Molecular Relationships and Genetic Diversity Analysis of Venetian Radicchio (Leaf Chicory, Cichorium intybus subsp. intybus var. sylvestre, 2n = 2x = 18) Biotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants Materials
2.2. Genomic DNA Isolation
2.3. Molecular Markers
2.4. Genetic Diversity and Relationships Analyses
2.5. Genetic Structure Analyses of the Core Collection
2.6. Genetic Diversity Statistics
2.7. Genetic Similarity Estimates
3. Results
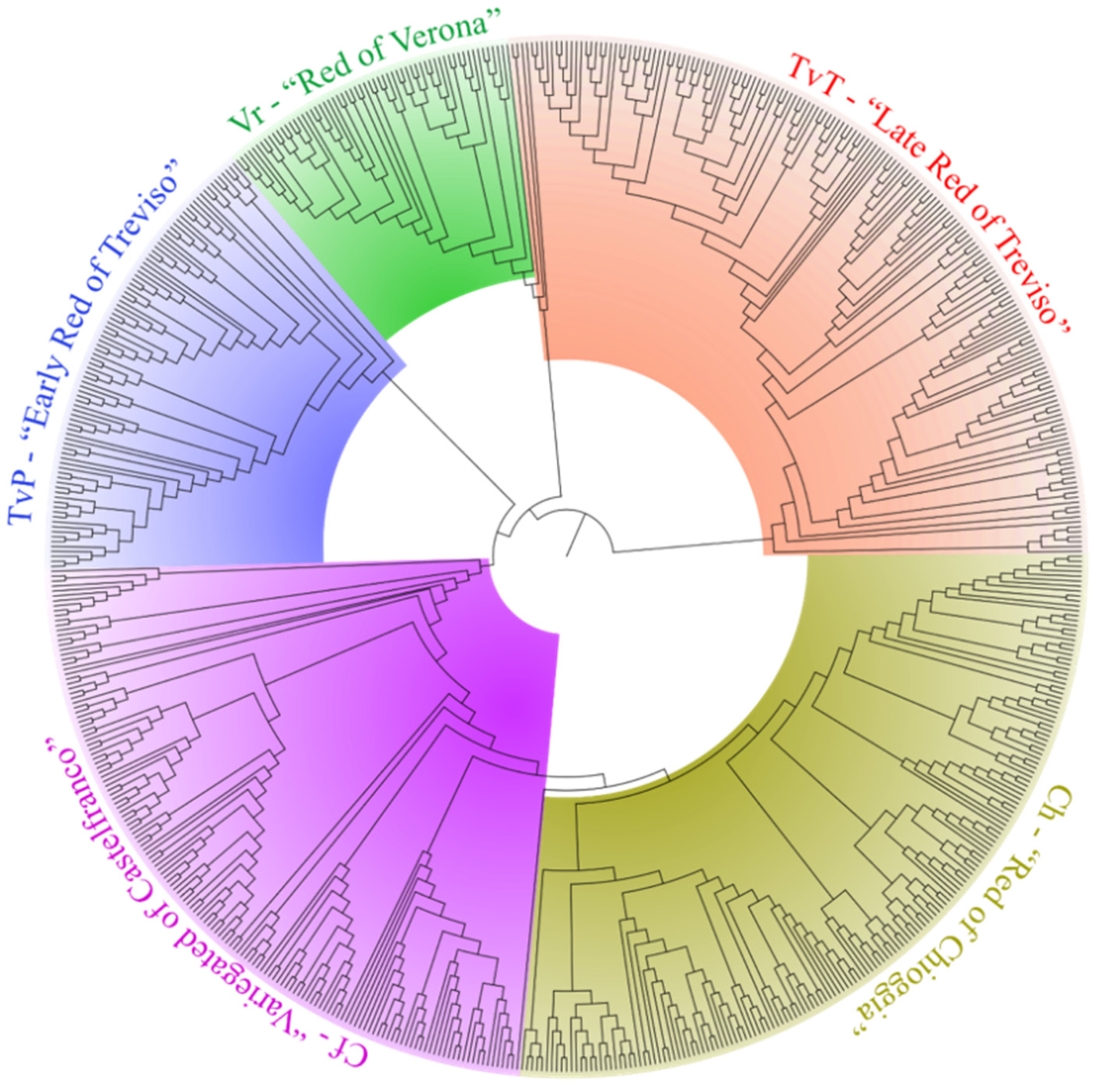
3.1. Genetic Relationships among Radicchio Populations and Biotypes
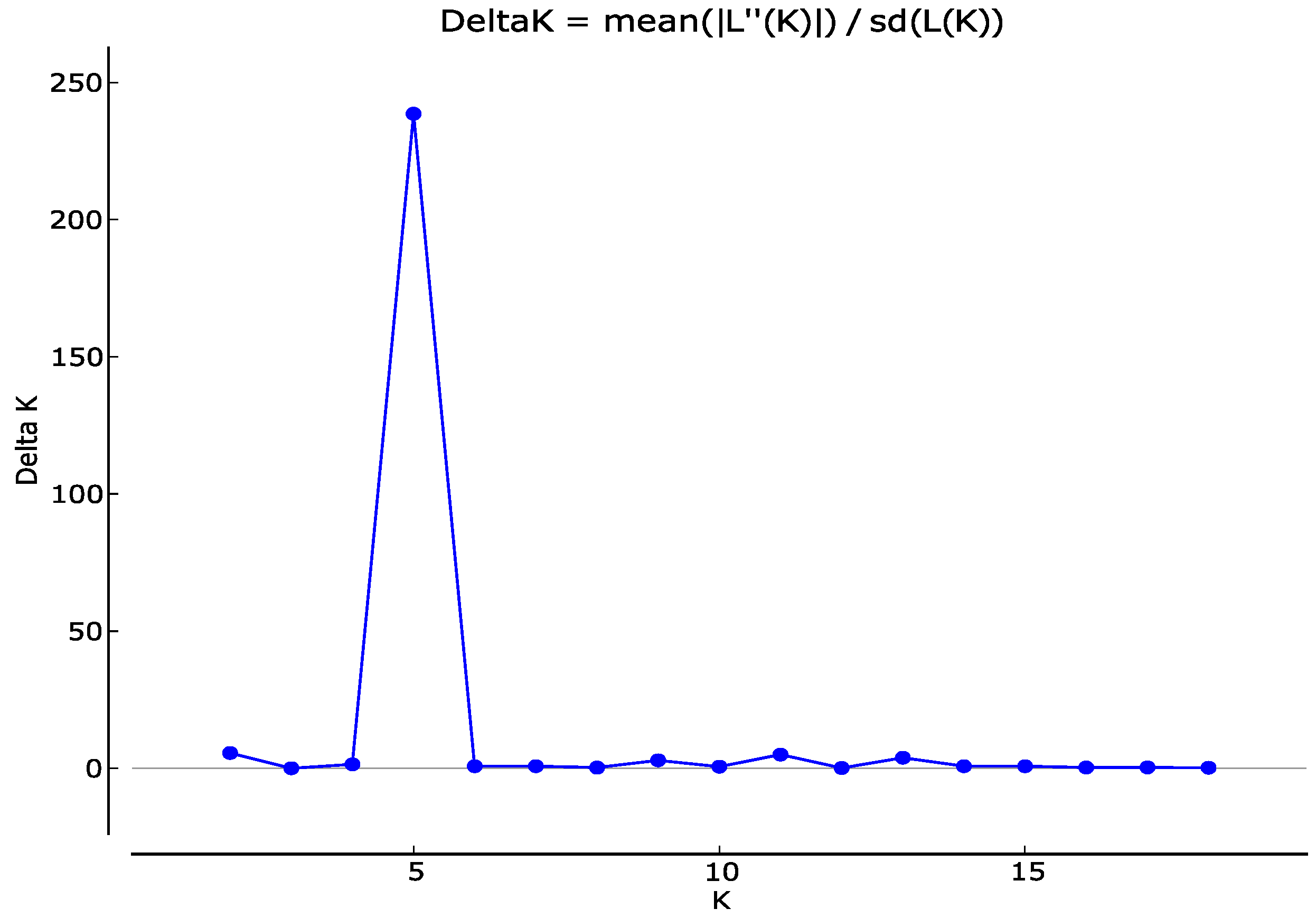
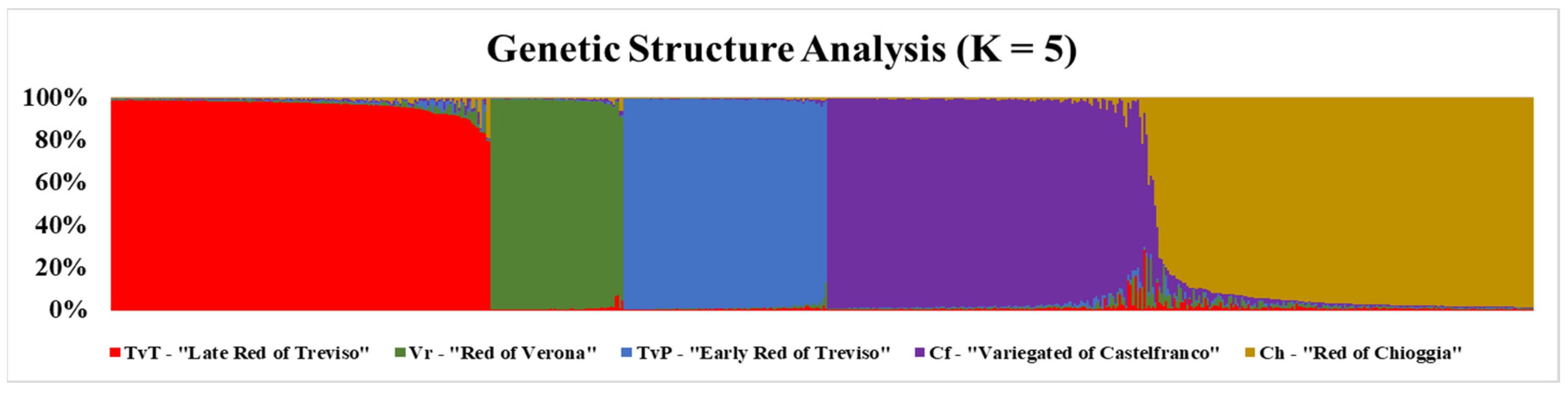
3.2. Genetic Structure of the Radicchio Core Collection and Biotype Clustering
3.3. Genetic Statistics of Populations and Biotypes
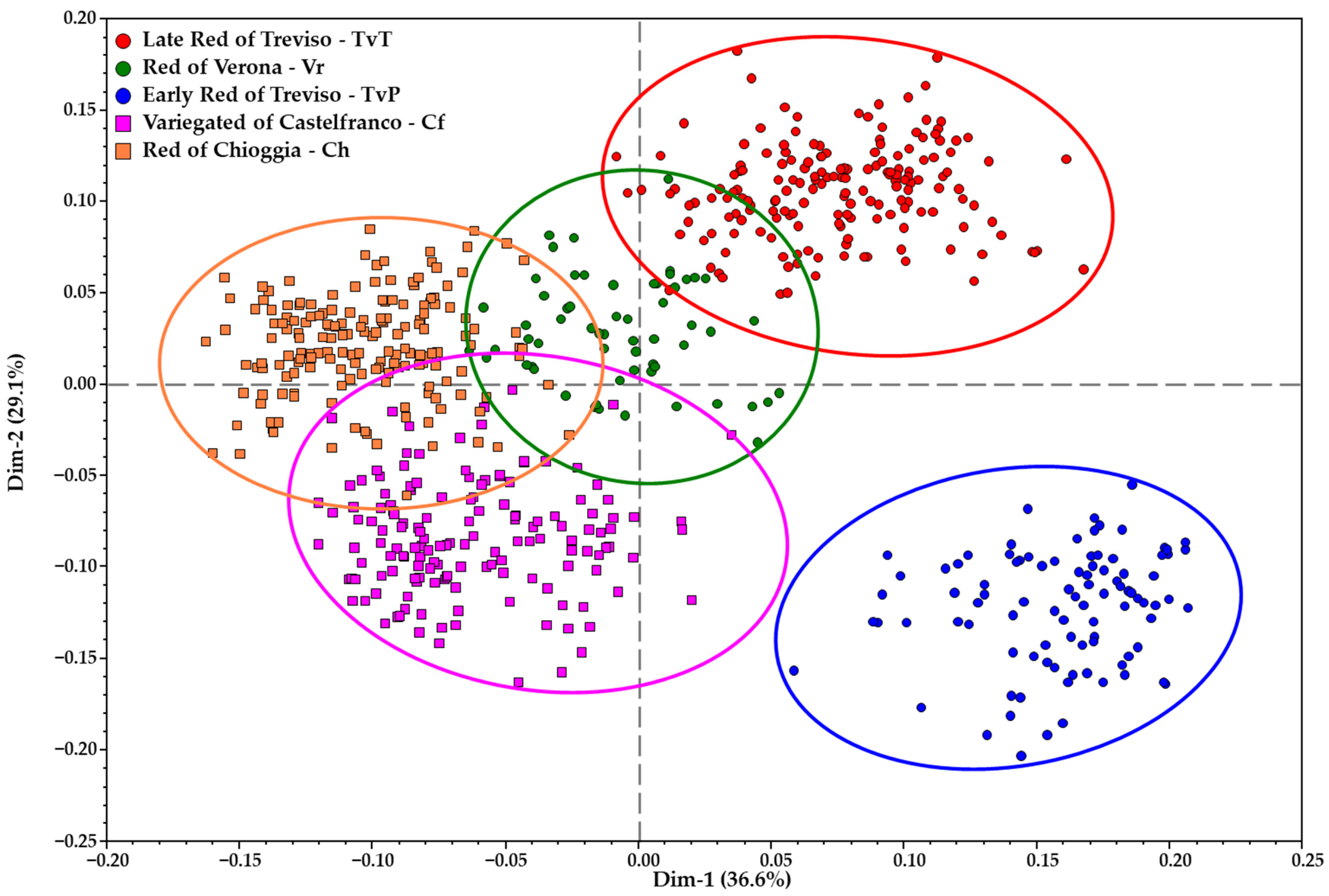
3.4. Genetic Similarity Analysis within and between Populations or Biotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Simko, I.; Jia, M.; Venkatesh, J.; Kang, B.-C.; Weng, Y.; Barcaccia, G.; Lanteri, S.; Bhattarai, G.; Foolad, M.R. Genomics and marker-assisted improvement of vegetable crops. Crit. Rev. Plant Sci. 2021, 40, 303–365. [Google Scholar] [CrossRef]
- Funk, V.A.; Susanna, A.; Steussy, T.F.; Bayer, R.J. Systematics, Evolution, and Biogeography of Compositae; International Association for Plant Taxonomy: Vienna, Austria, 2009. [Google Scholar]
- Susanna, A.; Baldwin, B.G.; Bayer, R.J.; Bonifacino, J.M.; Garcia-Jacas, N.; Keeley, S.C.; Mandel, J.R.; Ortiz, S.; Robinson, H.; Stuessy, T.F. The classification of the Compositae: A tribute to Vicki Ann Funk (1947–2019). Taxon 2020, 69, 807–814. [Google Scholar] [CrossRef]
- Bremer, K. Asteraceae: Cladistics and Classification; Timber Press: Portland, OR, USA, 1994. [Google Scholar]
