Pluridecadal Temporal Patterns of Tintinnids (Ciliophora, Spirotrichea) in Terra Nova Bay (Ross Sea, Antarctica)
Abstract
1. Introduction
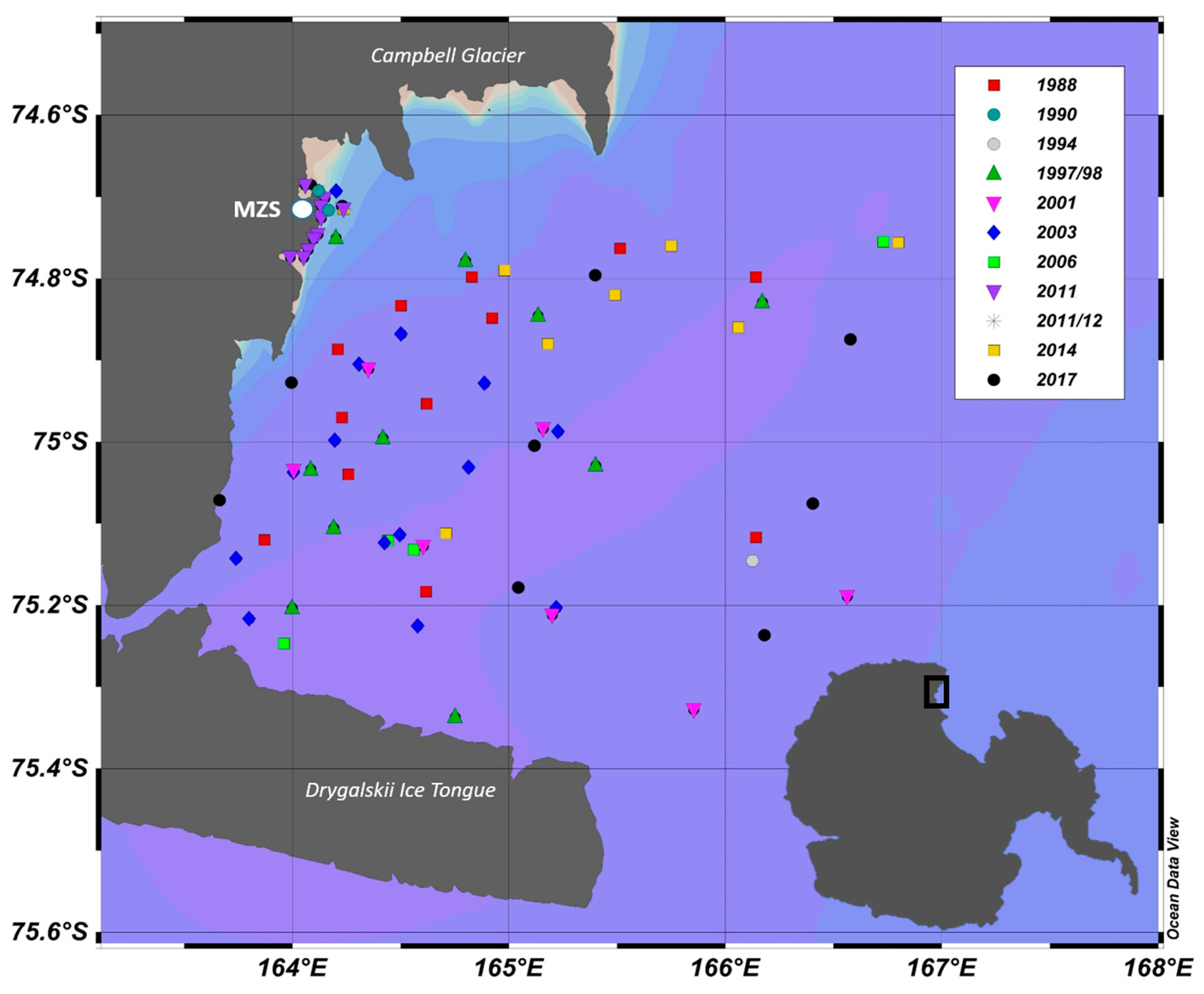
2. Material and Methods
3. Results
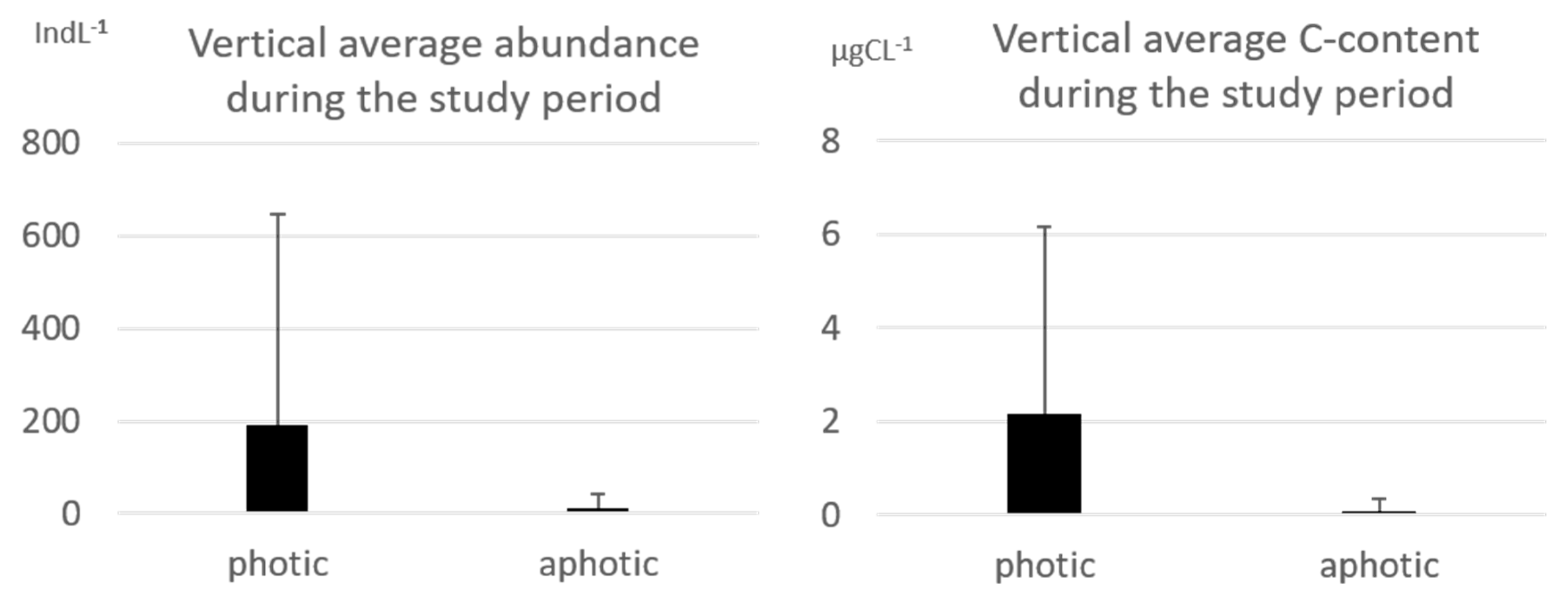
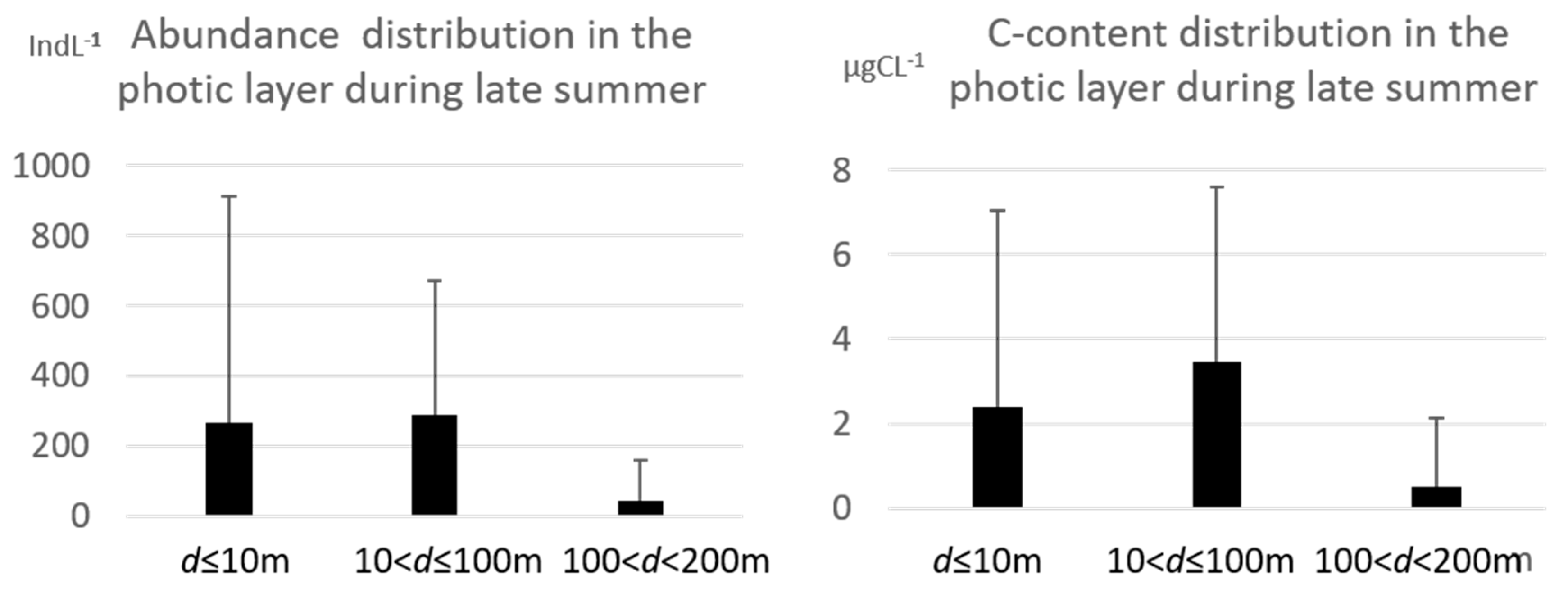
3.1. Tintinnid Abundance and Biomass
3.2. Tintinnids Composition
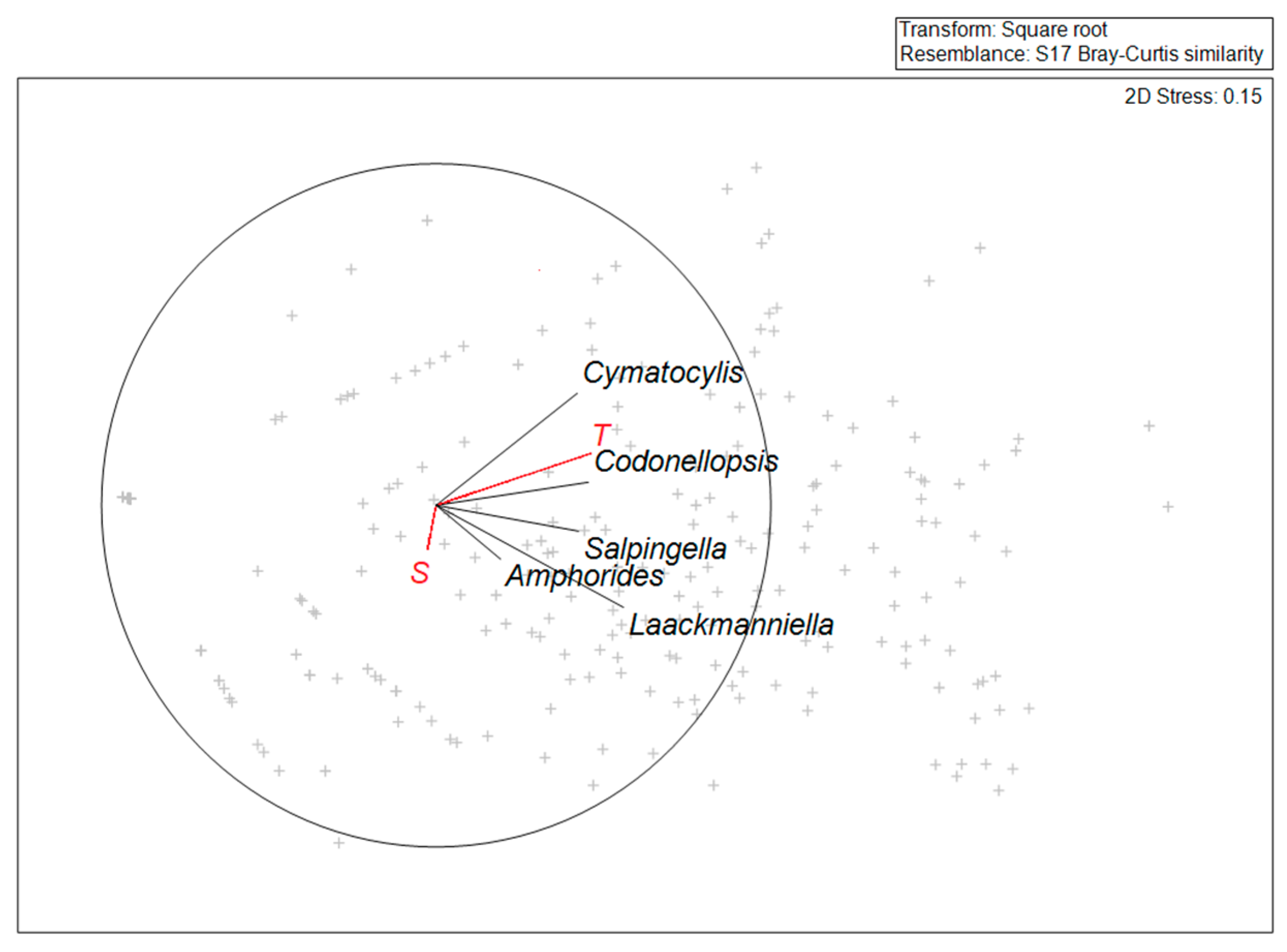
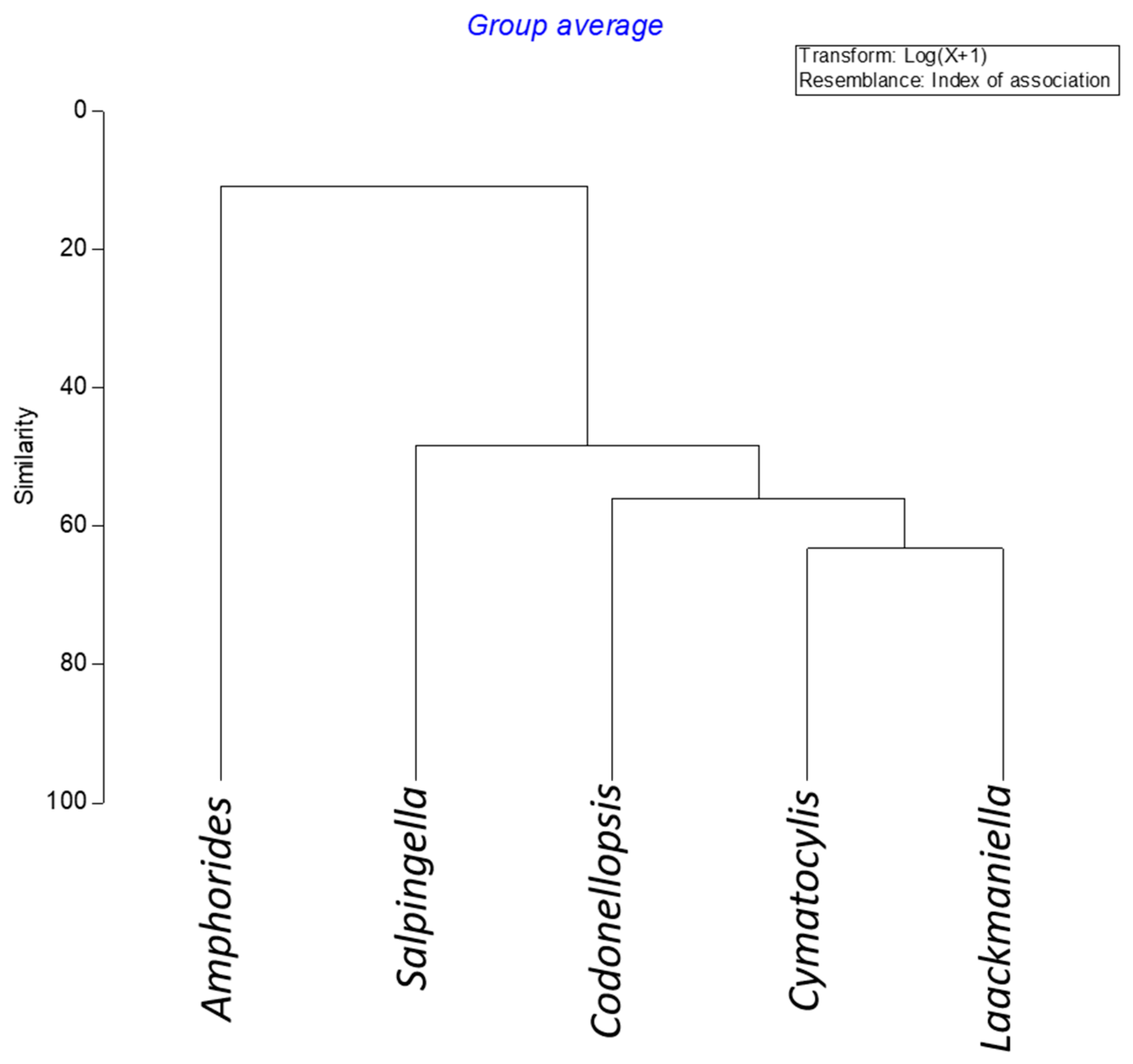
3.3. Tintinnid Community Structure
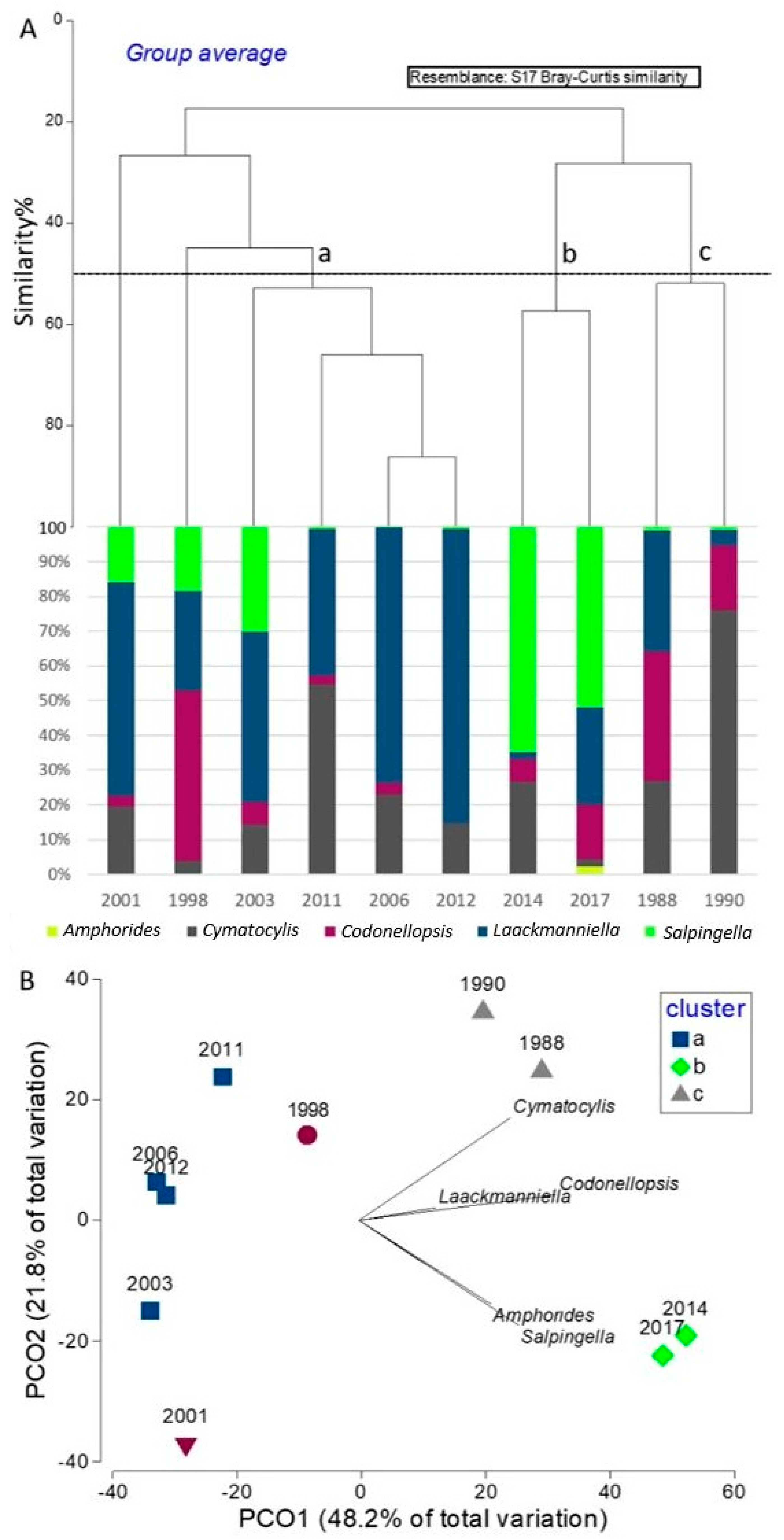
3.4. Plurennial Pattern of the Tintinnids Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, S.S.; Giulivi, C.F.; Dutrieux, P. Persistent Ross Sea freshening from imbalance West Antarctic ice shelf melting. J. Geophys. Res. Oceans 2022, 127, e2021JC017808. [Google Scholar] [CrossRef]
- Castagno, P.; Capozzi, V.; DiTullio, G.R.; Falco, P.; Fusco, G.; Rintoul, S.R.; Spezie, G.; Budillon, G. Rebound of shelf water salinity in the Ross Sea. Nat. Commun. 2019, 10, 5441. [Google Scholar] [CrossRef]
- Silvano, A.; Foppert, A.; Rintoul, S.R.; Holland, S.R.; Tamura, T.; Kimura, N.; Castagno, P.; Falco, P.; Budillon, G.; Haumann, F.A.; et al. Recent recovery of Antarctic Bottom Water formation in the Ross Sea driven by climate anomalies. Nat. Geosci. 2020, 10, 5441. [Google Scholar] [CrossRef]
- Lecomte, O.; Goosse, H.; Fichefet, T.; de Lavergne, C.; Barthélemy, A.; Zunz, V. Vertical ocean heat redistribution sustaining sea-ice concentration trends in the Ross Sea. Nat. Commun. 2017, 8, 258. [Google Scholar] [CrossRef]
- Jacobs, S.S.; Giulivi, C.F. Large multidecadal salinity trends near the Pacific-Antarctic continental margin. J. Clim. 2010, 23, 4508–4524. [Google Scholar] [CrossRef]
- Bromwich, D.H.; Kurtz, D.D. Katabatic wind forcing of the Terra Nova Bay polynya. J. Geophys. Res. 1984, 89, 3561–3572. [Google Scholar] [CrossRef]
- Kurtz, D.D.; Bromwich, D.H. A recurring atmospherically-forced polynya in Terra Nova Bay. In Oceanology of the Antarctic Continental Shelf; Jacobs, S.S., Ed.; Antarctic Research Series; American Geophysical Union: Washington, DC, USA, 1985; Volume 43, pp. 177–201. [Google Scholar]
- Kern, S. Wintertime Antarctic coastal polynya area: 1992–2008. Geophys. Res. Lett. 2009, 36, 1–5. [Google Scholar] [CrossRef]
- Rusciano, E.; Budillon, G.; Fusco, G.; Spezie, G. Evidence of atmosphere–sea ice–ocean coupling in the Terra Nova Bay polynya (Ross Sea—Antarctica). Cont. Shelf Res. 2013, 61–62, 112–124. [Google Scholar] [CrossRef]
