Abstract
The paper reports the record of Pseudochromadora gomoi sp.n. in circalittoral habitats (at depths of 47–59 m) of the Romanian Black Sea waters. The species is characterized by a unispiral amphidial fovea, the presence of lateral alae stretching from the posterior end of the cardia to the level of the cloacal opening, but also shows unique features found in other Desmodorida genera (i.e., the presence of a precloacal ventral ala in males similar to several Desmodora or Echinodesmodora species and eight subcephalic setae on the head capsule). The presence of a constrictor cuticle ring and orange elongated ducts in the anterior body part as in Pseudodesmodora lacrima, Leduc and Wharton, 2010, were also noted. The morphometric measurements of our species differ from most of the Pseudochromadora species reported in specialty literature. We extracted and counted all desmodorid individuals from 9 meiobenthic samples collected in June 2020, and performed a detailed morphological analysis of 12 individuals (5 females and 7 males) belonging to our species, also highlighting the dissimilarities with the related desmodorid species.
1. Introduction
The family Desmodoridae Filipjev, 1922, includes a variety of free-living, mostly marine nematodes, being recognized in general as a very heterogeneous group from a systematic point of view [1]. Several recent papers reviewed the taxonomic status of the group, e.g., [2,3,4], but there are still numerous gaps as a result of poorly described or controversial morphology of some taxa (e.g., Desmodorella Cobb, 1933, Croconema Cobb, 1920, and Desmodora de Man, 1889), lack of clear dichotomic keys, scarcity of molecular data, and the highly polyphyletic nature of Desmodoridae [5]. Lately, a new species belonging to Pseudochromadora Daday, 1899 (Desmodoridae Filipjev, 1922, Desmodorinae Filipjev, 1922), have been reported, e.g., [6,7,8,9], while others already known were thoroughly revised, e.g., [3,10].
To date, 13 valid species of Pseudochromadora Daday, 1899 [11], have been described worldwide, most reported from shallow tropical waters and only a few from cold habitats. Unlike its Desmodoridae relatives, no Pseudochromadora species has been recorded in deep-sea habitats up to the present [12,13,14]. The genus was raised in 1899 at the proposal of Dáday Jenő, who described the type taxon Pseudochromadora quadripapillata Daday, 1899, from Seleo Island, Papua New Guinea [15].
To the best of our knowledge, five genera belonging to the Desmodoridae Filipjev, 1922, have been reported in the Black Sea so far: Desmodora de Man, 1889; Chromaspirina Filipjev, 1918; Metachromadora Filipjev, 1918; Onyx Cobb, 189;, and Spirinia Gerlach, 1963 [16,17,18,19,20], with this being the first signaling of Pseudochromadora.
The current study aims to describe the new species Pseudochromadora gomoi sp.n. found on the Romanian Black Sea shelf, while also providing some considerations on its ecology.
2. Materials and Methods
A total of 9 sediment samples containing nematodes were collected using a Multicorrer device onboard R/V Mare Nigrum from the circalittoral zone of the Romanian Black Sea shelf in June 2020, at depths of 47–59 m. The top 5 cm of sediments of one core with a surface area of 78.5 cm2 area out of five cores collected simultaneously were cut out and washed with sea water through a 90 μm mesh sieve. The supernatant was subsequently fixed with 10% formalin, stained with a 2% rose bengal solution, and stored in a plastic jar until further laboratory analysis. All meiobenthic organisms, including nematodes, were picked out from the samples under a stereoscopic microscope. Nematodes were then transferred to glycerin using the [21] rapid method as modified by [22] and mounted on permanent slides. Nematodes were analyzed under a Zeiss Axio Lab A1 microscope furnished with 5 to 100x magnification objectives. Photographs of nematodes were taken with a Zeiss Axiocam 305 digital camera. For scanning electron microscope (SEM) analysis, the nematode samples were fixed in 4% glutaraldehyde in sodium cacodylate buffer (0.1 M, pH 7.4) in a refrigerator, at 4° for 24 h. The specimens were dehydrated in an ascending alcohol series: 30%, 50%, 70%, and 90%, and 3 changes of 100% ethanol (30 min for each change) [9,23]. The nematode specimens were transferred to a mixed solution of acetone and hexamethyldisilazane (HMDS) (3:1, 1:1, and 1:3), and finally subjected to 3 changes of 100% HMDS (30 min for each change). The samples in 100% HMDS were left overnight for the chemical evaporation and complete drying of the nematodes [24,25,26]. Finally, the samples were mounted on aluminum stubs covered with conductive double-sided adhesive carbon tabs. The nematodes were sputter-coated (SEM Coating Unit E5100) with gold for 60 s. The specimens were analyzed and photographed using a Phenom Pro scanning electron microscope (Phenom-World, Thermo Fisher Scientific, The Netherlands), at 10 kV and 15 kV acceleration voltages.