- Barcaccia, G.; Ghedina, A.; Lucchin, M. Current Advances in Genomics and Breeding of Leaf Chicory (Cichorium intybus L.). Agriculture 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Kiers, A.M. Endive, chicory, and their wild relatives. A systematic and phylogenetic study of Cichorium (Asteraceae). Gorteria Dutch Bot. Arch. Suppl. 2000, 5, 1–77. [Google Scholar]
- Lucchin, M.; Varotto, S.; Barcaccia, G.; Parrini, P. Chicory and Endive. In Vegetables I: Asteraceae, Brassicaceae Chenopodi-Caceae; Prohens, J., Nuez, F., Eds.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2008; Volume 1, pp. 3–48. [Google Scholar]
- Barcaccia, G.; Varotto, S.; Soattin, M.; Lucchin, M.; Parrini, P. Genetic and molecular studies of sporophytic self-incompatibility in Cichorium intybus L. In Proceedings of the Eucarpia Meeting on Leafy Vegetables Genetics and Breeding, Noordwijkerhout, The Netherlands, 19–21 March 2003; p. 154. [Google Scholar]
- Barcaccia, G.; Pallottini, L.; Soattin, M.; Lazzarin, R.; Parrini, P.; Lucchin, M. Genomic DNA fingerprints as a tool for identifying cultivated types of radicchio (Cichorium intybus L.) from Veneto, Italy. Plant Breed. 2003, 122, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Patella, A.; Scariolo, F.; Palumbo, F.; Barcaccia, G. Genetic Structure of Cultivated Varieties of Radicchio (Cichorium intybus L.): A Comparison between F1 Hybrids and Synthetics. Plants 2019, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palumbo, F.; Galla, G.; Martínez-Bello, L.; Barcaccia, G. Venetian Local Corn (Zea mays L.) Germplasm: Disclosing the Genetic Anatomy of Old Landraces Suited for Typical Cornmeal Mush Production. Diversity 2017, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, F.; Galla, G.; Barcaccia, G. Developing a Molecular Identification Assay of Old Landraces for the Genetic Authentication of Typical Agro-Food Products: The Case Study of the Barley ‘Agordino’. Food Technol. Biotechnol. 2017, 55, 29–39. [Google Scholar] [CrossRef]
