1. Introduction
Earthworms are the group of Oligochaeta worms that are known for their soil-dwelling properties and ecosystem functions that include soil turnover, soil fertility, and biomass [
1,
2]. Megascolecidae is the most diverse family of earthworms [
3] comprising over 1000 species [
3,
4], most of them being native to Asia and Australia [
4]. Out of many genera of the family
Megascolecidae, the genus
Megascolex was first described by Templeton [
5] under the name
M. caeruleus from the alpine regions of Ceylon (Sri Lanka), characterised by the presence of a row of small numerous spines or setae on each segment. Since then, 37 species and sub-species of
Megascolex have been reported from Sri Lanka [
6]. In India, the genus is comprised of 33 species [
7], out of which 24 species were presented in a checklist by Narayanan et al. [
8]. However, with the addition of
Megascolex lawsoni (Bourne, 1894) from the state [
9], the list was updated to 25 species with the presence of
M. insignis as the only near-endemic species which occurs both in India and Sri Lanka [
6]. The distribution of
Megascolex species in India has been reported from peninsular parts, particularly in the southern part of India [
9,
10,
11,
12]. Kerala is an important constituent part of the Western Ghats and houses 99 species of earthworms [
9]; however, just a few molecular studies have been conducted on
Drawida and
Moniligaster species [
13] and there are no molecular records for
Megascolex species.
Since earthworm taxonomy can be quite challenging particularly due to conservative morphological features [
14], the taxonomy and diversity assessments are unstable and in need of revision. With the availability of online databases such as the Barcode of Life Database (BOLD) and the National Centre for Biotechnology Information (NCBI) that hold thousands of DNA barcodes, there are opportunities to use these information resources to improve the study of the taxonomy, evolution, and ecology of many organisms [
15,
16,
17]. Nonetheless, in earthworms, these data have only been used to clarify a few taxonomic groups and the application of DNA barcoding to most of the Indian earthworm species is still deficient. This limits inferences about their actual diversity and correct classification [
18]. Progress in molecular taxonomy of some Indian genera/species complexes such as
Amynthas [
19],
Aporrectodea caliginosa species complex [
20],
Drawida and
Moniligaster [
13],
Eutyphoeus [
21,
22],
Kanchuria [
23], as well as the molecular diversity and genetic variability of some other earthworm species [
24,
25,
26,
27] has significantly added to work in the field over the past few years. Nevertheless, most of the species remained untouched by the molecular approach and have only been described by morpho-anatomical observations that require strong diagnostic features, extensive labour, and expertise to segregate complex species and cryptic species.
The present investigation aimed to provide the DNA barcodes as well as the morpho-anatomical descriptions using an integrative approach in Megascolex species collected from different sites of the Western Ghats in Kerala to unveil their phylogenetic relationships on the tree of life.
3. Results
3.1. Megascolex auriculata Aiyer, 1929
Megascolex auriculata Aiyer, 1929: 64.
Material examined: Sample ID: KERL0272A4; Process ID: IEW414-17; towards south of Periyar National Park, submerged area, Mlappara, Idukki (9°28′02.6′′ N 77°15′51.8′′ E), Kerala, India; 28 October 2015; Coll. Samuel W James and Shweta Yadav; BOLD accession No: ADH2012.
Description:
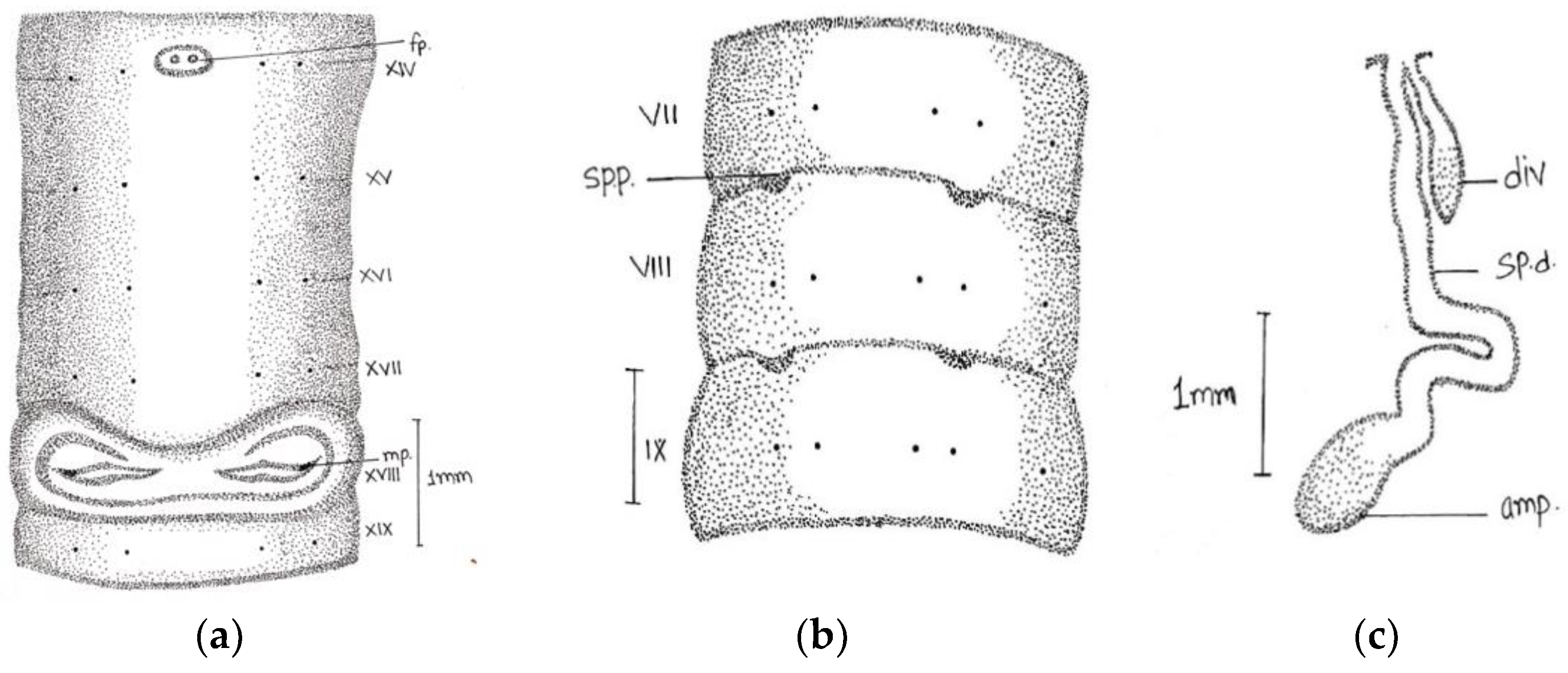
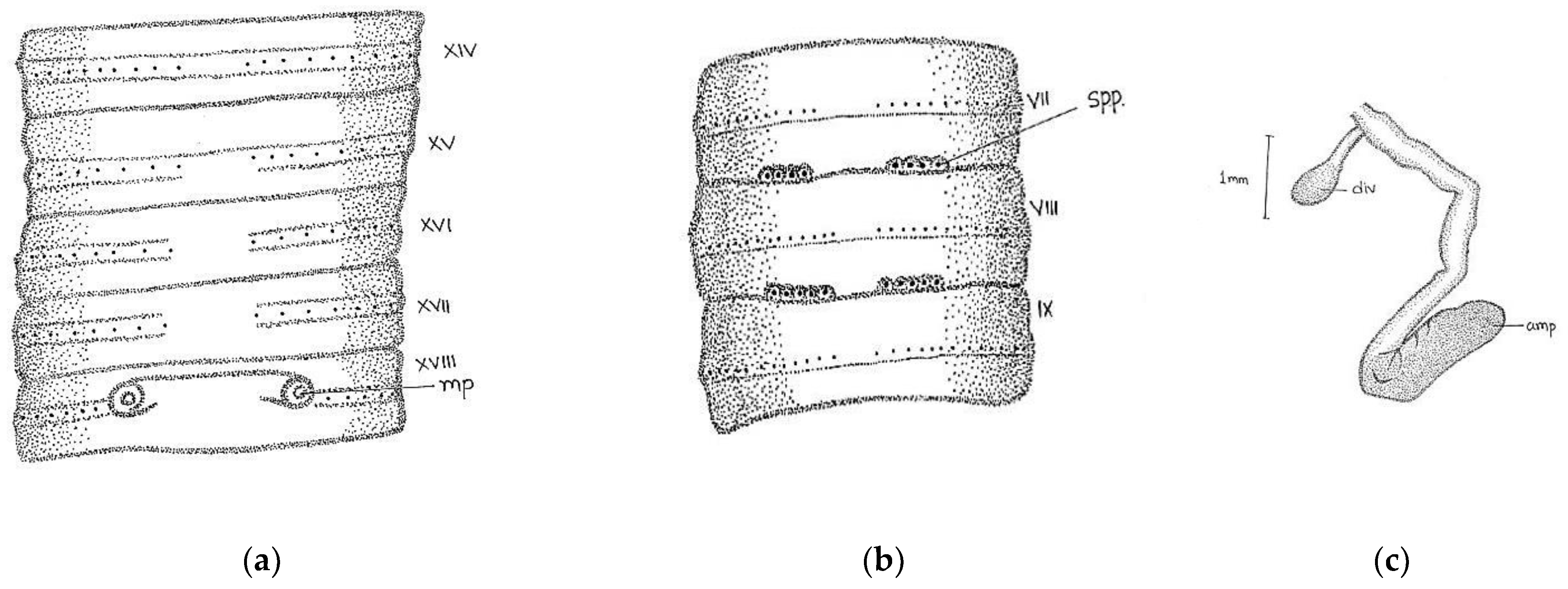
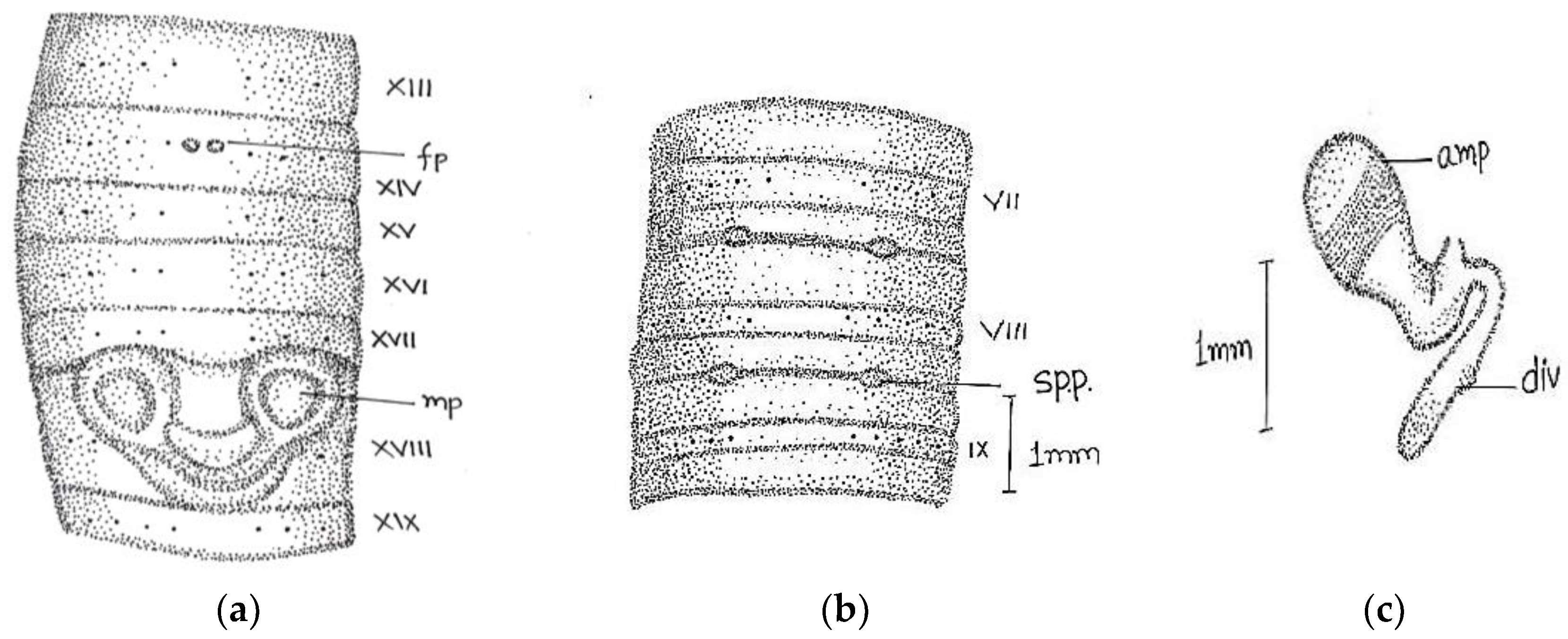
Length, 180 mm; diameter, 2 mm; segments, 318.