Measurements were taken with Zeiss Image software (ZEN 2.5—blue edition). Plates were prepared using CorelDRAW 2017 software. Drawings were completed based on the photographs and under the microscope. Species nomenclature was checked according to the World Register of Marine Species (WoRMS) [11].
Abbreviations of the measured variables presented in the description (Table 1) are:

Table 1.
Morphometrics of Pseudochromadora gomoi sp.n. and other Desmodoridae species based on literature. Data are presented as minimum–maximum values. All measurements are given in μm.
- L—body length (μm);
- M—maximum body diameter (μm);
- L. tail—tail length (μm);
- Am. W.—width of the amphidial fovea (μm);
- a—body length divided by maximum body diameter;
- b—body length divided by pharyngeal length;
- c—body length divided by tail length;
- Am. fovea c. b. d.—Width of the amphidial fovea as a percentage of corresponding body diameter (%);
- Ph. L—pharyngeal length (μm);
- Es. d.—esophagus diameter (μm);
- L. h. c.—length of head capsule (μm);
- L. c. s—length of cephalic setae (μm);
- Inn.l.s.—inner labial setae (μm);
- Out.l.s.—outer labial setae (μm);
- Subc. S.—subceph. Setae (μm);
- ph. b. d.—pharyngeal bulb diameter (μm);
- V (%)—distance of the vulva from the anterior end as a percentage of body length (%);
- Spic. arch—length of spicule along the arch (μm);
- Gub.—length of gubernaculum (μm);
- a.b.d.—anal body diameter (μm);
- V. ala L.—ventral ala length (μm);
- L. ala L.—lateral ala length (μm).
3. Results
Family Desmodoridae Filipjev, 1922
Subfamily Desmodorinae Micoletzky, 1924
Genus Pseudochromadora Daday, 1899
Diagnosis (after [2,3])
The Nematodes are characterized by a stout body and a short head capsule. Body annuli with distinct narrow spaces in between are present all over the body except for the head and tail tip. Lateral alae that extend from the posterior to the pharynx up to the beginning of the tail are characteristic. Along the body, six or eight longitudinal rows of short somatic setae are displayed. The head capsule could be divided in two or three parts, being separated into a slender labial region in the anterior part, followed by a thick cuticularized main part of the head capsule. A sutura can be noted between the regions of the head capsule. Four cephalic setae could be found either in the labial region or on the anterior rim of the head capsule. The amphids are usually unispiral (at least in females in the case of sexual dimorphism) and located on the main region of the head capsule. A cylindrical pharynx with a bipartite terminal bulb is present.
Males of most species have copulatory thorns and postcloacal thorns, arched spicules, and gubernaculum with capitulum.
Type species: Pseudochromadora quadripapillata Daday, 1899
3.1. Description
Type specimens
Slides (in glycerin) containing type specimens are deposited in the collection of the National Museum of Natural History Grigore Antipa Bucharest under the access numbers: 49994/a (holotype male), 49994/b (paratype male), and 49995 (allotype female).
Type locality
Black Sea (Romanian shelf) (St. MN205/90; coordinates: 30.23696667 E; 44.69308333 N; bottom depth: 59 m, bottom To: 8.93, S[PSU]: 18.53; and bottom [O2]: 8.31 mg/L)
Etymology. The species is named in the honor of Acad. Prof. Marian-Traian Gomoiu, a renowned Romanian biologist.
Pseudochromadora gomoi sp.n.
Material examined
3.1.1. Males (n = 7, 1 Holotype and 6 Paratypes)
The body length of males ranges between 1.35–1.85 mm, with maximum body diameter varying between 65.19 and 75.88 µm (Table 1). Two crowns of labial setiform papillae are displayed on the lips region: 6 (1–2 μm) on the inner circle and 6 (2.15–3.66 μm) on the outer one. The unispiral fovea amphidialis (11.85–14.83 µm diameter) is located centrally on the main region of the cephalic capsule (Figure 1). A faint amphidial plate could be observed (Figure 1B). The whole pharynx length and its thickness in the isthmus part vary between 208.62–234 μm and 29.32–40.83 µm, respectively, enlarging posteriorly into a bipartite bulb (51.29–57.89 µm). The nerve ring is situated in the posterior pharynx part (at about half of its length, 57%). The cardia (hardly seen in one single specimen) is almost oval. The tail is 92.96–125.04 µm and pointed (spinneret). The length of the spicule is 51.25–64.83 µm and the gubernaculum is 30.83–32.86 µm. A ventral ala (215.95–378.06 µm in length) starts at app. 2/3 of male body length and reaches near the spicule. No pre-or post-cloacal supplements were noted. The reproductive system is monorchic with one anterior outstretched testis located on the ventral part of the body (Figure 1C and Figure 2H). De Man’s indices are provided in Table 1.