- Sica, P.; Galvao, A.; Scariolo, F.; Maucieri, C.; Nicoletto, C.; Pilon, C.; Sambo, P.; Barcaccia, G.; Borin, M.; Cabrera, M.; et al. Effects of Drought on Yield and Nutraceutical Properties of Beans (Phaseolus spp.) Traditionally Cultivated in Veneto, Italy. Horticulturae 2021, 7, 17. [Google Scholar] [CrossRef]
- Nicolè, S.; Barcaccia, G.; Erickson, D.L.; Kress, J.W.; Lucchin, M. The coding region of the UFGT gene is a source of diagnostic SNP markers that allow single-locus DNA genotyping for the assessment of cultivar identity and ancestry in grapevine (Vitis vinifera L.). BMC Res. Notes 2013, 6, 502. [Google Scholar] [CrossRef] [Green Version]
- Hmmam, I.; Mariotti, R.; Ruperti, B.; Cultrera, N.; Baldoni, L.; Barcaccia, G. Venetian olive (Olea europaea) germplasm: Disclosing the genetic identity of locally grown cultivars suited for typical extra virgin oil productions. Genet. Resour. Crop Evol. 2018, 65, 1733–1750. [Google Scholar] [CrossRef]
- Gonthier, L.; Blassiau, C.; Morchen, M.; Cadalen, T.; Poiret, M.; Hendriks, T.; Quillet, M.C. High-density genetic maps for loci involved in nuclear male sterility (NMS1) and sporophytic self-incompatibility (S-locus) in chicory (Cichorium intybus L., Asteraceae). Theor. Appl. Genet. 2013, 126, 2103–2121. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.; Qi, P.; Pinto, V.B.; Devos, K.M.; Barcaccia, G. Construction of the First SNP-Based Linkage Map Using Genotyping-by-Sequencing and Mapping of the Male-Sterility Gene in Leaf Chicory. Front. Plant Sci. 2019, 10, 276. [Google Scholar] [CrossRef]
- Ghedina, A.; Galla, G.; Cadalen, T.; Hilbert, J.L.; Caenazzo, S.T.; Barcaccia, G. A method for genotyping elite breeding stocks of leaf chicory (Cichorium intybus L.) by assaying mapped microsatellite marker loci. BMC Res. Notes 2015, 8, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchedi, A. I radicchi di Treviso: Storia, coltivazione, forzatura e commercio. Ital. Agric. 1961, 98, 37–51. [Google Scholar]
