Arbuscular Mycorrhizal Fungi Associated with Roots Reveal High Diversity Levels at Different Elevations in Tropical Montane Rainforests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region and Sample Collection
2.2. DNA Extraction and PCR Amplification
2.3. Soil Properties Analysis
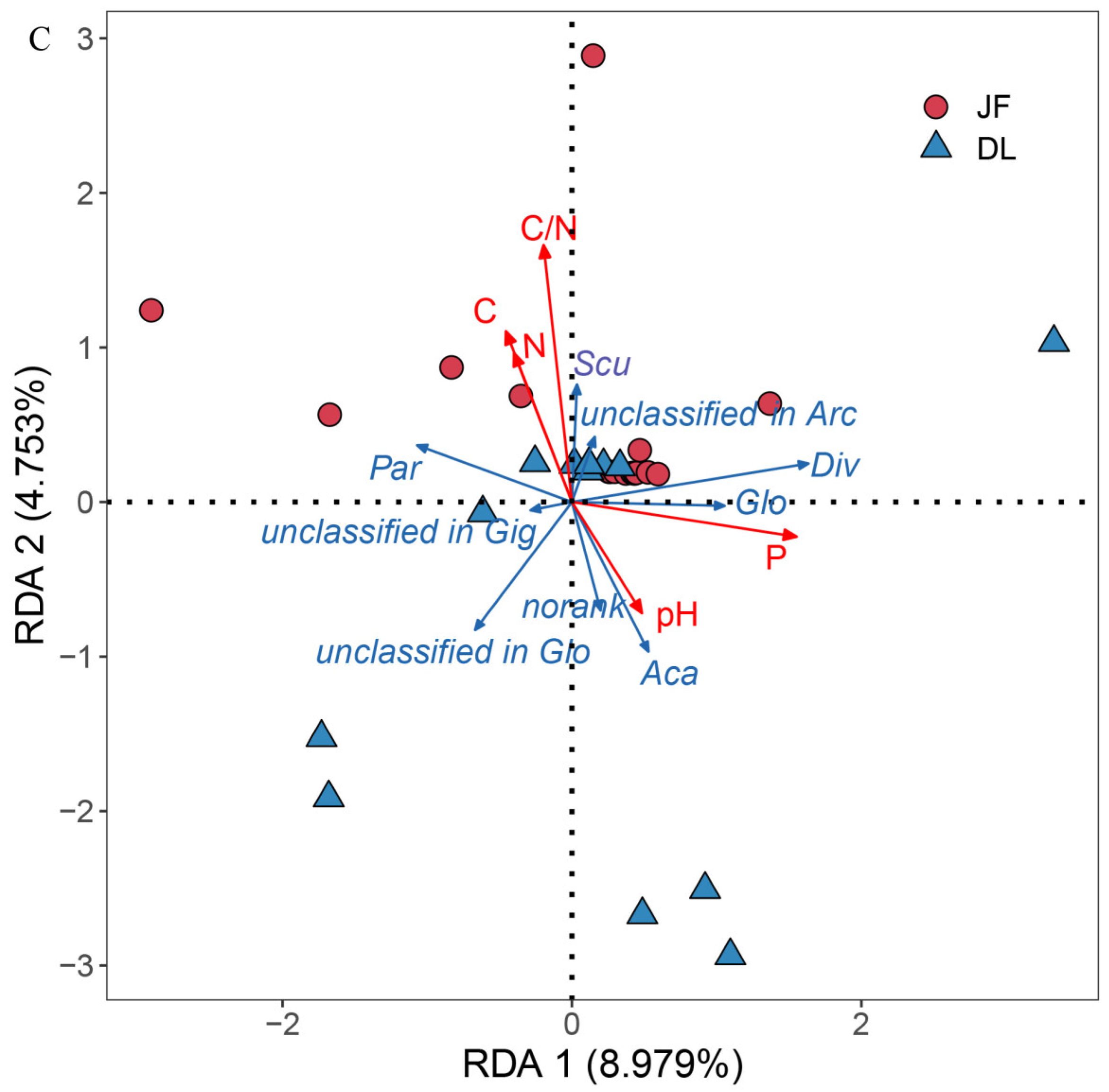
2.4. Statistical Data Analysis
3. Results
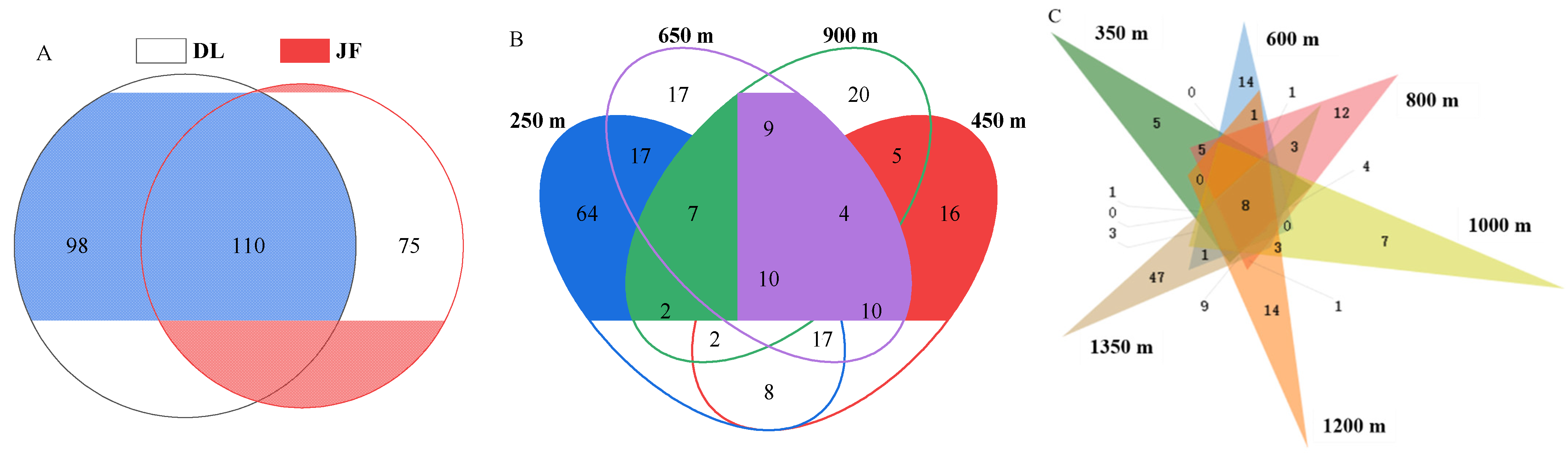
3.1. AMF Community Composition and Distribution
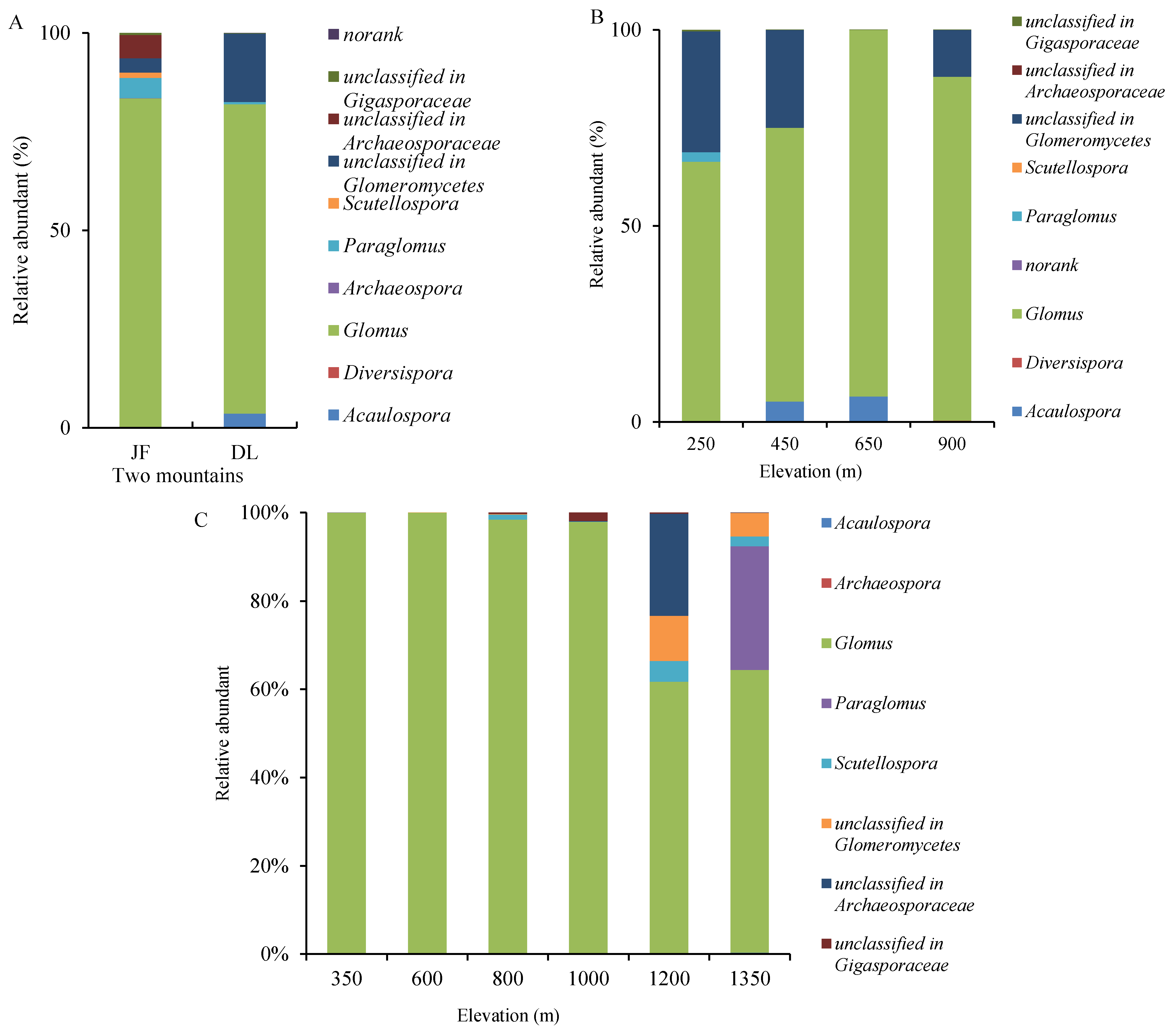
3.2. AMF Genus Relative Abundances and Occurrence Frequencies at Different Elevations at OTU Level
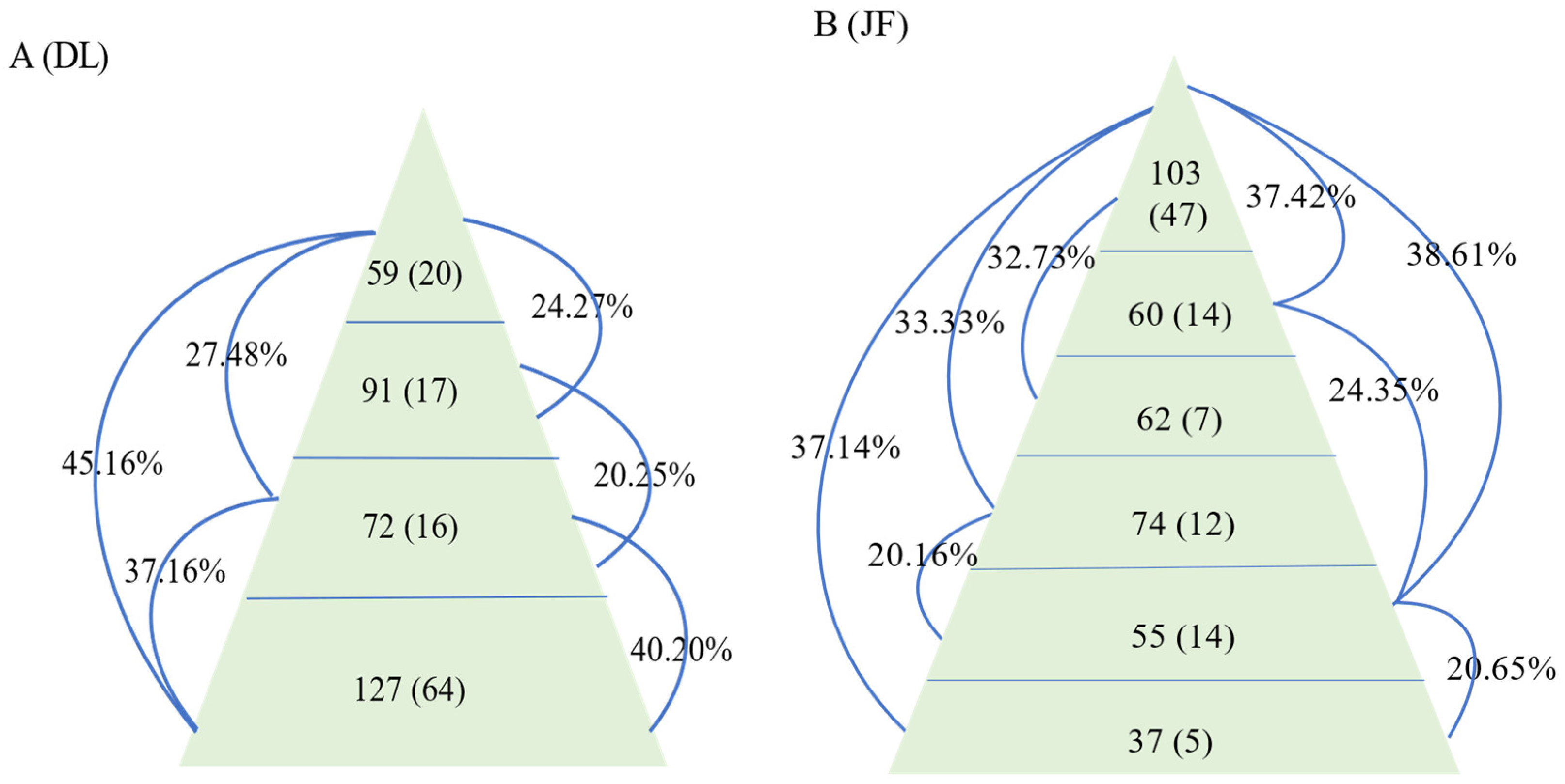
3.3. AMF Diversity at Different Elevations
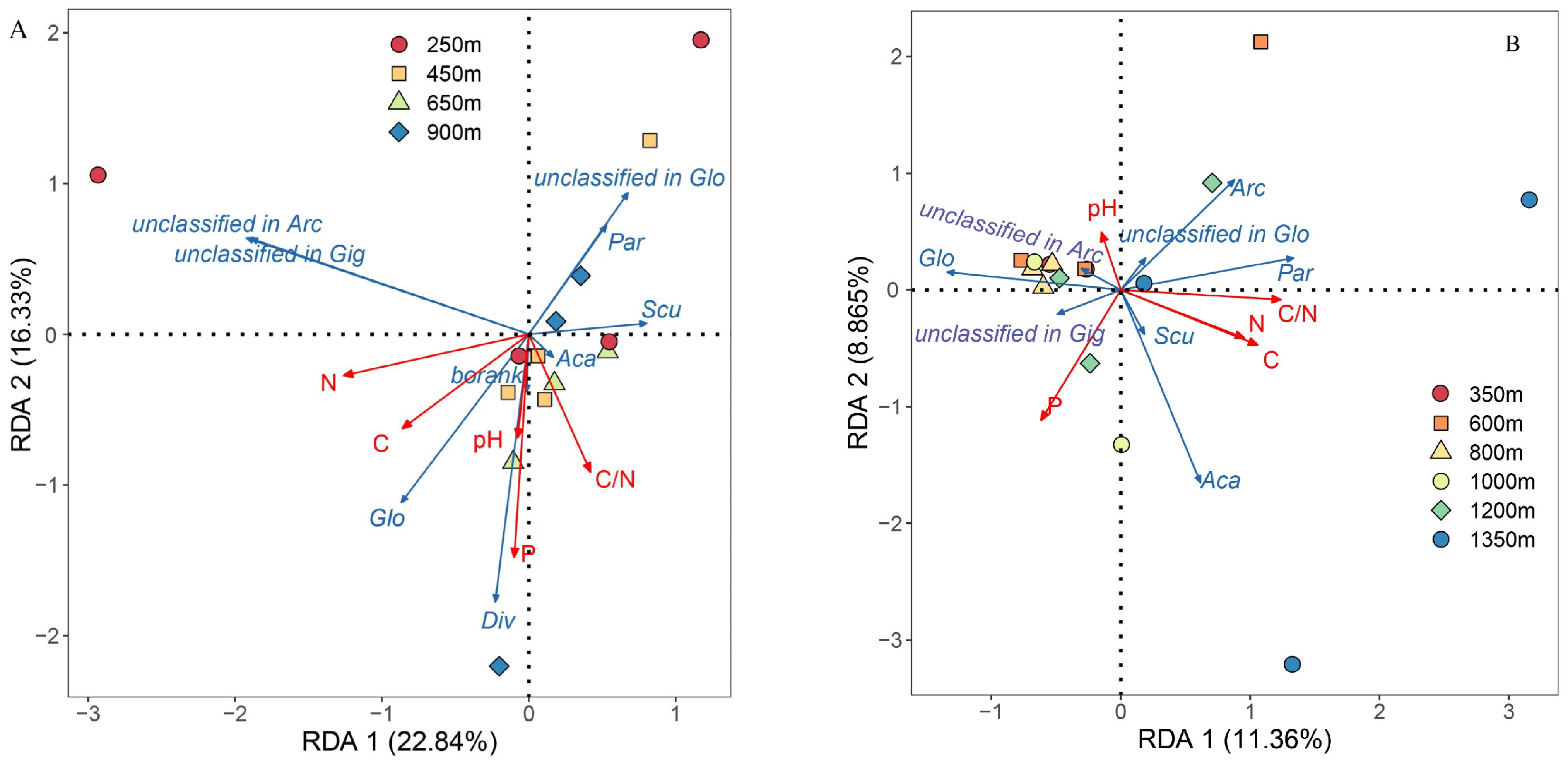
3.4. Effects of Soil Factors on AMF Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above-and belowground linkages shape responses of mountain vegetation to climate change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008; pp. 13–89. [Google Scholar]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.D.; Fernandes, M.F. Changes in microbial community structure and physiological profile in a kaolinitic tropical soil under different conservation agricultural practices. Appl. Soil Ecol. 2020, 152, 103545. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Malicka, M.; Magurno, F.; Posta, K.; Chmura, D.; Piotrowska-Seget, Z. Differences in the effects of single and mixed species of AMF on the growth and oxidative stress defense in Lolium perenne exposed to hydrocarbons. Ecotoxicol. Environ. Saf. 2021, 217, 112252. [Google Scholar] [CrossRef]
- Cotton, T.E.A. Arbuscular mycorrhizal fungal communities and global change: An uncertain future. FEMS Microbiol. Ecol. 2018, 94, 179. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Hedenec, P.; Nilsson, L.O.; Zheng, H.F.; Gundersen, P.; Schmidt, I.K.; Rousk, J.; Vesterdal, L. Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Soil Biol. Biochem. 2020, 149, 107933. [Google Scholar] [CrossRef]
