The Impact of the Honeybee Apis mellifera on the Organization of Pollination Networks Is Positively Related with Its Interactive Role throughout Its Geographic Range
Abstract
1. Introduction
2. Materials and Methods
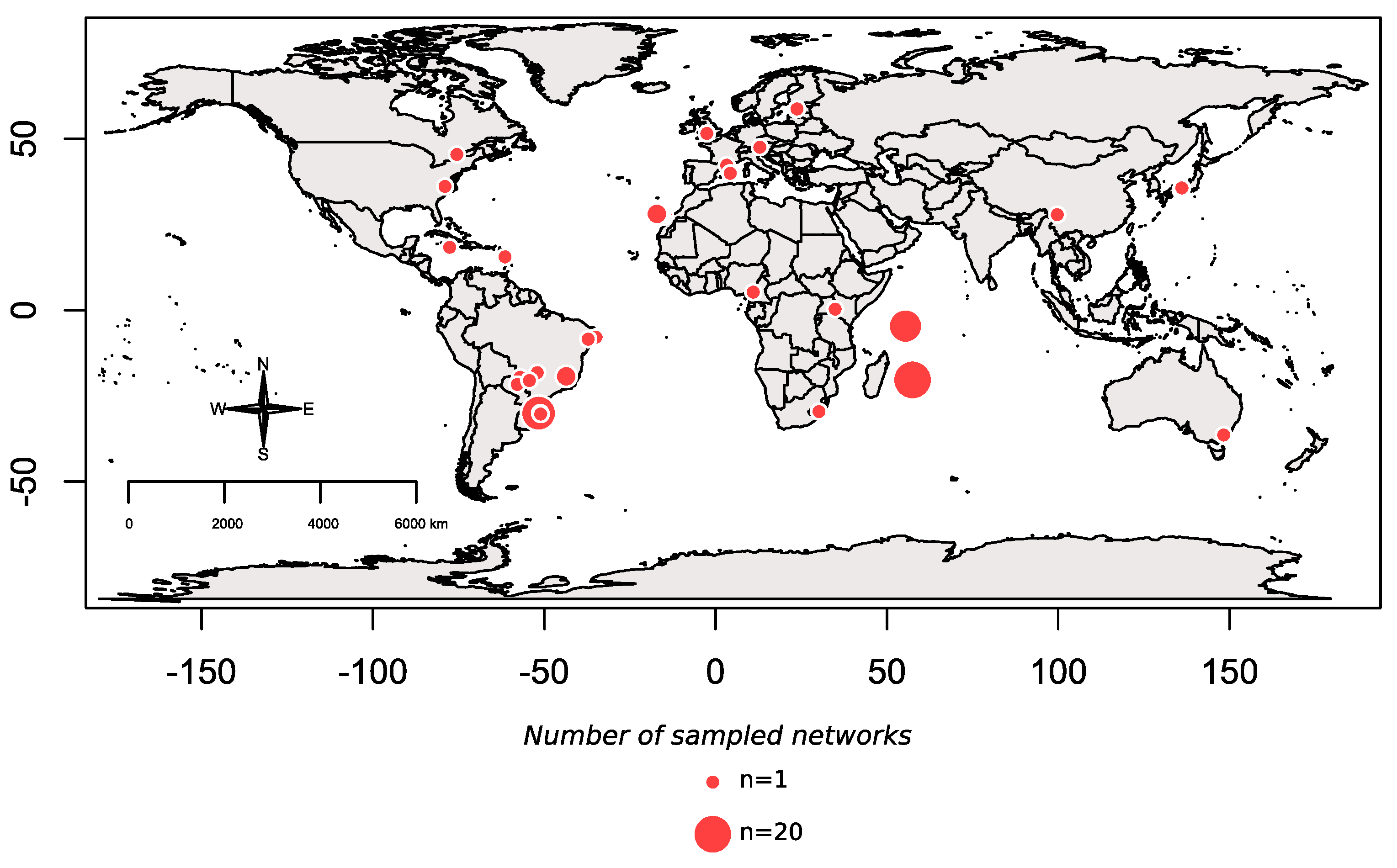
2.1. Interaction Network Dataset
2.2. The Interactive Role of Apis mellifera
2.3. Estimating Specialization, Niche Overlap, and Robustness
2.4. Weighted Impact of Apis mellifera on Pollination Networks
3. Data analysis
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeMaynadier, P.; Hunter, M.L. Keystone support. BioScience 1994, 44, 2. [Google Scholar] [CrossRef]
- Simberloff, D. Flagships, umbrellas, and keystones: Is single species management passé in the landscape era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Switanek, M.; Crailsheim, K.; Truhetz, H.; Brodschneider, R. Modelling seasonal effects of temperature and precipitation on honey bee winter mortality in a temperate climate. Sci. Total Environ. 2017, 579, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Gengler, N.; Francis, F. How humans reshaped diversity in honeybees (Apis mellifera L.): A review. Entomol. Faun. 2018, 2, id71. [Google Scholar]
- Medina, A.M.; Almeida-Neto, M. Grinnelian and Eltonian niche conservatism of the European honeybee (Apis mellifera) in its exotic distribution. Sociobiology 2020, 67, 239–246. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Giannini, T.C.; Garibaldi, L.A.; Acosta, A.L.; Silva, J.S.; Maia, K.P.; Saraiva, A.M.; Guimarães, P.R., Jr.; Kleinert, A.M. Native and non-native supergeneralist bee species have different effects on plant-bee networks. PLoS ONE 2015, 10, e0137198. [Google Scholar] [CrossRef]
- Stevenson, P.C. For antagonists and mutualists: The paradox of insect toxic secondary metabolites in nectar and pollen. Phytochem. Rev. 2020, 19, 603–614. [Google Scholar] [CrossRef]
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344. [Google Scholar] [CrossRef]
- Nicholls, E.; Hempel de Ibarra, N. Assessment of pollen rewards by foraging bees. Func. Ecol. 2017, 31, 76–87. [Google Scholar] [CrossRef]
- Dogantzis, K.A.; Zayed, A. Recent advances in population and quantitative genomics of honey bees. Curr. Opin. Insect. Sci. 2019, 31, 93–98. [Google Scholar] [CrossRef]
- Ilyasov, R.A.; Lee, M.L.; Takahashi, J.I.; Kwon, H.W.; Nikolenko, A.G. A revision of subspecies structure of western honey bee Apis mellifera. Saudi J. Biol. Sci. 2020, 27, 3615–3621. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do managed bees have negative effects on wild bees?: A systematic review of the literature. PLoS ONE 2017, 12, e0189268. [Google Scholar] [CrossRef]
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Winfree, R.; Aguilar, R.; Vázquez, D.P.; LeBuhn, G.; Aizen, M.A. A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 2009, 8, 2068–2076. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Rogers, S.; Cajamarca, P.; Tarpy, D.; Burrack, H. Honey bees and bumble bees respond differently to inter- and intra-specific encounters. Apidologie 2013, 44, 621–629. [Google Scholar] [CrossRef]
