Bryophytes Present in the Nests of Birds in Yanayacu Biological Station, Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Design and Data Collection
2.3. Analysis of Data
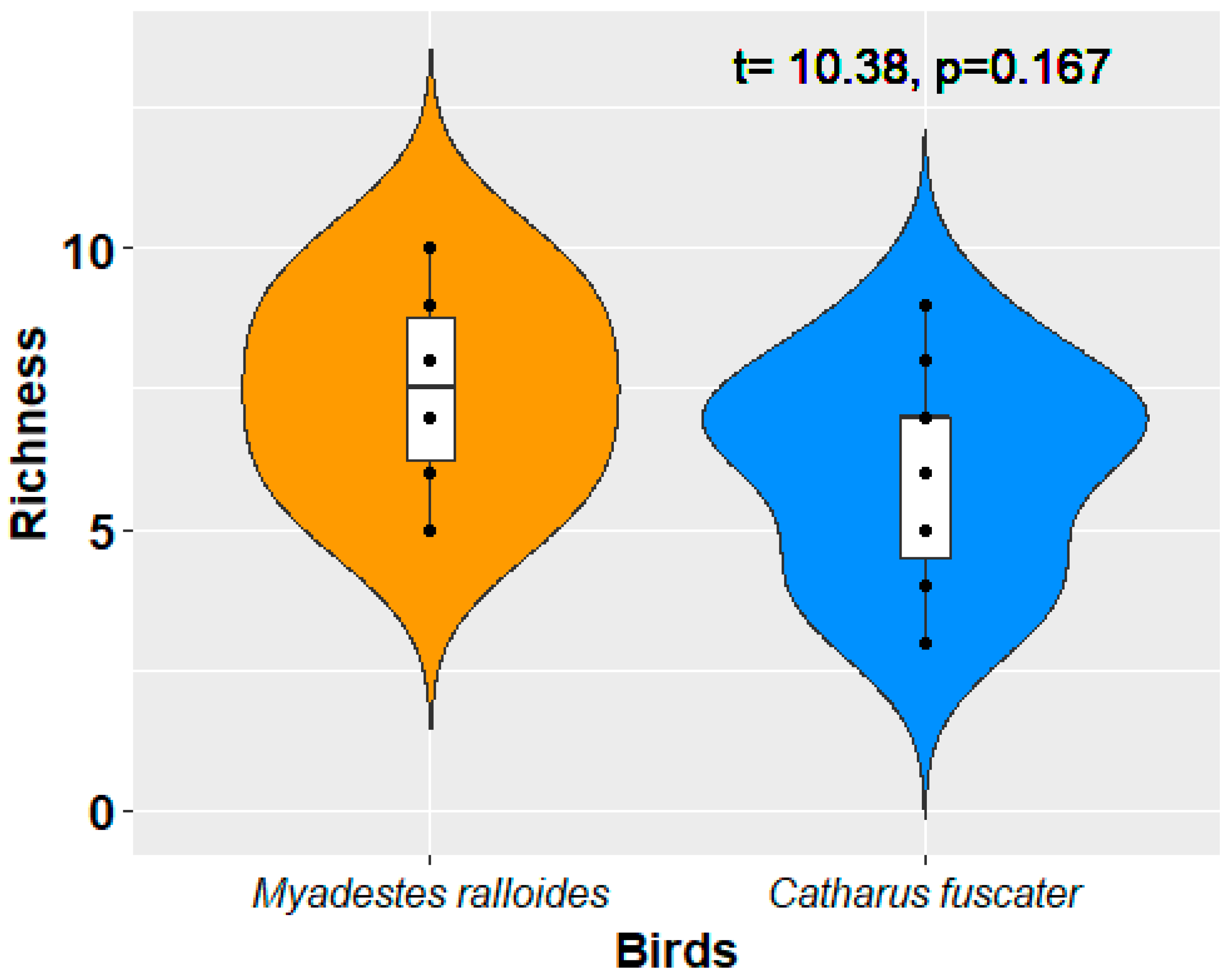
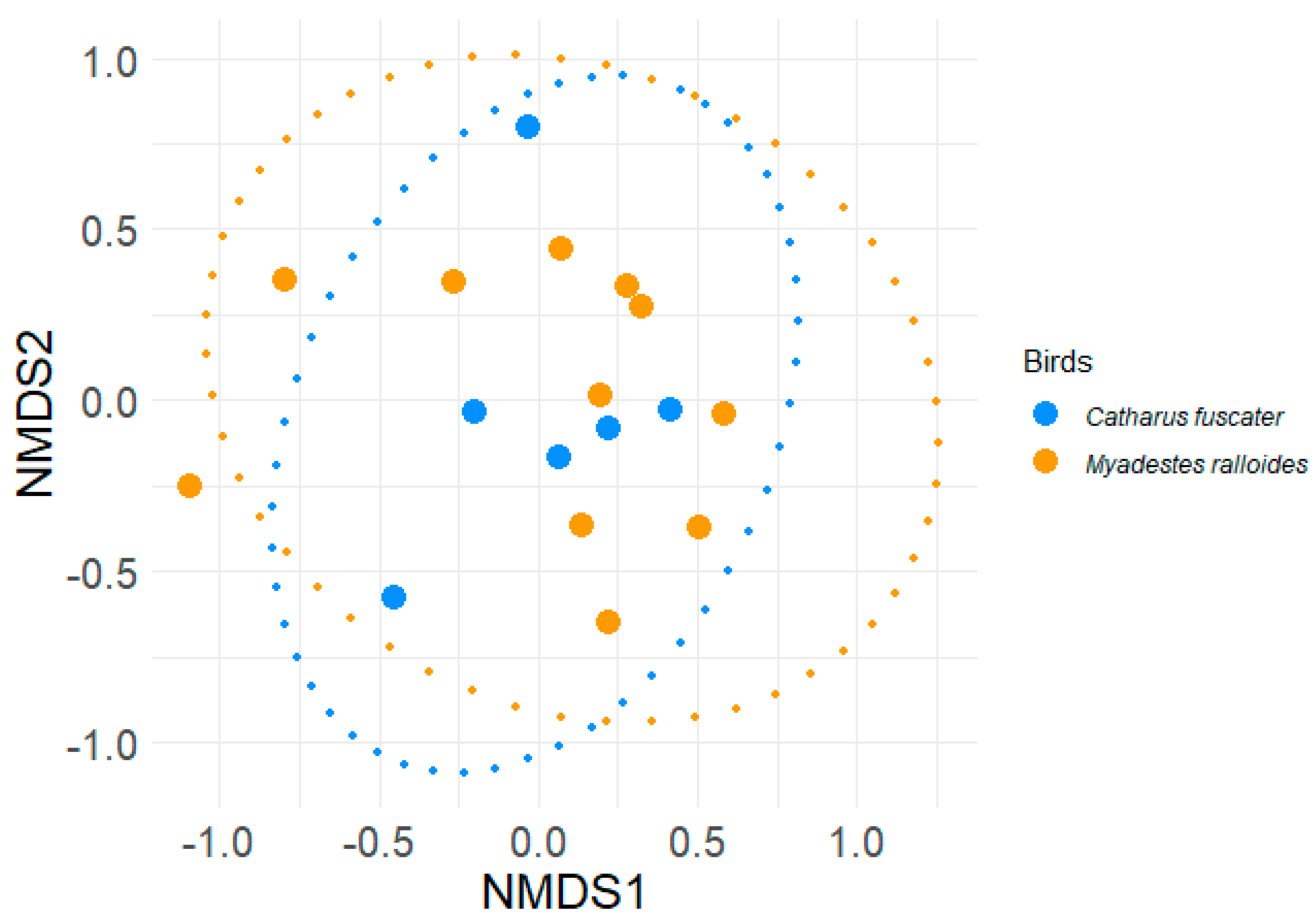
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Healy, S.D.; Morgan, K.V.; Bailey, I.E. Nest construction behaviour. In Nests, Eggs and Incubation: New Ideas about Avian Reproduction; Deeming, D.C., Reynolds, S.J., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 16–28. [Google Scholar]
- Fontúrbel, F.E.; Osorio, F.; Riffo, V.; Nuñez, M.; Bastias, R.; Carvallo, G.O. Mamma knows best. Ecology 2020, 101, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Calvelo, S.; Trejo, A.; Ojeda, V. Botanical composition and structure of hummingbird nests in different habitats from northwestern Patagonia (Argentina). J. Nat. Hist. 2006, 40, 589–603. [Google Scholar]
- McCormac, J.; Showman, R.E. Lichen composition in Blue-gray Gnatcatcher and Ruby-throated Hummingbird nests. Ohio Cardinal 2010, 33, 72–82. [Google Scholar]
- Osorio-Zuñiga, F.; Fontúrbel, F.E.; Rydin, H. Evidence of mutualistic synzoochory between cryptogams and hummingbirds. Oikos 2014, 123, 553–558. [Google Scholar] [CrossRef]
- Wesołowski, T.; Wierzcholska, S. Tits as bryologists: Patterns of bryophyte use in nests of three species cohabiting a primeval forest. J. Ornithol. 2018, 159, 733–745. [Google Scholar]
- Blem, C.R.; Blem, L.B. Composition and microclimate of Prothonotary Warbler nests. Auk 1994, 111, 197–200. [Google Scholar] [CrossRef]
- D’Alba, L.; Shawkey, M.D. Mechanisms of antimicrobial defense in avian eggs. J. Ornithol. 2015, 156, S399–S408. [Google Scholar]