- Qiu, Q.Y.; Bender, S.F.; Mgelwa, A.S.; Hu, Y.L. Arbuscular mycorrhizal fungi mitigate soil nitrogen and phosphorus losses: A meta-analysis. Sci. Total Environ. 2022, 807, 150857. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.Y.; Classen, A.T.; Zhao, K.; Chen, L.T.; Shi, Y.; Jiang, Y.X.; He, J.S. The links between ecosystem multifunctionality and above-and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, A.; Ainsaar, L.; Bâ, A.; Burla, S.; Diedhiou, A.G.; Hiiesalu, I.; Jairus, T. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2015, 349, 970–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Echeverria, S.; Teixeira, H.; Correia, M.; Timoteo, S.; Heleno, R.; Opik, M.; Moora, M. Arbuscular mycorrhizal fungi communities from tropical Africa reveal strong ecological structure. New Phytol. 2017, 213, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Q.; Cui, M.M.; Wang, C.; Wang, J.; Wang, S.L.; Sun, Z.J.; Ren, F.R.; Wan, S.Q.; Han, S.J. Elevated CO2, warming, N addition, and increased precipitation affect different aspects of the arbuscular mycorrhizal fungal community. Sci. Total Environ. 2022, 806, 150522. [Google Scholar] [CrossRef]
- Schroder, J.M.; Rodriguez, L.P.A.; Gunter, S. Research trends: Tropical dry forests: The neglected research agenda? For. Policy Econ. 2021, 122, 102333. [Google Scholar] [CrossRef]
- Pajares, S.; Bohannan, B.J.M. Ecology of nitrogen fixing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. 2016, 7, 1045. [Google Scholar] [CrossRef] [Green Version]
- Townsend, A.R.; Cleveland, C.C.; Houlton, B.Z.; Alden, C.B.; White, J.W.C. Multi-element regulation of the tropical forest carbon cycle. Front. Ecol. Environ. 2011, 9, 9–17. [Google Scholar] [CrossRef] [Green Version]
- da Silva, C.A.; Londe, V.; Andrade, S.A.L.; Joly, C.A.; Vieira, S.A. Fine root-arbuscular mycorrhizal fungi interaction in Tropical Montane Forests: Effects of cover modifications and season. For. Ecol. Manag. 2020, 476, 118478. [Google Scholar] [CrossRef]
- Niu, L.H.; Li, Y.; Wang, P.F.; Zhang, W.L.; Wang, C.; Wang, Q. Understanding the linkage between elevation and the activated-sludge bacterial community along a 3600-meter elevation gradient in China. Appl. Environ. Microbiol. 2015, 81, 6567–6576. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; Xu, M.; Li, X.L.; Christie, P.; Wagg, C.; Zhang, J.L. Linkages between changes in plant and mycorrhizal fungal community composition at high versus low elevation in alpine ecosystems. Environ. Microbiol. Rep. 2020, 12, 229–240. [Google Scholar] [CrossRef]