- Pimpini, F.; Chillemi, G. Evoluzione delle tecniche colturali e prospettive di sviluppo dei radicchi veneti. In Proceedings of the I Radicchi Veneti: 20 Anni di Esperienze Produttive e Commerciali, Verona, Italy, 1 December 1993; pp. 9–28. [Google Scholar]
- Pimpini, F.; Chillemi, G.; Lazzarin, R.; Bertolini, P.; Marchetti, C. Il Radicchio Rosso di Chioggia. Aspetti Tecnici ed Economici di Produzione e Conservazione; Veneto Agricoltura: Legnaro, Padova, 2001. [Google Scholar]
- Galla, G.; Ghedina, A.; Tiozzo, S.C.; Barcaccia, G. Toward a First High-quality Genome Draft for Marker-assisted Breeding in Leaf Chicory, Radicchio (Cichorium intybus L.). In Plant Genomics; Abdurakhmonov, I.Y., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Barcaccia, G.; Rosellini, D. A quick method for the isolation of plant DNA suitable for RAPD analysis. J. Genet. Breed. 1996, 50, 177–180. [Google Scholar]
- Barcaccia, G.; Mazzucato, A.; Belardinelli, A.; Pezzotti, M.; Lucretti, S.; Falcinelli, M. Inheritance of parental genomes in progenies of Poa pratensis L. from sexual and apomictic genotypes as assessed by RAPD markers and flow cytometry. Theor. Appl. Genet. 1997, 95, 516–524. [Google Scholar] [CrossRef]
- Barcaccia, G.; Mazzucato, A.; Albertini, E.; Zethof, J.; Gerats, A.; Pezzotti, M.; Falcinelli, M. Inheritance of parthenogenesis in Poa pratensis L.: Auxin test and AFLP linkage analyses support monogenic control. Theor. Appl. Genet. 1998, 97, 74–82. [Google Scholar] [CrossRef]
- Barcaccia, G.; Albertini, E.; Falcinelli, M. AFLP fingerprinting in Pelargonium peltatum: Its development and potential in cultivar identification. J. Hortic. Sci. Biotechnol. 1999, 74, 243–250. [Google Scholar] [CrossRef]