- Thomson, D.M. Local bumble bee decline linked to recovery of honeybees, drought effects on floral resources. Ecol. Lett. 2016, 19, 1247–1255. [Google Scholar] [CrossRef]
- Wojcik, V.A.; Morandin, L.A.; Davies Adams, L.; Rourke, K.E. Floral resource competition between honey bees and wild bees: Is there clear evidence and can we guide management and conservation? Environ. Entom. 2018, 47, 822–833. [Google Scholar] [CrossRef]
- Lindström, S.A.M.; Herbertsson, L.; Rundlöf, M.; Bommarco, R.; Smith, H.G. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc. R. Soc. B Biol. Sci 2016, 283, 20161641. [Google Scholar] [CrossRef]
- Torné-Noguera, A.; Rodrigo, A.; Osorio, S.; Bosch, J. Collateral effects of beekeeping: Impacts on pollen-nectar resources and wild bee communities. Basic Appl. Ecol. 2016, 17, 199–209. [Google Scholar] [CrossRef]
- Luna, P.; Dáttilo, W. Disentangling plant-animal interactions into complex networks: A multi-view approach and perspectives. In Plant-Animal Interactions: Sources of Biodiversity; Del-Claro, K., Torezan-Silingardi, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 261–281. [Google Scholar]
- Harvey, E.; Gounand, I.; Ward, C.L.; Altermatt, F. Bridging ecology and conservation: From ecological networks to ecosystem function. J. Appl. Ecol. 2017, 54, 371–379. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Morris, R.J. Ecological networks across environmental gradients. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 25–48. [Google Scholar] [CrossRef]
- Guimaraes, P.R., Jr. The structure of ecological networks across levels of organization. Ann. Rev. Ecol. Evol. Syst. 2020, 51, 433–460. [Google Scholar] [CrossRef]
- Burin, G.; Guimaraes, P.R., Jr.; Quental, T.B. Macroevolutionary stability predicts interaction patterns of species in seed dispersal networks. Science 2021, 372, 733–737. [Google Scholar] [CrossRef]
- Antoniazzi, R., Jr.; Dáttilo, W.; Rico-Gray, V. A useful guide of main indices and software used for ecological networks studies. In Ecological Networks in the Tropics: An Integrative Overview of Species Interactions from Some of the Most Species-Rich Habitats on Earth; Dáttilo, W., Rico-Gray, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 185–196. [Google Scholar]
- Delmas, E.; Besson, M.; Brice, M.H.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.J.; Gravel, D.; Guimarães, P.R., Jr.; Hembry, D.H.; Newman, E.A.; et al. Analysing ecological networks of species interactions. Biol. Rev. 2019, 94, 16–36. [Google Scholar] [CrossRef]
- Sazima, C.; Guimarães, P.R., Jr.; Dos Reis, S.F.; Sazima, I. What makes a species central in a cleaning mutualism network? Oikos 2010, 119, 1319–1325. [Google Scholar] [CrossRef]
- Guimarães, P.R., Jr.; Jordano, P.; Thompson, J.N. Evolution and coevolution in mutualistic networks. Ecol. Lett. 2011, 14, 877–885. [Google Scholar] [CrossRef]