- De Almeida Amélio, L.; Marchi Do Carmo, D.; Soares De Lima, J.; Fernandes Peralta, D. Bryophytes as a material to build birds’ nests in Brazil. Bol. Soc. Argent. 2017, 52, 199–208. [Google Scholar] [CrossRef]
- Torres Dowdall, J.R.; Osorio, F.; Suarez, G.M. Materiales utilizados por el Picaflor Rubí (Sephanoides sephaniodes) para la construccion de nidos en la selva Valdiviana, Chile. Ornitol. Neotr. 2007, 18, 433–437. [Google Scholar]
- Freile, J.F.; Restall, R.L. Birds of Ecuador; Bloomsbury Publishing Plc.: London, UK, 2018. [Google Scholar]
- Greeney, H.F.; Halupka, K. Nesting biology of the Andean Solitaire (Myadestes ralloides) in northeastern Ecuador. Ornitol. Neotrop. 2008, 19, 213–219. [Google Scholar]
- Greeney, H.F.; Dyrcz, A.; Mikusek, R.; Port, J. Cooperative breeding at a nest of Slaty-backed Nightingale-Thrushes (Catharus fuscater). Wilson J. Ornithol. 2015, 127, 323–325. [Google Scholar] [CrossRef]
- Dyrcz, A.; Port, J.; Mikusek, R.; Greeney, H.F. Parental care of nestlings in the Slaty-backed Nightingale-Thrush (Catharus fuscater) in eastern Ecuador. Ornitol. Neotrop. 2015, 26, 117–123. [Google Scholar] [CrossRef]
- Churchill, S.P.; Griffin, D.; Muñoz, J. A Checklist of the Mosses of the Tropical Andean Countries; Editorial CSIC; CSIC Press: Madrid, Spain, 2000; Volume 17. [Google Scholar]
- León-Yánez, S.; Gradstein, S.R.; Wegner, C. Hepáticas (Marchantiophyta) y antocerotas (Anthocerotophyta) del Ecuador: Catálogo. Herbario QCA; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2006. [Google Scholar]