- Barcaccia, G.; Albertini, E.; Rosellini, D.; Tavoletti, S.; Veronesi, F. Inheritance and mapping of 2 n-egg production in diploid alfalfa. Genome 2000, 43, 528–537. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cnops, G.; den Boer, B.; Gerats, A.; Van Montagu, M.; Van Lijsebettens, M. Chromosome landing at the Arabidopsis TORNADO1 locus using an AFLP-based strategy. Mol. Genet. Genom. 1996, 253, 32–41. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System Version 2.1: Owner Manual; Exeter Publishing: Setauket, NY, USA, 2000. [Google Scholar]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.F.; Foulds, L.R. Comparison of phylogenetic trees. Math. Biosci. 1981, 53, 131–147. [Google Scholar] [CrossRef]
- Felsenstein, J. Inferring Phylogenies; Sinauer Associates, Inc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Felsenstein, J. Phylogenies from Restriction Sites: A Maximum-Likelihood Approach. Evolution 1992, 46, 159–173. [Google Scholar] [CrossRef]
- Koopman, W.J.M. Phylogenetic signal in AFLP data sets. Syst. Biol. 2005, 54, 197–217. [Google Scholar] [CrossRef] [Green Version]
- Koopman, W.J.M.; Wissemann, V.; De Cock, K.; Van Huylenbroeck, J.; De Riek, J.; Sabatlno, G.J.H.; Visser, D.; Vosman, B.; Ritz, C.M.; Maes, B.; et al. AFLP markers as a tool to reconstruct complex relationships: A case study in Rosa (Rosaceae). Am. J. Bot. 2008, 95, 353–366. [Google Scholar] [CrossRef]
- Smith, T.A.; Mendelson, T.C.; Page, L.M. AFLPs support deep relationships among darters (Percidae: Etheostomatinae) consistent with morphological hypotheses. Heredity 2011, 107, 579–588. [Google Scholar] [CrossRef]
- Kingston, S.E.; Adams, L.D.; Rosel, P.E. Testing mitochondrial sequences and anonymous nuclear markers for phylogeny reconstruction in a rapidly radiating group: Molecular systematics of the Delphininae (Cetacea: Odontoceti: Delphinidae). BMC Evol. Biol. 2009, 9, 245. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Lanfear, R.; Ly, N.T.; Trifinopoulos, J.; Schrempf, D.; Schmidt, H.A. IQ-TREE Version 2.1.2: Tutorials and Manual Phylogenomic Software by Maximum Likelihood. 2021. Available online: http://www.iqtree.org/doc/iqtree-doc.pdf (accessed on 22 February 2022).