- Budillon, G.; Fusco, G.; Spezie, G. A study of surface heat fluxes in the Ross Sea (Antarctica). Antarct. Sci. 2000, 12, 243–254. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Annual changes in sea-ice, chlorophyll a, and primary production in the Ross Sea, Antarctica. Deep-Sea Res. 2004, 51, 117–138. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Phytoplankton dynamics within 37 Antarctic coastal polynya systems. J. Geophys. Res. 2003, 108, 3271. [Google Scholar] [CrossRef]
- Arrigo, K.R.; Worthen, D.L.; Schnell, A.; Lizotte, M.P. Primary production in Southern Ocean waters. J. Geophys. Res. 1998, 103, 587–600. [Google Scholar] [CrossRef]
- DiTullio, G.R.; Smith, W.O., Jr. Spatial patterns in phytoplankton biomass and pigment distributions in the Ross Sea. J. Geophys. Res. 1996, 101, 467–478. [Google Scholar] [CrossRef]
- Smith, W.O., Jr.; Gordon, L.I. Hyperproductivity of the Ross Sea (Antarctica) polynya during austral spring. Geophys. Res. Lett. 1997, 24, 233–236. [Google Scholar] [CrossRef]
- Smith, W.O.; Marra, J.; Hiscock, M.R.; Barber, R.T. The seasonal cycle of phytoplankton biomass and primary productivity in the Ross Sea. Antarctica. Deep-Sea Res. II 2000, 47, 3119–3140. [Google Scholar] [CrossRef]
- Nuccio, C.; Innamorati, M.; Lazzara, L.; Mori, G.; Massi, L. Spatial and temporal distribution of phytoplankton assemblages in the Ross Sea. In Ross Sea Ecology; Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin, Germany, 2000; pp. 231–245. [Google Scholar]
- Smith, W.O., Jr.; Ainley, D.G.; Arrigo, K.R.; Dinniman, M.S. The Oceanography and Ecology of the Ross Sea. Ann. Rev. Mar. Sci. 2014, 6, 469–487. [Google Scholar] [CrossRef]
- Xu, K.; Fu, F.X.; Hutchins, D.A. Comparative responses of two dominant Antarctic phytoplankton taxa to interactions between ocean acidification, warming, irradiance, and iron availability. Limnol. Oceanogr. 2014, 59, 1919–1931. [Google Scholar] [CrossRef]
- Mangoni, O.; Saggiomo, V.; Bolinesi, F.; Margiotta, F.; Budillon, G.; Cotroneo, Y.; Misic, C.; Rivaro, P.; Saggiomo, M. Phytoplankton blooms during austral summer in the Ross Sea, Antarctica: Driving factors and trophic implications. PLoS ONE 2017, 12, e0176033. [Google Scholar] [CrossRef]
- Bjørnsen, P.; Kuparinen, J. Growth and herbivory by heterotrophic dinoflagellates in the Southern Ocean, studied by microcosm experiments. Mar. Biol. 1991, 109, 397–405. [Google Scholar] [CrossRef]
- Kuparinen, J.; Bjørnsen, P. Bottom-up and top-down controls of the microbial food web in the Southern Ocean: Experiments with manipulated microcosms. Polar Biol. 1992, 12, 189–195. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.E. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek Inter. J. Gen. Mol. Microbiol. 2002, 81, 293–308. [Google Scholar]
