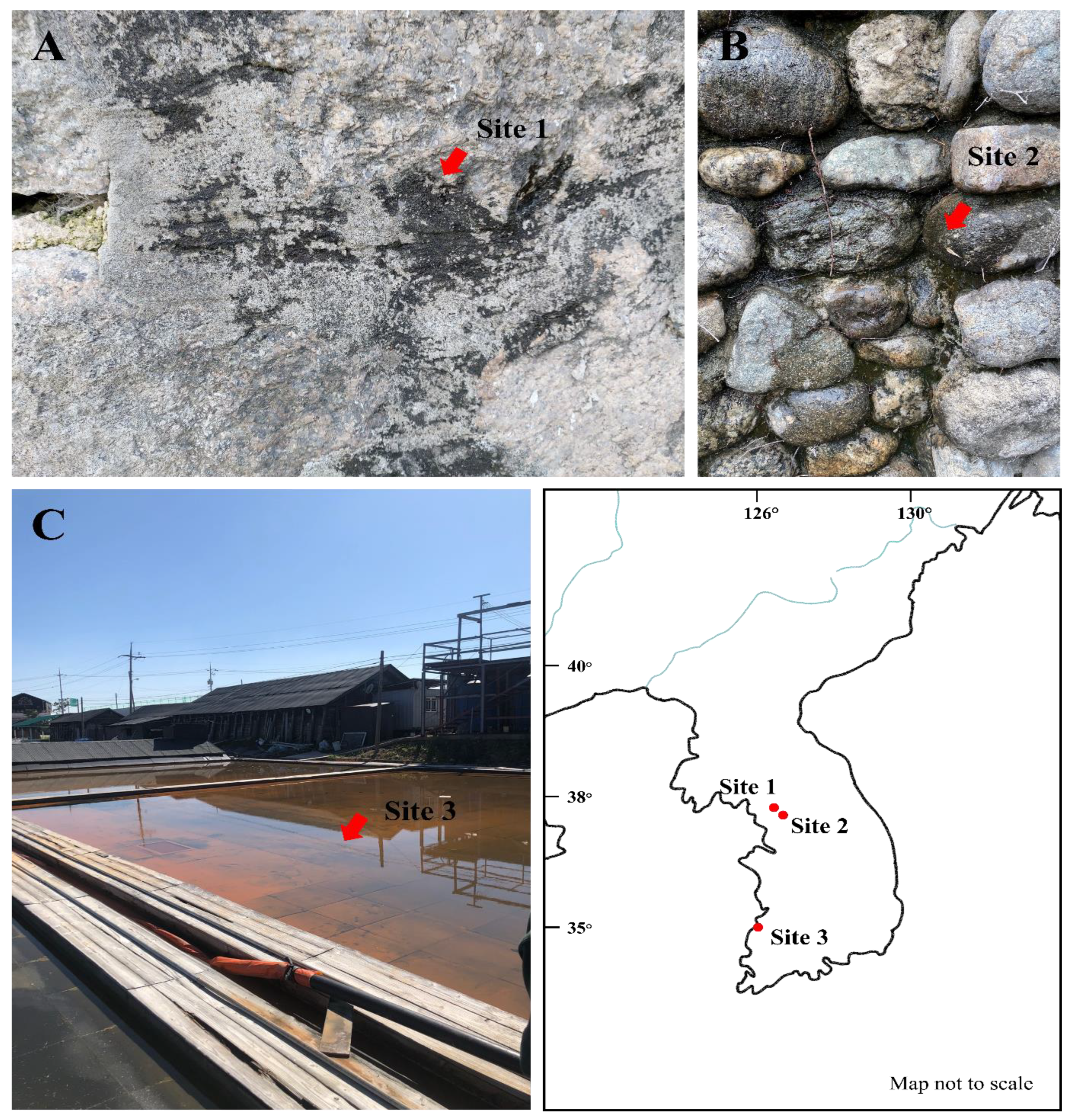
Three New Plectolyngbya Species (Leptolyngbyaceae, Cyanobacteria) Isolated from Rocks and Saltern of the Republic of Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Isolation and Culture Conditions
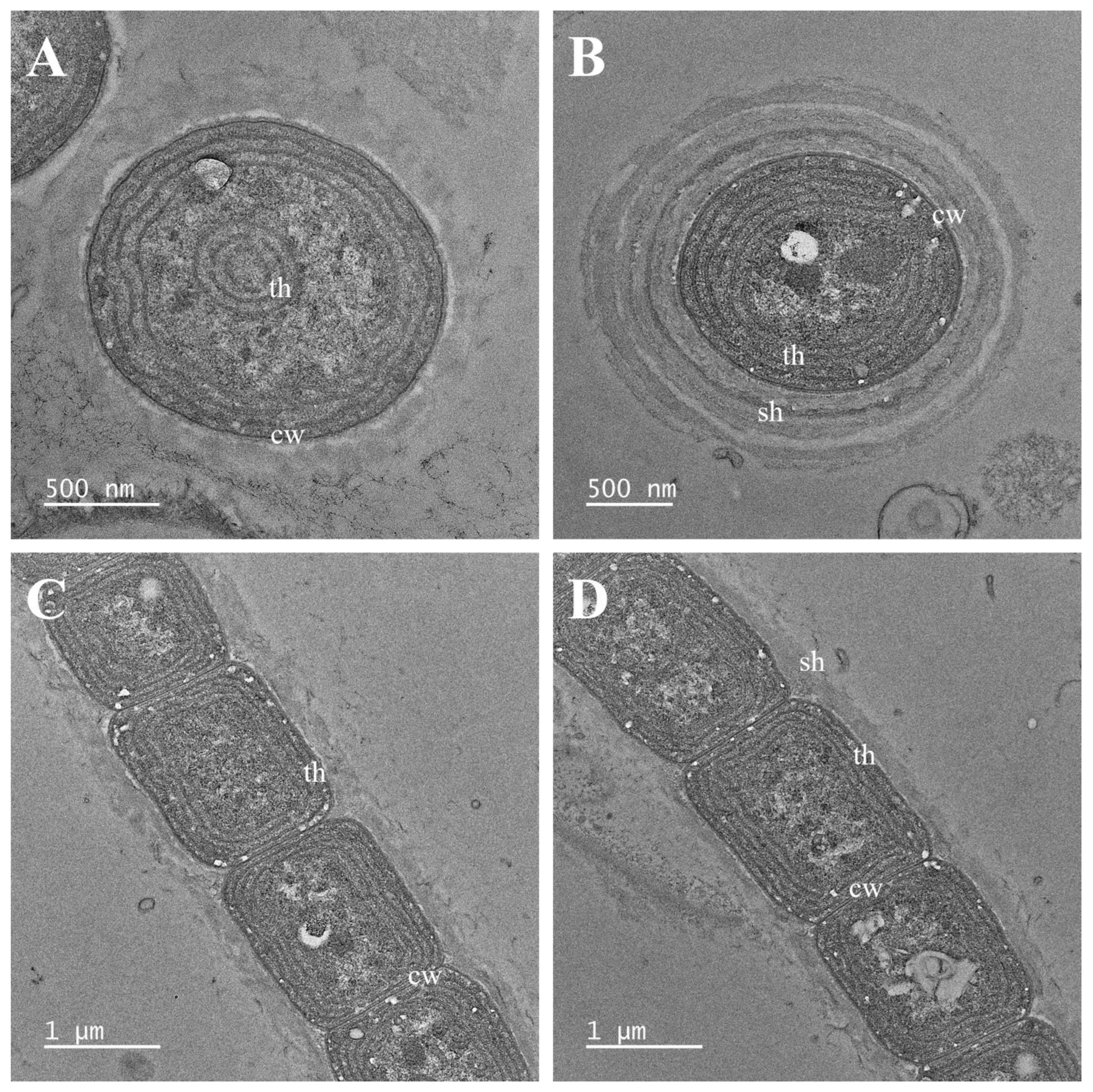
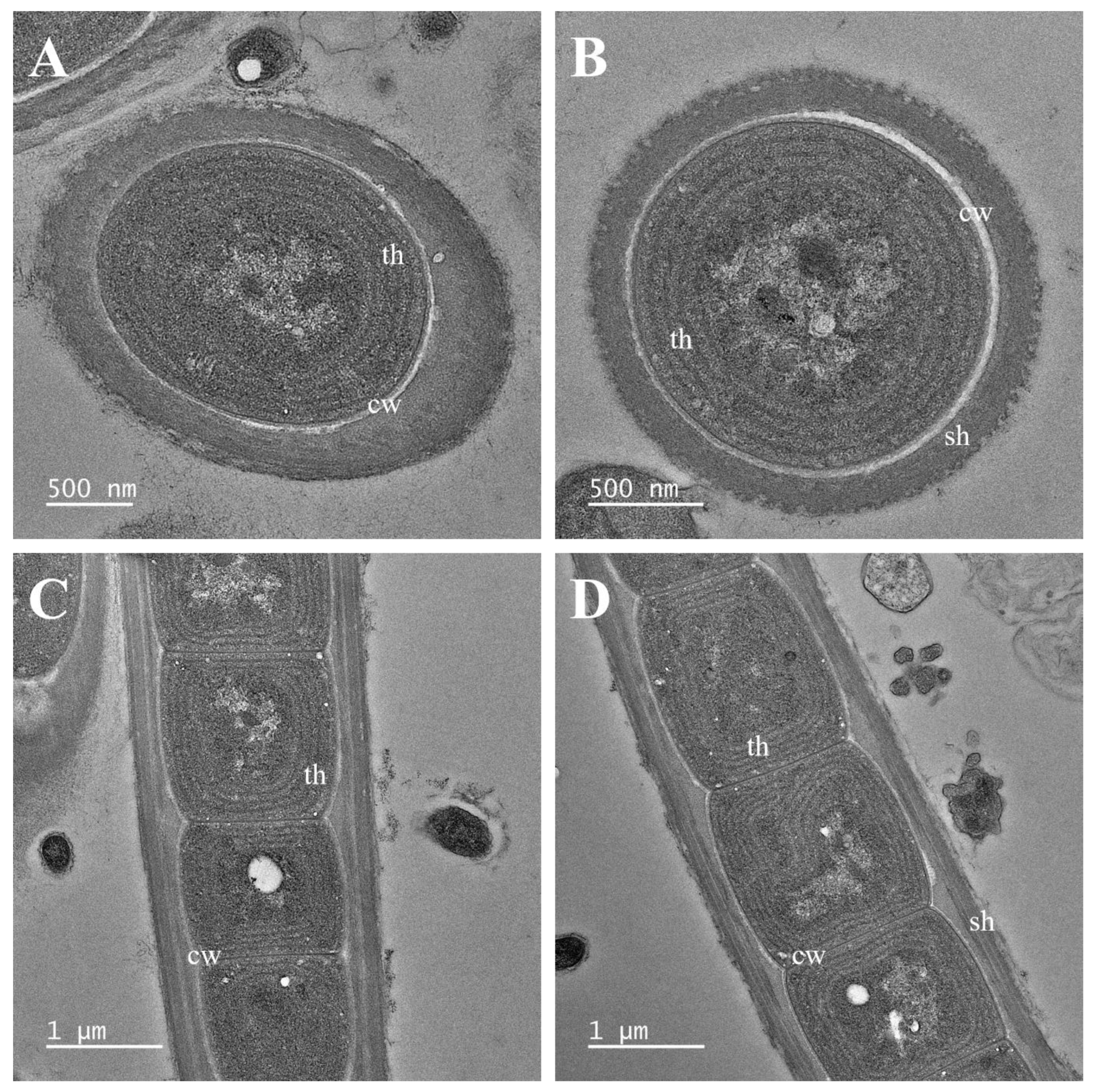
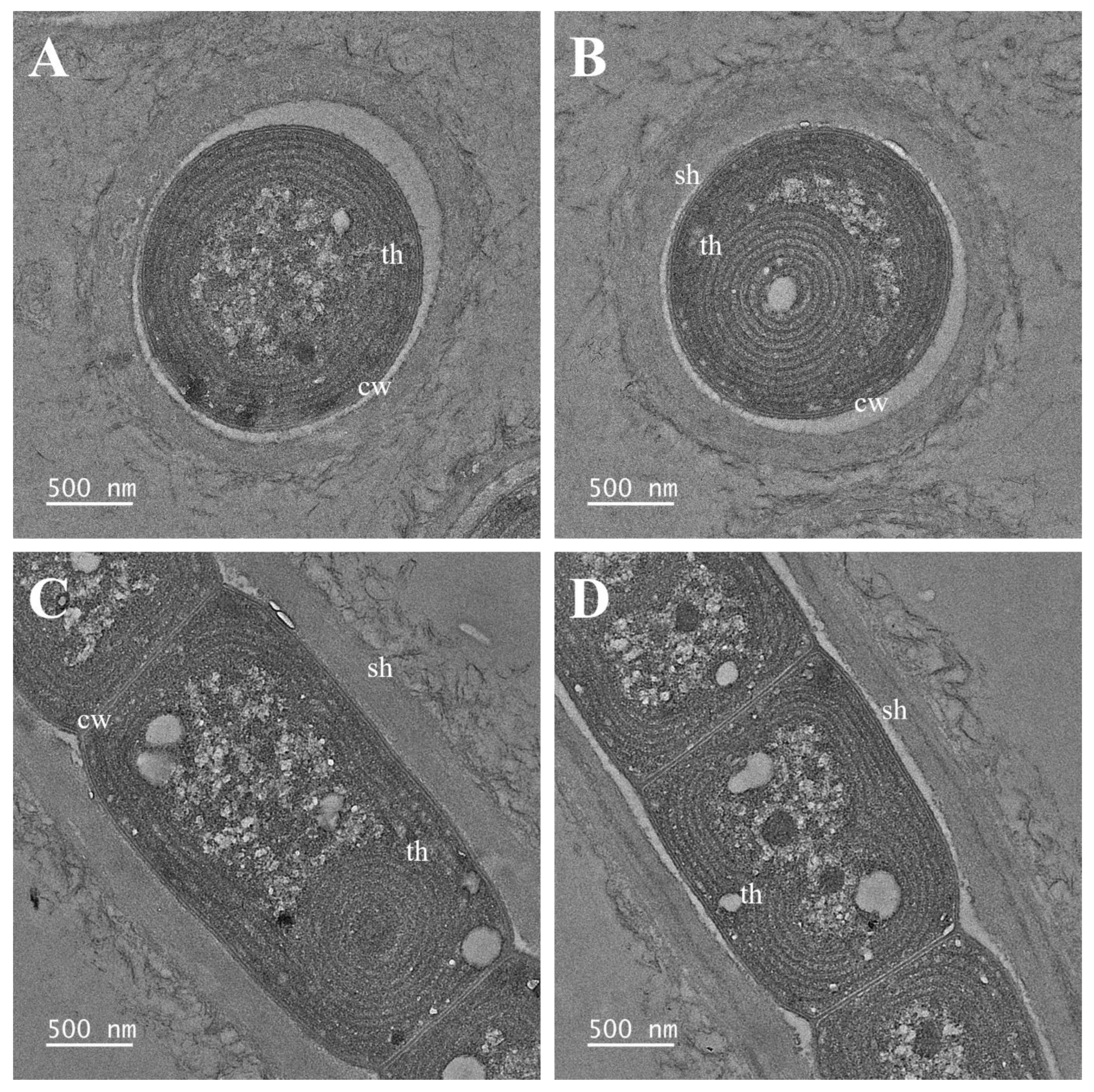
2.2. Morphological and Ultrastructure Analyses
2.3. Molecular Methods
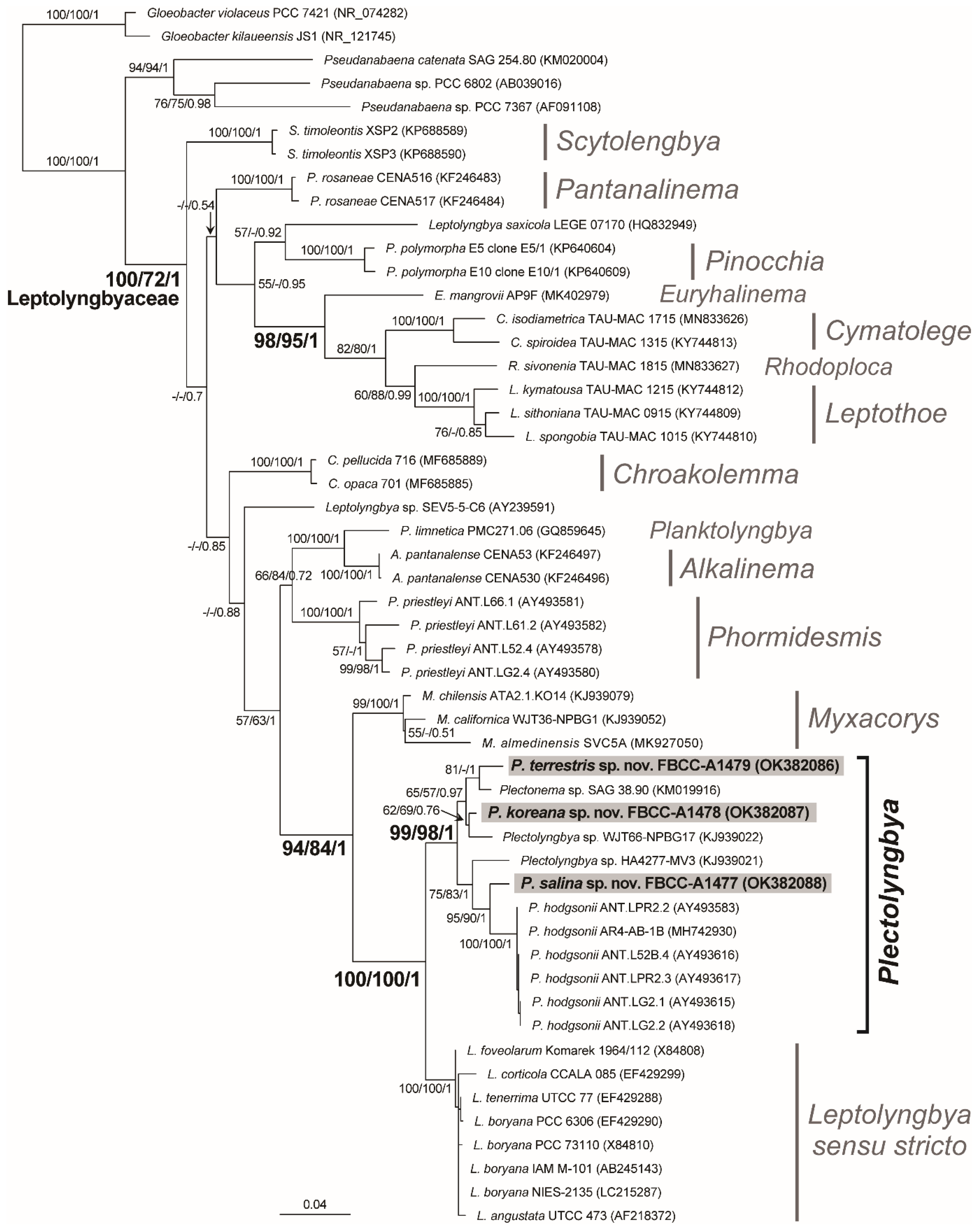
2.4. Phylogenetic Analysis
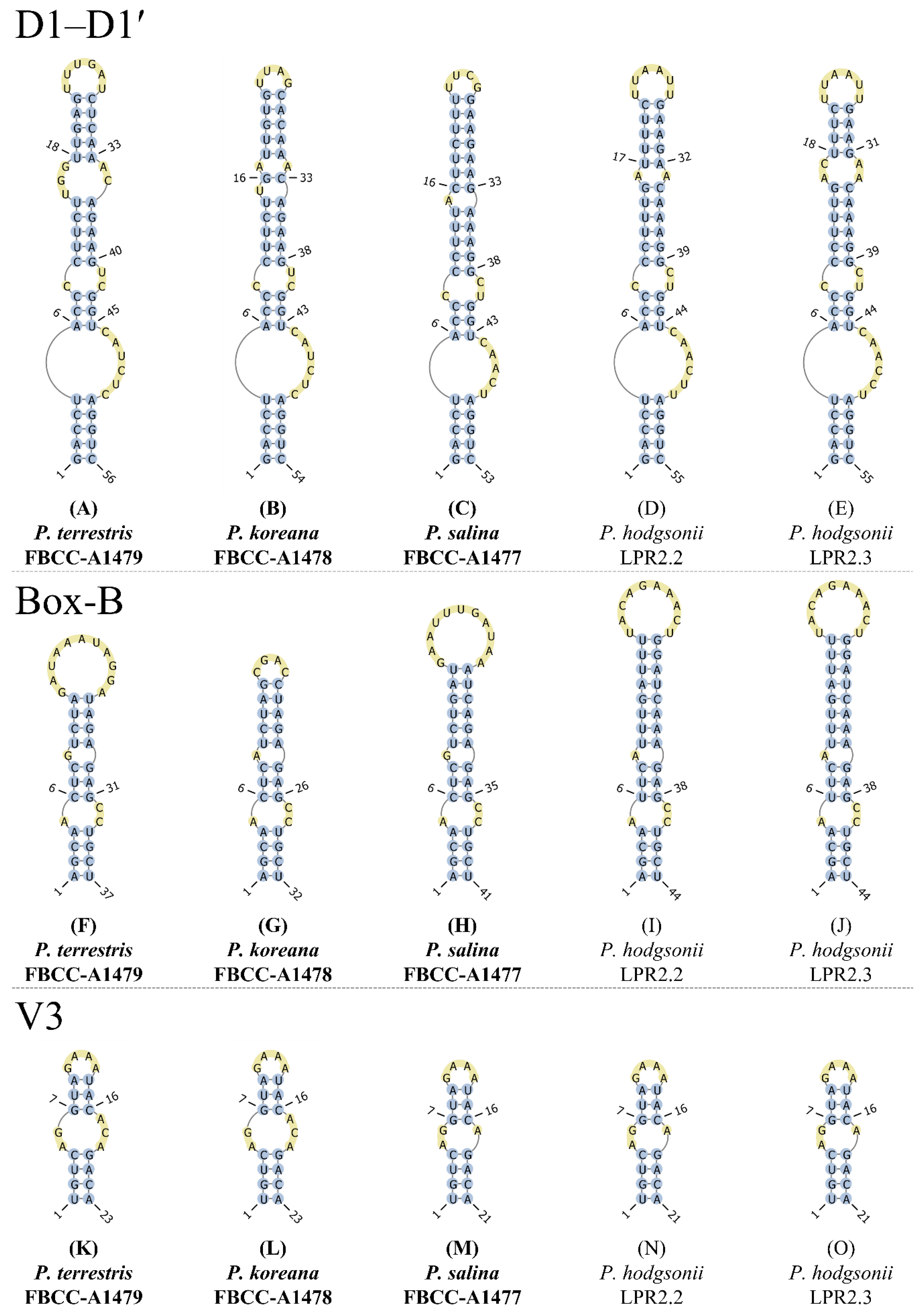
2.5. Structure Analyses of 16S–23S Internal Transcribed Spacer (ITS)
3. Results
3.1. Taxonomic Treatment
3.2. Characteristic of 16S rRNA and Phylogeny
3.3. Characterization of 16S–23S ITS Regions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitton, B.A.; Potts, M. Introduction to the cyanobacteria. In Ecology of Cyanobacteria II; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–13. [Google Scholar]
- Lindemann, S.R.; Moran, J.J.; Stegen, J.C.; Renslow, R.S.; Hutchison, J.R.; Cole, J.K.; Dohnalkova, A.C.; Tremblay, J.; Singh, K.; Malfatti, S.A.; et al. The epsomitic phototrophic microbial mat of Hot Lake, Washington: Community structural responses to seasonal cycling. Front. Microbiol. 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doğan, S.Ş.; Kocabaş, A. Metagenomic assessment of prokaryotic diversity within hypersaline Tuz Lake, Turkey. Microbiology 2021, 90, 647–655. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Nübel, U.; Muyzer, G. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Arch. Microbiol. 1998, 169, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Cyanobacteria in hypersaline environments: Biodiversity and physiological properties. Biodivers. Conserv. 2015, 24, 781–798. [Google Scholar] [CrossRef]
- Nägeli, C. Gattungen einzelliger algen, physiologisch und systematisch bearheitet. Neue Denkschriften Der Allgemeinen Schweizerischen Gesellschaft für Die Gesammten Naturwissenschaften. Allg. Schweiz. Nat. Ges. 1849, 10, 1–139. [Google Scholar]