Prostomium epilobous. Clitellum in xiv–1/2 xvii, dark-pigmented. First dorsal pore at 9/10.
Setae lumbricine loses orientation posterior. Setae
ab closely and
cd widely paired; the distance between
ab same throughout the body, while
cd varied after lxi segment and
d moved outwardly. At the extreme lower end, setae 9 or 10 in number and setae
e very close to
d without maintaining specific orientation. On xviii, two opposite-oriented separate trenches present. Male pores bounded with pit-like depression, encircled by circular lobe-like depression. Female pores paired in xiv between
aa encircled by whitish oval circle. Spermathecal pores two pairs in 7/8 and 8/9 in lines
b (
Figure 2).
Septa 6/7–9/10 very thick and 10/11–11/12 less thickened. Large barrel-shaped gizzard in vi. Oesophageal swelling at vii-xiii with numerous villi-like structures on its inner surface. Testes and funnels single pair in segment xi. Seminal vesicles large follicular in xii. Intestine begins in xvi. Last heart in xiii. The ectal half of the duct is wider than ental. Penial setae absent. The spermathecae are in viii and ix, ampullae long club-shaped with cylindrical diverticulum. Nephridia tufts are found, with up to six segments with one-two pair tufts, 8–9 tufts with long coiled tubes in the clitellar area, and 5–6 small-sized tufts in the post-clitellar region. Prostates racemose, loosely lobed in xviii-xxii segments, duct arises from the anterior part of the gland after 3–4 coils and opens outside. The duct emerges from the anterior portion of the gland after 3–4 coils and opens outside.
Distribution: The species is endemic to Kerala. Dist. Idukki, Kumili; Vandiperiyar Kottayam; Athirampuzha [
8,
10].
3.2. Megascolex cochinensis cochinensis Stephenson, 1915
Megascolex cochinensis Stephenson, 1915: 96–97.
Megascolex cochinensis cochinensis Stephenson. Blakemore 2007: 33.
Material examined: Sample ID: KERL0265A4; Process ID: IEW378-17; near waterfall, Adimali (10°02′03.8′′N 76°56′24.0′′E), Idukki, Kerala, India; 15 September 2015; Coll. Samuel W James and Shweta Yadav; BOLD Accession No: ADH2818. Sample ID: KEL17-773-27A25; Process ID: IEW727-17; Range Mannamangalam Peechi-Vazhani Wildlife Sanctuary (10°31′40.8′′N 76°22′26.0′′E), Thrissur, Kerala, India; 1 September 2017; Coll. Shweta Yadav; BOLD Accession No: ADL2102. Sample ID: KEL17-881-33A17; Process ID: IEW835-17; Vavlla sector, Chimmini Wildlife Sanctuary (10°26′55.3′′ N 76°27′32.8′′ E), Thrissur, Kerala, India; 1 September 2017; Coll. Shweta Yadav; BOLD Accession No: ADL1460. Sample ID: KEL17-882-33A18; Process ID: IEW836-17; Vavlla sector, Chimmini Wildlife Sanctuary (10°26′55.3′′ N 76°27′32.8′′ E), Thrissur, Kerala, India; 1 September 2017; Coll. Shweta Yadav; BOLD Accession No ADL1460. Sample ID: KEL17-886-33A22; Process ID: IEW840 17; Vavlla sector, Chimmini Wildlife Sanctuary (10°26′55.3′ N 76°27′32.8′′ E) Thrissur, Kerala, India; 1 September 2017; Coll. Shweta Yadav; BOLD Accession No ADL1460.
Description:
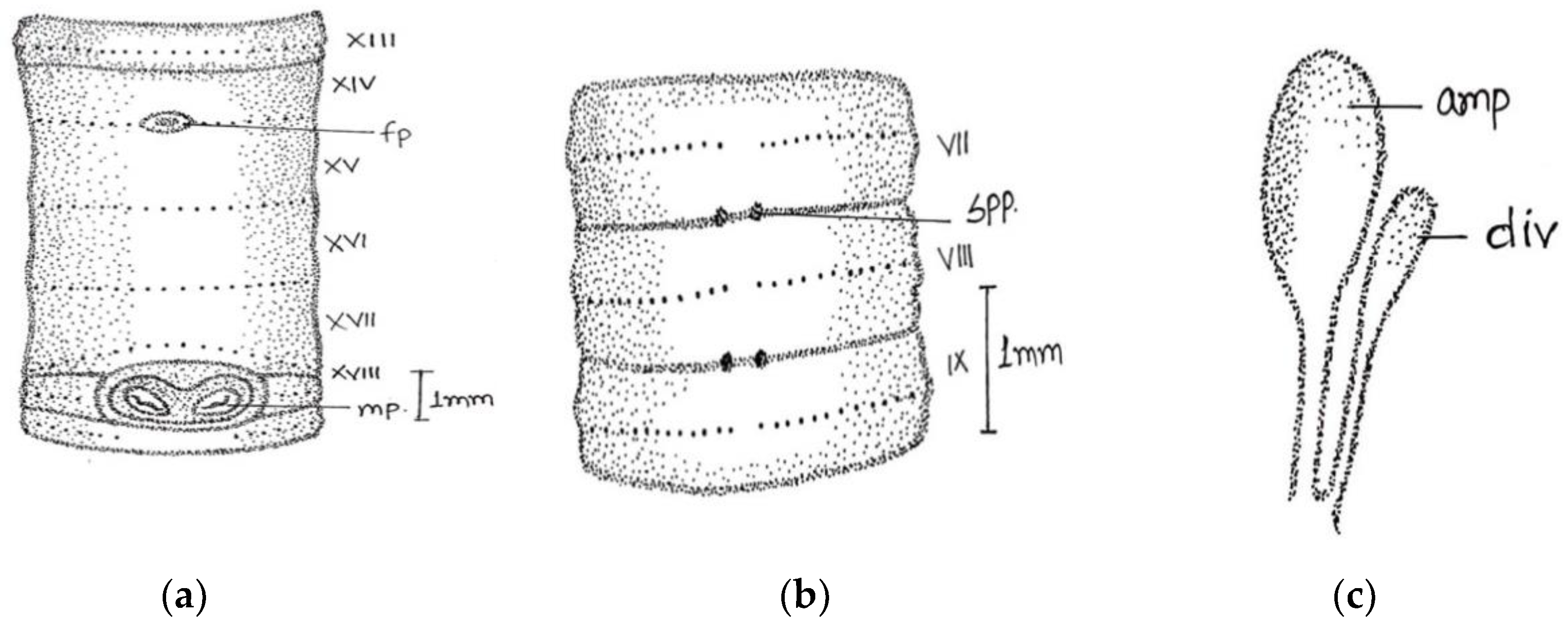
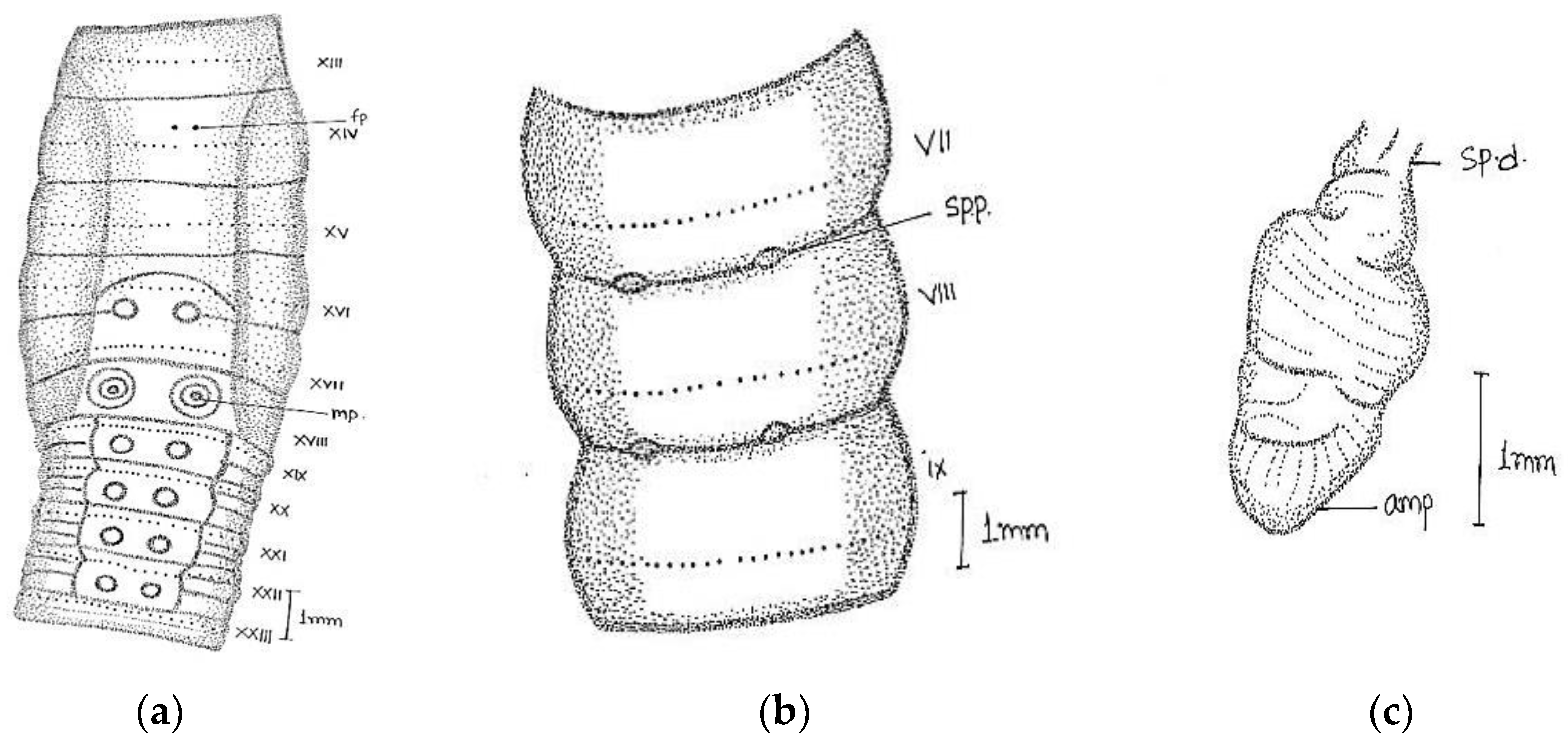
Large-sized worm; length, 190–208 mm; diameter 3–4 mm. Segments 125–180. Colour darkly pigmented in three specimens collected from same location and one non-pigmented collected from different location. Clitellum pale yellow in dark-coloured specimens extending over ½ xiii–1/3 xvii, setae present in clitellar region. Prostomium closed epilobic, first dorsal pore 5/6. Setae perichaetine, ring broken, intersetal intervals irregular, pre-clitellar
aa = 2 or 3
ab, clitellar
aa =
ab, post-clitellar
aa = 3
ab, setal counts were 46/v, 50/ix 54/xii, 42/xx, 36/xl. Male pores wavy slit-like on 18 segment, wavy slits approaching each other posteriorly within a light-coloured oval swollen area. Female pores single (in one specimen seems paired) mid-ventrally in line of setal ring in an oval whitish patch, on 14 segment extends laterally beyond
aa. Spermathecal pores two pairs in segments 7/8 and 8/9, at
a and sometimes on
a/
b or
b lines (
Figure 3).