- Calbet, A.; Landry, M.R. Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine system. Limnol. Oceanogr. 2004, 49, 51–57. [Google Scholar] [CrossRef]
- Schmoker, C.; Hernández-León, S.; Calbet, A. Microzooplankton grazing in the oceans: Impacts, data variability, knowledge gaps and future directions. J. Plankton Res. 2013, 35, 691–706. [Google Scholar] [CrossRef]
- Fonda Umani, S.; Monti, M.; Bergamasco, A.; Cabrini, M.; De Vittor, C.; Burba, N.; Del Negro, P. Plankton community structure and dynamics versus physical structure from Terra Nova Bay to Ross Ice Shelf (Antarctica). J. Mar. Syst. 2005, 55, 31–46. [Google Scholar] [CrossRef]
- Dolan, J.R. Morphology and ecology in tintinnid ciliates of the marine plankton: Correlates of lorica dimensions. Acta Protozool. 2010, 49, 235–244. [Google Scholar]
- Boltovskoy, D.; Dinofrio, E.O.; Alder, A.A. Intraspecific variability in Antarctic tintinnids: The Cymatocylis affinis/convallaria species group. J. Plankton Res. 1990, 12, 403–413. [Google Scholar] [CrossRef]
- Williams, R.; McCall, H.; Pierce, R.W.; Turner, J.T. Speciation of the tintinnid genus Cymatocylis by morphometric analysis of the loricae. Mar. Ecol. Prog. Ser. 1994, 107, 263–272. [Google Scholar] [CrossRef]
- Wasik, A.; Mikołajczyk, E. Annual cycle of tintinnids in Admiral Bay with an emphasis on seasonal variability in Cymatocylis affinis/convallaria lorica morphology. J. Plankton Res. 1994, 16, 1–8. [Google Scholar] [CrossRef]
- Kruse, S.; Jansen, S.; Krägefsky, S.; Bathman, U. Gut content analysis of three dominant Antarctic copepod species during an induced phytoplankton bloom EIFEX (European iron fertilization experiment). Mar. Ecol. 2009, 30, 301312. [Google Scholar] [CrossRef]
- Mauchline, J. The biology of mysids and euphausiids. In Advances in Marine Biology 18; Academic Press: London, UK, 1980. [Google Scholar]
- Buck, K.R.; Garrison, D.L.; Hopkins, T.L. Abundance and distribution of tintinnid ciliates in an ice edge zone during the austral autumn. Antarct. Sci. 1992, 4, 398. [Google Scholar] [CrossRef]
- Hopkins, T.L. Midwater food web in McMurdo sound, Ross Sea, Antarctica. Mar. Biol. 1987, 96, 93106. [Google Scholar] [CrossRef]
- Kellermann, A. Food and feeding ecology of postlarval and juvenile Pleurogramma antarcticum (Pisces; Notothenioidei) in the seasonal pack ice zone off the Antarctic Peninsula. Polar Biol. 1987, 7, 307315. [Google Scholar] [CrossRef]
- Laackmann, H. Antarktische Tintinninen. Zool. Anz. 1907, 3, 235–239. [Google Scholar]