- Gomont, M. Monographie des Oscillariées (Nostocacées Homocystées). Ann. Sci. Nat. Bot. Sér. 1892, 16, 91–264. [Google Scholar]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 3-Oscillatoriales. Arch. Hydrobiol. 1988, 80, 327–472. [Google Scholar]
- Taton, A.; Wilmotte, A.; Šmarda, J.; Elster, J.; Komárek, J. Plectolyngbya hodgsonii: A novel filamentous cyanobacterium from Antarctic lakes. Polar Biol. 2011, 34, 181–191. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. The halotolerance and phylogeny of cyanobacteria with tightly coiled trichomes (Spirulina Turpin) and the description of Halospirulina tapeticola gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 1265–1277. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Vazquez-Nion, D.; Rodriguez-Castro, J.; Lopez-Rodriguez, M.C.; Fernandez-Silva, I.; Prieto, B. Subaerial biofilms on granitic historic buildings: Microbial diversity and development of phototrophic multi-species cultures. Biofouling 2016, 32, 657–669. [Google Scholar] [CrossRef]
- Bornet, É.; Flahault, C. Revision des Nostocacées hétérocystées contenues dans les principaux herbiers de France (quatrième et dernier fragment). Ann. Des Sci. Nat. Bot. Septième Sér. 1886, 7, 177–262. [Google Scholar]
- Zammit, G.; Billi, D.; Albertano, P. The subaerophytic cyanobacterium Oculatella subterranea (Oscillatoriales, Cyanophyceae) gen. et sp. nov.: A cytomorphological and molecular description. Eur. J. Phycol. 2012, 47, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Bornet, É.; Flahault, C. Revision des Nostocacées hétérocystées contenues dans les principaux herbiers de France (Troisième fragment). Ann. Sci. Nat. Bot. Sept. Sér. 1886, 5, 51–129. [Google Scholar]
- Janet, M. Westiellopsis prolifica, gen. et sp. nov., a new member of the Stigonemataceae. Ann. Bot. 1941, 5, 167–170. [Google Scholar] [CrossRef]