Septa 4/5 and 5/6 are thin; 6/7–11/12 moderately thickened; 12/13–13/14 thickened. Gizzard large barrel-shaped in v. Calciferous glands absent. Intestine begins in xiv. Last hearts in xiii. Excretory system is micronephridal; large thick tufts from oesophagus to gizzard; 5–6 pairs of bushy tufts in clitellar region. Testes and male funnels free in x and xi. Seminal vesicles large in xi and xii attached to anterior septum. Prostates’ tightly packed lobules appear as a solid structure in xviii; duct thick, wide, and straight. Ovaries in xiii. Spermathecae two pairs in viii and ix, ampulla ovoid, duct long, ental part shiny. No penial setae.
Distribution: The species is near endemic and has been located in Thrissur: Forest Tramway (nr. Vazhachal); Dist. Kottayam, Athirampuzha [
8].
3.3. Megascolex filiciseta Stephenson, 1915
Megascolex filiciseta Stephenson, 1915: 94–96.
Material examined: Sample ID: KERL0272A2; Process ID: IEW412-17; towards south of Periyar National Park, submerged area, Mlappara, Idukki (9°28′02.6′′ N 77°15′51.8′′ E), Kerala, India; 28 October 2015; Coll. Samuel W James and Shweta Yadav; BOLD accession No: ADH2018.
Description:
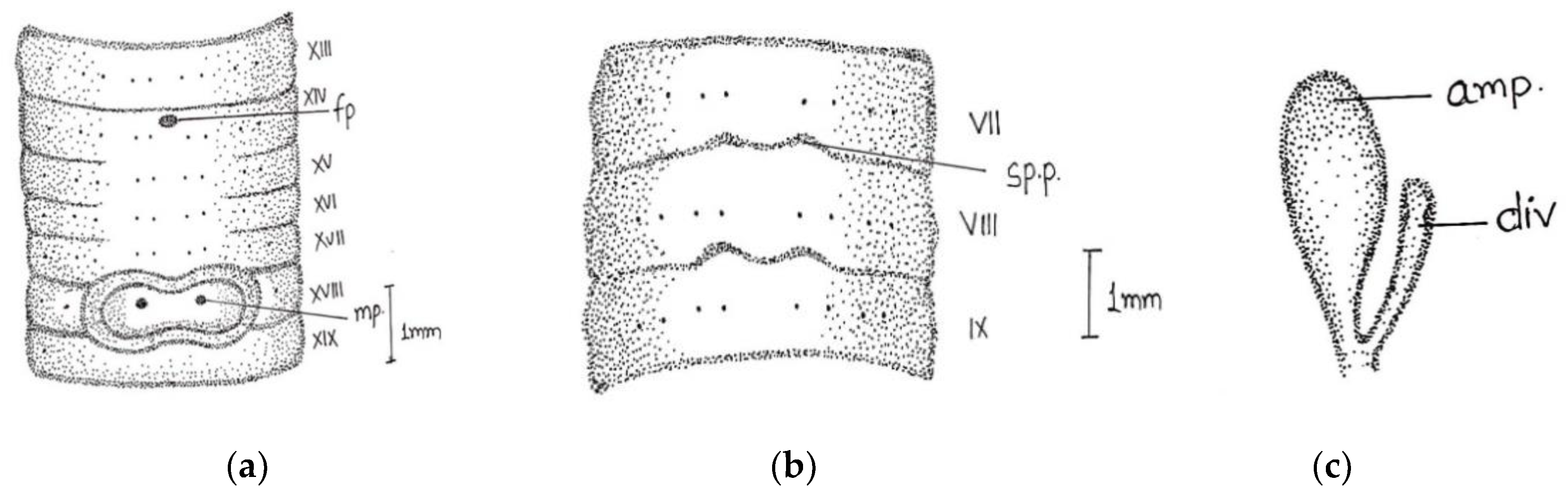
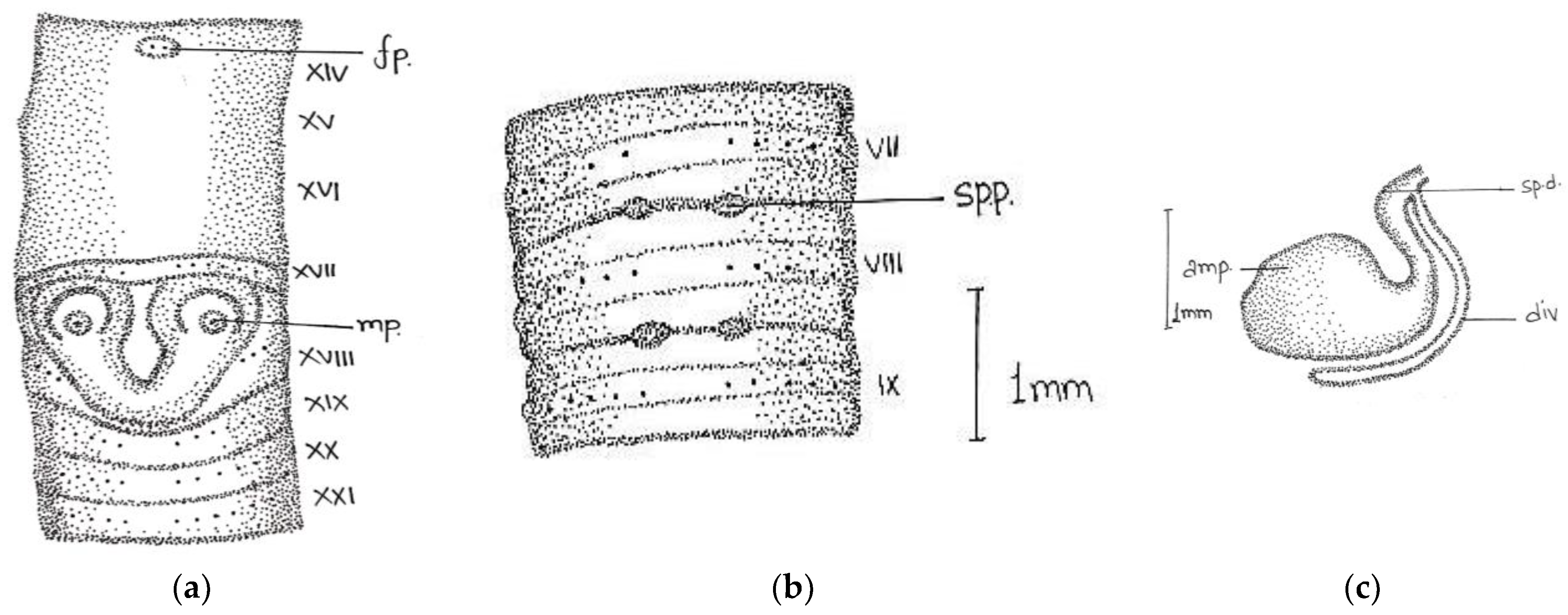
Length, 86 mm; diameter, 3 mm; segments, 140. Colour dorsally dark-bluish grey; extremely dark at posterior ends; 3–4 fine dark stripes present dorsally. Thick and stiff body wall. Clitellum not very clear; dorsally slight difference in colour observed in xiii-xvi. Prostomium epibolic marked by median groove, and major part hidden in transverse groove. First dorsal pore 5/6. In pre-clitellar region
aa = 2
ab, while distance between all setae was much reduced from clitellar region, it was difficult to observe the accurate distance between the setae; the longitudinal lines of setae broke dorsally while being ventrally distinct, numbers 28/v, 30/ix, and 22/xxv. Male pores in xviii on small porophores between
a and
b lines. Female pore was difficult to observe; appears to be in a whitish presetal ovoid patch present on xiv between
aa. Spermathecal pores minute; two pairs in 7/8 and 8/9 at
a (
Figure 4).
Septa 5/6–7/8 delicate; 8/9–13/14 relatively thickened. Gizzard large barrel-shaped in vi. Calciferous glands absent. Intestine begins in xvi. Last hearts in xiii. Excretory system micronephridial; bushy tufts present behind iv with numerous tubules emerging from the main stem; loops prominent behind xiv, not attached to septs. Testes and male funnels are free in x and xi; seminal vesicles small; lobed in ix and xii. Prostates small, flattened, and confined to xviii; duct not visible. Spermatheca two pairs in viii and ix close to ventral nerve cord; ampulla ovoid; duct short; cylindrical diverticulum almost half length of ampullae. Penial setae present; shaft bow-shaped; tapers towards the distal ends; tip slightly curved with stout teeth.
Distribution: Only known from the type locality, i.e., Palakkad: Parambikulam, Kerala [
8].
3.4. Megascolex konkanensis Fedarb, 1898
Megascolex konkanensis Fedarb, 1898: 434–436. Michaelsen 1910: 75; Stephenson 1916: 328.
Megascolex konkanensis konkanensis Fedarb. Blakemore, 2007: 34.
Material examined: Sample ID: KERL0267A4; Process ID: IEW381-17; Grassland, Parambikulam Road, Muthalamada, Palakkad (10°23′34.4′′ N 76°46′32.2′′ E), Kerala, India; 17 September 2015; Coll. Samuel W James and Shweta Yadav; BOLD accession No: ACU5977. Sample ID: KERL0267A6; Process ID: IEW383-17; Grassland, Parambikulam Road, Muthalamada, Palakkad (10°23′34.4′′ N 76°46′32.2′′ E), Kerala, India; 17 September 2015; Coll. Samuel W James and Shweta Yadav; BOLD accession No: ACU5977. Sample ID: KERL0270A5; Process ID: IEW397-17; Thenmala Reservoir, Shendurney Wildlife Sanctuary (8°53′03.7′′ N 77°10′03.4′′ E), Kollam, Kerala, India; 26 October 2015; Coll. Shweta Yadav; BOLD accession No: ACS2283. Sample ID: KERL0270A7; Process ID: IEW399-17; Thenmala Reservoir, Shendurney Wildlife Sanctuary (8°53′03.7′′ N 77°10′03.4′′ E), Kollam, Kerala, India; 26 October 2015; Coll. Shweta Yadav; BOLD accession No: ACS2283. Sample ID: KERL0271A1; Process ID: IEW411-17; Mlappara, Periyar National Park (9°27′22.6′′ N 77°13′53.8′′ E), Idukki, Kerala, India; 27 October 2015; Coll. Shweta Yadav; BOLD accession No: ACU6070.
