The Release and Spread of Basidiospores of Red-Listed Wood-Decay Fungus Fistulina hepatica in Oak Stands
Abstract
1. Introduction
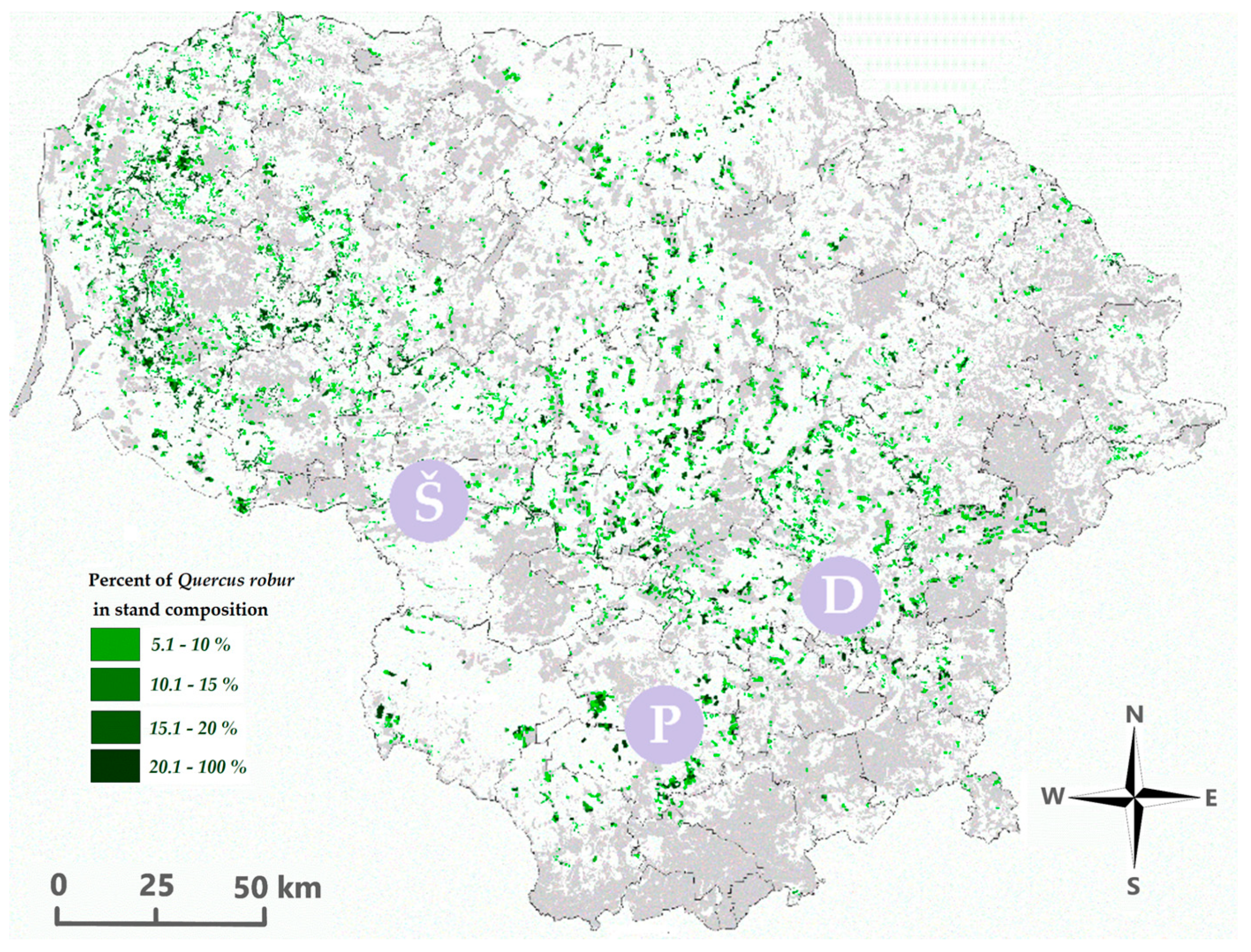
2. Materials and Methods
2.1. DNA Extraction, PCR Amplification, and Sequencing
2.2. Bioinformatics and Statistical Analysis
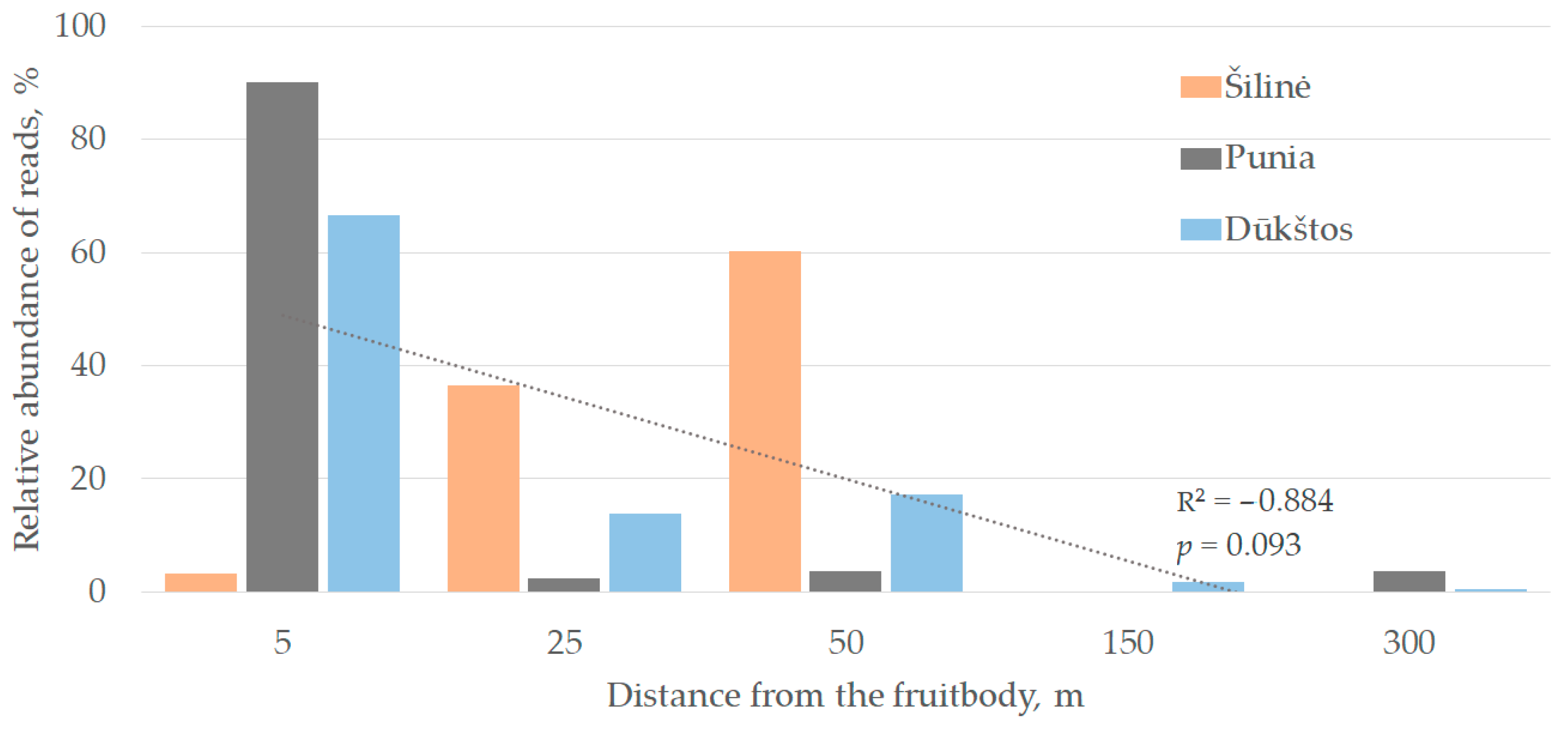
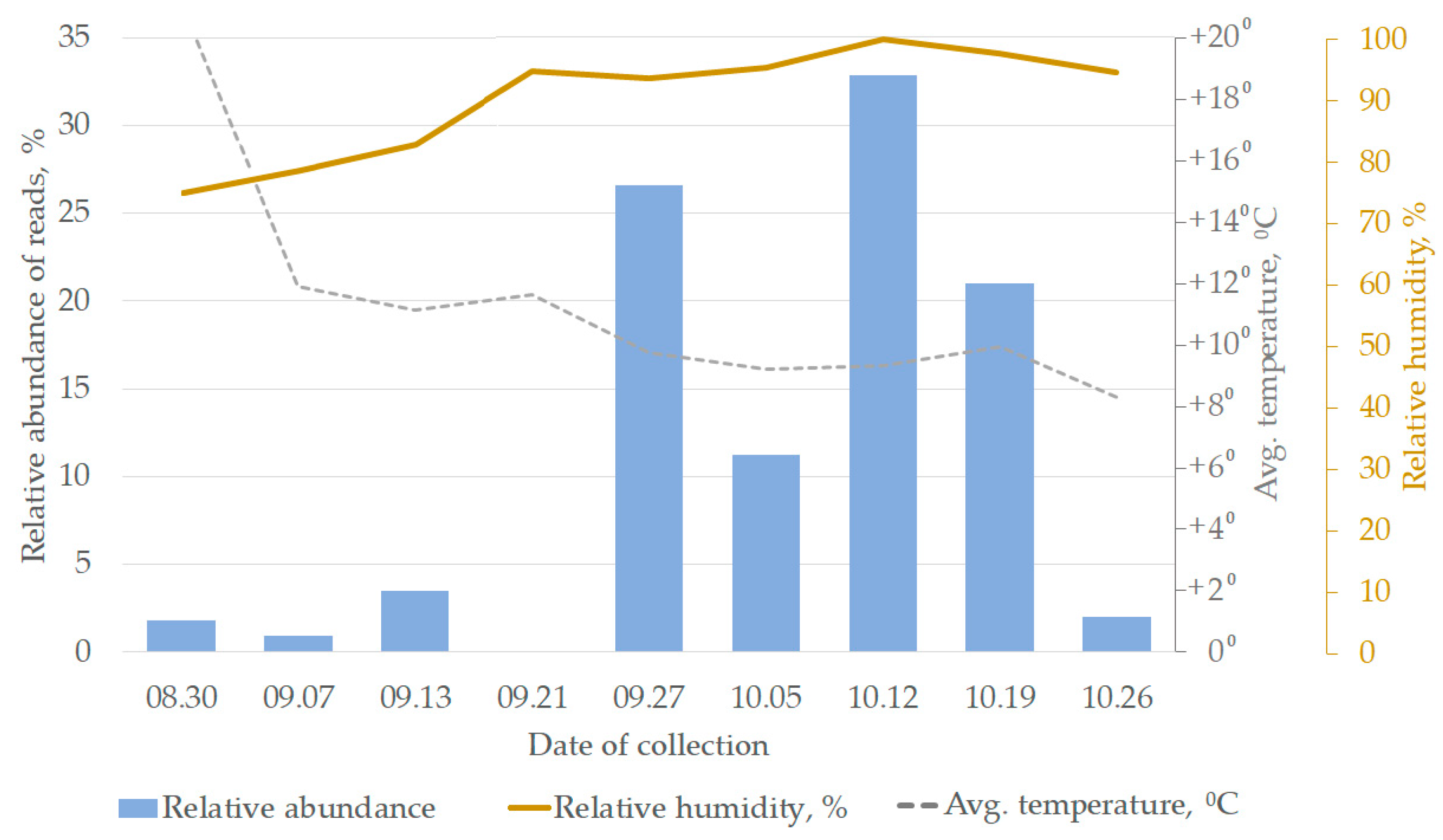
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackwell, M. The fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, B.R.; Joppa, L.N.; Pimm, S.L.; Laurance, W.F. What we know and don’t know about Earth’s missing biodiversity. Trends Ecol. Evol. 2012, 27, 501–510. [Google Scholar] [CrossRef]