- Dáttilo, W.; Lara-Rodríguez, N.; Jordano, P.; Guimarães, P.R., Jr.; Thompson, J.N.; Marquis, R.J.; Medeiros, L.P.; Ortiz-Pulido, R.; Marcos-García, M.A.; Rico-Gray, V. Unravelling Darwin’s entangled bank: Architecture and robustness of mutualistic networks with multiple interaction types. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161564. [Google Scholar] [CrossRef]
- Santos, G.M.M.; Aguiar, C.M.; Genini, J.; Martins, C.F.; Zanella, F.C.; Mello, M.A. Invasive Africanized honeybees change the structure of native pollination networks in Brazil. Biol. Invasions 2012, 14, 2369–2378. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019, 9, 4711. [Google Scholar] [CrossRef]
- Corcos, D.; Cappellari, A.; Mei, M.; Paniccia, D.; Cerretti, P.; Marini, L. Contrasting effects of exotic plant invasions and managed honeybees on plant-flower visitor interactions. Divers. Distrib. 2020, 26, 1397–1408. [Google Scholar] [CrossRef]
- Weaver, J.R.; Ascher, J.S.; Mallinger, R.E. Effects of short‚ Äêterm managed honey bee deployment in a native ecosystem on wild bee foraging and plant-pollinator networks. Insect Conserv. Divers. 2022, in press. [Google Scholar] [CrossRef]
- Cruz, C.P.; Luna, P.; Guevara, R.; Hinojosa-Díaz, I.A.; Villalobos, F.; Dáttilo, W. Climate and human influence shape the interactive role of the honeybee in pollination networks beyond its native distributional range. Basic Appl. Ecol. 2022, 63, 186–195. [Google Scholar] [CrossRef]
- Lima, G.; Leite, A.; Souza, C.; Castro, C.; de Santana Bezerra, E. A multilayer network in an herbaceous tropical community reveals multiple roles of floral visitors. Oikos 2020, 129, 1141–1151. [Google Scholar] [CrossRef]
- Oleques, S.; Vizentin-Bugoni, J.; Overbeck, G. Influence of grazing intensity on patterns and structuring processes in plant-pollinator networks in a subtropical grassland. Arthropod-Plant Interac. 2019, 13, 757–770. [Google Scholar] [CrossRef]
- Beal-Neves, M.; Vogel Ely, C.; Westerhofer-Esteves, M.; Blochtein, B.; Lahm, R.A.; Quadros, E.L.; Abreu-Ferreira, P.M. The influence of urbanization and fire disturbance on plant-floral visitor mutualistic networks. Diversity 2020, 12, 141. [Google Scholar] [CrossRef]
- Lara-Romero, C.; Seguí, J.; Pérez-Delgado, A.; Nogales, M.; Traveset, A. Beta diversity and specialization in plant–pollinator networks along an elevational gradient. J. Biogeogr. 2019, 46, 1598–1610. [Google Scholar] [CrossRef]
- Dicks, L.V.; Corbet, S.A.; Pywell, R.F. Compartmentalization in plant–insect flower visitor webs. J. Anim. Ecol. 2002, 71, 32–43. [Google Scholar] [CrossRef]
- Ollerton, J.; Johnson, S.D.; Cranmer, L.; Kellie, S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Ann. Bot. 2003, 92, 807–834. [Google Scholar] [CrossRef]
- Memmott, J. The structure of a plant-pollinator food web. Ecol. Lett. 1999, 2, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Inouye, D.W.; Pyke, G.H. Pollination biology in the Snowy Mountains of Australia: Comparisons with montane Colorado, USA. Aust. J. Ecol. 1988, 13, 191–210. [Google Scholar] [CrossRef]
- Motten, A.F. Pollination Ecology of the Spring Wildflower Community in the Deciduous Forests of Piedmont North Carolina. Ph.D. Thesis, Duke University, Duhram, NC, USA, 1982. [Google Scholar]