- Laackmann, H. Die Tintinnodeen der Deutschen Südpolar-Expedition 1901–1903. Dtsch. Südpolar-Exped. 1903, 1910, 341–496. [Google Scholar]
- Jiang, Y.; Yang, E.J.; Kim, S.Y.; Kim, Y.-N.; Lee, S.H. Spatial patterns in pelagic ciliate community responses to various habitats in the Amundsen Sea (Antarctica). Prog. Oceanogr. 2014, 128, 49–59. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Q.; Yang, E.J. Pelagic ciliate communities within the Amundsen Sea polynya and adjacent sea ice zone, Antarctica. Deep Sea Res. II 2016, 123, 69–77. [Google Scholar] [CrossRef]
- Monti-Birkenmeier, M.; Diociaiuti, T.; Fonda Umani, S.; Meyer, B. Microzooplankton composition in the winter sea ice of the Weddell Sea. Antarct. Sci. 2017, 29, 299–310. [Google Scholar] [CrossRef]
- Monti-Birkenmeier, M.; Diociaiuti, T.; Badewien, T.H.; Schulz, A.C.; Friedrichs, A.; Meyer, B. Microzooplankton composition in the Antarctic Peninsula region, with an emphasis on tintinnids. Polar Biol. 2021, 44, 1749–1764. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Dong, Y.; Zhao, Y.; Tao, Z.; Li, C.; Zhang, W.; Gregori, G. Planktonic ciliates in different water masses in open waters near Prydz Bay (East Antarctica) during austral summer, with an emphasis on tintinnid assemblages. Polar Biol. 2018, 41, 2355–2371. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.B.; Zhang, W.C.; Tao, Z.; Zhao, Y. Changes in tintinnid assemblages from Subantarctic zone to Antarctic zone along transect in Amundsen Sea (west Antarctica) in early austral autumn. J. Ocean Univ. China 2020, 19, 339–350. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommung der quantitativen Phytoplankton-Methodik. Mitt. Int. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Brandt, K. Die Tintinnodeen der Plankton-Expedition. Tefelerklarungen nebts kurzer Diagnose der neuen Arten. In Ergebn. Atlant. Ozean Planktonexpedition; Hensen, V., Ed.; Humboldt-Stift: Kiel und Leipzig, Germany, 1906; Volume 3, pp. 1–33. [Google Scholar]
- Brandt, K. Die Tintinnodeen der Plankton-Expedition. Sistematischer Teil. In Ergebn. Atlant. Ozean Planktonexpedition; Hensen, V., Ed.; Humboldt-Stift: Kiel und Leipzig, Germany, 1907; Volume 3, pp. 1–488. [Google Scholar]
- Kofoid, C.A.; Campbell, A.S. A conspectus of the marine and fresh-water Ciliata belonging to the suborder Tintinnoinea with descriptions of new species principally from the Agassiz Expedition to the eastern tropical Pacific, 1904–1905. Univ. Calif. Publ. Zool. 1929, 34, 1–403. [Google Scholar]
- Kofoid, C.A.; Campbell, A.S. Reports on the scientific results of the expedition to the Eastern Tropical Pacific. The Ciliata: The Tintinnoinea. Bull. Mus. Comp. Zool. Harvard Univ. 1939, 84, 1–473. [Google Scholar]