- Arnold, A.E. Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A fungal perspective on conservation biology. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef]
- Lofgren, L.A.; Stajich, J.E. Fungal biodiversity and conservation mycology in light of new technology, big data, and changing attitudes. Curr. Biol. 2021, 31, R1312–R1325. [Google Scholar] [CrossRef] [PubMed]
- Senn-Irlet, B.; Heilmann-Clausen, J.; Genney, D.; Dahlberg, A. Guidance for Conservation of Macrofungi in Europe; ECCF: Strasbourg, France, 2007. [Google Scholar]
- Ryvarden, L.; Gilbertson, R.L. European Polypores, Part 2; Fungiflora: Oslo, Norway, 1994; p. 507. [Google Scholar]
- Butin, H. Tree Diseases and Disorders; Oxford University Press: New York, NY, USA, 1995; p. 84. [Google Scholar]
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer: Berlin/Heidelberg, Germany, 2000; p. 193. [Google Scholar]
- Wojewoda, W. Checklist of Polish Larger Basidiomycetes. In Biodiversity of Poland, 1st ed.; Mirek, Z., Ed.; Instytut Botaniki im. W. Szafera, Polska Akademia Nauk: Krakow, Poland, 2003; Volume 7. [Google Scholar]
- Kujawa, A.; Michalak, M.; Konik, J. Dęby w krajobrazie rolniczym jak o siedlisko zastępcze dla ozorka dębowego Fistulina hepatica (Schaeff.) With. Przegląd Przyrodniczy. Chrońmy Przyr. Ojczystą 2018, 29, 21–27. Available online: https://docplayer.pl/61710618-Stanowiska-ozorka-debowego-fistulina-hepatica-schaeff-with-w-srodkowo-wschodniej-polsce.html (accessed on 25 September 2023).
