in Table S7. The emergence rate of M. sieversii seedlings in the forest edges and gaps of treatment W are significantly higher than that of control treatment C (T-test, p = 0.001, p = 0.015, respectively). The survival rate of M. sieversii seedlings in the forest edges and gaps reached 100% for treatment W (T-test, p = 0.001, p = 0.001, respectively). In the forest understory, there was no significant difference in the seedling emergence and survival rates between treatment W and control treatment C.

Table 2.
Emergence and survival of Malus sieversii seedlings. Treatment W is the post-digestion seeds in the cattle feces; treatment C is the control seeds in the soil. Values are mean ± SE (n = 30).
4. Discussion
Seed dispersal effectiveness by frugivores depends on both quantity and quality [29]. In terms of quantity, a higher frequency of animal visits results in a greater number of seeds dispersed per visit, thereby increasing the seed dispersal range and the chances of plant population renewal [56,57]. In terms of quality, the seeds are dispersed by animals to suitable habitats far from the mother tree and the seed germination rate increases after passing through the animal digestive tract, both of which can contribute to the seeds successfully establishing new individuals in the new environment [27].
4.1. Seed Dispersal Quantity of Cattle
Within seed dispersal networks, various animal groups display unique characteristics in the efficient dispersal of seeds, resulting in variations in their contributions to the reproduction of plants [29]. In this study, six different types of animals were observed visiting the ground fruits of M. sieversii (Figure 3). It was confirmed that all five of the animals that consumed the fruits were frugivorous, which supports previous research on the role of frugivores in seed dispersal [58]. However, the red fox studied in this research did not exhibit any predatory behavior toward the ground fruits. This could be attributed to its dietary preferences and feeding habits, as previous studies have shown that red foxes tend to prefer small mammals, birds, and insects as their main food, and display less predatory behavior toward fruits [59]. Further analysis of the animal visitation records revealed that cattle were frequently active around M. sieversii trees and showed a preference for the fruits. This preference may be due to the appealing scent or nutritional compounds of the fruits [3]. Additionally, compared to other animals, cattle exhibited significantly higher total visits, visits with fruit removal, and fruit removal rates (Table 1). These findings further emphasize the role of cattle in the seed dispersal of M. sieversii and confirm their positive contribution to the effectiveness of seed dispersal. This supports the previous results of Sengupta (2022) who found that cattle demonstrate a high rate of predation and possess effective seed dispersal capabilities while consuming fruits [24]. Cattle could visit and consume the fruits of M. sieversii on the ground at any time during the day (Table 1), which supports previous research on cattle as seed dispersers [1]. Cattle have the potential to engage in seed dispersal during various time periods, thereby widening the temporal window and increasing opportunities for seed dispersal, which is crucial for the success of seed dispersal.
4.2. Seed Dispersal Quality of Cattle
Long-distance dispersal commonly refers to the dispersal of seeds of more than 100 m by wind, water, or animals [60]. This process plays a vital role in ecosystem functioning and is strongly linked to species diversity, genetic diversity, and population dispersal. In this study, the dispersal of M. sieversii seeds by large-sized cattle was found to be consistent with the characteristics of long-distance dispersal. Through their predation and excretory activities, cattle could disperse seeds to distant locations from the parent tree, thereby facilitating the migration of species and enhancing genetic diversity within populations. This supports the previous results of Schupp (1993) who found that larger avian and mammalian species typically exhibit longer dispersal distances and higher effectiveness in dispersal [61].
Understanding the activity rhythm of animals is beneficial for the rational utilization of natural resources and provides valuable guidance for managing grazing livestock and advancing animal husbandry [62]. In much of the research, it was found that livestock in grazing systems have a wide range of movement, with cattle often covering distances of several hundreds of meters within an hour [63]. In our study, a quantitative analysis of cattle movement patterns revealed an average hourly displacement distance of approximately 176.95 m. Additionally, it was found that cattle exhibited higher activity levels during specific time intervals, with the highest activity levels observed from 11:00 to 13:00 and 19:00 to 21:00. This supports the previous results of Hou et al. (2014) who found a peak time of cattle activity of 18:00–20:00 [63]. The feeding behavior of livestock mainly occurs during the day, especially around sunrise and sunset, with rare foraging at night [62]. Our study findings can provide a reference value for local forestry management departments to manage grazing time on the basis of protecting M. sieversii.