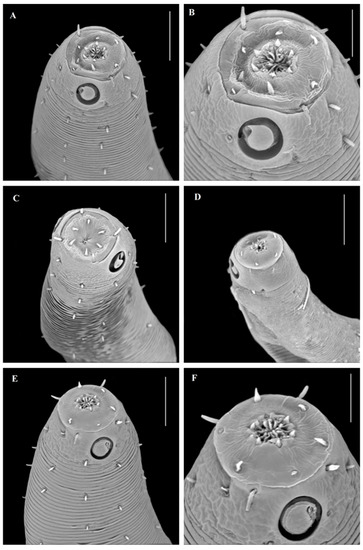
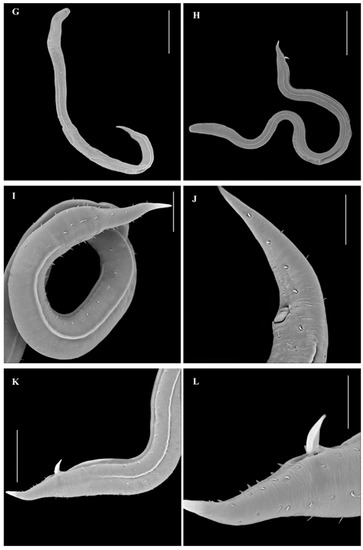
Figure 1.
Pseudochromadora gomoi sp.n. SEM images—(A) detail on the head structure (lip, suture and main cephalic region of the head) (male); (B) detail of outer labial and body setae; (C) fragment of constrictor cuticle ring in the anterior body part of a juvenile female; (D) detail on the cheilorabdhia and related mouth structure; (E) female: entire body; (F) female: cloacal and tail region; (G) male: entire body; (H) male: detail of tail and spicule region; and (I) male: detail on ventral and lateral alae in the posterior body region. Scale: (B,F): 10 µm; (A,C,D,E): 20 µm; (J,L): 30 µm; (I): 50 µm; (K): 80 µm; and (G,H): 200 µm.
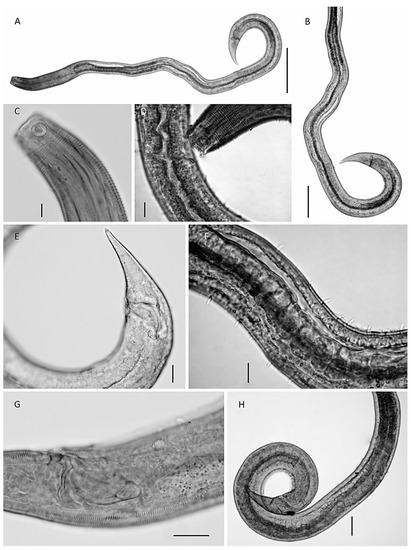
Figure 2.
Pseudochromadora gomoi sp.n.—paratype male (A–H). (A) the entire male aspect; (B) detail of the reproductive system; (C) detail on the fovea amphidialis; (D) detail of the ventral alae; (E) detail of the tail region and terminal part of the lateral alae; (F) detail of the cuticule setae; (G) detail of the spicule apparatus and part of the ventral alae; (H) detail of the ventral ala and the reproductive system; (A,B)—scale 100 µm; (C)—scale 10 µm; (D,F,H)—scale 50 µm; and (E,G)—scale 20 µm.
Type material
Holotype: adult male. The body length is 1744.86 μm; maximum body diameter: 84.823 μm. The head slightly tapers to its end. Both sides of the cephalic region are thickened. Two crowns of six labial setiform papillae each are displayed on the lips region (the size of the inner papillae: 1–2 μm, and of the outer ones: 3.25 μm), respectively. Four setae (4.85 μm) are located on the suture between the labial and cephalic regions. A total of eight additional subcephalic sensillae (3.32 μm) are placed on the cephalic capsule approximately at the level of the amphids and continue with the longitudinal rows of body setae (Figure 1E). The unispiral fovea amphidialis (12.52 µm diameter) (Table 1) with a short turn that goes backward is located centrally on the main region of the cephalic capsule. Lips are fringed, not clearly delineated. The mouth is equipped with a crown of 10–12 cheilorhabdia, with every inner labial seta being flanked by two rabdia. The buccal cavity is narrow, with one dorsal tooth and two minute ventrosublateral teeth (Figure 4D). Orange elongated ducts were noted in the head and upper part of the pharynx region (Figure 3C). The pharynx length is 228.301 μm, enlarging posteriorly into a bipartite bulb (61.312 µm). Except for the head and tail tip, the body cuticle is coarsely ringed with numerous setae. Eight longitudinal rows of somatic setae (2–5 μm long) run along almost the entire body length continuing in less numerous rows in the tail region (six rows). The tail is 117.417 µm and pointed (spinneret). Spicules are curved, with capitulum and velum; gubernaculum is conspicuous and almost straight and no apophysis was observed. The length of the spicule is 58.806 µm; gubernaculum is 21.52 µm. No copulatory or postcloacal thorns were seen. Slender undulatory lateral alae extending along the body from the posterior of pharyngeal cardia end to the spicula/cloacal opening are present. A ventral ala (364.12 µm in length) starts at app. 2/3 of male body length and reaches near the spicule. No supplements could be distinguished within the ventral alae thickness.