Description:
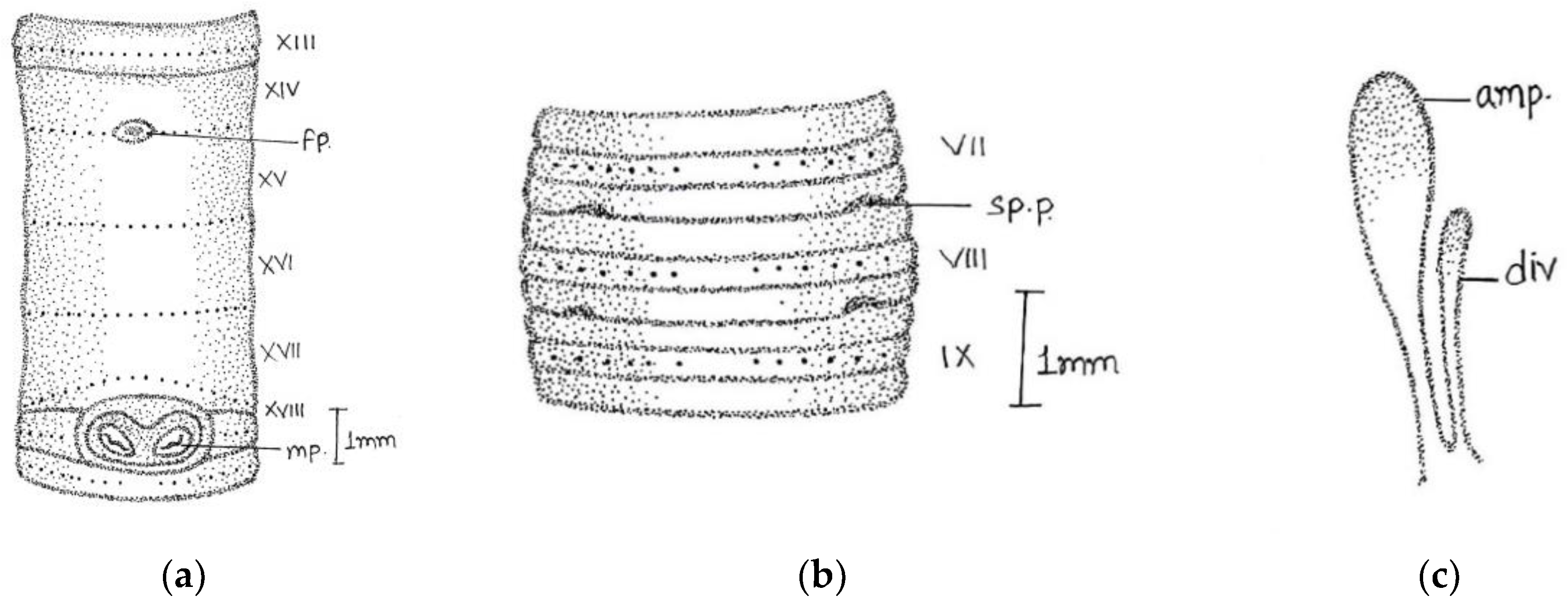
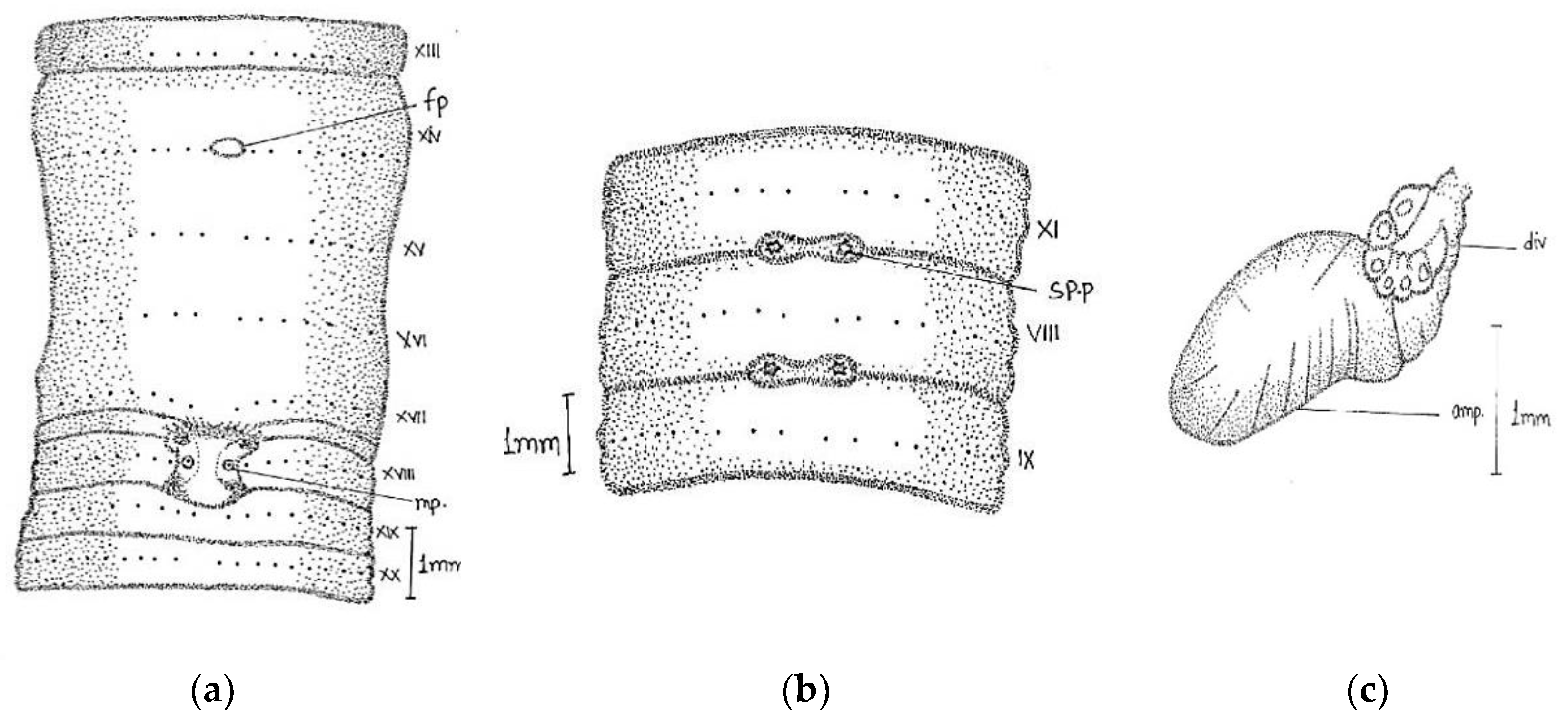
Large-sized worm; length, 170–316 mm; diameter, 4–5 mm; segments, 260–316. Colour grey with bluish irregular marks. Prostomium epilobic with two short longitudinal grooves on the dorsal surface of the first segment. First dorsal pore in 5/6. Dorsal setal gaps; before clitellum
zz equal to two times larger than
yz, while behind clitellum less than a gap
zz = 1/2
yz. Ventrally in front of clitellum
aa = 2
ab and behind
aa = 3
ab/4
ab. Setae visible from ii. The distance interval between
ab and
bc is also irregular. Setal numbers were 36/vi, 32/ix, 38/xii, and 34/xxi. Clitellum 14–17. Male pores on xviii on transverse oval papillae placed towards lateral side of the segment (the actual pore unrecognizable); papillae enlarged dumbbell-shaped; surrounded by darker area of corresponding shape; occupy xvii–ix segments; anteriorly a little close towards the middle line. Female pores minute in oval white space between
aa in xiv, while exact pore is not visible. Spermathecal pores tiny in 7/8 and 8/9 in line with setae
e (
Figure 5).
Septa 5/6–11/12 moderately thickened. Gizzard large in vi. Pharynx large with one pair mucous glands in v. Last heart in xiii. Intestine begins in xviii. Spermatheca two pairs in viii and ix; pear-shaped elongated ampullae with little small/equal-sized duct. In two specimens, the anterior part of duct as wide as ampullae. The club-shaped large diverticulum; length equal to or little larger than duct. Well-developed globular seminal vesicles in xi-xii approaching towards each other. Prostate large, mop-like, and bushy with numerous finger-shaped lobules. The duct was thick, glistening, and straight-inwardly directed, without muscular sac. Without calciferous glands and penial setae.
Distribution: The species is native peregrine to Kerala and has been reported from different regions of the country: Dist. Kozhikode: Tiruvallur (Thiruvallur), Calicut (Kozhikode); Dist. Palakkad: Chitoor (Chittur); Dist. Malappuram: Tirur, Kanjikode, Palghat (Palakkad); Dist. Ernakulam: Kalady; Dist. Thrissur: Kavalai; Dist. Kottayam: Athirampuzha, Kottayam; Dist. Kollam: Kulattupuzha (Kulathupuzha), Maddathoray (Madathara), Pathanapuram, Quilon (Kollam), Shasthancottah (Sasthamkotta); Dist. Alappuzha: Kerumaadi; Dist. Thiruvananthapuram: Trivandrum (Thiruvananthapuram); Travancore [
8].
3.5. Megascolex polytheca polytheca Stephenson, 1915
Megascolex polytheca Stephenson, 1915: 89–90.
Megascolex polytheca polytheca Stephenson. Blakemore 2007: 36.
Material examined: Sample ID: KERL0276A5; Process ID: IEW441-17; Reserve Forest, Kanan Devan Hills (10°06′39.2′′ N 77°05′28′′ E), Munnar Kerala, India; 27 October 2015, Coll. Shweta Yadav; BOLD accession No: ADH2014. Sample ID: KERL0264A5; Process ID: IEW373-17; (10°6′56.16′′ N 77°5′13.56′′ E), Eravikulam National Park, Munnar, Kerala, India; 14 September 2015, Coll. Shweta Yadav, BOLD accession No: ADH2014.
Description:
Length, 105–140 mm; diameter, 3 mm; segments, 159–194. Colour grey, slightly darker at anterior end. The anterior part of the body before clitellum relatively thick and stout with sharply demarcated segments, while post-clitellar region smoother and cylindrical. Setae on segment iii-xii are arranged on raised segmental equators. Clitellum not profoundly distinguishable; in 13–17 with visible setal rings. Prostomium epilobous with hinder end of open tongue, and a transverse groove at the front end of the tongue present. First dorsal pore at 4/5. Setae ring closed dorsally
zz = 1/2
yz and ventrally
aa = 3
ab, behind the male aperture
aa = 4
ab or 5
ab,
ab >
bc. Setae
a and
b are relatively larger than other setae. Setae
a to
e are in regular longitudinal lines. Setal numbers counted were 51/v, 52/ix, 53/xiii, 48/xx, and 46/xxxi. Male pores on large circular raised papillae, which are enclosed within biconcave depressions in xviii. The papillae are confined to xviii in one specimen, while in the other partly in xvii. A dark-coloured oval spot present at
aa in xiv, while female aperture not distinguished. Spermathecal pores 4–7 in numbers 7/8 and 8/9. On separating the lips of the groove, a row of 4–7 white dots visible on each side. These points are surrounded by a dark area and begin internally between the lines
b or
c(
Figure 6).