The effects of the animal digestive tract on seed germination are crucial in evaluating seed dispersal effectiveness. This includes three possible outcomes: promotion, inhibition, or no effect [64,65,66]. This study found that the germination rate of M. sieversii seeds significantly increased after they passed through the cattle digestive tract. This improvement may be due to the elimination of seed germination inhibitors during digestion or an increased permeability of the seed coat, making it easier to break. This supports the previous results of Soltani et al. (2018) who found that the germination rate of medium-sized seeds with physiological dormancy increases after passing through the digestive tract [65].
Effective seed dispersal depends not only on the increased germination rate of seeds after passing through the digestive tract but also on animals dispersing seeds in habitats that are conducive to successful seedling establishment [67,68]. This study found that cattle can disperse seeds of M. sieversii to various habitats (forest edges, gaps, and understory) (Figure S2). This result is similar to that of Campos et al. (2011) who found that seeds held in the cattle’s digestive tract can be dispersed to grasslands, shrubs, and forests [69]. Furthermore, this study found that compared to the seedling emergence and survival rates of M. sieversii seeds dispersed to the forest understory through seed rain without seed dispersers those dispersed to the forest edges and gaps by cattle were significantly higher and those dispersed to the forest understory by cattle had no significant difference. This difference could be attributed to the more favorable lighting conditions provided by forest edges and gaps, which are conducive to M. sieversii seedling emergence and survival [40]. Additionally, cattle feces may create suitable temperature and humidity conditions that promote seedling emergence [70]. Another factor that may contribute to the lower survival rate in the forest understory is the Janzen–Connell effect, which means that the same species of seedlings adjacent to the mother tree are attacked by specific soil pathogens, resulting in a lower survival rate as the distance from the mother tree decreases [71]. However, the presence of cattle can help disperse the seeds of M. sieversii to habitats that are far away from the mother tree, which may potentially avoid the negative impact of the Janzen–Connell effect. All these factors indicate that the seed dispersal of M. sieversii by cattle increases the chances of successful seedling establishment in the wild.
5. Conclusions
This study highlights the significant role of cattle in the effective dispersal of M. sieversii seeds in the wild. While cattle disperse a significantly higher number of M. sieversii seeds compared to other animal species that visit ground fruits, they also have the ability to disperse seeds to different habitats far from the mother tree, promoting seed germination and seedling establishment. Based on these findings, we suggest that local forestry management departments allow reasonable cattle grazing during the M. sieversii fruiting period in the wild fruit forest. This will facilitate the long-distance dispersal of M. sieversii seeds. Future research can explore the seed dispersal effectiveness of various animals on M. sieversii, which can help evaluate the role of different animals as seed spreaders in biodiversity conservation and maintaining ecosystem functioning in the wild fruit forest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15121205/s1, Figure S1. Cattle feces containing Malus sieversii seeds and seedlings observed in the wild; Figure S2. Malus sieversii seeds dispersed to three habitats by cattle; Table S1. Visiting records of various animal species to Malus sieversii ground fruits; Table S2. Removal information of 20 Malus sieversii fruits placed on the ground by various animals; Table S3. Average dispersal distance of cattle to Malus sieversii seeds (m); Table S4. Average movement distance of cattle every hour (m); Table S5. Average movement distance of cattle at different times (m); Table S6. Cumulative daily germination number of seeds after cold stratification. Table S7. Emergence rate and survival rate of Malus sieversii seedlings in different treatments.
Author Contributions
Conceptualization, X.S. and D.T.; methodology, S.B. and X.S.; investigation, S.B., J.X. and Y.L.; data curation, S.B. and J.X.; writing—original draft preparation, S.B. and X.S.; writing—review and editing, S.B. and X.S.; visualization, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31960229, and the Natural Science Foundation of Xinjiang Uygur Autonomous Region of China, grant number 2017D01B17.
Institutional Review Board Statement
All experimental procedures involving animals were approved by the Animal Welfare and Ethics Committee of Xinjiang Agricultural University, Urumqi, Xinjiang, China (animal protocol number: 2021081).
