Bithyniid Abundance in the South of Western Siberia Water-Courses and Water Reservoirs (Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mollusk Sampling and Examination
3. Results
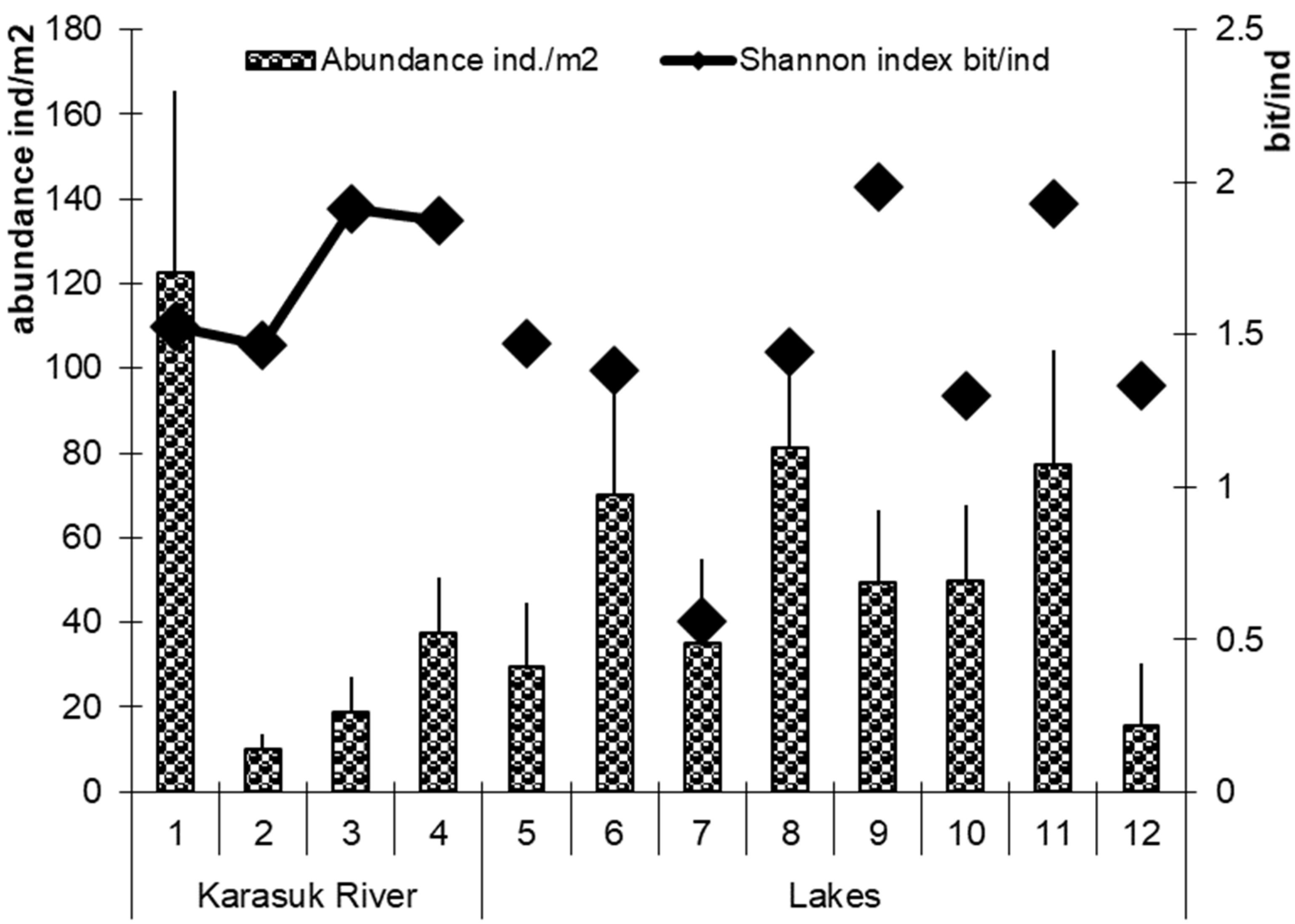
3.1. Diversity of Gastropods in Water Bodies of Northern Kulunda
3.2. Bithyniid Abundance in the Four Major Basins Situated in the Novosibirsk Region
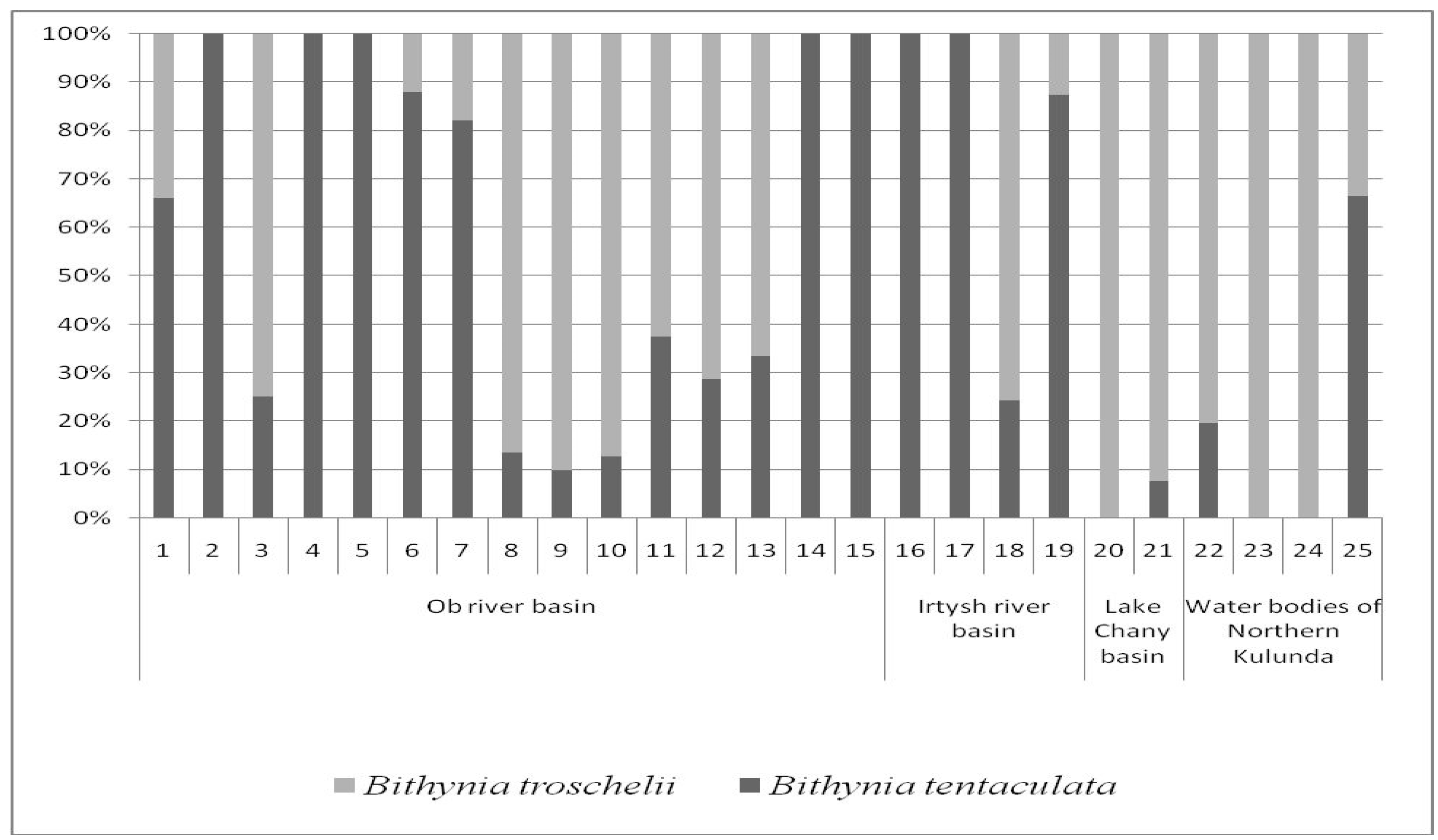
3.2.1. Ob River Basin
3.2.2. Irtysh River Basin
3.2.3. Lake Chany Basin
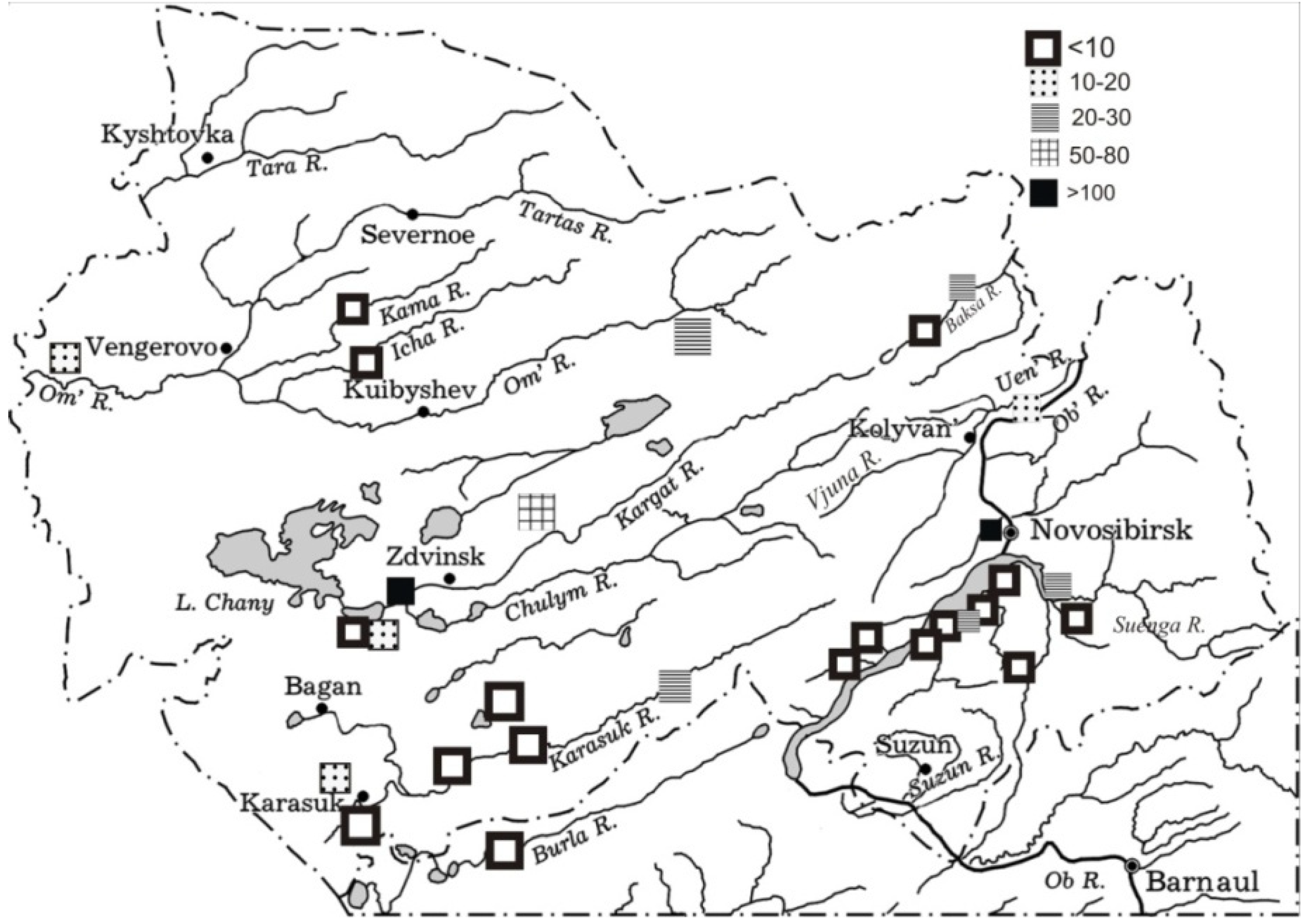
3.2.4. Water Bodies of Northern Kulunda
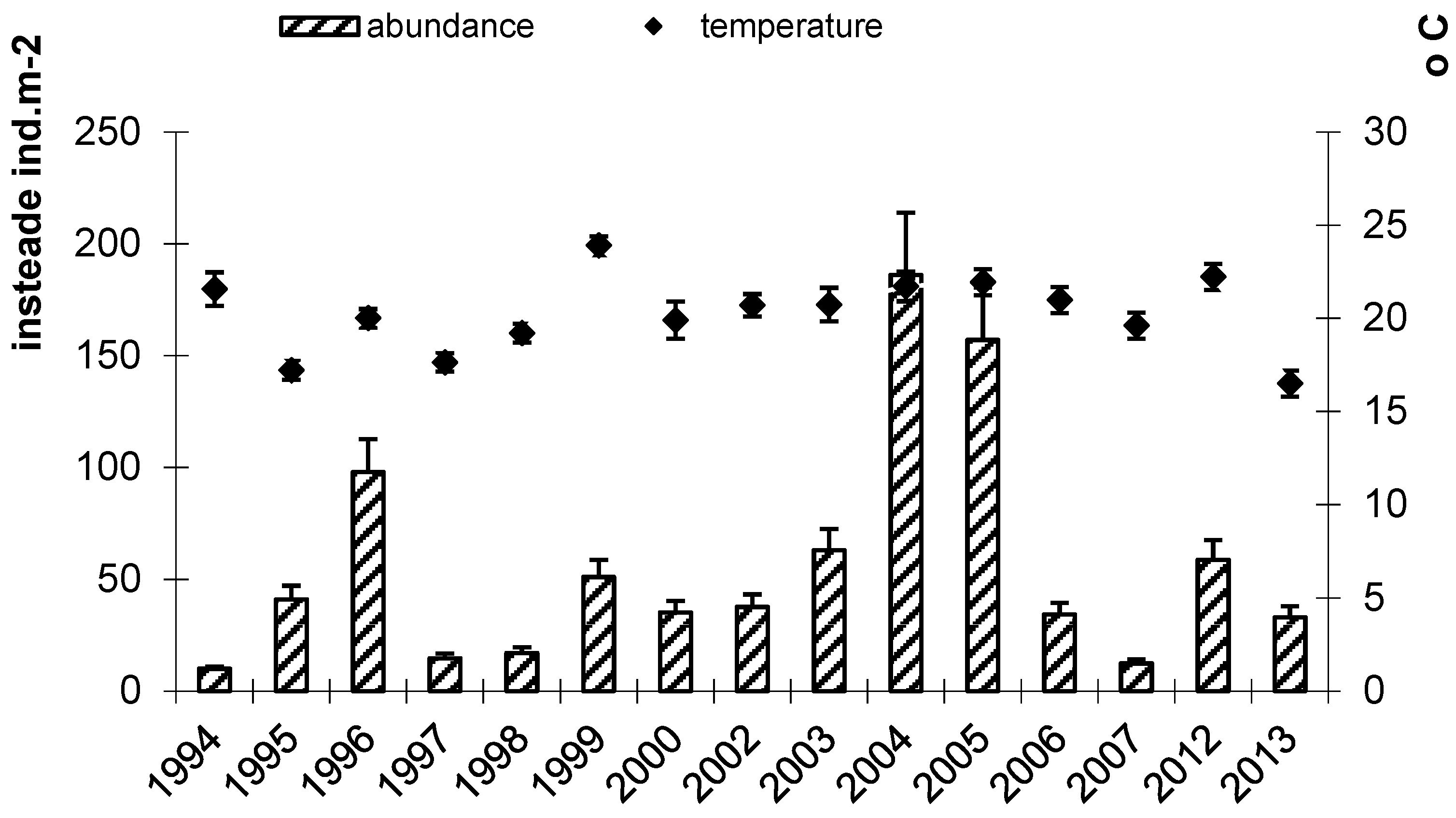
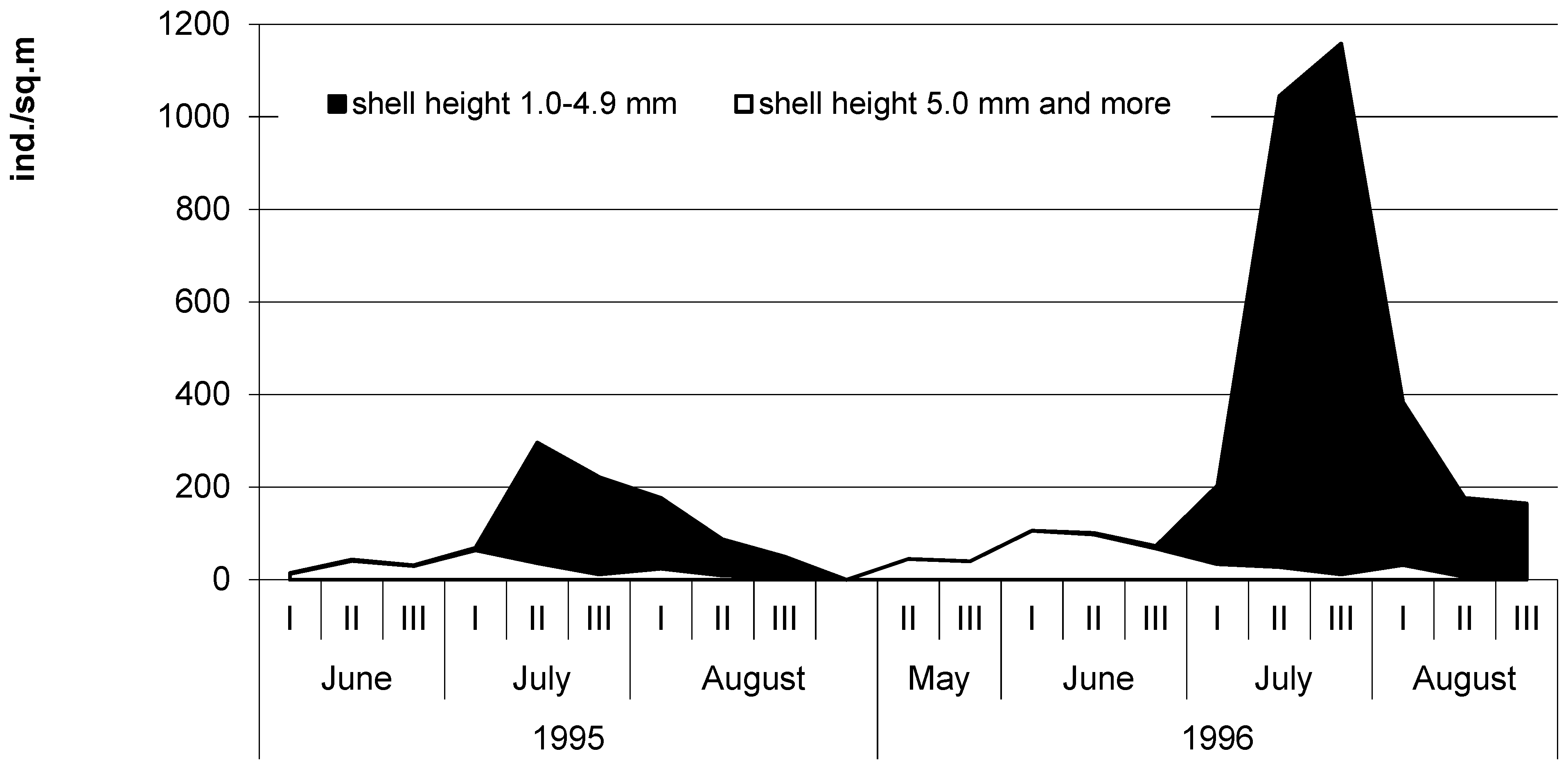
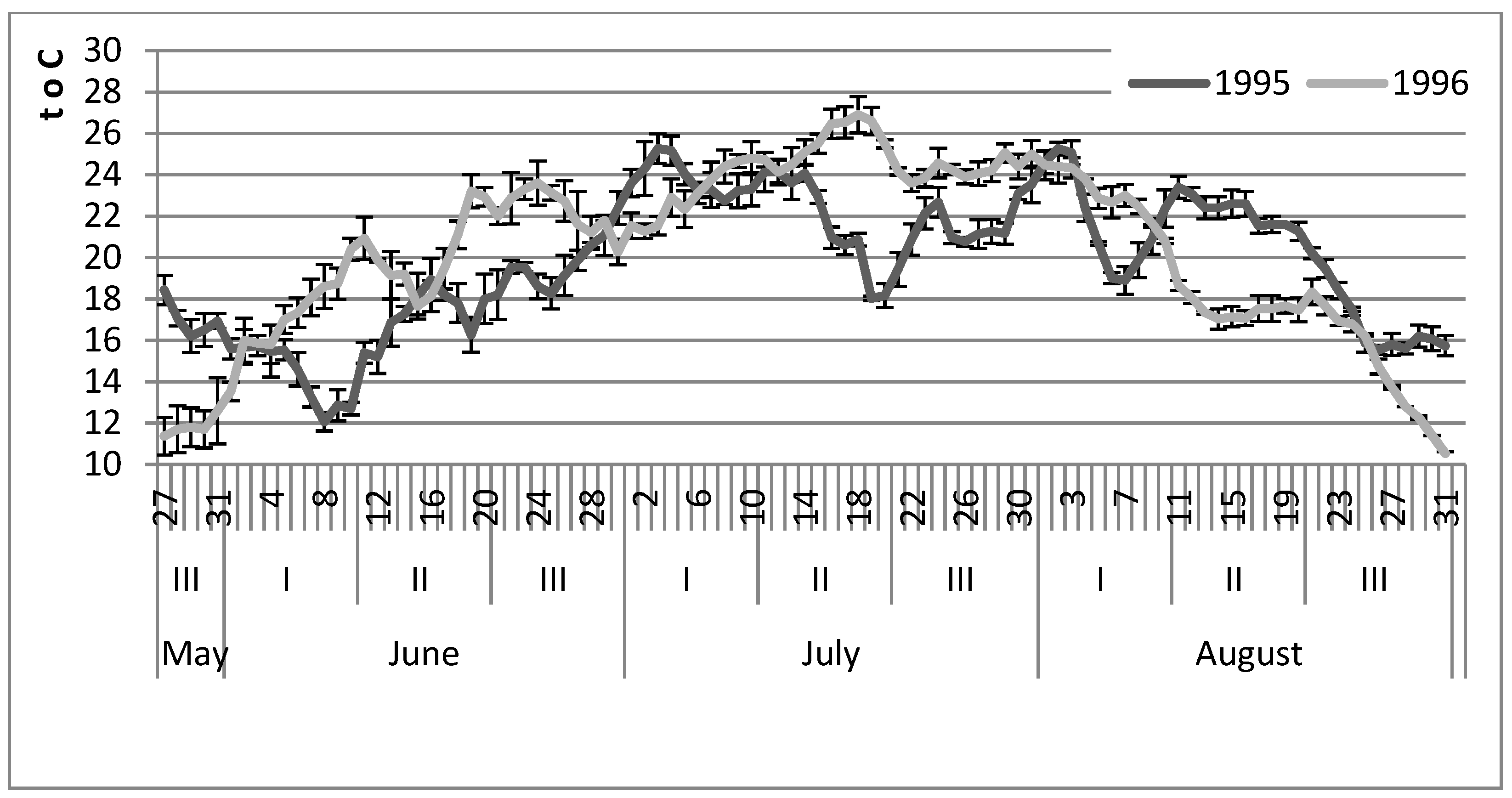
3.3. The Seasonal Dynamics of Bithyniid Snail Abundance in the South of Western Siberia