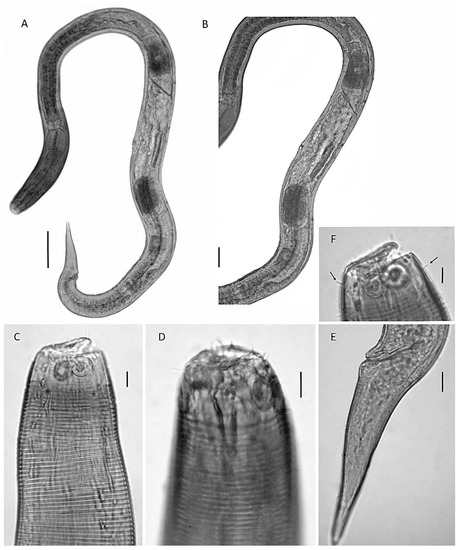
Figure 3.
Pseudochromadora gomoi sp.n.—allotype female. (A) the entire female aspect; (B) detail on the reproductive system; (C) the anterior body region with ducts in the head and the isthmus part of the pharynx; (D) detail of the big dorsal tooth and the two minute ventrosublateral teeth; (E) the tail with the cloacal opening; (F) the lip region cut off revealing the slim sutura and subcephalic setae on the head capsule region (arrows); (A,B)—scale 50 µm; and (C–F)—scale 10 µm.
3.1.2. Female (n = 5)
Females’ body lengths range between 1.37 and 2.1 mm and the maximum body diameter is between 58.98 and 100.58 µm. The reproduction system is didelphic–amphidelphic with reflexed ovaries. The female vulva is small, slit-like, located at 57–66% distance from the anterior end. The tail is conical, 109.44 –- 140.44 µm in length, and pointed (spinneret) (Table 1).
Allotype: female. Body length: 1401.17 µm; maximum body diameter: 82.811 µm. The cephalic structure (including the number and arrangement of labial and cephalic setae), the slender lateral alae, and the orange elongated ducts in the upper part of the head and pharynx regions (Figure 3C) are similar to males. No sexual dimorphism of amphids was observed. The female vulva is small, slit-like, located at 59.16% distance from the anterior end. The reproduction system is didelphic–amphidelphic with reflexed ovaries (Figure 3A,B and Figure 4A). The tail is conical, 93.618 µm in length, and pointed (spinneret).
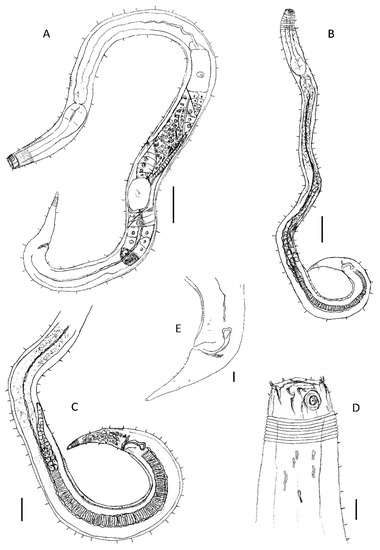
Figure 4.
(A) female: overview of female body with details on the didelphic–amphidelphic reproductive system with reflexed ovaries; (B) male: overview of male body; (C) detail of the monorchic reproductive system with one anterior outstretched testis D detail on the head region (teeth and amphidial fovea); (A–C)—scale 50 µm; and (D,E)—scale 10 µm.
3.2. Differential Diagnostic
Pseudochromadora gomoi sp.n. resembles Desmodora (Pseudochromadora) pontica Filipjev, 1922, by the presence of additional setae on the cephalic capsule and precloacal ventral ala in males but differs from it by the lack of the tubular supplements located within ala thickness as reported by [27].