Data Availability Statement
Readers can obtain all research data from this article upon reasonable request.
Acknowledgments
We thank all the staff of the Ili Botanical Garden, Xinjiang, for their support and help in our work. At the same time, we would like to thank Shi Lei from Xinjiang Agricultural University for identifying the animals captured by our camera. In addition, we would like to express our gratitude to the anonymous reviewers for their valuable revision comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Jordano, P.; Forget, P.-M.; Lambert, J.E.; Böhning-Gaese, K.; Traveset, A.; Wright, S.J. Frugivores and seed dispersal: Mechanisms and consequences for biodiversity of a key ecological interaction. Biol. Lett. 2011, 7, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.C.; Smith, T.B. Closing the seed dispersal loop. Trends Ecol. Evol. 2002, 17, 379–386. [Google Scholar] [CrossRef]
- Mouissie, A.M.; Lengkeek, W.; Diggelen, R.V. Estimating adhesive seed-dispersal distances: Field experiments and correlated random walks. Funct. Ecol. 2005, 19, 478–486. [Google Scholar] [CrossRef]
- Casper, B.B.; Heard, S.B.; Apanius, V. Ecological correlates of single-seededness in a woody tropical flora. Oecologia 1992, 90, 212–217. [Google Scholar] [CrossRef]
- Traveset, A.; Robertson, A.W.; Rodríguez-Pérez, J. A Review on the Role of Endozoochory in Seed Germination. In Seed Dispersal: Theory and Its Application in a Changing World; CABI Pub.: Wallingford, UK, 2007; pp. 78–103. ISBN 978-1-84593-165-0. [Google Scholar]
- Brochet, A.L.; Guillemain, M.; Fritz, H.; Gauthier-Clerc, M.; Green, A.J. Plant dispersal by teal (Anas crecca) in the Camargue: Duck guts are more important than their feet: Plant dispersal by teal. Freshw. Biol. 2010, 55, 1262–1273. [Google Scholar] [CrossRef]
- Vander Wall, S.B.; Beck, M.J. A comparison of frugivory and scatter-hoarding seed-dispersal syndromes. Bot. Rev. 2012, 78, 10–31. [Google Scholar] [CrossRef]
- McConkey, K.R. Seed dispersal by primates in Asian habitats: From species, to communities, to conservation. Int. J. Primatol. 2018, 39, 466–492. [Google Scholar] [CrossRef]
- Zwolak, R.; Sih, A. Animal personalities and seed dispersal: A conceptual review. Funct. Ecol. 2020, 34, 1294–1310. [Google Scholar] [CrossRef]
- Naniwadekar, R.; Mishra, C.; Datta, A. Fruit resource tracking by hornbill species at multiple scales in a tropical forest in India. J. Trop. Ecol. 2015, 31, 477–490. [Google Scholar] [CrossRef]
- Butler, H.C.; Johnson, S.D. Seed dispersal by monkey spitting in Scadoxus (Amaryllidaceae): Fruit selection, dispersal distances and effects on seed germination. Austral. Ecol. 2022, 47, 1029–1036. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Jordano, P.; García, C.; Godoy, J.A.; García-Castaño, J.L. Differential contribution of frugivores to complex seed dispersal patterns. Proc. Natl. Acad. Sci. USA 2007, 104, 3278–3282. [Google Scholar] [CrossRef]
- Spiegel, O.; Nathan, R. Incorporating dispersal distance into the disperser effectiveness framework: Frugivorous birds provide complementary dispersal to plants in a patchy environment. Ecol. Lett. 2007, 10, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Calviño-Cancela, M.; Martín-Herrero, J. Effectiveness of a varied assemblage of seed dispersers of a fleshy-fruited plant. Ecology 2009, 90, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.P.; Morris, W.F.; Jordano, P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants: Total effect of animal mutualists on plants. Ecol. Lett. 2005, 8, 1088–1094. [Google Scholar] [CrossRef]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. A general framework for effectiveness concepts in mutualisms. Ecol. Lett. 2017, 20, 577–590. [Google Scholar] [CrossRef]