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamli, H.; Azmai, S.S.H.; Hamed, A.; Abdulla-Al-Asif, S. Diversity and Habitat Characteristics of Local Freshwater Gastropoda (Caenogastropoda) from Sarawak, Malaysia. Singap. J. Sci. Res. 2020, 10, 23–27. [Google Scholar] [CrossRef]
- Strong, E.E.; Gargominy, O.W.; Ponder, F.; Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Zhadin, V.I. Mollusks of Fresh and Brackish Waters of the USSR. In Opredelitel’ po Faune SSSR (Identification Guide to the Fauna of the USSR); Sovetskaya Nauka: Moscow, Russia, 1952; Volume 46, pp. 1–376. [Google Scholar]
- Berezkina, G.V.; Starobogatov, Y.I. Ekologiya Razmnozheniya i Kladki Yaits Presnovodnykh Legochnykh Mollyuskov (Ecology of Breeding and Egg Clusters by Freshwater Pulmonary Mollusks); Zoological Institute AN SSSR: Leningrad, Russia, 1988; Volume 174, pp. 1–307. [Google Scholar]
- Kruglov, N.D. Molluski Semeistva Prudovikov (Lymnaeidae, Gastropoda, Pulmonata) Evropyi Severnoi Azii (Osobennosti Ekologii i Parazitologicheskoe znachenie) (Mollusks of the Family Lymnaeidae (Gastropoda, Pulmonata) in Europe and North Asia (Characteristics of Ecology and Parasitological Role)); Smolensk: Izd Smolensk Gos Ped Univ: Smolensk, Russia, 2005; p. 507. [Google Scholar]
- Novikov, E.A. Freshwater Mollusks in the Basin of Middle Flow of the Ob River. In Extended Abstract of Dissertation Ph.D. Thesis (Biol.); Tomsk State University: Tomsk, Russia, 1971; pp. 1–20. [Google Scholar]
- Dolgin, V.N. Ecological and Faunistic Characteristic of the Freshwater Mollusks in Siberia. In Extended Abstract of Doktorskaya Thesis Dissertation (Biol.); Tomsk State University: Tomsk, Russia, 2001; pp. 1–423. [Google Scholar]