- Dvořák, P.; Hašler, P.; Poulíčková, A. Phylogeography of the Microcoleus vaginatus (Cyanobacteria) from Three Continents—A Spatial and Temporal Characterization. PLoS ONE 2012, 7, e40153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hašler, P.; Dvořák, P.; Johansen, J.R.; Kitner, M.; Ondřej, V.; Poulíčková, A. Morphological and molecular study of epipelic filamentous genera Phormidium, Microcoleus and Geitlerinema (Oscillatoriales, Cyanophyta/cyanobacteria). J. Czech Phycol. Soc. 2012, 12, 341–356. [Google Scholar] [CrossRef] [Green Version]
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: www.algaebase.org (accessed on 3 November 2022).
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Johansen, J.R.; Kovácik, L.; Casamatta, D.A.; Iková, K.F.; Kaštovský, J. Utility of 16S-23S ITS sequence and secondary structure for recognition of intrageneric and intergeneric limits within cyanobacterial taxa: Leptolyngbya corticola sp. nov. (Pseudanabaenaceae, Cyanobacteria). Nova Hedwig. 2011, 92, 283–302. [Google Scholar] [CrossRef]
- Vaz, M.G.M.V.; Andreote, A.P.D.; Malone, C.F.S.; Sant’Anna, C.L.; Barbiero, L.; Fiore, M.F. Pantanalinema gen. nov. and Alkalinema gen. nov.: Novel pseudanabaenacean genera (Cyanobacteria) isolated from saline-alkaline lakes. Int. J. Syst. Evol. Microbiol. 2015, 65, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Genuário, D.B.; de Souza, W.R.; Monteiro, R.T.R.; Sant Anna, C.L.; Melo, I.S. Amazoninema gen. nov., (Synechococcales, Pseudanabaenaceae) a novel cyanobacteria genus from Brazilian Amazonian rivers. Int. J. Syst. Evol. Microbiol. 2018, 68, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Perkerson, R.B.; Johansen, J.R.; Kovácik, L.; Brand, J.; Kaštovský, J.; Casamatta, D.A. A unique pseudanabaenalean (Cyanobacteria) genus Nodosilinea gen. nov. based on morphological and molecular data. J. Phycol. 2011, 47, 1397–1412. [Google Scholar] [CrossRef]