- Zhang, M.G.; Shi, Z.Y.; Yang, M.; Lu, S.C.; Cao, L.B.; Wang, X.G. Molecular diversity and distribution of arbuscular mycorrhizal fungi at different elevations in Mt. Taibai of Qinling Mountain. Front. Microbiol. 2021, 12, 609386. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.C.; da Silva, D.K.A.; de Melo, M.A.C.; Escobar, I.E.C.; Oehl, F.; da Silva, G.A. Edaphic factors influence the distribution of arbuscular mycorrhizal fungi along an altitudinal gradient of a Tropical Mountain. Microb. Ecol. 2019, 78, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ni, Y.; Liang, W.; Wang, J.; Chu, H. Distinct soil bacterial communities along a small-scale elevational gradient in alpine tundra. Front. Microbiol. 2015, 6, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, C.P.; Callaway, R.M.; Hart, M.M.; Pither, J.; Klironomos, J. Phylogenetic structure of arbuscular mycorrhizal fungal communities along an elevation gradient. Mycorrhiza 2017, 27, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.L.; Veresoglou, S.D.; Chen, Y.L.; Rillig, M.C.; Xiang, D.; Ondrej, D.; Hao, Z.P.; Liu, L.; Deng, Y.; Hu, Y.J.; et al. Plant community, geographic distance and abiotic factors play different roles in predicting AMF biogeography at the regional scale in northern China. Environ. Microbiol. 2016, 8, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.C.; Geng, Q.H.; Zhang, H.G.; Bian, C.Y.; Chen, H.Y.H.; Jiang, D.L.; Xu, X. Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytol. 2020, 229, 2957–2969. [Google Scholar] [CrossRef]
- Chen, D.X.; Li, Y.D.; Liu, H.P.; Xu, H.; Xiao, W.F.; Luo, T.S.; Zhou, Z.; Lin, M.X. Biomass and carbon dynamics of a tropical mountain rain forest in China. Sci. China Life Sci. 2010, 53, 798–810. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society Agronomy and Soil Science Society America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Marinho, F.; da Silva, I.R.; Oehl, F.; Maia, L.C. Checklist of arbuscular mycorrhizal fungi in tropical forests. Sydowia 2019, 70, 107–127. [Google Scholar] [CrossRef]