Septa 4/5 very delicate; 5/6 and 6/7 slightly thickened; 7/8–11/12 highly thickened. The large barrel-shaped gizzard in v. Calciferous glands absen;, oesophagus dilated deep yellow and vascular in xii. Intestine begins in xix. The last heart in xiii. Micronephridial excretory system. Spermathecae are small, circular, and 4–7 in number. Ampullae of spermathecae club-shaped organ with a long stalk with backwardly directed dilated end and more or less parallel to each other in a close set row. Male funnels in x and xi and seminal vesicles attached to anterior walls of xi and xii and made up of ovoid lobules. The ovaries are composed of finger-like lobes and break up at their free ends into strings of ova. Prostates large in xviii composed of small lobes and closely compacted together; duct short, muscular, stout, and widened near its termination.
Distribution: This species is endemic to Kerala. Thrissur: Kavalai in forest tramway [
8].
3.6. Megascolex polytheca zonatus Stephenson, 1915
Megascolex polytheca var. zonatus Stephenson, 1915: 90–91.
Megascolex polytheca zonatus Stephenson. Blakemore 2007: 36.
Material examined: Sample ID: KERL0273A3; Process ID: IEW418-17; (10°6′56.88′′N 77°5′20.4′′E), Eravikulam National Park, Kannan Devan Hills, Kerala, India; 29 October 2015, Coll. Shweta Yadav; BOLD accession No: ADH2015. Sample ID: KERL0273A4; Process ID: IEW419-17; near Agraharam resort (10°6′56.88′′ N 77°5′20.4′′ E), Eravikulam National Park, Kannan Devan Hills, Kerala, India; 29 October 2015, Coll. Shweta Yadav; BOLD accession No: ADH2015.
Description:
Length, 112–118 mm; diameter, 2.5–2.75 mm; segments, 115–122. Colour grey. The anterior part of the body before clitellum relatively thick and stout with sharply demarcated segments, while post-clitellar region relatively constant in diameter. Setae perichaetine, clitellum browner than body in 13–17 with visible setal ring. Prostomium similar to
M.p. polytheca epilobous with hinder end of the tongue open, and a transverse groove at the front end of the tongue present. First dorsal pore at 5/6. Setae ring closed dorsally
zz = 1/2
yz and ventrally
aa = 2
ab, behind the male aperture
aa = 3
ab,
ab >
bc. Setae
a and
b are relatively larger than other setae. Setae
a to
e are in regular longitudinal lines. Setal numbers counted were 48/v, 50/ix, 52/xiii, 48/xx, and 48/xxxi. Male pores on large circular in lines with setae b; raised papillae which are enclosed within biconcave depressions in xviii segment. The male region quite similar to
M.
polytheca polytheca occupies xviii. A dark-coloured oval spot present at
aa in xiv, while female aperture not distinguished. Spermathecal pores numerous in 7/8 and 8/9. On separating the lips of the groove, a row of 4–7 white dots visible on each side. These points are surrounded by a dark area and begin internally between the lines
b or
c (
Figure 7).
Spermathecae small, five in each side of spermathecal groove. Each is a club-shaped organ with a long stalk with backwardly directed dilated end and more or less parallel to each other in a close set row. Male funnels in x and xi and seminal vesicles attached to anterior walls of xi and xii and made up of ovoid lobules. The ovaries are composed of finger-like lobes and break up at their free ends into strings of ova. Prostates large in xviii composed of small lobes and closely compacted together; duct short, muscular, stout, and widened near its termination.
The internal anatomy agrees closely with M. polytheca polytheca, however, the spermathecae are relatively smaller than M. polytheca polytheca, with four spermathecae on each side of the spermathecal groove. The ampulla of the spermathecae is distinguishable from the duct, is ovoid, dark purple in colour, and can be easily differentiated from M. p. polytheca; the diverticulum is club-shaped and simple; duct is cylindrical.
Distribution: The species is endemic to Kerala; Dist. Palakkad: Parambikula [
8].
3.7. Megascolex ratus Cognetti de Martiis, 1911
Megascolex ratus Cognetti, 1911: 500–502. Michaelsen 1913: 87; Stephenson 1916: 327; Aiyer 1929: 68.
Material examined: Sample ID: KERL0268A4; Process ID: IEW387-17; Neyyar Dam and Wildlife Sanctuary (8°32′00.4′′N 77°08′53.5′′E), Thiruvanathapuram, Kerala, India; 26 October 2015; Coll. Shweta Yadav; BOLD accession No: ADH2820. Sample ID: KERL0270A1; Process ID: IEW395-17; Rosemala, Shendurney Wildlife Sanctuary (8°53′03.7′′ N 77°10′03.4′′ E), Kollam, Kerala, India; 24 October 2015; Coll. Shweta Yadav; BOLD accession No: ADH2016. Sample ID: KERL0270A9; Process ID: IEW401-17; Rosemala, Shendurney Wildlife Sanctuary (8°53′03.7′′ N 77°10′03.4′′ E), Kollam, Kerala, India; 26 October 2015; Coll. Shweta Yadav; BOLD accession No: ADH2016. Sample ID: KERL0274A1; Process ID: IEW422-17; Mannoorkara, close to Peppara Dam Wildlife Sanctuary, (8°38′35.2′′ N 77°10′50.5′′ E), Thiruvananthapuram, Kerala, India; 26 November 2015; Coll. Shweta Yadav; BOLD accession No: ADH2819. Sample ID: KERL0274A9; Process ID: IEW427-17; Mannoorkara, close to Peppara Dam Wildlife Sanctuary (8°38′35.2′′N 77°10′50.5′′E), Thiruvananthapuram, Kerala, India; 26 November 2015; Coll. Shweta Yadav; BOLD accession No: ADH2019. Sample ID: KERL0275A1; Process ID: IEW430-17; Mannoorkara, close to Neyyar Wild Life Sanctuary (8°33′00.2′′ N 77°14′33.0′′ E), Vazhichal, Kerala, India; 27 November 2015; Coll. Shweta Yadav; BOLD accession No: ADH2820.
Description:
Length, 140–350 mm; diameter, 7–8 mm; segments, 160–180. Colour dorsally violet-brown, ventrally grey. Prostomium short, broad, tanylobous, dorsally with longitudinal furrows that do not reach the posterior margin. Segments x-xiv biannulate. Setae more crowded in the ventral line than in the dorsal region, circles of setae are interrupted in the mid-ventral line of genital region where aa = 1/2 ab. There is no dorsal break, while in pre-clitellar
aa = 2
ab and behind the clitellum
aa = 3
ab. At x segment 108 setae and at xviii 130 setae. Clitellum prominent in one specimen out of seven collected specimens while setae present in entire clitellar region. Clitellum saddle-shaped occupying xiv- xviii and without intersegmental furrows. On xiv it is little extended towards xiii. Male pores on xviii, in the lines of setae
h; between the male pores the setae absent. Male pores are placed on whitish tubercles supported by swollen papillae. Paired genital markings in 17, 19–22, sometimes also in 16 and 23. Female pores in xiv presetal on
a, transversely extended. Spermathecal pores two pairs in intersegmental furrows 7/8 and 8/9 in the lines of the setae
f (
Figure 8).
Septa 7/8 and 8/9 thickened. Gizzard large in vi. Intestine begins in xiv. Last hearts in xiii. Two pair of testes in x and xi encapsulated in a large lobulated structure, which is compressed between strong septa. Prostates with strong, muscular cylindrical duct, which terminates into little folded lobular structure. Two pairs of Spermathecae in viii and ix with sac-shaped transversely striped ampullae, broader distally and rounded proximally. The duct opens into a tiny diverticulum, which is enclosed in the duct wall.
Distribution: The species is near endemic to Kerala and has been reported in Dist. Thiruvananthapuram: Bonaccord (Bonacaud, Bonakkad), Chimungi (Chemmunji), Coorloon, Mukkunni Reserve Forest, Trivandrum (Thiruvananthapuram) [
8].
3.8. Megascolex travancorensis travancorensis Michaelsen, 1910
Megascolex travancorensis Michaelsen, 1910: 72–73.
Megascolex travancorensis travancorensis Michaelsen. Blakemore 2007: 37.
Material examined: Sample ID: KERL0275A2; Process ID: IEW431-17; close to Neyyar Wild Life Sanctuary (8°33′0.216′′ N 77°14′33′′ E), Vazhichal, Kerala, India; 27 October 2017; Coll. Shweta Yadav; BOLD accession No: ADH2010. Sample ID: KERL0269A3; Process ID: IEW389-17; Peppara Wildlife Sanctuary (8°37′14.7′′ N 77°10′3.36′′ E), Thiruvananthapuram, Kerala, India; 25 October 2017; Coll. Shweta Yadav; BOLD accession No: ADH2017.
Description:
Length, 118–122 mm; diameter, 3.0–3.2 mm; segments, 182–198. Colour grey. Prostomium epilobic. Setae ii-viii enlarged and closely paired anteriorly, while onwards loses orientation, more or less in longitudinal lines. Setal counts were 12/v, 12/xiii, 16/xxii, 20 xl. Clitellum not distinct. Dorsal pores start at 4/5. Male pores in xviii on raised egg-shaped cushions in lines
b, within longitudinal curved slits in each cushion, run posterior to anterior from the base of circular rings. Female pore tiny in xiv, very hard to recognise. Spermathecal pores hard to recognise, apparently two pairs in 7/8 and 8/9 in lines
b (
Figure 9).
Septa 6/7–12/13 thickened. Gizzard in vi. Last hearts in xiii. Micronephridial system. Two pairs of testis funnels free in x and xi. Seminal vesicles small; densely packed racemose in xi and xii. Prostates fairly large, elongated, rectangular, and deeply incised with a cracked uneven surface occupying xvi-xxii; duct fairly long, shiny, thick distal part goes straight forward, and proximal part goes back to the irregular curve. Proximal part of the duct longer and covered by the glandular part. Spermathecae large, ampullae pear-shaped, dark in colour, distally narrowed, and severely bent at the opening. The duct is narrow than ampullae. A slender club-shaped distally somewhat bent diverticulum present. No penial setae.
Distribution: The species is endemic to Kerala and reported so far from Dist. Kollam: Kottarakkara, Kulathupuzha, Dist.Thiruvananthapuram: Killipalam, Pallode (Palode) [
8].
3.9. Megascolex papparensis sp. nov.
LSIDurn:lsid:zoobank.org:act:BC2E4221-2984-4697-BC73-EEF09894E051
Holotype: Clitellate specimen (Sample ID: KERL0274A14; Process ID: IEW366-17); registration number: ZSI CZRC T/21; BOLD accession No: ADH2299, Mannoorkara, close to Peppara Dam and Wildlife Sanctuary (8°38′35.2′′ N 77°10′50.5′′ E), Thiruvananthapuram, Kerala, India; 26 November 2017, Coll. Shweta Yadav.
Paratypes: Clitellate specimen (Sample ID: KERL0274A14-1; Process ID: IEW366-17); registration number: DHSGV-ZDM-272015-013; BOLD accession number: ADH2299, Mannoorkara, close to Peppara Dam and Wildlife Sanctuary (8°38′35.2′′ N 77°10′50.5′′ E), Thiruvananthapuram, Kerala, India; 26 November 2017, Coll. Shweta Yadav.
Clitellate specimen (Sample ID: KERL0274A14-2; Process ID: IEW366-17); registration number: DHSGV-ZDM-272015-014; BOLD accession No: ADH2299; collection site similar to other paratypes.