- Jahodářová, E.; Dvořák, P.; Hašler, P.; Poulíčková, A. Revealing hidden diversity among tropical cyanobacteria: The new genus Onodrimia (Synechococcales, Cyanobacteria) described using the polyphasic approach. Phytotaxa 2017, 326, 28–40. [Google Scholar] [CrossRef]
- Turicchia, S.; Ventura, S.; Komárková, J.; Komárek, J. Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize. 2. Diversity of oscillatorialean genera. Nova Hedwig. 2009, 89, 165–200. [Google Scholar] [CrossRef]
- Song, G.-F.; Jiang, Y.-G.; Li, R.-H. Scytolyngbya timoleontis, gen. et sp. nov. (Leptolyngbyaceae, Cyanobacteria): A novel false branching cyanobacteria from China. Phytotaxa 2015, 224, 72–84. [Google Scholar] [CrossRef]
- Sciuto, K.; Moro, I. Detection of the new cosmopolitan genus Thermoleptolyngbya (Cyanobacteria, Leptolyngbyaceae) using the 16S rRNA gene and 16S-23S ITS region. Mol. Phylogenet. Evol. 2016, 105, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, K.; Moschin, E.; Moro, I. Cryptic cyanobacterial diversity in the giant cave (Trieste, Italy): The new genus Timaviella (Leptolyngbyaceae). Cryptogam. Algol. 2017, 38, 285–323. [Google Scholar] [CrossRef]
- Zimba, P.V.; Huang, I.-S.; Foley, J.E.; Linton, E.W. Identification of a new-to-science cyanobacterium, Toxifilum mysidocida gen. nov. & sp. nov. (Cyanobacteria, Cyanophyceae). J. Phycol. 2017, 53, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier: Heidelberg, Germany, 2005; pp. 1–749. [Google Scholar]
- Gomont, M. Monographie des Oscillariées (Nostocacées homocystées). Ann. Sci. Nat. Bot. Sér. 1892, 15, 263–368. [Google Scholar]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.C.B.G.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Lee, N.-J.; Seo, Y.; Ki, J.-S.; Lee, O.-M. A study of newly recorded genus and species for aerial cyanobacteria Wilmottia murrayi (Oscillatoriales, Cyanobacteria) in Korea. Korean J. Environ. Biol. 2019, 37, 260–267. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeong, M.S.; Kim, D.-Y.; Her, S.; Wie, M.-B. Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2015, 90, 204–214. [Google Scholar] [CrossRef]