- Motten, A.F. Pollination ecology of the spring wildflower community of a temperate deciduous forest. Ecol. Monog. 1986, 56, 21–42. [Google Scholar] [CrossRef]
- Small, E. Insect pollinators of the Mer Bleue peat bog of Ottawa. Can. Field-Nat. 1976, 90, 22–28. [Google Scholar]
- Ingversen, T.T. Plant–pollinator interactions on Jamaica and Dominica: The centrality, asymmetry and modularity of networks. Master’s Thesis, University of Aarhus, Aarhus, Denmark, 2006. [Google Scholar]
- Kakutani, T.; Inoue, T.; Kato, M.; Ichihashi, H. Insect-flower relationship in the campus of Kyoto University, Kyoto: An overview of the flowering phenology and the seasonal pattern of insect visits. Contrib. Biol. Lab. Kyoto Univ. 1990, 27, 465–521. [Google Scholar]
- Kato, M.; Miura, R. Flowering phenology and anthophilous insect community at a threatened natural lowland marsh at Nakaikemi in Tsuruga, Japan. Contrib. Biol. Lab. Kyoto Univ. 1996, 29, 1–48. [Google Scholar]
- Bartomeus, I.; Vilà, M.; Santamaria, L. Contrasting effects of invasive plants in plant-pollinator networks. Oecologia 2008, 155, 761–770. [Google Scholar] [CrossRef]
- Bezerra, E.L.S.; Machado, I.C.S.; Mello, M.A.R. Pollination networks of oil-flowers: A tiny world within the smallest of all worlds. J. Anim. Ecol. 2009, 78, 1096–1101. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.N.; Muff, S.; Memmott, J.; Müller, C.B.; Caflisch, A. The robustness of pollination networks to the loss of species and interactions: A quantitative approach incorporating pollinator behaviour. Ecol. Lett. 2010, 13, 442–452. [Google Scholar] [CrossRef]
- Kaiser-Bunbury, C.N.; Vázquez, D.P.; Stang, M.; Ghazoul, J. Determinants of the microstructure of plant-pollinator networks. Ecology 2014, 95, 3314–3324. [Google Scholar] [CrossRef]
- Trøjelsgaard, K.; Jordano, P.; Carstensen, D.W.; Olesen, J.M. Geographical variation in mutualistic networks: Similarity, turnover and partner fidelity. Proc. R. Soc. B. 2015, 282, 20142925. [Google Scholar] [CrossRef]
- Benadi, G.; Hovestadt, T.; Poethke, H.J.; Blüthgen, N. Specialization and phenological synchrony of plant–pollinator interactions along an altitudinal gradient. J. Anim. Ecol. 2014, 83, 639–650. [Google Scholar] [CrossRef]
- Hagen, M.; Kraemer, M. Agricultural surroundings support flower–visitor networks in an Afrotropical rain forest. Biol. Conserv. 2010, 143, 1654–1663. [Google Scholar] [CrossRef]
- Carstensen, D.W.; Trøjelsgaard, K.; Ollerton, J.; Morellato, L.P.C. Local and regional specialization in plant–pollinator networks. Oikos 2018, 127, 531–537. [Google Scholar] [CrossRef]
- Souza, C.S.; Maruyama, P.K.; Aoki, C.; Sigrist, M.R.; Raizer, J.; Gross, C.L.; de Araujo, A.C. Temporal variation in plant–pollinator networks from seasonal tropical environments: Higher specialization when resources are scarce. J. Ecol. 2018, 106, 2409–2420. [Google Scholar] [CrossRef]