- Alder, V.A. Tintinnoinea. In South Atlantic Zooplankton; Boltovskoy, D., Ed.; Backhuys: Leiden, The Netherlands, 1999; pp. 321–384. [Google Scholar]
- Petz, W. Ciliates. In Antarctic Marine Protists; Scott, F.J., Marchant, H.J., Eds.; Camberra ABRS: Hobart, Australia, 2005; pp. 347–448. [Google Scholar]
- Verity, P.G.; Langdon, C. Relationship between lorica volume, carbon, nitrogen, and ATP content of tintinnids in Narragansett Bay. J. Plankton Res. 1984, 6, 859–868. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Getting Started with PRIMER v7. PRIMER-E; Plymouth Marine Laboratory: Plymouth, UK, 2015. [Google Scholar]
- Pierce, R.W.; Turner, J.T. Global biogeography of marine tintinnids. Mar. Ecol. Progr. Ser. 1993, 94, 11–26. [Google Scholar] [CrossRef]
- Monti, M.; Fonda Umani, S. Distribution of the main microzooplankton taxa in the Ross Sea (Antarctica): Austral summer 2004. In Ross Sea Ecology; Faranda, F.M., Guglielmo, L., Ianora, A., Eds.; Springer: Berlin, Germany, 2000; pp. 275–289. [Google Scholar]
- Gowing, M.M.; Garrison, D.L. Larger microplankton in the Ross Sea: Abundance, biomass and flux in the austral summer. In Biogeochemistry of the Ross Sea; DiTullio, G.R., Dunbar, R.B., Eds.; Wiley: Hoboken, NJ, USA, 2003; pp. 243–260. [Google Scholar]
- Thompson, G.A.; Alder, V.A. Patterns in tintinnid species composition and abundance in relation to hydrological conditions of the southwestern Atlantic during austral spring. Aquat. Microb. Ecol. 2005, 40, 85–101. [Google Scholar] [CrossRef]
- Monti, M.; Fonda Umani, S. Tintinnids in Terra Nova Bay—Ross Sea during two austral summer (1987/88 and 1989/90). Acta Protozool. 1995, 34, 193–201. [Google Scholar]
- Dolan, J.R.; Pierce, R.W.; Yang, E.J.; Kim, S.Y. Southern Ocean biogeography of tintinnid ciliates of the marine plankton. J. Eukaryot. Microbiol. 2012, 59, 511–519. [Google Scholar] [CrossRef]
- Fonda Umani, S.; Accornero, A.; Budillon, G.; Capello, M.; Tucci, S.; Cabrini, M.; Del Negro, P.; Monti, M.; De Vittor, C. Particulate matter and plankton dynamics in the Ross Sea Polynya of Terra Nova Bay during the Austral Summer 1997/98. J. Mar. Syst. 2002, 36, 29–49. [Google Scholar] [CrossRef]
- Andreoli, C.; Tolomio, C.; Moro, I.; Radice, M.; Moschin, E.; Bellato, S. Diatoms and dinoflagellates in Terra Nova Bay (Ross Sea-Antarctica) during austral summer 1990. Polar Biol. 1995, 15, 465–475. [Google Scholar] [CrossRef]
- Arrigo, K.R.; DiTullio, G.R.; Dunbar, R.B.; Robinson, D.H.; van Woert, M.; Worthen, D.L.; Denise, L.W.; Lizotte, M.P. Phytoplankton taxonomic variability in nutrient utilization and primary production in the Ross Sea. J. Geophys. Res. 2000, 105, 8827–8846. [Google Scholar] [CrossRef]