- Rippka, R.; Waterbury, J.; Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974, 100, 419–436. [Google Scholar] [CrossRef]
- Saw, J.H.W.; Schatz, M.; Brown, M.V.; Kunkel, D.D.; Foster, J.S.; Shick, H.; Christensen, S.; Hou, S.; Wan, X.; Donachie, S.P. Cultivation and complete genome sequencing of Gloeobacter kilaueensis sp. nov., from a lava cave in Kilauea Caldera, Hawaii. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lauterborn, R. Die sapropelische Lebewelt. Ein Beitrag zur Biologic des Faulschlamms nattirlicher Gewässer. Verh. Naturh. Med. Ver. Heidelb. 1915, 13, 395–481. [Google Scholar]
- Zammit, G. Systematics and biogeography of sciophilous cyanobacteria; an ecological and molecular description of Albertania skiophila (Leptolyngbyaceae) gen. & sp. nov. Phycologia 2018, 57, 481–491. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Han, K. PseudoViewer3: Generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 2009, 25, 1435–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinou, D.; Voultsiadou, E.; Panteris, E.; Zervou, S.-K.; Hiskia, A.; Gkelis, S. Leptothoe, a new genus of marine cyanobacteria (Synechococcales) and three new species associated with sponges from the Aegean Sea. J. Phycol. 2019, 55, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Komárková-Legnerová, J.; Cronberg, G. New and recombined filamentous cyanophytes from lakes in South Scania, Sweden. Arch. Hydrobiol. 1992, 95, 21–31. [Google Scholar]
- Konstantinou, D.; Voultsiadou, E.; Panteris, E.; Gkelis, S. Revealing new sponge-associated cyanobacterial diversity: Novel genera and species. Mol. Phylogenet. Evol. 2021, 155, 1–17. [Google Scholar] [CrossRef]