- Xu, X.H.; Chen, C.; Zhang, Z.; Sun, Z.H.; Chen, Y.H.; Jiang, J.D.; Shen, Z.G. The influence of environmental factors on communities of arbuscular mycorrhizal fungi associated with Chenopodium ambrosioides revealed by MiSeq sequencing investigation. Sci. Rep. 2017, 7, 45134. [Google Scholar] [CrossRef]
- Kotilinek, M.; Hiiesalu, I.; Kosnar, J.; Smilauerov, M.; Smilauer, P.; Altman, J.; Dvorsky, M.; Kopecky, M.; Dolezal, J. Fungal root symbionts of high-altitude vascular plants in the Himalayas. Sci. Rep. 2017, 7, 6562. [Google Scholar] [CrossRef] [Green Version]
- Hazard, C.; Gosling, P.; van der Gast, C.J.; Mitchell, D.T.; Doohan, F.M.; Bending, G.D. The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J. 2013, 7, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.Y.; Wang, F.Y.; Zhang, K.; Chen, Y.L. Diversity and distribution of arbuscular mycorrhizal fungi along altitudinal gradients in Mount Taibai of the Qinling Mountains. Can. J. Microbiol. 2014, 60, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, X.Z.; Zhang, Z.M.; Zhao, Y.; Yang, J.T.; Zhu, Y.W. Species diversity and drivers of arbuscular mycorrhizal fungal communities in a semi-arid mountain in China. Peer J. 2017, 5, e4155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.L.; Zhang, K.X.; Sun, X.; He, X.L. Dynamics of arbuscular mycorrhizal fungi and glomalin in the rhizosphere of Gymnocarpos przewalskii in Northwest Desert, China. Appl. Soil Ecol. 2022, 170, 104251. [Google Scholar] [CrossRef]
- O’Malley, M.A. ‘Everything is everywhere: But the environment selects’: Ubiquitous distribution and ecological determinism in microbial biogeography. Stud. Hist. Philos. Biol. Biomed. Sci. 2008, 39, 314–325. [Google Scholar] [CrossRef]
- Sousa, N.M.F.; Veresoglou, S.D.; Oehl, F.; Rillig, M.C.; Maia, L.C. Predictors of Arbuscular Mycorrhizal Fungal Communities in the Brazilian Tropical Dry Fores. Microb. Ecol. 2018, 75, 447–458. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. Mycorrhizae and succession in plantings of beachgrass in sand dunes. Am. J. Bot. 1997, 84, 118–130. [Google Scholar] [CrossRef]
- Yang, M.; Shi, Z.Y.; Bede, S.M.; Zhang, M.G.; Cao, L.B. Alterations to arbuscular mycorrhizal fungal community composition is driven by warming at specific elevations. Peer J. 2021, 9, e11792. [Google Scholar] [CrossRef]