- Garrison, D.L.; Mathot, S.; Gowing, M.M.; Kunze, H.; Lessard, E.J. Phytoplankton and microzooplankton community structure in the Ross Sea polynya: November and December 1994. Antarct. J. U. S. 1995, 30, 212–214. [Google Scholar]
- Seibel, B.A.; Dierssen, H.M. Cascading trophic impacts of reduced biomass in the Ross Sea, Antarctica: Just a tip of the iceberg? Biol. Bull. 2003, 205, 93–97. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Putt, M.; Moisan, T. Nano- and microplankton dynamics during the spring Phaeocystis sp. bloom in McMurdo Sound, Antarctica. J. Mar. Biol. Assoc. U. K. 1995, 75, 815–832. [Google Scholar] [CrossRef]
- Gowing, M.M.; Garrison, D.L. Austral winter distribution of large tintinnid and large sarcodinid protozooplankton in the ice-edge zone of the Weddel/Scotia Seas. J. Mar. Syst. 1991, 2, 131–141. [Google Scholar] [CrossRef]
- Hansen, P.J. Dinophysis—A planktonic dinoflagellate genus which can act as a prey and a predator of a ciliate. Mar. Ecol. Progr. Ser. 1991, 69, 201–204. [Google Scholar] [CrossRef]
- Alder, V.A.; Boltovskoy, D. The ecology of larger microzooplankton in the Weddell-Scotia Confluence Area: Horizontal and vertical distribution patterns. J. Mar. Res. 1993, 51, 323–344. [Google Scholar] [CrossRef]
- Stammerjohn, S.E.; Smith, R.C. Opposing Southern Ocean climate patterns as revealed by trends in regional sea ice coverage. Clim. Change 1997, 37, 617–639. [Google Scholar] [CrossRef]
- Harangozo, S.A.; Connolley, W.M. The role of the atmospheric circulation in the record minimum extent of open water in the Ross Sea in the 2003 austral summer. Atmosphere-Ocean 2006, 44, 83–97. [Google Scholar] [CrossRef][Green Version]
- Montes-Hugo, M.A.; Yuan, X. Climate patterns and phytoplankton dynamics in Antarctic latent heat polynyas. J. Geophys. Res. 2012, 117, C05031. [Google Scholar] [CrossRef]
- Schine, C.M.S.; van Dijken, G.L.; Arrigo, K.R. Spatial analysis of trends in primary production and relationship with large-scale climate variability in the Ross Sea, Antarctica (1997–2013). J. Geophys. Res. Oceans 2016, 121, 368–386. [Google Scholar] [CrossRef]
- Bolinesi, F.; Saggiomo, M.; Ardini, F.; Castagno, P.; Cordone, A.; Fusco, G.; Rivaro, P.; Saggiomo, V.; Mangoni, O. Spatial-related community structure and dynamics in phytoplankton of the Ross Sea, Antarctica. Front. Mar. Sci. 2020, 7, 1092. [Google Scholar] [CrossRef]