- Chakraborty, S.; Maruthanayagam, V.; Achari, A.; Pramanik, A.; Jaisankar, P.; Mukherjee, J. Euryhalinema mangrovii gen. nov., sp. nov. and Leptoelongatus litoralis gen. nov., sp. nov. (Leptolyngbyaceae) isolated from an Indian mangrove forest. Phytotaxa 2019, 422, 58–74. [Google Scholar] [CrossRef]
- Pietrasiak, N.; Osorio-Santos, K.; Shalygin, S.; Martin, M.P.; Johansen, J.R. When Is a lineage a species? a case study in Myxacorys gen. nov. (Synechococcales: Cyanobacteria) with the description of two new species from the Americas. J. Phycol. 2019, 55, 976–996. [Google Scholar] [CrossRef]
- Lee, N.-J.; Seo, Y.; Ki, J.-S.; Lee, O.-M. Morphology and molecular description of Wilmottia koreana sp. nov. (Oscillatoriales, Cyanobacteria) isolated from the Republic of Korea. Phytotaxa 2020, 447, 237–251. [Google Scholar] [CrossRef]
- Kim, D.-H.; Choi, H.J.; Ki, J.-S.; Lee, O.-M. Pinocchia daecheonga sp. nov. (Synechococcales, Cyanobacteria) isolated from a Daecheong lake in Geum river, Republic of Korea. Phytotaxa 2021, 510, 135–147. [Google Scholar] [CrossRef]
- Mai, T.; Johansen, J.R.; Pietrasiak, N.; Bohunická, M.; Martin, M.P. Revision of the Synechococcales (Cyanobacteria) through recognition of four families including Oculatellaceae fam. nov. and Trichocoleaceae fam. nov. and six new genera containing 14 species. Phytotaxa 2018, 365, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Oh, H.-S.; Park, S.-C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.H.; Kováčik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef] [Green Version]
- da Silva Malone, C.F.; Rigonato, J.; Laughinghouse, H.D.; Schmidt, E.C.; Bouzon, Z.L.; Wilmotte, A.; Fiore, M.F.; Sant’Anna, C.L. Cephalothrix gen. nov. (Cyanobacteria): Towards an intraspecific phylogenetic evaluation by multilocus analyses. Int. J. Syst. Evol. Microbiol. 2015, 65, 2993–3007. [Google Scholar] [CrossRef] [PubMed]