- Polato, N.R.; Gill, B.A.; Shah, A.A.; Gray, M.M.; Casner, K.L.; Barthelet, A.; Messer, P.W.; Simmons, M.P.; Guayasamin, J.M.; Encalada, A.C. Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proc. Natl. Acad. Sci. USA 2018, 115, 12471–12476. [Google Scholar] [CrossRef] [Green Version]
- Bonfim, J.A.; Vasconcellos, R.L.F.; Gumiere, T.; Mescolotti, D.D.C.; Oehl, F.; Cardoso, E.J.B. Diversity of arbuscular mycorrhizal fungi in a Brazilian atlantic forest toposequence. Microbiol. Ecol. 2016, 71, 164–177. [Google Scholar] [CrossRef]
- Guo, Y.X.; Ren, C.J.; Yi, J.J.; Doughty, R.; Zhao, F.Z. Contrasting Responses of Rhizosphere Bacteria, Fungi and Arbuscular Mycorrhizal Fungi Along an Elevational Gradient in a Temperate Montane Forest of China. Front. Microbiol. 2020, 11, 2042. [Google Scholar] [CrossRef] [PubMed]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; Gebert, F.; et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 2016, 7, 13736. [Google Scholar] [CrossRef] [Green Version]
- Mommer, L.; Cotton, T.E.A.; Raaijmakers, J.M.; Termorshuizen, A.J.; van Ruijven, J.; Hendriks, M.; van Rijssel, S.Q.; van de Mortel, J.E.; van der Paauw, J.W.; Schijlen, E.G.W.M. Lost in diversity: The interactions between soilborne fungi, biodiversity and plant productivity. New Phytol. 2018, 218, 542–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashi, S.; Singh, B.; Keitel, C.; Adams, M. Soil carbon and nitrogen stocks in forests along an altitudinal gradient in the eastern Himalayas and a meta-analysis of global data. Glob. Chang. Biol. 2016, 22, 2255–2268. [Google Scholar] [CrossRef]
- Carrara, J.E.; Walter, C.A.; Hawkins, J.S.; Peterjohn, W.T.; Averill, C.; Brzostek, E.R. Interactions among plants, bacteria, and fungi reduce extracellular enzyme activities under long-term N fertilization. Glob. Chang. Biol. 2018, 24, 2721–2734. [Google Scholar] [CrossRef] [PubMed]
- Jeske, E.S.; Tian, H.; Hanford, K.; Walters, D.T.; Drijber, R.A. Long-term nitrogen fertilization reduces extraradical biomass of arbuscular mycorrhizae in a maize (Zea mays L.) cropping system. Agric. Ecosyst. Environ. 2018, 255, 111–118. [Google Scholar] [CrossRef]