- Robinson, O.; Dylus, D.; Dessimoz, C. Phylo.io: Interactive Viewing and Comparison of Large Phylogenetic Trees on the Web. Mol. Biol. Evol. 2016, 33, 2163–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [Google Scholar] [CrossRef]
- Kimura, M.; Crow, J.F. The number of alleles that can be maintained in a finite population. Genetics 1964, 49, 725–738. [Google Scholar] [CrossRef]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [Green Version]
- Mcdermott, J.M.; Mcdonald, B.A. Gene Flow in Plant Pathosystems. Annu. Rev. Phytopathol. 1993, 31, 353–373. [Google Scholar] [CrossRef]
- Lewontin, R.C. The apportionment of human diversity. In Evolutionary Biology; Dobzhansky, T., Hecht, M.K., Steere, W.C., Eds.; Springer: New York, NY, USA, 1972; pp. 381–398. [Google Scholar]
- Yeh, F.C.; Yang, R.; Boyle, T.B.; Ye, Z.; Mao, J.X. POPGENE, the User-Friendly Shareware for Population Genetic Analysis; University of Alberta: Edmonton, AB, Canada, 1998; Volume 10, pp. 295–301. [Google Scholar]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Mitton, J.B. Gene Flow. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 192–196. [Google Scholar]
| Markers Type | Primer Name | Primer Sequence |
|---|---|---|
| RAPD | OP-P01 | GTAGCACTCC |
| OP-Q17 | GAAGCCCTTG | |
| OP-Q03 | GGTCACCTCA | |
| OP-A08 | GTGACGTAGG | |
| OP-M10 | TCTGGCGCAC | |
| AFLP | PstI+AA/MseI+CAA | GACTGCGTACATGCAGAA |
| GACGATGAGTCCTGAGAGTAACAA | ||
| PstI+AT/MseI+CAA | GACTGCGTACATGCAGAT | |
| GACGATGAGTCCTGAGAGTAACAA | ||
| PstI+AG/MseI+CAG | GACTGCGTACATGCAGAG | |
| GACGATGAGTCCTGAGAGTAACAG |
| Population ID | N | npl | %pl | na | ne | H’ | I | GS | H’T | H’S | DST | GST | Nm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TvT1 | 18 | 28 | 22.1% | 1.221 | 1.144 | 0.080 | 0.120 | 0.951 | 0.007 | 0.080 | 0.924 | 0.041 | |
| TvT2 | 30 | 23 | 18.1% | 1.181 | 1.124 | 0.065 | 0.096 | 0.962 | 0.044 | 0.043 | 0.494 | 0.512 | |
| TvT3 | 30 | 25 | 19.7% | 1.197 | 1.120 | 0.067 | 0.101 | 0.961 | 0.030 | 0.057 | 0.656 | 0.262 | |
| TvT4 | 36 | 19 | 15.0% | 1.150 | 1.094 | 0.049 | 0.074 | 0.971 | 0.025 | 0.062 | 0.715 | 0.199 | |
| TvT5 | 28 | 17 | 13.4% | 1.134 | 1.096 | 0.049 | 0.072 | 0.970 | 0.027 | 0.059 | 0.684 | 0.231 | |
| TvT6 | 32 | 25 | 19.7% | 1.197 | 1.126 | 0.065 | 0.098 | 0.962 | 0.024 | 0.063 | 0.723 | 0.191 | |
| Overall TvT | 174 | 44 | 34.7% | 1.347 | 1.136 | 0.084 | 0.133 | 0.958 | 0.086 | 0.067 | 0.019 | 0.225 | 1.727 |
| s.d. | 0.478 | 0.274 | 0.152 | 0.223 | 0.014 | 0.024 | 0.016 | ||||||
| Vr1 | 35 | 23 | 18.1% | 1.181 | 1.107 | 0.061 | 0.092 | 0.965 | 0.041 | 0.028 | 0.406 | 0.731 | |
| Vr2 | 26 | 25 | 19.7% | 1.197 | 1.111 | 0.060 | 0.093 | 0.964 | 0.032 | 0.038 | 0.542 | 0.422 | |
| Overall Vr | 61 | 27 | 21.3% | 1.213 | 1.119 | 0.070 | 0.105 | 0.962 | 0.070 | 0.063 | 0.006 | 0.090 | 5.032 |
| s.d. | 0.411 | 0.277 | 0.151 | 0.220 | 0.013 | 0.023 | 0.019 | ||||||
| TvP1 | 30 | 22 | 17.3% | 1.173 | 1.099 | 0.053 | 0.080 | 0.968 | 0.030 | 0.048 | 0.614 | 0.314 | |