| No. | Taxa and Accession Number | [1] | [2] | [3] | [4] | [5] | [6] | [7] | [8] | [9] | [10] | [11] | [12] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [1] | Plectolyngbya terrestris FBCC-A1479 (OK382086) | - | 98.61 | 98.12 | 97.82 | 97.05 | 96.45 | 96.59 | 96.93 | 96.09 | 96.61 | 96.76 | 96.60 |
| [2] | Plectonema sp. SAG 38.90 (KM019916) | 20 | - | 98.75 | 98.26 | 97.31 | 97.07 | 96.52 | 96.45 | 95.58 | 96.09 | 96.39 | 96.22 |
| [3] | Plectolyngbya koreana FBCC-A1478 (OK382087) | 27 | 18 | - | 98.78 | 97.48 | 97.35 | 97.07 | 97.00 | 95.92 | 96.44 | 96.83 | 96.68 |
| [4] | Plectolyngbya sp. WJT66-NPBG17 (KJ939022) | 25 | 20 | 14 | - | 97.04 | 97.30 | 97.39 | 96.26 | 95.57 | 96.08 | 96.01 | 95.90 |
| [5] | Plectolyngbya sp. HA4277-MV3 (KJ939021) | 34 | 31 | 29 | 34 | - | 97.40 | 97.40 | 95.48 | 95.05 | 95.39 | 95.30 | 95.18 |
| [6] | Plectolyngbya salina FBCC-A1477 (OK382088) | 51 | 42 | 38 | 31 | 30 | - | 98.12 | 96.10 | 95.67 | 96.01 | 95.96 | 95.77 |
| [7] | Plectolyngbya hodgsonii ANT.LPR2.2 (AY493583) | 49 | 50 | 42 | 30 | 30 | 27 | - | 95.75 | 95.41 | 95.75 | 95.67 | 95.47 |
| [8] | Leptolyngbya foveolarum Komárek 1964/112 (X84808) | 44 | 51 | 43 | 43 | 52 | 56 | 61 | - | 99.22 | 99.83 | 99.86 | 99.55 |
| [9] | L. corticola CCALA 085 (EF429299) | 45 | 51 | 47 | 51 | 57 | 50 | 53 | 9 | - | 99.22 | 99.28 | 98.91 |
| [10] | L. tenerrima UTCC 77 (EF429288) | 39 | 45 | 41 | 45 | 53 | 46 | 49 | 2 | 9 | - | 99.91 | 99.55 |
| [11] | L. boryana NIES-2135 (LC215287) | 45 | 50 | 44 | 44 | 52 | 56 | 60 | 2 | 8 | 1 | - | 99.70 |
| [12] | L. angustata UTCC 473 (AF218372) | 45 | 50 | 44 | 45 | 53 | 56 | 60 | 6 | 12 | 5 | 4 | - |
| Plectolyngbya hodgsonii ANT.LPR2 T | P. terrestris FBCC-A1479 | P. koreana FBCC-A1478 | P. salina FBCC-A1477 | |
|---|---|---|---|---|
| Filament | Slightly or irregularly coiled | Straight or curved | Straight or curved | Straight or waved and sometimes gently twisted |