- Shi, G.X.; Yang, Y.; Liu, Y.J.; Uwamungu, J.Y.; Liu, Y.M.; Wang, Y.B.; Feng, H.Y.; Yao, B.Q.; Zhou, H.K. Effect of elymus nutans on the assemblage of arbuscular mycorrhizal fungal communities enhanced by soil available nitrogen in the restoration succession of revegetated grassland on the Qinghai-Tibetan Plateau. Land Degrad. Dev. 2022, 33, 931–944. [Google Scholar] [CrossRef]
- Chen, K.; Huang, G.; Li, Y.K.; Zhang, X.R.; Lei, Y.H.; Li, Y.; Xiong, J.; Sun, Y.F. Illumina MiSeq Sequencing Reveals Correlations among Fruit Ingredients, Environmental Factors, and AMF Communities in Three Lycium Barbarum Producing Regions of China. Microbiol. Spectr. 2022, 10, e0229321. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, P.F.; Li, J.; Ren, L.F.; Bai, W.M.; Tian, Q.Y.; Sun, W.; Zhang, W.H. Arbuscular mycorrhizal fungal communities associated with two dominant species differ in their responses to long-term nitrogen addition in temperate grasslands. Funct. Ecol. 2018, 32, 1575–1588. [Google Scholar] [CrossRef]
- Gao, D.M.; Pan, X.J.; Zhou, X.G.; Wei, Z.; Li, N.H.; Wu, F.Z. Phosphorus fertilization and intercropping interactively affect tomato and potato onion growth and rhizosphere arbuscular mycorrhizal fungal community. Arch. Agron. Soil Sci. 2021, 67, 919–933. [Google Scholar] [CrossRef]
- Kaur, S.; Campbell, B.J.; Suseela, V. Root metabolome of plant-arbuscular mycorrhizal symbiosis mirrors the mutualistic or parasitic mycorrhizal phenotype. New Phytol. 2022, 234, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Wang, W.Q.; Yang, J.; Qin, S.F.; Yang, Y.S.; Sun, C.Y.; Pei, G.; Zeeshan, M.; Liao, H.L.; Liu, L.; et al. Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 2022, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.C.; Zhao, B.Y.; Zheng, S.; Zhang, X.W.; Wang, X.L.; Dong, W.T.; Xie, Q.J.; Wang, G.; Xiao, Y.P.; Chen, F.; et al. Phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 2021, 184, 5527. [Google Scholar] [CrossRef]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 21, 1420–1427. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the law of the minimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- Avio, L.; Njeru, E.M.; Oehl, F.; Turrini, A.; Bocci, G.; Barberi, P.; Giovannetti, M.; Sbrana, C. Small-scale soil heterogeneity affects the distribution of arbuscular mycorrhizal fungal species in a hot-spot field in a Mediterranean site. Appl. Soil Ecol. 2020, 154, 103631. [Google Scholar] [CrossRef]