- Gradstein, S.R. The Liverworts and Hornworts of Colombia and Ecuador; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Bignal, K.L.; Ashmore, M.R.; Headley, A.D. Effects of air pollution from road transport on growth and physiology of six transplanted bryophyte species. Environ. Pollut. 2008, 156, 332–340. [Google Scholar] [CrossRef]
- Nöske, N.M.; Hilt, N.; Werner, F.A.; Brehm, G.; Fiedler, K.; Sipman, H.J.; Gradstein, S.R. Disturbance effects on diversity of epiphytes and moths in a montane forest in Ecuador. Basic. Appl. Ecol. 2008, 9, 4–12. [Google Scholar] [CrossRef]
- Medina, J.; Quizhpe, W.; Deleg, J.; Gonzalez, K.; Aguirre, Z.; Aguirre, N.; Benítez, A. Are Juglans neotropica Plantations Useful as a Refuge of Bryophytes Diversity in Tropical Areas? Life 2021, 11, 434. [Google Scholar] [CrossRef]
- Freile, J.F.; Brinkhuizen, D.M.; Greenfield, P.J.; Lysinger, M.; Navarrete, L.; Nilsson, J.; Boyla, K.A. Lista de Las Aves del Ecuador/Checklist of the Birds of Ecuador; Comité Ecuatoriano de Registros Ornitológicos: Quito, Ecuador, 2015. [Google Scholar]
- Churchill, S.P.; Linares, C.E. Prodromus Bryologiae Novo-Granatensis: Introduccion a la Flora de Musgos de Colombia; Instituto de Ciencias Naturales: Bogota, Colombia, 1995. [Google Scholar]
- Gradstein, S.R.; da Costa, D.P. The Hepaticae and Anthocerotae of Brazil; New York Botanical Garden Press: New York, NY, USA, 2003; Volume 87, pp. 1–318. [Google Scholar]
- Collar, N.J. Family Turdidae (thrushes). In Handbook of the Birds of the World; Cornell Lab of Ornithology: Ithaca, NY, USA, 2005; Volume 10, pp. 514–807. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘Vegan’. Community Ecology Package, Version 2.4-4. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 24 August 2019).