| Cell shape | Cylindrical | Cylindrical or slightly barrel-shaped | Cylindrical or slightly barrel-shaped | Cylindrical or slightly barrel-shaped |
| Sheath | Thin, colorless, attached or slightly widened, diffluent | Thin, colorless, attached | Thin, colorless, attached or slightly widened, diffluent | Thin, colorless, attached or slightly widened, diffluent |
| Apical cell | Rounded | Conical or rounded | Conical or rounded and rarely circled | Conical or rounded |
| False branching | Obligatory solitary or in pairs | Facultative (old trichome) Solitary or in pairs | Obligatory solitary or in pairs | Obligatory Solitary or in pairs |
| Necridic cell | No data | Present | Present | Present |
| Thylakoids | Parietal, circular | Parietal, circular | Parietal, circular | Parietal, circular |
| Habitat | Periphytic and metaphytic | Subaerophytic | Subaerophytic | Halophytic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Lee, N.-J.; Kim, J.-H.; Yang, E.-C.; Lee, O.-M. Three New Plectolyngbya Species (Leptolyngbyaceae, Cyanobacteria) Isolated from Rocks and Saltern of the Republic of Korea. Diversity 2022, 14, 1013. https://doi.org/10.3390/d14121013
Kim D-H, Lee N-J, Kim J-H, Yang E-C, Lee O-M. Three New Plectolyngbya Species (Leptolyngbyaceae, Cyanobacteria) Isolated from Rocks and Saltern of the Republic of Korea. Diversity. 2022; 14(12):1013. https://doi.org/10.3390/d14121013
Chicago/Turabian StyleKim, Do-Hyun, Nam-Ju Lee, Jee-Hwan Kim, Eun-Chan Yang, and Ok-Min Lee. 2022. "Three New Plectolyngbya Species (Leptolyngbyaceae, Cyanobacteria) Isolated from Rocks and Saltern of the Republic of Korea" Diversity 14, no. 12: 1013. https://doi.org/10.3390/d14121013
APA StyleKim, D.-H., Lee, N.-J., Kim, J.-H., Yang, E.-C., & Lee, O.-M. (2022). Three New Plectolyngbya Species (Leptolyngbyaceae, Cyanobacteria) Isolated from Rocks and Saltern of the Republic of Korea. Diversity, 14(12), 1013. https://doi.org/10.3390/d14121013