- Boltovskoy, D.; Adler, V.A. Microzooplankton and tintinnid species-specific assemblage structures: Patterns of distribution and year-to-year variations in the Weddell Sea (Antarctica). J. Plankton Res. 1992, 14, 1405–1423. [Google Scholar] [CrossRef]

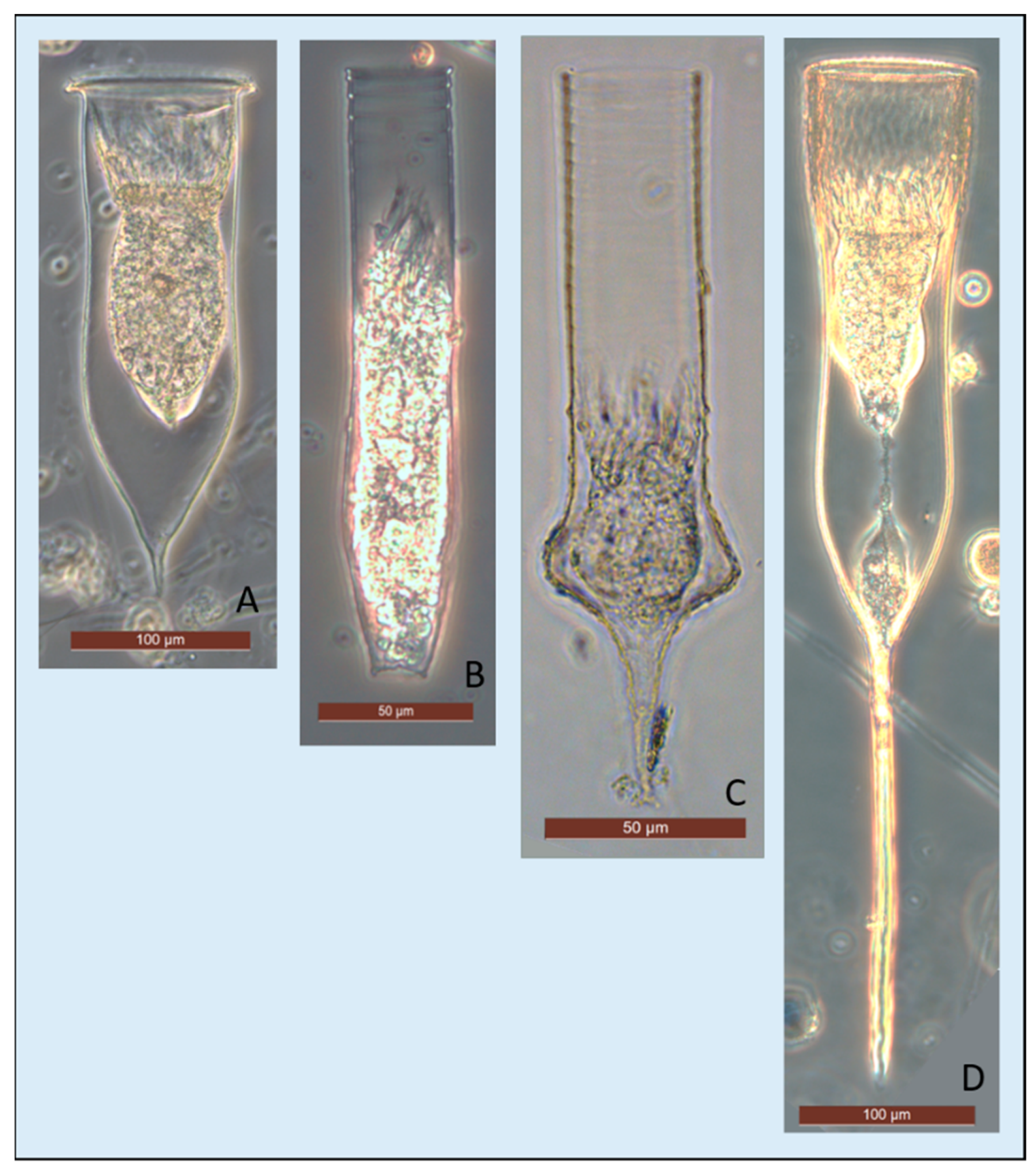

| Species | Total Length (µm) | LOD (µm) |
|---|---|---|
| Amphorides laackmanni (Jörgensen) Strand, 1928 | 50–70 | 15–20 |
| Cymatocylis convallaria Laackmann, 1910 | 110–140 | 80–100 |
| Cymatocylis cristallina Laackmann, 1909 | 180–200 | 60–80 |
| Cymatocylis drygalskii (Laackmann) Laackmann, 1907 | 180–340 | 80–100 |
| Cymatocylis nobilis (Laackmann) Laackmann, 1910 | 180–200 | 60–80 |
| Cymatocylis vanhoeffeni (Laackmann) Laackmann, 1910 | 300–550 | 80–100 |
| Cymatocylis spp. | 110–350 | 60–100 |
| Codonellopsis gaussi (Laackmann, 1907) Kofoid and Campbell, 1929 | 125–200 | 30–35 |
| Codonellopsis glacialis (Laackmann, 1907) Kofoid and Campbell, 1929 | 80–105 | 30–40 |
| Codonellopsis spp. | 80–200 | 25–40 |
| Laackmanniella naviculaefera (Laackmann, 1907) Kofoid and Campbell, 1929 | 125–250 | 40–50 |
| Laackmanniella spp. | 125–250 | 40–50 |
| Salpingella spp. | 70–200 | 15–20 |
| 1988 | 1990 | 1994 | 1997–1998 | 2001 | 2003 | 2006 | 2011 | 2011–2012 | 2014 | 2017 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphorides laackmanni | * | * | |||||||||||
| Cymatocylis convallaria | * | * | * | * | * | ||||||||
| Cymatocylis cristallina | * | * | |||||||||||
| Cymatocylis drygalskii | * | * | * | * | * | * | * | * | * | * | * | ||
| Cymatocylis nobilis | * | ||||||||||||
| Cymatocylis vanhoeffeni | * | * | * | * | * | ||||||||
| Cymatocylis spp. | * | * | * | * | * | * | * | ||||||
| Codonellopsis gaussi | * | * | * | * | * | * | * | * | * | * | * | ||
| Codonellopsis glacialis | * | * | * | * | * | ||||||||
| Codonellopsis spp. | * | * | |||||||||||
| Laackmanniella naviculaefera | * | * | * | * | * | * | * | * | * | * | * | * | |
| Laackmanniella spp. | * | * | * | ||||||||||
| Salpingella spp. | * | * | * | * | * | * | * | * | * | * | * | * | |
| av.a. indL−1 | sd | Max indL−1 | Occurrence % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d ≤ 10 m | 10 < d ≤ 100 m | 100 < d < 200 m | d ≤ 10 m | 10 < d ≤ 100 m | 100 < d < 200 m | d ≤ 10 m | 10 < d ≤ 100 m | 100 < d < 200 m | d ≤ 10 m | 10 < d ≤ 100 m | 100 < d < 200 m | |
| Amphorides | 0 | 5 | 1 | 0 | 21 | 4 | 0 | 133 | 32 | 0 | 8 | 9 |
| Cymatocylis | 50 | 563 | 17 | 131 | 103 | 76 | 1100 | 470 | 714 | 69 | 80 | 63 |
| Codonellopsis | 58 | 32 | 7 | 150 | 60 | 46 | 1076 | 300 | 446 | 62 | 49 | 21 |
| Laackmanniella | 33 | 91 | 19 | 71 | 129 | 42 | 413 | 535 | 269 | 63 | 85 | 62 |