- Haurez, B.; Tagg, N.; Petre, C.-A.; Brostaux, Y.; Boubady, A.; Doucet, J.-L. Seed dispersal effectiveness of the western lowland gorilla (Gorilla gorilla gorilla) in Gabon. Afr. J. Ecol. 2018, 56, 185–193. [Google Scholar] [CrossRef]
- Quintero, E.; Pizo, M.A.; Jordano, P. Fruit resource provisioning for avian frugivores: The overlooked side of effectiveness in seed dispersal mutualisms. J. Ecol. 2020, 108, 1358–1372. [Google Scholar] [CrossRef]
- Cárdenas, S.; Echeverry-Galvis, M.Á.; Stevenson, P.R. Seed dispersal effectiveness by oilbirds (Steatornis caripensis) in the Southern Andes of Colombia. Biotropica 2021, 53, 671–680. [Google Scholar] [CrossRef]
- Jerozolimski, A.; Ribeiro, M.B.N.; Martins, M. Are tortoises important seed dispersers in Amazonian forests? Oecologia 2009, 161, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A. Animal-mediated seed dispersal in India: Implications for conservation of India’s biodiversity. Biotropica 2022, 54, 1320–1330. [Google Scholar] [CrossRef]
- Pérez-Méndez, N.; Jordano, P.; Valido, A. Downsized mutualisms: Consequences of seed dispersers’ body-size reduction for early plant recruitment. Perspect. Plant Ecol. 2015, 17, 151–159. [Google Scholar] [CrossRef]
- Naniwadekar, R.; Rathore, A.; Shukla, U.; Chaplod, S.; Datta, A. How far do Asian forest hornbills disperse seeds? Acta Oecologica 2019, 101, 103482. [Google Scholar] [CrossRef]
- Li, N.; Zhong, M.; Leng, X.; Wang, A.; Fang, S.B.; An, S.Q. Seed dispersal effectiveness of plant by frugivores: A review. Chin. J. Ecol. 2015, 34, 2041–2047. [Google Scholar] [CrossRef]
- Camargo, P.H.S.A.; Martins, M.M.; Feitosa, R.M.; Christianini, A.V. Bird and ant synergy increases the seed dispersal effectiveness of an ornithochoric shrub. Oecologia 2016, 181, 507–518. [Google Scholar] [CrossRef]
- González-Castro, A.; Calviño-Cancela, M.; Nogales, M. Comparing seed dispersal effectiveness by frugivores at the community level. Ecology 2015, 96, 808–818. [Google Scholar] [CrossRef]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed dispersal effectiveness revisited: A conceptual review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef]
- Chu, J.Y.; Feng, L.J.; Hou, Y.X.; Lu, B.; Wang, Q.; Zhou, L.; Wang, J. Analysis on population damage of Malus sieversii. Non Wood For. Res. 2022, 40, 265–273. [Google Scholar] [CrossRef]
- Duan, N.; Bai, Y.; Sun, H.; Wang, N.; Ma, Y.; Li, M.; Wang, X.; Jiao, C.; Legall, N.; Mao, L.; et al. Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat. Commun. 2017, 8, 249. [Google Scholar] [CrossRef]
- Lamboy, W.F.; Yu, J.; Forsline, P.L.; Weeden, N.F. Partitioning of allozyme diversity in wild populations of Malus sieversii L. and implications for germplasm collection. J. Am. Soc. Hortic. Sci. 1996, 121, 982–987. [Google Scholar] [CrossRef]
- Yang, M.; Li, F.; Long, H.; Yu, W.; Yan, X.; Liu, B.; Zhang, Y.; Yan, G.; Song, W. Ecological distribution, reproductive characteristics, and in situ conservation of Malus sieversii in Xinjiang, China. HortScience 2016, 51, 1197–1201. [Google Scholar] [CrossRef]
- Mierkamili, M.; Liu, Z.Q.; Ma, X.D.; Zhang, H.X.; Tian, Z.P. Survival status, problems and conservation strategies of Malus sieversii. Guihaia 2021, 41, 2100–2109. [Google Scholar] [CrossRef]
- Cornille, A.; Giraud, T.; Smulders, M.J.M.; Roldán-Ruiz, I.; Gladieux, P. The domestication and evolutionary ecology of apples. Trends Genet. 2014, 30, 57–65. [Google Scholar] [CrossRef]