- Marčiulynas, A.; Sirgedaitė-Šėžienė, V.; Menkis, A. Fungi Inhabiting Stem Wounds of Quercus robur following Bark Stripping by Deer Animals. Forests 2023, 14, 2077. [Google Scholar] [CrossRef]
- Yurkewich, J.I.; Castaño, C.; Colinas, C. Chestnut red stain: Identification of the fungi associated with the costly discolouration of Castanea sativa. For. Pathol. 2017, 47, e12335. [Google Scholar] [CrossRef]
- Niemelä, T. Suomen Kääpien Määritysopas; Guide to the Polypores of Finland: Valkeakoski, Finland, 2006; p. 16. [Google Scholar]
- Regué, A.; Bassié, L.; de-Miguel, S.; Colinas, C. Environmental and stand conditions related to Fistulina hepatica heart rot attack on Castanea sativa. For. Pathol. 2019, 49, e12517. [Google Scholar] [CrossRef]
- McCartney, A.; West, J. Dispersal of fungal spores through the air. In Food Mycology: A Multifaceted approach to Fungi and Food; Samson, R.A., Dijksterhuis, J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 65–81. [Google Scholar]
- Money, N.P.; Fischer, M.W. Biomechanics of spore release in phytopathogens. Plant Relatsh. Mycota 2009, 5, 115–133. [Google Scholar] [CrossRef]
- Wyatt, T.T.; Wösten, H.A.; Dijksterhuis, J. Fungal spores for dispersion in space and time. Adv. Appl. Microb. 2013, 85, 43–91. [Google Scholar] [CrossRef]
- Tucker, K.; Stolze, J.L.; Kennedy, A.H.; Money, N.P. Biomechanics of conidial dispersal in the toxic mold Stachybotrys chartarum. Fungal Genet. Biol. 2007, 44, 641–647. [Google Scholar] [CrossRef]
- Golan, J.J.; Pringle, A. Long-distance dispersal of fungi. Microb. Spect. 2017, 5, 4–5. [Google Scholar] [CrossRef]
- Werth, S.; Wagner, H.H.; Gugerli, F.; Holderegger, R.; Csencsics, D.; Kalwij, J.M.; Scheidegger, C. Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 2006, 87, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Shinn, E.A.; Smith, G.W.; Prospero, J.M.; Betzer, P.; Hayes, M.L.; Garrison, V.; Barber, R.T. African dust and the demise of Caribbean coral reefs. Geophys. Res. Lett. 2000, 27, 3029–3032. [Google Scholar] [CrossRef]
- Castaño Soler, C.; Bonet Lledos, J.A.; Oliva Palau, J.; Farré Martinez, G.; Martínez de Aragón, J.; Parladé Izquierdo, X.; Alday, J.G. Rainfall homogenizes while fruiting increases diversity of spore deposition in Mediterranean conditions. Fungal Ecol. 2019, 41, 279–288. [Google Scholar] [CrossRef]
- Schweigkofler, W.; O’Donnell, K.; Garbelotto, M. Detection and quantification of airborne conidia of Fusarium circinatum, the causal agent of pine pitch canker, from two California sites by using a real-time PCR approach combined with a simple spore trapping method. Appl. Environ. Microbiol. 2004, 70, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Garbelotto, M.; Smith, T.; Schweigkofler, W. Variation in rates of spore deposition of Fusarium circinatum, the causal agent of pine pitch canker, over a 12-month-period at two locations in Northern California. Phytopathology 2008, 98, 137–143. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Lynikienė, J.; Marčiulynienė, D.; Gedminas, A.; Menkis, A. Seasonal and site-specific patterns of airborne fungal diversity revealed using passive spore traps and high-throughput DNA sequencing. Diversity 2023, 15, 539. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bodeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandstrom-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Edman, M.; Gustafsson, M.; Stenlid, J.; Ericson, L. Abundance and viability of fungal spores along a forestry gradient—Responses to habitat loss and isolation? Oikos 2004, 104, 35–42. [Google Scholar] [CrossRef]