- Rozek, K.; Rola, K.; Blaszkowski, J.; Leski, T.; Zubek, S. How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil? For. Ecol. Manag. 2020, 465, 118091. [Google Scholar] [CrossRef]
- Bucking, H.; Kafle, A. Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: Current knowledge and research gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.H.; Shen, Y.K.; Li, Q.; Xiao, W.F.; Song, X.Z. Arbuscular mycorrhizal fungal colonization and soil pH induced by nitrogen and phosphorus additions affects leaf C:N:P stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Plant Soil 2021, 461, 421–440. [Google Scholar] [CrossRef]
- Caruso, T. Disentangling the factors shaping arbuscular mycorrhizal fungal communities across multiple spatial scales. New Phytol. 2018, 220, 954–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.Y.; Qiu, Y.P.; Zhang, K.C.; Yang, F.; Chen, M.F.; Luo, X.; Yan, X.B.; Wang, P.; Zhang, Y.; Chen, H.H.; et al. Climate warming promotes deterministic assembly of arbuscular mycorrhizal fungal communities. Glob. Chang. Biol. 2022, 28, 1147–1161. [Google Scholar] [CrossRef] [PubMed]

| Occurrence Frequency (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | DL | JF | Total | ||||||||
| 250 m | 450 m | 650 m | 900 m | 350 m | 600 m | 800 m | 1000 m | 1200 m | 1350 m | ||
| Acaulospora | 66.67 | 25 | 100 | 33.33 | 0 | 0 | 0 | 66.67 | 33.33 | 66.67 | 40 |
| Archaeospora | 0 | 0 | 0 | 0 | 0 | 33.33 | 0 | 0 | 0 | 0 | 33.33 |
| Diversispora | 0 | 0 | 0 | 33.33 | 0 | 0 | 0 | 0 | 0 | 0 | 33.33 |
| Glomus | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Norank | 0 | 0 | 66.67 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 66.67 |
| Paraglomus | 33.33 | 25 | 0 | 0 | 50 | 0 | 0 | 33.33 | 33.33 | 33.33 | 20 |
| Scutellospora | 0 | 0 | 33.33 | 0 | 0 | 33.33 | 66.67 | 33.33 | 33.33 | 66.67 | 26.67 |
| Unclassified in Glomeromycetes | 33.33 | 50 | 33.33 | 66.67 | 0 | 66.67 | 33.33 | 0 | 33.33 | 33.33 | 40 |
| Unclassified in Archaeosporaceae | 33.33 | 0 | 0 | 0 | 0 | 0 | 0 | 33.33 | 33.33 | 33.33 | 13.33 |