Pseudochromadora gomoi sp.n. resembles other Pseudochromadora species by the presence of lateral alae extending far from the posterior of the cardia to the anterior of the anal/cloacal opening, the shape and central position of unispiral amphidial fovea, and the number and arrangement of setiform papillae in the labial region.
It differs from other Pseudochromadora species by the presence of eight additional setae on the head capsule, a ventral ala in the posterior body region of males, orange ducts in the anterior body region (head and pharynx), the lack of pre- or post-cloacal copulatory thorns and of sexual dimorphism, and by several morphometric parametrics (detailed in Table 2 and hereinafter).

Table 2.
Details on the location of sampling stations, average density of Pseudochromadora gomoi sp.n. (given as numbers of individuals in a square meter) (average densities are square root transformed) and environmental parameters.
It differs from P. quadripapillata Daday, 1899, [15] by the absence of precloacal supplements (31–36 small internal cup-shaped supplements connected with gland cells in the derma in the latter), but it resembles it by the lack of copulatory thorns.
It differs from P. benepapillata (Timm, 1961) Datta, Ganguly, and Chakraborty, 2018, [10] by the rounded labial region (vs. the hat-shaped); the lack of sexual dimorphism of amphidial fovea, and of copulatory thorns; the presence of subcephalic setae; longer cephalic setae (up to ~5 μm vs. 2 μm); and longer gubernaculum (30.83–32.86 vs. 13–15 µm) and spicules (51.25–64.83 μm vs. 48–53 μm) (Table 2).
Pseudochromadora gomoi sp.n. differs from P. buccobulbosa Verschelde and Vincx, 1995, [7] by the lack of a dorsal plug in the buccal cavity and of a swollen pharyngostome.
It differs from P. securis Verschelde Nicholas and Vincx, 2006, and P. galeata Verschelde, Nicholas and Vincx, 2006, [3] by the lack of a complex teeth armature but it resembles them by the presence of a cheilostome.
It differs from P. reathae Leduc and Wharton, 2010, [8] by the lack of pre-cloacal supplements (8–9 cone-shaped projections flanked by two cuticularized pieces connected with paired gland cells), and the pharynx shape (straight vs. bent).
Pseudochromadora gomoi sp.n. differs from P. interdigitatum Muthumbi, Verschelde and Vincx, 1995, [28] in the way the body annules split up and interdigitate with the lateral alae and the lack of copulatory thorns.
Pseudochromadora gomoi sp.n. differs from most of the genus species by the morphometric measurements (Table 1). Hence, none of the nine Pseudochromadora species’ body size taken into analysis has more than 1 mm in length as opposed to our species that reached up to 1.85 mm in males and 2.1 mm in females. Similarly, the maximum body width of our species was greater than of other species. In general, the females were larger than males, ranging between 58.98 –100.58 μm in the former and 65.2 and 75.88 μm in the latter, respectively, compared with the other Pseudochromadora species of which maximum body width varied from 34–37 μm (P. securis) to 49–62 μm (P. interdigitatum) in females and from 32–33 μm (P. reathae) to 40–44 μm (P. interdigitatum) in males. The amphidial fovea of both males and females is also wider in our species (10.04—13.1 μm in females and 11.85–14.83 μm in males) than in others, except for P. rossica [23] (18–27 μm males; 19–25 μm females) and P. interdigitatum males (10–14 μm). In general, the amphidial fovea width in males was 6–8 μm, whereas in females it was very slightly smaller (P. buccobulbosa [7], P. thinaiica [9], P. coomansi [7], P. buccobulbosa [7], P. galeata [3], P. reathae [8], and P. securis [3]. De Man’s parameters were also different, especially c (body length divided by tail length).
3.3. Ecology
The species was found in June 2020 in nine stations within the Terebellides stroemii Sars, 1835, and Modiolula phaseolina (Philippi, 1844) habitats on mixed sediments (mud, sand, and shells) (for other details on the environmental parameters see Table 2) at depths between 47–59 m, in the circalittoral bionomic zone of the Romanian Black Sea shelf. Its average densities ranged between 382.2 and 101,907.8 ± 32,907.15 ind.m−2, with the highest abundances reached at station 90 and the lowest at station 31 (Table 2). Species accompanying P. gomoi sp.n. were mainly from Linhomoeidae, Commesomatidae, Desmoscolecidae, and Sphaerolaimidae as well as a rich population of harpacticoids (i.e., Sarsamphiascus sinuatus (Sars G.O., 1906), Enhydrosoma sordidum Monard, 1926, Stenhelia (Delavalia) reflexa (Brady and Robertson, 1876), Alteutha Baird, 1846).