- Montero-Castaño, A.; Vilà, M. Influence of the honeybee and trait similarity on the effect of a non-native plant on pollination and network rewiring. Func. Ecol. 2017, 31, 142–152. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Memmott, J.; Vaughan, I.P.; Li, H.D.; Ren, Z.X.; Lázaro, A.; Zhou, W.; Xu, X.; Wang, W.-J.; Liang, H.; et al. The impact of a native dominant plant, Euphorbia jolkin ii, on plant–flower visitor networks and pollen deposition on stigmas of co-flowering species in subalpine meadows of Shangri-La, SW China. J. Ecol. 2021, 109, 2107–2120. [Google Scholar] [CrossRef]
- Motivans-Švara, E.; Ştefan, V.; Sossai, E.; Feldmann, R.; Aguilon, D.J.; Bontsutsnaja, A.; E-Vojtkó, A.; Kilian, I.C.; Lang, P.; Mõtlep, M.; et al. Effects of different types of low-intensity management on plant-pollinator interactions in Estonian grasslands. Ecol. Evol. 2021, 11, 16909–16926. [Google Scholar] [CrossRef]
- Miranda, P.N.; da Silva Ribeiro, J.E.L.; Luna, P.; Brasil, I.; Delabie, J.H.C.; Dáttilo, W. The dilemma of binary or weighted data in interaction networks. Ecol. Complex. 2019, 38, 1–10. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open J. Ecol. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 15 February 2022).
- Borrett, S.R. Throughflow centrality is a global indicator of the functional importance of species in ecosystems. Ecol. Indic. 2013, 32, 182–196. [Google Scholar] [CrossRef]
- Brandes, U. On variants of shortest-path betweenness centrality and their generic computation. Social Net. 2008, 30, 136–145. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Evol. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Olesen, J.M. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 2006, 312, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Katz, L. A new status index derived from sociometric analysis. Psychometrika 1953, 18, 39–43. [Google Scholar] [CrossRef]
- Medeiros, L.P.; Garcia, G.; Thompson, J.N.; Guimarães, P.R., Jr. The geographic mosaic of coevolution in mutualistic networks. Proc. Natl. Acad. Sci. USA 2018, 115, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Maia, K.P.; Rasmussen, C.; Olesen, J.M.; Guimarães, P.R., Jr. Does the sociality of pollinators shape the organisation of pollination networks? Oikos 2019, 128, 741–752. [Google Scholar] [CrossRef]
- Horn, H.S. Measurement of “overlap” in comparative ecological studies. Am. Nat. 1966, 100, 419–424. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef]
- Burgos, E.; Ceva, H.; Perazzo, R.P.; Devoto, M.; Medan, D.; Zimmermann, M.; Delbue, A.M. Why nestedness in mutualistic networks? J. Theor Bol. 2007, 249, 307–313. [Google Scholar] [CrossRef]
- Silva, J.R.C.; da Silva Mouga, D.M.D.; Dec, E. The bee community (Hymenoptera, Apidae) of Ilha Grande, Babitonga bay, Santa Catarina State, Brazil: Structure, insularity and interaction network. Sociobiology 2022, 69, e7360. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: London, UK, 2017. [Google Scholar]
- Batra, S.W. Solitary bees. Sci. Am. 1984, 250, 120–127. [Google Scholar] [CrossRef]