Description:
Length, 85 mm; diameter, 3 mm; segments, 196. Prostomium epiobic with raised growth. Colour light grey. Setae paired in longitudinal lines
aa = 2
ab = 3
ab in pre-clitellar region,
aa = 1.5
ab in post-clitellar region, while setae absent in clitellar region. Setal counts were 12/v, 17/xii, 20/xx, and 21/xxx. Clitellum dark-brown coloured in 13–17. Dorsal pores start at 5/6. Male pores ca ½ circumference apart in a groove in xviii on oval glandular papillae, both papillae united by v-shaped groove at the distal end. The male region is slightly extended, sometimes appears in xix, while on deep observation it remains in xviii and touches the boundary of xix. Female pores paired in light-coloured oval patch in
aa. Spermathecal pores two pairs in 7/8 and 8/9 in
a lines (
Figure 10).
Septa 6/7–12/13 thickened. Gizzard large in vi. Last hearts in xiii. Funnels free in xi and xii. Seminal vesicles compact in xi and xii. Prostate large, occupies xviii-xxiv segments; duct proximally very thin and curved. Spermathecae ampulla oval and slightly bent at anterior end; duct long and slightly bent; diverticulum shorter than ampulla.
Etymology: “papparensis” is derived from its type of habitation, Peppara Dam, Kerala.
Remarks: Particularly in terms of the prostatic duct, this species is similar to
Megascolex travancorensis var.
quilonensis [
44]. However, the structure of spermathecae varies, especially in diverticulum length.
Variations: The main variation observed between holotype and paratype was the size of the prostate. In the holotype, the prostate covered six segments (xviii–xxiv) and in the paratypes it occupied eight segments, xvii-xxiv and xviii-xxv, respectively. Further, the colour of the clitellum in the holotype was darker as compared to paratypes, and in the holotype the clitellum occupied xiii-xvii segments and in paratypes it occupied xiii ½, xvi ½, or xvii segments.
3.10. Megascolex triangularis Stephenson, 1925
Megascolex triangularis Stephenson, 1925: 56–57.
Material examined: Sample ID: KERL0264A6; Process ID: IEW374-17; Reserve Forest (10°06′56.2′′ N 77°05′13.6′′ E), Kannan Devan Hills, Kerala, India; 29 October 2017; Coll. Shweta Yadav; BOLD accession No: ADH2301; Sample ID: KERL0273A1; Process ID: IEW416-17; Reserve Forest (10°06′56.9′′ N 77°05′20.4′′ E), Kannan Devan Hills, Kerala, India; 29 October 2017; Coll. Shweta Yadav; BOLD accession No: ADH2301.
Description:
Length, 138–150 mm; diameter, 5–6 mm; segments, 190–210. Dorsal pores start in 6/7. Clitellum 14–17. Prostomium? Colour dark brownish. Setae more than 50 per segment. Male pores paired, slit-like structure, situated at
a in centre of the curved grooves, which are concave laterally and span 17/18 to 18/19. Female pores in 14th in whitish oval patch. Spermathecal pores two pairs in 7/8 and 8/9 at
ab lines (
Figure 11).
Septa 5/6–11/12 thickened. Gizzard large in v. Intestine begins in iv. No calciferous glands present. Male funnels in 10 and 11. Seminal vesicles in 11 and 13. Last heart in 13. Prostate racemose in 18; no prostatic duct seen. Spermathecae ampulla large ovate; duct short and wide without any demarcation; diverticula multiloculate. Penial setae absent.
Distribution: The species is known from the original description; Dist. Thrissur: Kavalai, Kerala [
8].
3.11. Megascolex vazhichlensis sp. nov.
LSID. urn:lsid:zoobank.org:act:7C257761-1B02-4100-A738-9C39A2D5B857
Material examined: Holotype Clitellate specimen (Sample ID: KERL0275A3; Process ID: IEW449-17); registration number: ZSI CZRC T/22; BOLD accession No: ADH2821. Forest land (8°33′00.2′′ N 77°14′33.0′′ E), Vazhichal Kerala, India; 27 November 2017, Coll. Shweta Yadav.
Description:
Length, 175 mm; diameter, 3 mm; colour mild grey; segments, 256; secondary annulation in vii, viii, and ix. The anterior end is truncated (not tapering); prostomium is small and triangular, the pointed posterior angle directed upwards. First dorsal pore in 4/5. In pre-clitellar region
aa > 2
ab; in clitellar region
aa = 1/2
ab = 2
ab. The ventral setae are in definite longitudinal lines; setae of viii and ix are relatively small. The dorsal setae are in irregular intervals
zz = 4–5
yz. The numbers were 16/v,18/xi, 21/xx, and 30/xxx. Setae at irregular intervals in post-clitellar region with smaller dorsal break in posterior region
ca zz = 3
yz. Clitellum ½ xiii–xvi. On segment xviii, two projected U-shaped grooves connecting two male pore papillae. The two grooves are united horizontally at the base. It is difficult to observe exact position of male aperture, apparently in lines of
b and
c. The female pores (?) paired represented by two separate white spots between
aa. The spermathecal apertures are small in 7/8 and 8/9 in lines of setae
b, while slits laterally extended (
Figure 12).
Septa 5/6 very thin; 6/7–10/11 relatively thickened; from 13/14 considerably thickened. Gizzard large barrel-shaped in v. Intestine begins in xvi. The last hearts in segment xiii. The micronephridia are present as large tufts from v to ix; thick covering of micro nephridia present in clitellar region and behind the prostates. Testes and funnels are free in x and xi. Seminal vesicles composed of lobules, attached to the anterior walls of segments xi and xii. Those in xi are small and those in xii are moderate in size. Prostates are flat, occupy xvi-ix; duct long, thick, curved, shining, and backwardly directed. Ovaries are not visible. Spermathecae are sausage-shaped, bent towards their free end, and dilated at the extremity. The duct is short, moderately stout, half as thick as the ampulla, single posteriorly curved elongated and club-shaped; shining diverticulum present.