4. Discussion
At a first glance, Pseudochromadora gomoi sp.n. found in 2020 in the Romanian waters resembles Desmodora pontica Filipjev, 1922, described by [16] and lately by [29] who amended the diagnosis of the species D. pontica Filipjev, 1922, from [1] and [3]. Since its first description in Sevastopol Bay by [16], D. pontica occurrence in the Black Sea has been documented over time by several researchers [19,20,30,31,32]. Other species belonging to Desmodora de Man, 1889, have been occasionally mentioned in the Black Sea, e.g., Desmodora cf. conica Vitiello, 1971, in the deep hypoxic waters of the Bosphorus strait [19], but according to our best knowledge, this is the first report of a Pseudochromadora species in the Black Sea.
It is worth mentioning that in the samples collected in June 2020, D. pontica Filipjev, 1922, specimens were not found, though these have been previously reported in the study area [18,32]. Instead, there were large populations of Pseudochromadora gomoi sp.n., mostly inhabiting mixed sediments (mud, sand, and shells) at depths between 47–59 m (Table 2).
Pseudochromadora gomoi sp.n. exhibits a combination of diagnostic features of species from the Desmodora genus (e.g., D. pontica Filipjev, 1922, D. inflexa Wieser, 1954, D. dimorpha Hopper, 1961, D. cuddlesae Inglis, 1963, D. striatocephala Tchesunov, 2008, D. porosum Moura, Silva, and Esteves, 2014, and D. veronicae Moura, Silva, and Esteves, 2014), such as the presence of ventral alae, the additional subcephalic setae on the head capsule, and also characters from Pseudochromadora Daday, 1899, such as the occurrence of the lateral alae and eight rows of somatic setae along the body and the position of fovea amphidialis on the centrally main part of the head capsule.
Our species differs from most of the Pseudochromadora Daday, 1899, species described up to now by the presence of a ventral ala and subcephalic setae, the lack of copulatory thorns in males, a faint amphidial plate, orange ducts at the level of the head and anterior pharyngeal part as well as of a constrictor ring in the anterior body region noted in one juvenile female (only observed in SEM), characteristics evinced, for example, in Pseudodesmodora lacrima Leduc and Wharton, 2010. It shows also significant different morphometric characters (i.e., body size, amphidial fovea width, and de Man’s indices).
Nevertheless, the difficulty to discriminate between the species belonging to Desmodoridae is widely recognized. For example, “conflicting’’ characteristics have also been identified in the case of Desmodora campbelli Allgen, 1932. The species has undergone several revisions over time and in spite of this it is currently accepted as valid and has not yet been assigned to a specific genus and therefore is regarded as a taxon inquirendum [2,33]. Hence, the species shows slender lateral alae (character for Pseudochromadora) but has subcephalic setae (character for Desmodora) of variable sizes (distinct or faint depending on the species provenience) [2]. The authors of [34] found a genetic variation within populations of Desmodora cf. campbelli morphotypes comparable with or sometimes even higher than between populations whilst distinct discontinuities in the variation of several morphological features, including body size, amphid shape, male copulatory organs, and cuticle ornamentation were evinced. This intraspecific morphological variation seems to be a characteristic of several deep-sea species of other genera (e.g., Paracanthoncus) [35].
Another example could be given for the problematic species belonging to Pseudochromadora itself, P. cazca, that after numerous revisions of its taxonomic status since its discovery by [36], it has only recently been definitively assigned by [2] to the Pseudochromadora genus. Nevertheless, no less than twenty revisions of Desmodoridae have been documented [29], pointing out the necessity for advanced studies on the taxonomy and phylogeny of this nematode group.
5. Conclusions
We report the occurrence of Pseudochromadora gomoi sp.n. identified in the circalittoral habitats of the Romanian Black Sea waters. Although most characters of the new species comply with the general description of the Pseudochromadora genus (i.e., the presence of the lateral alae extended from the posterior to the pharynx up to the beginning of the tail), we evinced features typical for other species belonging to Desmodoridae. Therefore, we deem that further molecular comparative studies are required to fully elucidate the taxonomy and phylogeny of the species.