| TvP2 | 35 | 21 | 16.5% | 1.165 | 1.099 | 0.059 | 0.089 | 0.965 | 0.003 | 0.075 | 0.959 | 0.021 | |
| TvP3 | 28 | 24 | 18.9% | 1.189 | 1.113 | 0.068 | 0.102 | 0.959 | 0.050 | 0.029 | 0.368 | 0.859 | |
| Overall TvP | 93 | 32 | 25.2% | 1.252 | 1.130 | 0.078 | 0.120 | 0.953 | 0.078 | 0.060 | 0.018 | 0.229 | 1.687 |
| s.d. | 0.436 | 0.274 | 0.154 | 0.226 | 0.015 | 0.024 | 0.015 | ||||||
| Cf1 | 22 | 24 | 18.9% | 1.189 | 1.126 | 0.066 | 0.099 | 0.960 | 0.045 | 0.043 | 0.489 | 0.523 | |
| Cf2 | 36 | 16 | 12.6% | 1.126 | 1.075 | 0.043 | 0.065 | 0.975 | 0.024 | 0.064 | 0.724 | 0.190 | |
| Cf3 | 29 | 21 | 16.5% | 1.165 | 1.112 | 0.060 | 0.089 | 0.966 | 0.042 | 0.046 | 0.523 | 0.456 | |
| Cf4 | 31 | 25 | 19.7% | 1.197 | 1.111 | 0.076 | 0.111 | 0.956 | 0.025 | 0.063 | 0.715 | 0.199 | |
| Cf5 | 33 | 20 | 15.8% | 1.158 | 1.094 | 0.054 | 0.080 | 0.970 | 0.016 | 0.072 | 0.814 | 0.114 | |
| Overall Cf | 151 | 37 | 29.1% | 1.291 | 1.142 | 0.085 | 0.131 | 0.952 | 0.088 | 0.060 | 0.028 | 0.319 | 1.068 |
| s.d. | 0.456 | 0.282 | 0.159 | 0.233 | 0.014 | 0.026 | 0.015 | ||||||
| Ch1 | 23 | 21 | 16.5% | 1.167 | 1.101 | 0.057 | 0.087 | 0.966 | 0.002 | 0.107 | 0.982 | 0.009 | |
| Ch3 | 35 | 22 | 17.3% | 1.175 | 1.109 | 0.065 | 0.096 | 0.963 | 0.053 | 0.057 | 0.519 | 0.463 | |
| Ch4 | 35 | 27 | 21.3% | 1.213 | 1.130 | 0.073 | 0.109 | 0.960 | 0.056 | 0.054 | 0.493 | 0.515 | |
| Ch5 | 23 | 26 | 20.5% | 1.205 | 1.124 | 0.067 | 0.101 | 0.963 | 0.047 | 0.062 | 0.569 | 0.379 | |
| Ch6 | 26 | 32 | 25.2% | 1.205 | 1.124 | 0.079 | 0.121 | 0.954 | 0.050 | 0.059 | 0.543 | 0.421 | |
| Ch7 | 31 | 32 | 25.2% | 1.252 | 1.196 | 0.084 | 0.127 | 0.952 | 0.048 | 0.061 | 0.561 | 0.391 | |
| Overall Ch | 173 | 46 | 36.2% | 1.362 | 1.181 | 0.110 | 0.170 | 0.946 | 0.109 | 0.079 | 0.030 | 0.274 | 1.323 |
| s.d. | 0.483 | 0.304 | 0.170 | 0.249 | 0.017 | 0.029 | 0.016 | ||||||
| Mean Overall | 652 | 61 | 48.0% | 1.480 | 1.216 | 0.130 | 0.202 | 0.931 | 0.132 | 0.068 | 0.064 | 0.488 | 0.524 |
| s.d. | 0.502 | 0.328 | 0.178 | 0.257 | 0.020 | 0.032 | 0.011 |
| TvT1 | 0.951 | |||||||||||||||||||||
| TvT2 | 0.946 | 0.962 | ||||||||||||||||||||
| TvT3 | 0.946 | 0.958 | 0.961 | |||||||||||||||||||
| TvT4 | 0.950 | 0.958 | 0.962 | 0.971 | ||||||||||||||||||
| TvT5 | 0.952 | 0.960 | 0.961 | 0.971 | 0.970 | |||||||||||||||||
| TvT6 | 0.946 | 0.950 | 0.953 | 0.956 | 0.957 | 0.962 | ||||||||||||||||
| Vr1 | 0.925 | 0.932 | 0.929 | 0.931 | 0.933 | 0.932 | 0.965 | |||||||||||||||
| Vr2 | 0.922 | 0.933 | 0.931 | 0.933 | 0.935 | 0.928 | 0.960 | 0.964 | ||||||||||||||
| TvP1 | 0.911 | 0.922 | 0.923 | 0.922 | 0.923 | 0.920 | 0.919 | 0.901 | 0.968 | |||||||||||||
| TvP2 | 0.915 | 0.935 | 0.928 | 0.932 | 0.932 | 0.922 | 0.933 | 0.919 | 0.941 | 0.965 | ||||||||||||
| TvP3 | 0.903 | 0.919 | 0.915 | 0.919 | 0.918 | 0.909 | 0.933 | 0.906 | 0.947 | 0.955 | 0.959 | |||||||||||
| Cf1 | 0.908 | 0.923 | 0.927 | 0.929 | 0.926 | 0.921 | 0.919 | 0.920 | 0.910 | 0.919 | 0.913 | 0.960 | ||||||||||
| Cf2 | 0.913 | 0.930 | 0.929 | 0.929 | 0.927 | 0.918 | 0.931 | 0.934 | 0.912 | 0.925 | 0.920 | 0.941 | 0.975 | |||||||||