Etymology: “vazhichlensis” is derived from its type of habitation, the Vazhichal Kerala.
Remarks: The species resembles Megascolex papparensis sp. nov., particularly in the male region, where the prostate of M. vazhichlensis sp. nov occupies four segments (xvi–xix) with a consistently thickened duct. A large-sized prostate (xviii-xxiv) with a thin curved ental end are characteristic of Megascolex papparensis sp. Further, spermathecae of Megascolex papparensis sp. nov. have oval-shaped and slightly bent ampullae and M. vazhichlensis sp. nov. have club-shaped ampullae.
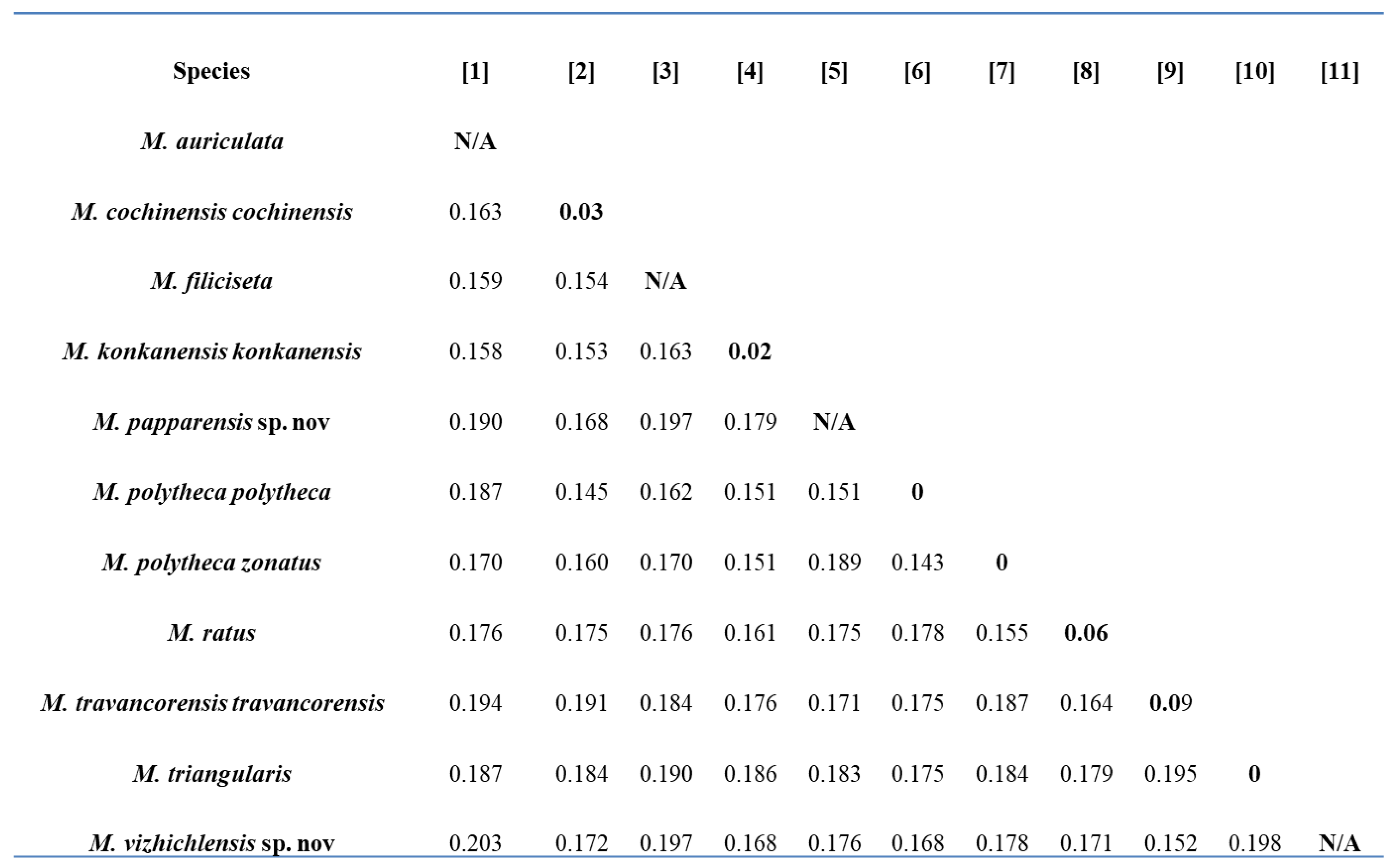
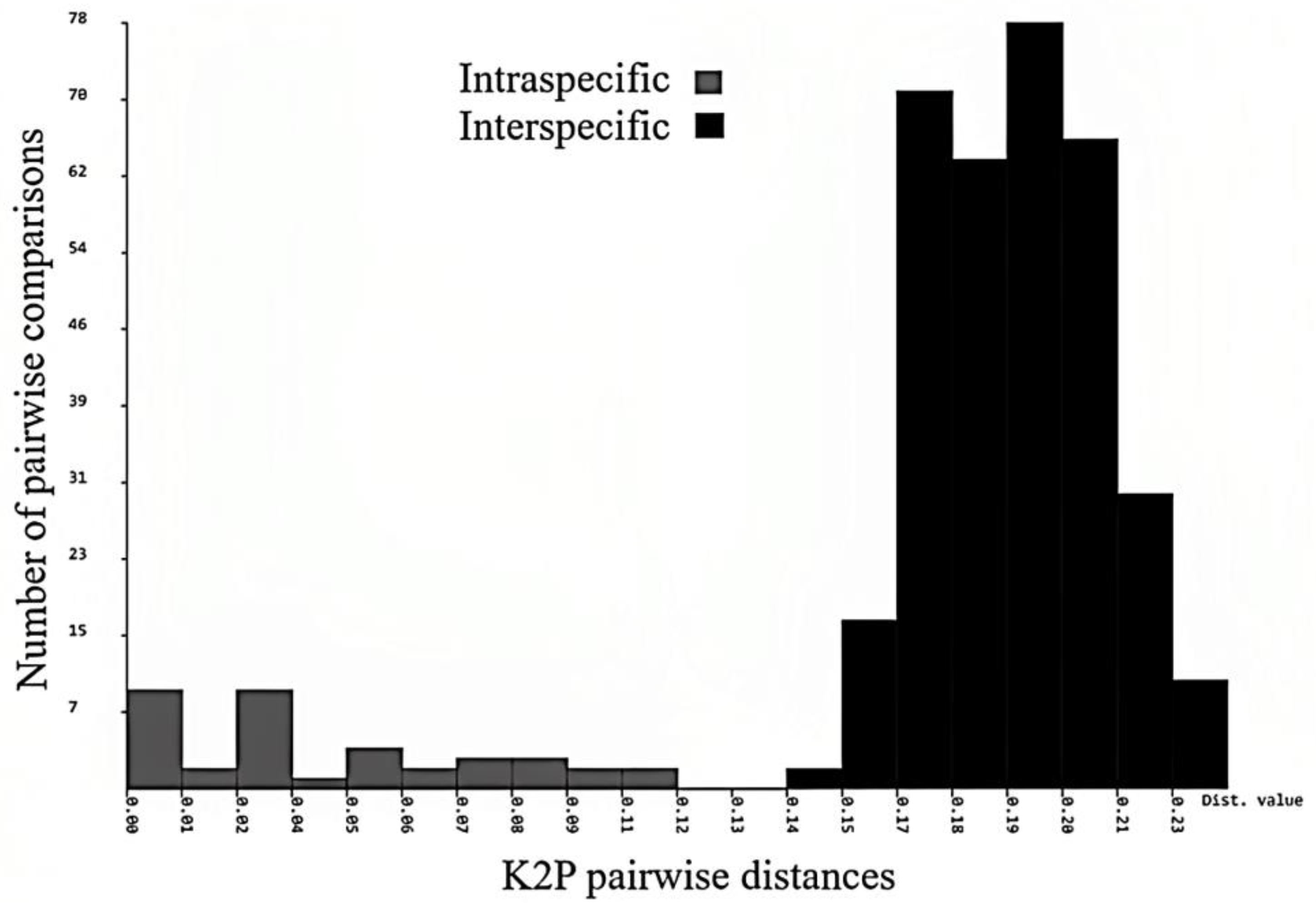
3.12. Intraspecific and Interspecific Genetic Distances
The mean nucleotide sequence composition was A = 28.6; T = 30.4; G = 18.6; and C = 22.4 with GC% = 40.04, GC% at codon 1 = 53.25, GC% at codon 2 = 42.26, and GC% at codon 3 = 24.80, which has been reported in many earthworm species [
13,
45]. The values of uncorrected pairwise genetic distances are provided in
Figure 13. In
Megascolex species, the mean intraspecific distance recorded was 1.232% and the minimum interspecific distance recorded was 15.18%. The minimum interspecific distance recorded was between
M. polytheca polytheca and
M. polytheca zonatus (14.3%) and between
M. polytheca polytheca and
M. cochinensis cochinensis (14.5%). Contrary to this, the maximum interspecific distance was found between
M. vazhichlensis sp. nov and
M. auriculta (20.3%) and between
M. vazhichlensis sp. nov and
M. triangularis (19.8%), respectively. Additionally, we recorded highest intraspecific genetic distance in
M. travancorensis travancorensis (9.0%), followed by
M. ratus (6.0%). The result of the cluster sequencing analysis indicated no overlapping interactions between genetic distances among
Megascolex species (
Table 2).
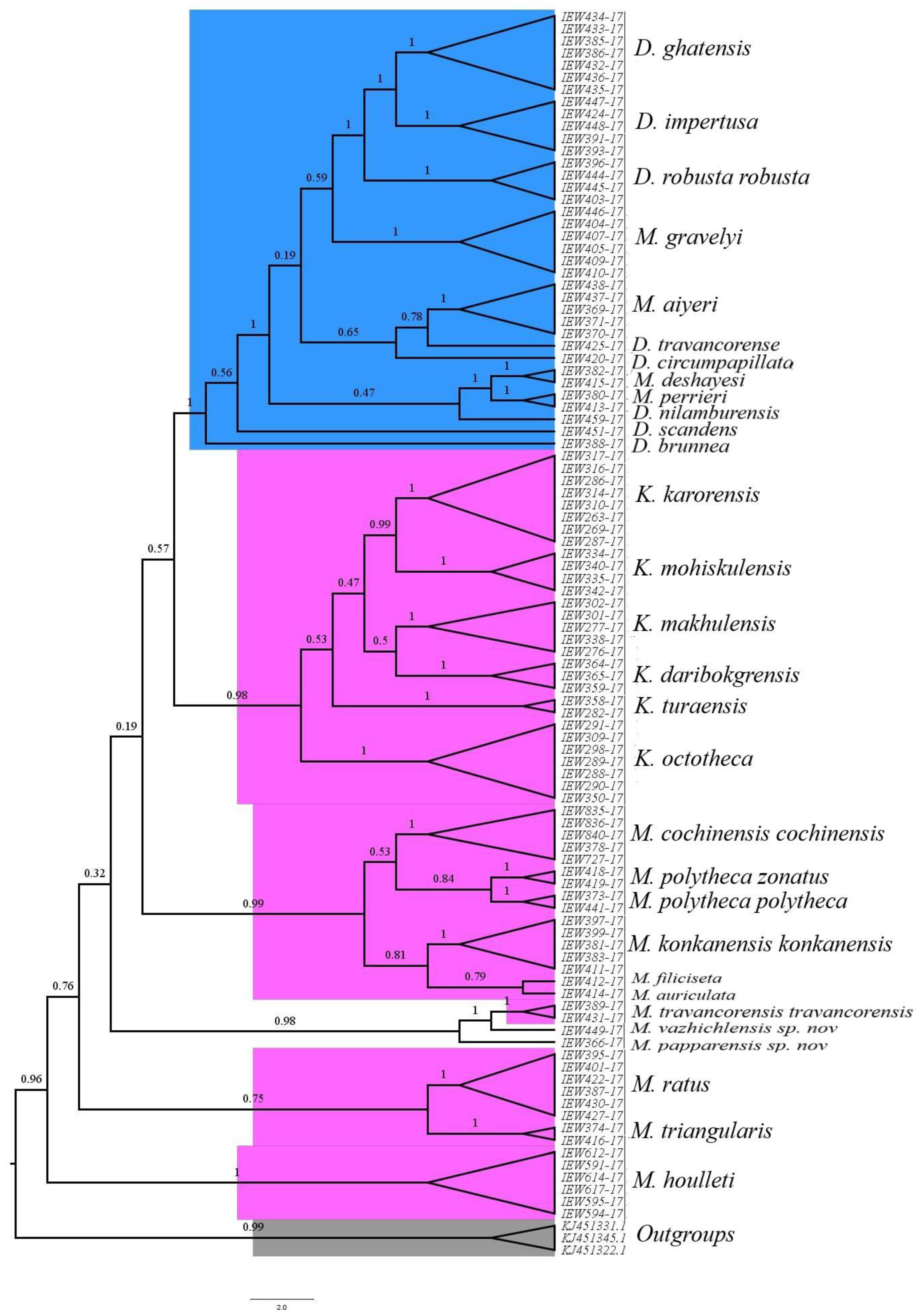
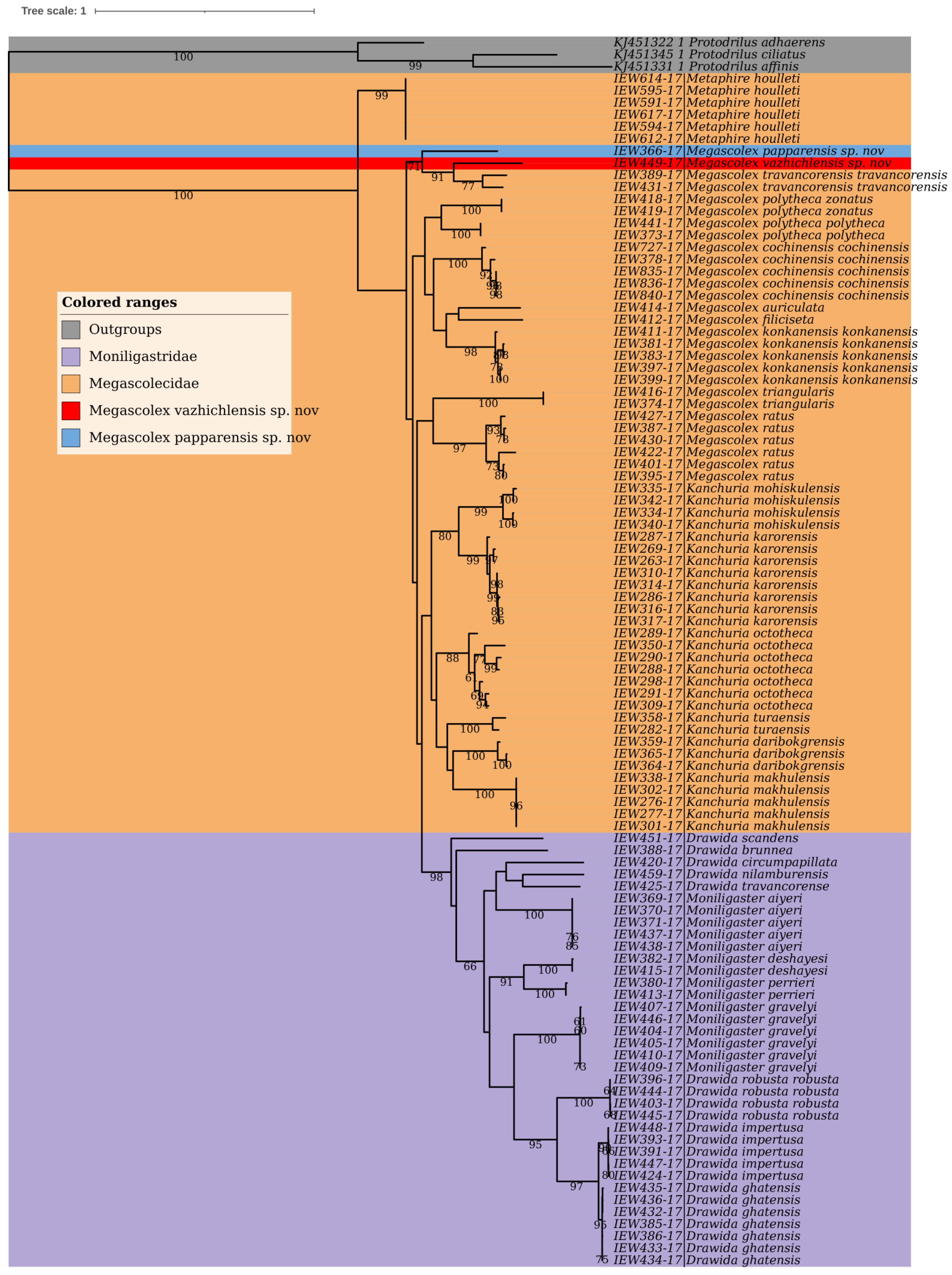
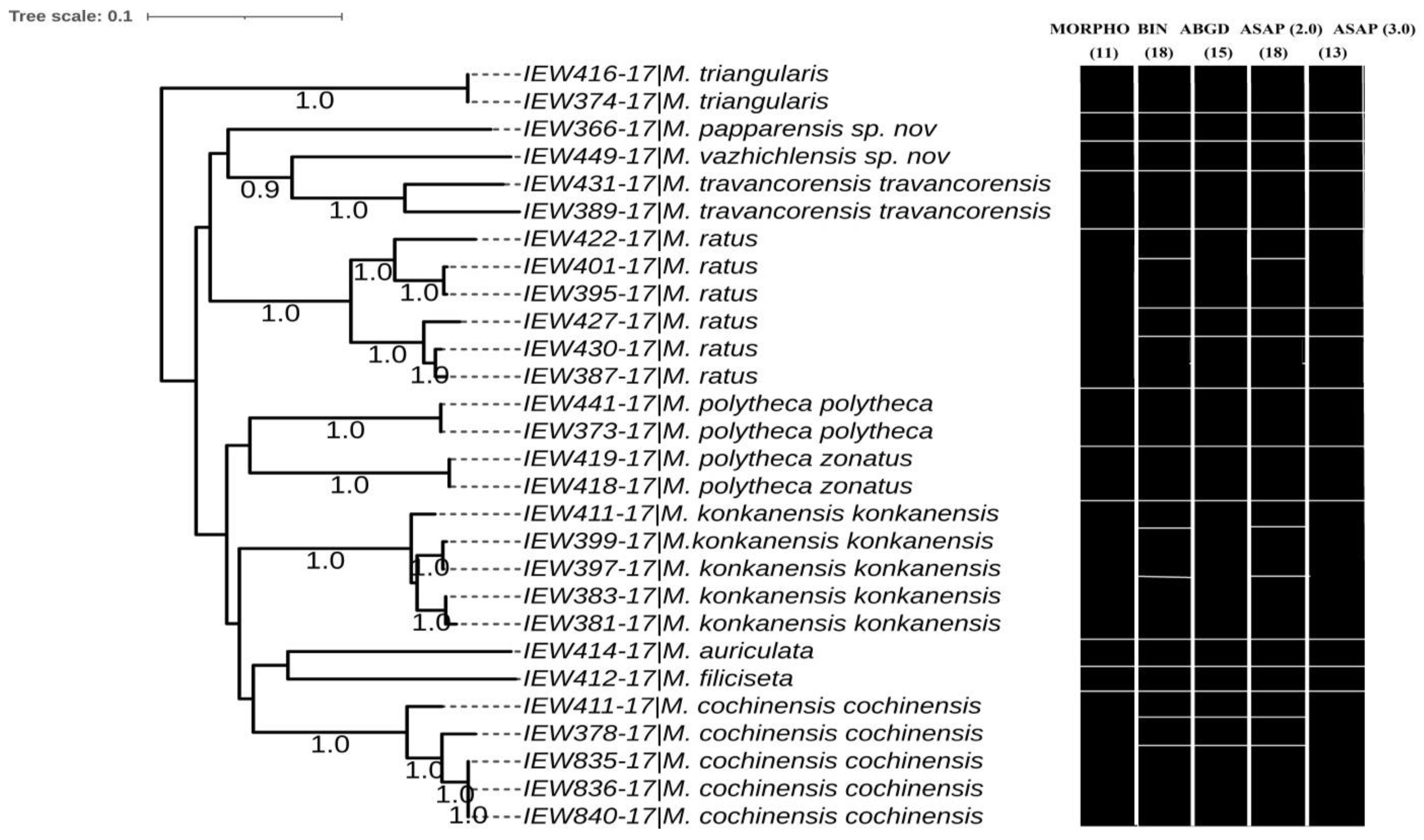
3.13. Phylogenetic Studies and Species Delimitation
The phylogenetic studies based on the COI dataset fell into distinct clusters in BI (
Figure 14) and ML trees (
Figure 15). Except for the species represented by single COI sequences, the phylogenetic analysis shows full support for each taxon as they were fully recovered on both BI and ML trees with strong supports. Moreover, there were minor differences in topologies of BI and ML trees. Furthermore, the three species delimitation methods produced different OTUs (
Figure 16). The ABGD method found 15 OTUs, while ASAP analysis at 2.0 and 3.0 ASAP scores gave 18 and 13 OTUs, respectively. The BIN analysis also revealed 18 OTUs. Additionally, the ABGD depicted a clear barcode gap of 12–14% within
Megascolex species (
Figure 17).
4. Discussion
In the present investigation, the collected species from the Kerala part of the Western Ghats were discriminated into nine species, namely,
Megascolex auriculata,
M. cochinensis cochinensis,
M. filiciseta,
M. ratus,
M. travancorensis travancorensis,
M. triangularis,
M. konkanensis konkanensis,
M. polytheca polytheca, and
M. polytheca zonatus, based on their morpho-anatomical observations. In addition, two new species were added to the genus, i.e.,
M. papparensis sp. nov. and
M. vazhichlensis sp. nov. This segregation was further confirmed based on the DNA barcoding approach. In DNA barcoding, the reliability depends on a clear discontinuity between values of intraspecific and interspecific genetic divergences. In our report, the ABGD reflects the clear and no overlap barcode gap of 12–14%, which supports the validation and accuracy of DNA barcoding in the delimitation of
Megascolex species. Moreover, in DNA barcoding, the species were considered distinct if their maximum intraspecific distances were less than the distances to their nearest neighbours (NN). In our cluster sequencing report, the maximum intraspecific genetic distance in each species was less than the distance to their nearest neighbour. Furthermore, based on such a limited COI dataset, certain conclusions were inferred. Firstly, looking at the family levels in both BI and ML phylogenetic trees, the species of the family Moniligastridae formed a distinct monophyletic clade in both phylogenetic trees (BI and ML), within a phylogenetic
Megascolecidae (a monophyletic family of Crassiclitellata), to which the studied
Megascolex species belongs. These results are not in accordance with the findings of James et al [
46], which showed that Moniligastridae is the sister taxon to the Crassiclitellata. Secondly, the genus
Megascolex appeared paraphyletic as revealed by BI and ML phylogenetic trees. On the BI tree, three clades were found with good posterior probability supports. Clade one with a high posterior probability of 0.98 was composed of
M. polytheca polytheca,
M. polytheca zonatus,
M. konkanensis konkanensis,
M. cochinensis cochinensis,
M. filiciseta, and
M. auriculata, respectively. Subsequently, the second clade with a good posterior probability value of 0.75 was formed by
M. travancorensis travancorensis,
M. ratus, and
M. triangularis species. The last clade consisted of
M.
travancorensis travancorensis,
M. papparensis sp. nov., and
M. vazhichlensis sp. nov with a higher posterior probability value of 0.99. Although similar clades were observed on the ML tree, only the clade formed by