Author Contributions
Conceptualization, M.M.; methodology, M.M. and R.M.; SEM—R.M.; software, R.M. and A.T.; validation, M.M.; investigation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, M.M., R.M., S.M., A.T., and T.B.; supervision, M.M.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian Ministry of Research, Innovation, and Digitization in the framework of the national CORE Program (projects: PN 19200302, PN19200101, and PN 19200401), the Program Development of the National R&D system—AMBI AQUA—No. 23PFE, the European Union’s Horizon 2020 BRIDGE-BS project under grant agreement no. 101000240, and the DOORS project under grant agreement no. 101000518.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Decraemer, W.; Smol, N. Orders Chromadorida, Desmodorida and Desmoscolecida. In Freshwater Nematodes: Ecology and Taxonomy; Abebe, E.-A., Transpurger, W., Andrassy, I., Eds.; CABI Publishing: Cambridge, UK, 2006; pp. 497–573. [Google Scholar]
- Verschelde, D.; Gourbault, N.; Vincx, M. Revision of Desmodora with descriptions of new desmodorids (Nematoda) from hydrothermal vents of the Pacific. J. Mar. Biol. Assoc. UK 1998, 78, 75–112. [Google Scholar] [CrossRef]
- Verschelde, D.; Nicholas, W.; Vincx, M. A review of the genera Croconema Cobb, 1920 and Pseudochromadora Daday, 1899 (Nematoda, Desmodoroidea): New species from the coasts of Kenya and Australia. Hydrobiologia 2006, 571, 17–40. [Google Scholar] [CrossRef]
- Tchesunov, A.V. Order Desmodorida De Coninck, 1965. In Handbook of Zoology Gastrotricha, Cycloneuralia and Gnathifera. Nematoda; Schmidt–Rhaesa, A., Ed.; De Gruyter: Berlin/Boston, Germany, 2014; Volume 2, pp. 399–432. [Google Scholar]
- van Megen, H.; van den Elsen, S.; Holterman, M.; Karssen, G.; Mooyjman, P.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 2009, 6, 927–950. [Google Scholar] [CrossRef]
- Gourbault, N.; Vincx, M. Two new species of brood protecting Desmodoridae (Nematoda) from Guadeloupe. Nematologica 1990, 36, 131–143. [Google Scholar] [CrossRef]
- Verschelde, D.; Vincx, M. Psammonema gen.n. and Pseudochromadora Daday, 1889 (Nematoda, Desmodoridae) from sandy sediments of Gazi, Kenya. Bull. de L’Institut Royal Des Sci. Nat. de Belgique 1995, 65, 11–39. [Google Scholar]
- Leduc, D.; Wharton, D.A. New free-living marine nematode species (Nematoda: Desmodoridae) from the coast of New Zealand. Zootaxa 2010, 2611, 45. [Google Scholar] [CrossRef]
- Zograf, J.K.; Skripova, E.R.; Semenchenko, A.A.; Vu, V.D.; Nguyen, T.-L.; Phan, T.H.; Mordukhovich, V.V. A novel free-living marine nematode species Pseudochromadora thinaiica sp. n. (Nematoda: Desmodoridae) from the seagrass bed of Vietnam. Russ. J. Nematol. 2021, 29, 169–182. [Google Scholar]
- Datta, T.K.; Ganguly, A.; Chakraborty, S.K. Pseudochromadora benepapillata (Timm 1961) comb. n. (Desmodoridae: Nematoda): Revision of its taxonomic status and distribution. Zootaxa 2018, 4425, 165–174. [Google Scholar] [CrossRef] [PubMed]
- WoRMS Editorial Board. World Register of Marine Species. 2014. Available online: http://www.marinespecies.org (accessed on 20 May 2022).
- Cavalcanti, M.; Da Silva, M.C.; Da Fonsêca-Genevois, V. Spirodesma magdae nov. gen. nov. sp. (Nematoda: Desmodoridae) from the Brazilian deep sea (Campos Basin, Rio de Janeiro, Brazil). Zootaxa 2009, 2096, 109–118. [Google Scholar] [CrossRef]
- Leduc, D. New free–living nematode species and records (Chromadorea: Plectida and Desmodorida) from the edge and axis of Kermadec Trench, Southwest Pacific Ocean. PeerJ 2021, 9, e12037. [Google Scholar] [CrossRef]
- Fadeeva, N.; Mordukhovich, V.; Zograf, J. Free-living marine nematodes of Desmodorella and Zalonema (Nematoda: Desmodoridae) with description of two new species from the deep sea of the North Western Pacific. Zootaxa 2016, 4175, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Daday, J. Uj–guineai szabadon elö nematodok. Mathematikai És Természettudományi Értesitö 1899, 17, 557–572. [Google Scholar]
- Filipjev, I.N. Free–living marine nematodes of the Sevastopol area. Trans. Zool. Lab. Sevastop. Biol. Stn. Russ. Acad. Sci. 1918, 1–255, (Translated from Russian). [Google Scholar]