- Panyushkina, I.; Mukhamadiev, N.; Lynch, A.; Ashikbaev, N.; Arizpe, A.; O’Connor, C.; Abjanbaev, D.; Mengdibayeva, G.; Sagitov, A. Wild apple growth and climate change in southeast Kazakhstan. Forests 2017, 8, 406. [Google Scholar] [CrossRef]
- Cui, X.N.; Liu, D.G.; Liu, A.H. Research progress in integrated management of Agrilus mali. Plant Prot. 2015, 41, 16–23. [Google Scholar]
- Shan, Q.; Wang, Z.; Ling, H.; Zhang, G.; Yan, J.; Han, F. Unreasonable human disturbance shifts the positive effect of climate change on tree-ring growth of Malus sieversii in the origin area of world cultivated apples. J. Clean. Prod. 2021, 287, 125008. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Xu, J.; Shi, X.J. Effects of different light intensities on seed germination and seedling growth of Malus sieversii. J. Xinjiang Agric. Univ. 2021, 44, 401–406. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wen, Z.B.; Wang, Q. Population genetic structure of Malus sieversii and environmental adaptations. Chin. J. Plant Ecol. 2022, 46, 1098–1108. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Xu, J.; Shi, X.J. Responses of seedling growth and biomass allocation of Malus sieversii to precipitation amount and precipitation interval. Arid Zone Res. 2023, 40, 102–110. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Bai, S.; Lv, Y.; Shi, X.; Tan, D. Recovery and germination of Malus sieversii (Ledeb.) M. Roem. (Rosaceae) seeds after ingestion by cattle, horses, and sheep. Sustainability 2022, 14, 13930. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.M.; Zhou, X.B. Ecological stoichiometry of surface soil nutrient and its influencing factors in the wild fruit forest in Yili region, Xinjiang, China. Chin. J. Appl. Ecol. 2016, 27, 10. [Google Scholar] [CrossRef]
- Zhang, X.S. On the eco-geographical characters and the problems of classification of the wild fruit-tree forest in the Ili valley of Sinkiang. J. Integr. Plant Biol. 1973, 2, 239–253. [Google Scholar]
- Yan, G.R. Study on the wild fruit trees and its conservation of Tianshan mountain in Xinjiang. Wild Plant Res. 2001, 20, 13–14. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2005; ISBN 978-0-8018-8221-0. [Google Scholar]
- Campos, C.M.; Campos, V.E.; Miguel, F.; Cona, M.I. Management of protected areas and its effect on an ecosystem function: Removal of Prosopis flexuosa seeds by mammals in Argentinian drylands. PLoS ONE 2016, 11, e0162551. [Google Scholar] [CrossRef] [PubMed]
- Miguel, F.; Cona, M.I.; Campos, C.M. Seed removal by different functional mammal groups in a protected and grazed landscape of the Monte, Argentina. Seed Sci. Res. 2017, 27, 174–182. [Google Scholar] [CrossRef]
- Prasad, S.; Pittet, A.; Sukumar, R. Who really ate the fruit? A novel approach to camera trapping for quantifying frugivory by ruminants. Ecol. Res. 2010, 25, 225–231. [Google Scholar] [CrossRef]
- Blake, S.; Wikelski, M.; Cabrera, F.; Guezou, A.; Silva, M.; Sadeghayobi, E.; Yackulic, C.B.; Jaramillo, P. Seed dispersal by Galápagos tortoises. J. Biogeogr. 2012, 39, 1961–1972. [Google Scholar] [CrossRef]
- Qin, W.; Xiao, Y.Q.; Yan, J.J.; Ma, Y. Comparative study on fruit and seed morphology of 17 types of Malus sieversii (Led.) Roem. J. Xinjiang Agric. Univ. 2014, 37, 373–378. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier/AP: San Diego, CA, USA, 2014; ISBN 978-0-12-416677-6. [Google Scholar]
- Liu, Z.Q.; Dong, H.G.; Yu, T.; Chen, W.M. Study on the germination characteristics of Malus sieversii seeds and the field transplanting of seedlings of different seedling ages. J. Anhui Agric. Sci. 2021, 49, 54–56. [Google Scholar] [CrossRef]