M.
travancorensis travancorensis,
M. papparensis sp. nov., and
M. vazhichlensis sp. nov show good clade support of 75, whereas the other two clades show weak supports. Additionally, in a phylogenetic tree study, a model of short internal and long external branches was detected in species, namely,
M. ratus and
M.
travancorensis travancorensis, which is due to the high genetic divergence commonly found in some earthworm species. We followed three SD approaches to delimit species, and the results show incongruence in OTUs (BIN (18), ABGD (15), ASAP 2.0 (18), and ASAP 3.0 (13)). All the approaches show congruence in terms of the number of OTUs for species
M. triangularis,
M. polytheca polytheca, and
M. polytheca zonatus, respectively. Contrary to this,
M. travancorensis travancorensis was split by BIN, ASAP 2.0, and ASAP 3.0 into putative species, although it was merged by ABGD analysis. Similarly, in
M. cochinensis cochinensis,
M. konkanensis konkanensis, and
M. ratus, the BIN, ASAP 2.0, and ASAP 3.0 split the individuals of these species into putative species. Generally, the incongruence in OTUs was seen in species of
M.cochinensis cochinensis,
M. konkanensis konkanensis, and
M. ratus with high intraspecific genetic divergence. Moreover, the high intraspecific divergence in
M. travancorensis travancorensis,
M. cochinensis cochinensis,
M. konkanensis konkanensis, and
M. ratus was retained in each of them as single species due to their lesser intraspecific distance compared to their nearest neighbours (NN).
The high divergence in
M. cochinensis cochinensis could be explained in that some of the individuals of this species were collected from Chimmini Wild Life Sanctuary, Kerala, and a few others were taken from Peechi-Vazhani Wildlife Sanctuary and Adimali Idukki Waterfall, respectively, with an average distance of 100 km and varied habitats. Such variations in habitats may perhaps induce selection [
47], and, due to poor dispersal capability [
48], they show large intraspecific divergence. A similar case is with
M. konkanensis konkanensis and
M. ratus, in which the individuals of these species differed in the habitats where they had been collected. Moreover, owing to a long evolutionary history [
49], a direct effect of selection forces the soil-dwelling invertebrates to evolve to survive in specific habitats and stabilizing selection may perhaps select forms that diverge from the morphological optimum [
47]. Conversely, the high genetic divergence found in earthworms can be explained by poor dispersal capability as they travel only limited distances/year [
48], excluding the occurrence of passive dispersal by waterways or vertebrate predators [
50]. This low dispersal ability is generally reflected as isolation by distance flow, where the genetic segregation is extremely linked to the geographical distance of the species.
The taxa which occur naturally, occupy limited geographical ranges, and are restricted to a specific geographical region are termed endemic species [
51]. India is known to host diverse endemic fauna and flora because of its two major hotspots, namely the Himalayas and the Western Ghats. The Western Ghats mountain range located in the southwestern portion of India along with Sri Lanka is known for its biodiversity hotspots [
52,
53] and contains several endemic species. The level of endemism in these regions is quite common among various taxa such as fishes, land snails, trees, amphibians, odonates, and reptiles [
54,
55]. This sort of endemism is also true for earthworms that exceptionally show a high level of endemism (71.6% in Sri Lanka and 77% in the Western Ghats) as reported by Narayanan et al. [
6,
56]. The
Megascolex species with their origins in Gondwanaland are ancient lineages with 36 and 25 species [
9] in Sri Lanka and Kerala, a part of Western Ghats, respectively. It is noteworthy that the earthworm fauna of Sri Lanka possesses a close relation to the Western Ghats mountains of India, especially in the Kerala state [
8]. Given the Indian mainland and the present Sri Lanka island were together before the breakup of the Gondwana land [
57], comparative studies may highlight an understanding of the evolutionary history of
Megascolex species, which could be achieved with the supplementations of their molecular data. Since the high rate of endemism is of immense bio-geographical significance nonetheless, the endemism of species is predominantly influenced by poor regional survey and taxonomic impediments and, therefore, the status of given species may alter with its distribution range and expansion. In such cases, the present study serves as a barcode reference library to infer the study of their molecular taxonomy and phylogenetic relationships. Moreover, the integrative methods are essential to work on the species that are considered difficult to discriminate exclusively on taxonomical features alone. Therefore, involving integrative methods not only provides species delimitations but also deciphers their molecular phylogeny, evolution, and population ecology. Such studies have already begun in Southeast Asian countries [
58,
59], America [
60,
61], and Europe [
62,
63], which has opened new avenues in the fields of their ecology, conservation, and sustainable development. Therefore, to achieve such objectives, there is an urgent demand for molecular data on Indian earthworms that are not only diverse in the country but also endemic to the sub-continent.