- Vinarski, M.V.; Andreev, N.I.; Andreeva, S.I.; Karimov, A.V.; Lazutkina, E.A. Latitudinal changes in the diversity of freshwater gastropods (Mollusca: Gastropoda) in waterbodies of western Siberia. Inland Water Biol. 2012, 5, 83–90. [Google Scholar] [CrossRef]
- Yurlova, N.I.; Vodyanitskaya, S.N. Long Therm Changes of Species Composition and Abundance of Pulmonata Snails (Gastropoda) in the Lake Chany (South of West Siberia). Sib. Ekol. Zhurnal 2005, 2, 255–266. (In Russian) [Google Scholar]
- Serbina, E.A. Characteristics of the seasonal development of Schistogonimus rarus (Trematoda: Prosthogonimidae). An essay on quantitative estimation of the trematode in the ecosystem of the Malye Chany lake (south of Western Siberia). Parazitologiya 2008, 42, 53–65. (In Russian) [Google Scholar]
- Yanygina, L.V.; Vizer, A.M. Long-term dynamics and current distribution of the River snail (Viviparus viviparus) in the Novosibirsk reservoir. Vestnik Tomskogo gosudarstvennogo universiteta. Biol. Tomsk State Univ. J. Biol. 2020, 49, 149–165. [Google Scholar] [CrossRef]
- Serbina, E.A. Bithyniid Snails as Hosts of Opisthorchiidae and Notocotylidae in the south of western Siberia. Russ. Parasitol. Res. 2022, 121, 2367–2377. [Google Scholar] [CrossRef]
- Filimonova, L.V.; Shalyapina, V.I. Cercariae of Trematodes in the Prosobranch Mollusc Bithynia Inflate from Lakes of North Kulunda; Trudy Akademiia nauk SSSR; Gel’Mintologicheskaia Laboratoriia: Moscow, Russia, 1980; Volume 30, pp. 113–124. (In Russian) [Google Scholar]
- Sidorov, E.G. Natural Foci of Opisthorchiasis; Nauka Kaz: Alma-Ata, Kazakhstan, 1983; pp. 1–240. (In Russian) [Google Scholar]