- Filipjev, I.N. New data on free nematodes of the Black Sea (Novye Dannye o Svobodnykh Nematodakh Chernogo Moria) Tr. Stavropol’skogo Sel’skokhoziaistvennogo Inst. 1922, 1, 13–184. [Google Scholar]
- Băcescu, M.; Müller, G.; Gomoiu, M.-T. Cercetari de ecologie bentala in Marea Neagra. Analiza cantitativă, calitativă şi comparată a faunei bentale pontice. Ecol. Mar. 1971, 4, 1–357. [Google Scholar]
- Sergeeva, N.G.; Ürkmez, D.; Revkova, T. Meiobenthic nematodes at the deep oxic/anoxic boundary of the Black Sea (Istanbul Strait Outlet Area) with new records for Turkey. Reg. Stud. Mar. Sci. 2021, 46, 101904. [Google Scholar] [CrossRef]
- Ürkmez, D.; Sezgin, M.; Karaҫuha, M.E.; Öksüz, I. Meiobenthic Assemblages from the Southwestern Coast of the Black Sea, Iğneada (Turkey) Biologia 2016, 71, 1017–1026. Biologia 2016, 71, 1017–1026. [Google Scholar] [CrossRef]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef]
- De Grisse, A. Redescription ou modifications de quelques techniques utililisés dans l’étude des nématodes phytoparasitaires. Mededelingen van de Rijksfaculteit Landbouwetenschappen Gent 1969, 34, 351–369. [Google Scholar]
- Mordukhovich, V.V.; Fadeeva, N.P.; Semenchenko, A.A.; Zograf, J.K. New species of Pseudochromadora Daday, 1899 (Nematoda: Desmodoridae) from Russky Island (the Sea of Japan). Russ. J. Nematol. 2015, 23, 125–135. [Google Scholar]
- Eisenbach, J.D. Techniques for preparing nematodes for scanning electron microscopy. An Advanced Treatise on Meloidogyne. J. Nematol. 1986, 18, 479. [Google Scholar]
- Cesaroni, L.; Guidi, L.; Balsamo, M.; Semprucci, F. Scanning electron microscopy in the taxonomical study of free-living marine nematodes. Microscopie 2017, 28, 31–38. [Google Scholar]
- Rahman, F.H.A. Chemical Drying of Nematodes for Scanning Electron Microscopy Observations. World J. Agric. Soil Sci. 2021, 7, 1–5. [Google Scholar]
- Boucher, G. Nematodes des sables fins infralittoraux de la Pierre Noire (Manche occidentale). I. Desmodorida. Bulletin Du Muséum National D’histoire Naturelle 1975, 195, 101–128. [Google Scholar]
- Muthumbi, A.; Verschelde, D.; Vincx, M. New Desmodoridae (Nematoda: Desmodoroidea): Three new species from Ceriops mangrove sediments (Kenya) and one related new species from the North Sea. Cah. Biol. Mar. 1995, 36, 181–195. [Google Scholar]
- Armenteros, M.; Ruiz-Abierno, A.; Decraemer, W. Revision of Desmodorinae and Spiriniinae (Nematoda: Desmodoridae) with redescription of eight known species. Eur. J. Taxono. 2014, 96, 1–32. [Google Scholar] [CrossRef]
- Filipjev, I.N. Encore sur les Nématodes libres de la mer Noire. Tr. Stravrop. Skh. Inst. Zool. 1922, 1, 83–184. [Google Scholar]
- Mureşan, M. Diversity and distribution of free–living nematodes within periazoic level on the Romanian Shelf of the Black Sea. Geo–Eco-Marina 2014, 20, 19–28. [Google Scholar]
- Mureşan, M. Assessment of free–living marine nematodes community from the NW Romanian Black Sea Shelf. Geo–Eco–Marina 2012, 18, 133–145. [Google Scholar]
- Ingels, J.; Vanhove, S.; De Mesel, I.; Vanreusel, A. The biodiversity and biogeography of the free-living nematode genera Desmodora and Desmodorella (family Desmodoridae) at both sides of the Scotia Arc. Polar Biol. 2006, 29, 936–949. [Google Scholar] [CrossRef]
- Hauquier, F.; Leliaert, F.; Rigaux, A.; Derycke, S.; Vanreusel, A. Distinct genetic differentiation and species diversification within two marine nematodes with different habitat preference in Antarctic sediments. BMC Evolut. Biol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Apolônio, S.; Decraemer, W.; Moens, T.; Amadeu, P.; Derycke, S. Low genetic by high morphological variation overmore than 1000 km coastline refutes omnipresence of cryptic diversity in marine nematodes. BMC Evolut. Biol. 2017, 17, 71. [Google Scholar]
- Gerlach, S.A. Brasilianische Meeres–Nematoden I. Boletim do Instituto Oceanográfico. Bol. Inst. Paul. Oceanogr. S. Paulo. 1956, 5, 3–69. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).