- Rydgren, K.; Indreeide, B.; Slagsvold, T.; Lampe, H.M. Nest building in titmice Paridae: Selectivity in bryophyte use. Ecol. Evol. 2023, 13, e9852. [Google Scholar] [CrossRef]
- Andreas, B.K. Use of bryophytes by Carolina chickadees (Poecile carolinensis) in nest construction. Evansia 2010, 27, 23–29. [Google Scholar] [CrossRef]
- Breil, D.A.; Moyle, S.M. Bryophytes used in construction of bird nests. Bryologist 1976, 79, 95–98. [Google Scholar] [CrossRef]
- Glądalski, M.; Wolski, G.J.; Bańbura, M.; Kaliński, A.; Markowski, M.; Skwarska, J.; Wawrzyniak, J.; Bańbura, J. Differences in use of bryophyte species in tit nests between two contrasting habitats: An urban park and a forest. Zool. J. Linn. 2021, 88, 807–815. [Google Scholar] [CrossRef]
- Asakawa, Y. Biologically active compounds from bryophytes. Pure Appl. Chem. 2007, 79, 557–580. [Google Scholar] [CrossRef]



| Myadestes ralloides | Catharus fuscater | ||
|---|---|---|---|
| Liverworts | |||
| Lepidoziaceae | Bazzania hookeri (Lindenb.) Trevis. | 3 | 3 |
| Frullaniaceae | Frullania peruviana Gottsche | 4 | 2 |
| Lejeuneaceae | Lejeunea sp.1 | 0 | 2 |
| Lejeuneaceae | Lejeunea sp.2 | 1 | 1 |
| Metzgeriaceae | Metzgeria leptoneura Spruce | 4 | 2 |
| Monocleaceae | Monoclea gottschei Lindb. | 1 | 0 |
| Lejeuneaceae | Omphalanthus filiformis (Sw.) Nees | 3 | 2 |
| Plagiochilaceae | Plagiochila sp.1 | 8 | 5 |
| Plagiochilaceae | Plagiochila sp.2 | 3 | 0 |
| Pallaviciniaceae | Symphyogyna aspera F.A.McCormick | 1 | 0 |
| Trichocoleaceae | Trichocolea flaccida (Spruce) Spruce | 5 | 5 |
| Trichocoleaceae | Trichocolea paraphyllina (Spruce) Steph. | 4 | 0 |
| Mosses | |||
| Dicranaceae | Campylopus sp. | 0 | 1 |
| Hookeriaceae | Hypnella pilifera (Hook. F. and Wilson) A. Jaeger | 0 | 2 |
| Bartramiaceae | Leiomela bartramioides (Hook.) Paris | 1 | 0 |
| Bartramiaceae | Leiomela deciduifolia Herzog | 1 | 0 |
| Meteoriaceae | Meteoridium remotifolium (Müll. Hal.) Manuel | 8 | 5 |
| Phyllogoniaceae | Phyllogonium viscosum (P. Beauv.) Mitt. | 0 | 1 |
| Lembophyllaceae | Porotrichodendron lindigii (Hampe) W.R. Buck | 0 | 1 |
| Neckeraceae | Porotrichum mutabile Hampe | 3 | 3 |
| Prionodontaceae | Prionodon densus (Sw. ex Hedw.) Müll. Hal. | 5 | 1 |
| Racopilaceae | Racopilum intermedium Hampe | 1 | 0 |
| Meteoriaceae | Squamidium nigricans (Hook.) Broth. | 0 | 4 |
| Thuidiaceae | Thuidium tomentosum Schimp. | 10 | 5 |
| Pilotrichaceae | Trachyxiphium guadalupense (Brid.) W.R. Buck | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, Á.; Jaramillo, E.; Yangua-Solano, E.; Greeney, H.F. Bryophytes Present in the Nests of Birds in Yanayacu Biological Station, Ecuador. Diversity 2023, 15, 1123. https://doi.org/10.3390/d15111123
Benítez Á, Jaramillo E, Yangua-Solano E, Greeney HF. Bryophytes Present in the Nests of Birds in Yanayacu Biological Station, Ecuador. Diversity. 2023; 15(11):1123. https://doi.org/10.3390/d15111123
Chicago/Turabian StyleBenítez, Ángel, Edison Jaramillo, Erika Yangua-Solano, and Harold F. Greeney. 2023. "Bryophytes Present in the Nests of Birds in Yanayacu Biological Station, Ecuador" Diversity 15, no. 11: 1123. https://doi.org/10.3390/d15111123
APA StyleBenítez, Á., Jaramillo, E., Yangua-Solano, E., & Greeney, H. F. (2023). Bryophytes Present in the Nests of Birds in Yanayacu Biological Station, Ecuador. Diversity, 15(11), 1123. https://doi.org/10.3390/d15111123