| Unclassified in Gigasporaceae | 33.33 | 25 | 66.67 | 33.33 | 0 | 0 | 66.67 | 100 | 33.33 | 66.67 | 43.33 |
| Mountain | Elevation (m) | N (%) | C (%) | C/N | P (g/kg) | pH |
|---|---|---|---|---|---|---|
| DL | 250 | 0.20 ± 0.05 ab | 2.23 ± 0.43 ab | 11.81 ± 0.89 ab | 0.26 ± 0.05 a | 4.86 ± 0.27 bc |
| 450 | 0.19 ± 0.03 ab | 2.12 ± 0.45 ab | 10.90 ± 0.74 ab | 0.46 ± 0.03 a | 5.57 ± 0.15 ab | |
| 650 | 0.12 ± 0.01 b | 1.46 ± 0.14 b | 12.13 ± 0.26 b | 0.36 ± 0.01 a | 5.81 ± 0.13 a | |
| 900 | 0.23 ± 0.01 a | 3.19 ± 0.21 a | 13.65 ± 0.53 a | 0.49 ± 0.01 a | 4.61 ± 0.43 c | |
| JF | 350 | 0.23 ± 0.05 b | 3.32 ± 0.60 b | 14.78 ± 0.24 bc | 0.36 ± 0.05 a | 5.63 ± 0.21 ab |
| 600 | 0.19 ± 0.03 b | 3.09 ± 0.72 b | 16.13 ± 1.72 ab | 0.21 ± 0.10 a | 5.88 ± 0.49 a | |
| 800 | 0.17 ± 0.01 b | 2.89 ± 0.30 b | 16.39 ± 0.39 ab | 0.36 ± 0.06 a | 4.63 ± 0.14 b | |
| 1000 | 0.12 ± 0.01 b | 1.54 ± 0.22 b | 13.20 ± 0.35 c | 0.17 ± 0.03 a | 5.13 ± 0.45 ab | |
| 1200 | 0.29 ± 0.06 b | 4.80 ± 1.21 b | 16.69 ± 0.29 ab | 0.31 ± 0.03 a | 4.83 ± 0.08 ab | |
| 1350 | 0.62 ± 0.16 a | 11.13 ± 2.64 a | 17.97 ± 0.69 a | 0.31 ± 0.15 a | 4.91 ± 0.24 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Shi, Z.; Xu, X.; Wang, X. Arbuscular Mycorrhizal Fungi Associated with Roots Reveal High Diversity Levels at Different Elevations in Tropical Montane Rainforests. Diversity 2022, 14, 587. https://doi.org/10.3390/d14080587
Zhang M, Shi Z, Xu X, Wang X. Arbuscular Mycorrhizal Fungi Associated with Roots Reveal High Diversity Levels at Different Elevations in Tropical Montane Rainforests. Diversity. 2022; 14(8):587. https://doi.org/10.3390/d14080587
Chicago/Turabian StyleZhang, Mengge, Zhaoyong Shi, Xiaofeng Xu, and Xugang Wang. 2022. "Arbuscular Mycorrhizal Fungi Associated with Roots Reveal High Diversity Levels at Different Elevations in Tropical Montane Rainforests" Diversity 14, no. 8: 587. https://doi.org/10.3390/d14080587
APA StyleZhang, M., Shi, Z., Xu, X., & Wang, X. (2022). Arbuscular Mycorrhizal Fungi Associated with Roots Reveal High Diversity Levels at Different Elevations in Tropical Montane Rainforests. Diversity, 14(8), 587. https://doi.org/10.3390/d14080587