| Salpingella | 127 | 99 | 3 | 452 | 236 | 9 | 3250 | 975 | 68 | 51 | 50 | 45 |
| av.a. indL−1 | sd | Max indL−1 | Occurrence % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| on | int | off | on | int | off | on | int | off | on | int | off | |
| Amphorides | 1 | 2 | 0 | 8 | 12 | 0 | 133 | 83 | 0 | 4 | 6 | 0 |
| Cymatocylis | 34 | 26 | 64 | 79 | 65 | 212 | 442 | 470 | 1100 | 70 | 56 | 61 |
| Codonellopsis | 15 | 28 | 70 | 50 | 79 | 199 | 446 | 704 | 1076 | 35 | 43 | 45 |
| Laackmanniella | 27 | 45 | 42 | 57 | 100 | 79 | 346 | 535 | 266 | 67 | 61 | 58 |
| Salpingella | 12 | 80 | 170 | 68 | 280 | 562 | 716 | 2782 | 3250 | 32 | 49 | 71 |
| Amphorides | Cymatocylis | Codonellopsis | Laackmanniella | Salpingella | Tintinnid Abundance | |
|---|---|---|---|---|---|---|
| Temperature | 0.0558 | 0.3772 | 0.4837 | 0.2516 | 0.1516 | 0.4929 |
| Salinity | −0.0838 | −0.0305 | −0.0976 | 0.0008 | −0.0238 | −0.0824 |
| Cluster A | ||||
| Average similarity: 62.70 | ||||
| av.a. | av.sim. | contrib.% | cum.% | |
| Laackmanniella | 24.64 | 46.98 | 74.94 | 74.94 |
| Cymatocylis | 11.74 | 13.62 | 21.72 | 96.66 |
| Cluster B | ||||
| Average similarity: 57.35 | ||||
| av.a. | av.sim. | contrib.% | cum.% | |
| Salpingella | 386.69 | 45.99 | 80.20 | 80.20 |
| Codonellopsis | 68.86 | 7.20 | 12.55 | 92.75 |
| Cluster C | ||||
| Average similarity: 51.87 | ||||
| av.a. | av.sim. | contrib.% | cum.% | |
| Cymatocylis | 78.28 | 36.51 | 70.40 | 70.40 |
| Codonellopsis | 57.18 | 11.92 | 22.99 | 93.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti-Birkenmeier, M.; Diociaiuti, T.; Castagno, P.; Budillon, G.; Fonda Umani, S. Pluridecadal Temporal Patterns of Tintinnids (Ciliophora, Spirotrichea) in Terra Nova Bay (Ross Sea, Antarctica). Diversity 2022, 14, 604. https://doi.org/10.3390/d14080604
Monti-Birkenmeier M, Diociaiuti T, Castagno P, Budillon G, Fonda Umani S. Pluridecadal Temporal Patterns of Tintinnids (Ciliophora, Spirotrichea) in Terra Nova Bay (Ross Sea, Antarctica). Diversity. 2022; 14(8):604. https://doi.org/10.3390/d14080604
Chicago/Turabian StyleMonti-Birkenmeier, Marina, Tommaso Diociaiuti, Pasquale Castagno, Giorgio Budillon, and Serena Fonda Umani. 2022. "Pluridecadal Temporal Patterns of Tintinnids (Ciliophora, Spirotrichea) in Terra Nova Bay (Ross Sea, Antarctica)" Diversity 14, no. 8: 604. https://doi.org/10.3390/d14080604
APA StyleMonti-Birkenmeier, M., Diociaiuti, T., Castagno, P., Budillon, G., & Fonda Umani, S. (2022). Pluridecadal Temporal Patterns of Tintinnids (Ciliophora, Spirotrichea) in Terra Nova Bay (Ross Sea, Antarctica). Diversity, 14(8), 604. https://doi.org/10.3390/d14080604