| Cf3 | 0.913 | 0.930 | 0.933 | 0.934 | 0.932 | 0.925 | 0.933 | 0.926 | 0.919 | 0.926 | 0.920 | 0.953 | 0.948 | 0.966 | ||||||||
| Cf4 | 0.914 | 0.930 | 0.932 | 0.933 | 0.931 | 0.924 | 0.935 | 0.929 | 0.922 | 0.930 | 0.923 | 0.942 | 0.945 | 0.953 | 0.956 | |||||||
| Cf5 | 0.913 | 0.926 | 0.931 | 0.933 | 0.931 | 0.924 | 0.936 | 0.932 | 0.914 | 0.921 | 0.913 | 0.942 | 0.954 | 0.952 | 0.951 | 0.970 | ||||||
| Ch1 | 0.923 | 0.934 | 0.932 | 0.934 | 0.936 | 0.929 | 0.930 | 0.932 | 0.911 | 0.913 | 0.905 | 0.928 | 0.946 | 0.939 | 0.935 | 0.942 | 0.966 | |||||
| Ch3 | 0.925 | 0.937 | 0.936 | 0.935 | 0.936 | 0.932 | 0.932 | 0.943 | 0.903 | 0.918 | 0.905 | 0.933 | 0.941 | 0.935 | 0.934 | 0.938 | 0.953 | 0.963 | ||||
| Ch4 | 0.918 | 0.928 | 0.930 | 0.931 | 0.930 | 0.930 | 0.929 | 0.925 | 0.904 | 0.912 | 0.902 | 0.928 | 0.934 | 0.935 | 0.929 | 0.936 | 0.953 | 0.960 | 0.960 | |||
| Ch5 | 0.915 | 0.925 | 0.928 | 0.928 | 0.927 | 0.928 | 0.931 | 0.927 | 0.897 | 0.907 | 0.897 | 0.934 | 0.942 | 0.936 | 0.933 | 0.941 | 0.953 | 0.954 | 0.952 | 0.963 | ||
| Ch6 | 0.911 | 0.920 | 0.922 | 0.922 | 0.921 | 0.923 | 0.924 | 0.917 | 0.900 | 0.905 | 0.891 | 0.922 | 0.925 | 0.932 | 0.928 | 0.937 | 0.942 | 0.942 | 0.939 | 0.939 | 0.954 | |
| Ch7 | 0.913 | 0.925 | 0.929 | 0.929 | 0.929 | 0.926 | 0.926 | 0.919 | 0.903 | 0.908 | 0.898 | 0.929 | 0.926 | 0.937 | 0.931 | 0.931 | 0.939 | 0.940 | 0.940 | 0.936 | 0.937 | 0.952 |
| TvT1 | TvT2 | TvT3 | TvT4 | TvT5 | TvT6 | Vr1 | Vr2 | TvP1 | TvP2 | TvP3 | Cf1 | Cf2 | Cf3 | Cf4 | Cf5 | Ch1 | Ch3 | Ch4 | Ch5 | Ch6 | Ch7 |
| TvT | 0.958 | ||||
| Vr | 0.931 | 0.962 | |||
| TvP | 0.922 | 0.913 | 0.953 | ||
| Cf | 0.927 | 0.927 | 0.920 | 0.952 | |
| Ch | 0.928 | 0.926 | 0.905 | 0.934 | 0.946 |
| TvT | Vr | TvP | Cf | Ch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, A.; Scariolo, F.; Negrisolo, E.; Lucchin, M.; Barcaccia, G. Molecular Relationships and Genetic Diversity Analysis of Venetian Radicchio (Leaf Chicory, Cichorium intybus subsp. intybus var. sylvestre, 2n = 2x = 18) Biotypes. Diversity 2022, 14, 175. https://doi.org/10.3390/d14030175
Basso A, Scariolo F, Negrisolo E, Lucchin M, Barcaccia G. Molecular Relationships and Genetic Diversity Analysis of Venetian Radicchio (Leaf Chicory, Cichorium intybus subsp. intybus var. sylvestre, 2n = 2x = 18) Biotypes. Diversity. 2022; 14(3):175. https://doi.org/10.3390/d14030175
Chicago/Turabian StyleBasso, Andrea, Francesco Scariolo, Enrico Negrisolo, Margherita Lucchin, and Gianni Barcaccia. 2022. "Molecular Relationships and Genetic Diversity Analysis of Venetian Radicchio (Leaf Chicory, Cichorium intybus subsp. intybus var. sylvestre, 2n = 2x = 18) Biotypes" Diversity 14, no. 3: 175. https://doi.org/10.3390/d14030175
APA StyleBasso, A., Scariolo, F., Negrisolo, E., Lucchin, M., & Barcaccia, G. (2022). Molecular Relationships and Genetic Diversity Analysis of Venetian Radicchio (Leaf Chicory, Cichorium intybus subsp. intybus var. sylvestre, 2n = 2x = 18) Biotypes. Diversity, 14(3), 175. https://doi.org/10.3390/d14030175