- Zhang, Z.Z. Study on Sexual Regeneration Limitation of Malus sieversii Population. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2022. [Google Scholar]
- Campos, C.M.; Velez, S.; Miguel, M.F.; Papú, S.; Cona, M.I. Studying the quantity component of seed dispersal effectiveness from exclosure treatments and camera trapping. Ecol. Evol. 2018, 8, 5470–5479. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.G.N.; Koroiva, R.; Cassimiro, C.A.L.; Batista, F.R.C. Endangered globose cactus Melocactus lanssensianus P. J. Braun depends on lizards for effective seed dispersal in the Brazilian Caatinga. Plant Ecol. 2021, 222, 14. [Google Scholar] [CrossRef]
- Jordano, P. Fruits and Frugivory. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CABI Pub.: Wallingford, UK, 2000; pp. 125–166. ISBN 0-85199-432-6. [Google Scholar]
- Castañeda, I.; Doherty, T.S.; Fleming, P.A.; Stobo-Wilson, A.M.; Woinarski, J.C.Z.; Newsome, T.M. Variation in red fox Vulpes vulpes diet in five continents. Mammal Rev. 2022, 52, 328–342. [Google Scholar] [CrossRef]
- Cain, M.L.; Milligan, B.G.; Strand, A.E. Long-distance seed dispersal in plant populations. Am. J. Bot. 2000, 87, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Schupp, E.W. Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio 1993, 1, 15–29. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, Z.X.; Li, D.S. A review about grazing behavior of domestic ruminants. J. Inn. Mong. Agric. Univ. Nat. Sci. Ed. 2000, 2, 109–116. [Google Scholar] [CrossRef]
- Hou, L.L.; Wang, X.; Zhang, X.; Yan, Y.C.; Yan, R.R.; Cheng, L.; Xin, X.P. The effect of grazing intensity on beef cattle’s behavior. Acta Agrestia Sin. 2021, 29, 1974–1982. [Google Scholar]
- Traveset, A. Effect of seed passage through vertebrate frugivores’ guts on germination: A review. Perspect. Plant Ecol. Evol. Syst. 1998, 1, 151–190. [Google Scholar] [CrossRef]
- Soltani, E.; Baskin, C.C.; Baskin, J.M.; Heshmati, S.; Mirfazeli, M.S. A meta-analysis of the effects of frugivory (endozoochory) on seed germination: Role of seed size and kind of dormancy. Plant Ecol. 2018, 219, 1283–1294. [Google Scholar] [CrossRef]
- Illescas-Gallegos, E.; Rodríguez-Trejo, D.A.; Villanueva-Morales, A.; Borja-de La Rosa, M.A.; Ordóñez-Candelaria, V.R.; Ortega-Aragón, L.A. Factors influencing physical dormancy and its elimination in two legumes. Rev. Chapingo Ser. Cienc. For. Am. 2021, 27, 413–429. [Google Scholar] [CrossRef]
- Carlo, T.A.; García, D.; Martínez, D.; Gleditsch, J.M.; Morales, J.M. Where do seeds go when they go far? Distance and directionality of avian seed dispersal in heterogeneous landscapes. Ecology 2013, 94, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.M.; García, D.; Martínez, D.; Rodriguez-Pérez, J.; Herrera, J.M. Frugivore behavioural details matter for seed dispersal: A multi-species model for Cantabrian thrushes and trees. PLoS ONE 2013, 8, e65216. [Google Scholar] [CrossRef]
- Campos, C.M.; Campos, V.E.; Mongeaud, A.; Borghi, C.E.; De Los Ríos, C.; Giannoni, S.M. Relationships between Prosopis flexuosa (Fabaceae) and cattle in the Monte desert: Seeds, seedlings and saplings on cattle-use site classes. Rev. Chil. Hist. Nat. 2011, 84, 289–299. [Google Scholar] [CrossRef]
- Venier, P.; Carrizo García, C.; Cabido, M.; Funes, G. Survival and germination of three hard-seeded Acacia species after simulated cattle ingestion: The importance of the seed coat structure. S. Afr. J. Bot. 2012, 79, 19–24. [Google Scholar] [CrossRef]
- Bayandala; Mishanbieke, J. Study on the effect of distance restriction on the survival of Malus Sieversii seedlings in Xinjiang. Hunan Agric. Sci. 2019, 11, 75–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).