- Gregory, P.H. Distribution of airborne pollen and spores and their long distance transport. Pure Appl. Geophys. 1978, 116, 309–315. [Google Scholar] [CrossRef]
- Stenlid, J. Regional differentiation in Heterobasidion annosum. In Proceedings of the International Conference on Root and Butt Rots, SLU, Uppsala, Sweden, 9–6 August 1993; Johansson, M., Stenlid, J., Eds.; pp. 243–248. [Google Scholar]
- Gáper, J. Discharge of basidiospores from Fistulina hepatica fruitbodies in the natural environment. Czech Mycol. 1996, 49, 41–48. Available online: http://www.czechmycology.org/_cm/CM49106.pdf (accessed on 19 September 2023). [CrossRef]
- Nuss, I. Ecology of polypores. Biblioth. Mycol. 1975, 45, 1–258. (In German) [Google Scholar]
- Martinez-Bracero, M.; Markey, E.; Clancy, J.H.; McGillicuddy, E.J.; Sewell, G.; O’Connor, D.J. Airborne Fungal Spore Review, New Advances and Automatisation. Atmosphere 2022, 13, 308. [Google Scholar] [CrossRef]
- Hasnain, S.M.; Al-Frayh, A.; Khatija, F.; Al-Sedairy, S. Airborne Ganoderma basidiospores in a country with desert environment. Grana 2004, 43, 111–115. [Google Scholar] [CrossRef][Green Version]
- Pasanen, A.-L.; Pasanen, P.; Jantunen, M.J.; Kalliokoski, P. Significance of Air Humidity and Air Velocity for Fungal Spore Release into the Air. Atmos. Environ. Part A Gen. Top. 1991, 25, 459–462. [Google Scholar] [CrossRef]
- Katial, R.K.; Zhang, Y.; Jones, H.J.; Dyer, P.D. Atmospheric mold spore counts in relation to meteorological parameters. Int. J. Biometeorol. 1997, 41, 17–22. [Google Scholar] [CrossRef]
- Grinn-Gofroń, A.; Strzelczak, A. Hourly Predictive Artificial Neural Network and Multivariate Regression Tree Models of Alternaria and Cladosporium Spore Concentrations in Szczecin (Poland). Int. J. Biometeorol. 2009, 53, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Soukup, F. A contribution to the knowledge of sporulation of some polypores I. Lesnictví 1988, 33, 145–158. Available online: http://www.czechmycology.org/_cm/CM421.pdf#page=3 (accessed on 22 September 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marčiulynas, A.; Menkis, A. The Release and Spread of Basidiospores of Red-Listed Wood-Decay Fungus Fistulina hepatica in Oak Stands. Diversity 2023, 15, 1110. https://doi.org/10.3390/d15111110
Marčiulynas A, Menkis A. The Release and Spread of Basidiospores of Red-Listed Wood-Decay Fungus Fistulina hepatica in Oak Stands. Diversity. 2023; 15(11):1110. https://doi.org/10.3390/d15111110
Chicago/Turabian StyleMarčiulynas, Adas, and Audrius Menkis. 2023. "The Release and Spread of Basidiospores of Red-Listed Wood-Decay Fungus Fistulina hepatica in Oak Stands" Diversity 15, no. 11: 1110. https://doi.org/10.3390/d15111110
APA StyleMarčiulynas, A., & Menkis, A. (2023). The Release and Spread of Basidiospores of Red-Listed Wood-Decay Fungus Fistulina hepatica in Oak Stands. Diversity, 15(11), 1110. https://doi.org/10.3390/d15111110