- Arroyo-Correa, B.; Burkle, L.A.; Emer, C. Alien plants and flower visitors disrupt the seasonal dynamics of mutualistic networks. J. Ecol. 2020, 108, 1475–1486. [Google Scholar] [CrossRef]
- Traveset, A.; Richardson, D.M. Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol. Evol. 2006, 21, 208–216. [Google Scholar] [CrossRef]
- Aizen, M.A.; Morales, C.L.; Morales, J.M. Invasive mutualists erode native pollination webs. PLoS Biol. 2008, 6, e31. [Google Scholar] [CrossRef]
- Prendergast, K.S.; Ollerton, J. Impacts of the introduced European honeybee on Australian bee-flower network properties in urban bushland remnants and residential gardens. Austral Ecol. 2022, 47, 35–53. [Google Scholar] [CrossRef]
- Carman, K.; Jenkins, D.G. Comparing diversity to flower-bee interaction networks reveals unsuccessful foraging of native bees in disturbed habitats. Biol. Conserv. 2016, 202, 110–118. [Google Scholar] [CrossRef]
- Richardson, D.M.; Allsopp, N.; D'Antonio, C.M.; Milton, S.J.; Rejmánek, M. Plant invasions-the role of mutualisms. Biol. Rev. 2000, 75, 65–93. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Aizen, M.A.; Alcántara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; García, M.B.; García, D.; Gómez, J.M.; Jordano, P.; et al. Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 2015, 29, 299–307. [Google Scholar] [CrossRef]
- Cappellari, A.; Bonaldi, G.; Mei, M.; Paniccia, D.; Cerretti, P.; Lorenzi, M. Functional traits of plants and pollinators explain resource overlap between honeybees and wild pollinators. Oecologia 2022, 198, 1019–1029. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dáttilo, W.; Cruz, C.P.; Luna, P.; Ratoni, B.; Hinojosa-Díaz, I.A.; Neves, F.S.; Leponce, M.; Villalobos, F.; Guevara, R. The Impact of the Honeybee Apis mellifera on the Organization of Pollination Networks Is Positively Related with Its Interactive Role throughout Its Geographic Range. Diversity 2022, 14, 917. https://doi.org/10.3390/d14110917
Dáttilo W, Cruz CP, Luna P, Ratoni B, Hinojosa-Díaz IA, Neves FS, Leponce M, Villalobos F, Guevara R. The Impact of the Honeybee Apis mellifera on the Organization of Pollination Networks Is Positively Related with Its Interactive Role throughout Its Geographic Range. Diversity. 2022; 14(11):917. https://doi.org/10.3390/d14110917
Chicago/Turabian StyleDáttilo, Wesley, Carlos Pinilla Cruz, Pedro Luna, Brenda Ratoni, Ismael A. Hinojosa-Díaz, Frederico S. Neves, Maurice Leponce, Fabricio Villalobos, and Roger Guevara. 2022. "The Impact of the Honeybee Apis mellifera on the Organization of Pollination Networks Is Positively Related with Its Interactive Role throughout Its Geographic Range" Diversity 14, no. 11: 917. https://doi.org/10.3390/d14110917
APA StyleDáttilo, W., Cruz, C. P., Luna, P., Ratoni, B., Hinojosa-Díaz, I. A., Neves, F. S., Leponce, M., Villalobos, F., & Guevara, R. (2022). The Impact of the Honeybee Apis mellifera on the Organization of Pollination Networks Is Positively Related with Its Interactive Role throughout Its Geographic Range. Diversity, 14(11), 917. https://doi.org/10.3390/d14110917