- Serbina, E.A.; Yurlova, N.I. Involvement of Codiella troscheli (Mollusca, Prosobranchia) in the life cycle of Metorchis albidus (Trematoda: Opisthorchidae). Med. Parazitol. Parazit. Bolezn. 2002, 3, 21–23. (In Russian) [Google Scholar]
- Serbina, E.A. Cercariae Opisthorchis felineus and Metorchis bilis from first intermediate hosts for the first time in basin of Chany lake (Novosibirsk region, Russia) is found. Russ. J. Parasitol. 2016, 37, 421–429. (In Russian) [Google Scholar] [CrossRef]
- Glöer, P. Bithynia leachii troschelii (Paasch 1842)—Die östliche Rasse von B. leachii (Sheppard 1823) (Gastropoda: Orthogastropoda: Bithyniidae). Arch. Molluskenkd. Int. J. Malacol. 2002, 130, 259–265. [Google Scholar] [CrossRef]
- Starobogatov, Y.I. Class Gastropoda. In Identification Key to the Freshwater Invertebrates in European; Gidrometeoizdat: Leningrad, Russia, 1977; pp. 152–174. (In Russian) [Google Scholar]
- Falniowski, A.; Glöer, P.; Szarowska, M. Bithynia troschelii (Paasch, 1842), a giant of unknown origin? Folia Malacol. 2004, 12, 137–139. [Google Scholar] [CrossRef]
- Glöer, P.; Falniowski, A.; Szarowska, M. Bithynia leachii (Sheppard, 1823) and B. troschelii (Paasch, 1842), two distinct species? Heldia 2005, 6, 49–56. [Google Scholar]
- Beer, S.A.; Lifshits, A.V.; Maslova, L.K.; Zavoikin, V.D. Locality of distribution and ecology of mollusks Bithynia inflata in the north of the Tomsk region. Message 1. Influence of floods on the nature of the distribution of bitinia in floodplain water bodies. The role of abiotic factors. Med. Parazitol. Parazit. Bolezni. 1976, 1, 74–82. (In Russian) [Google Scholar]
- Petney, T.N.; Sithithaworn, P.; Andrews, R.H.; Kiatsopit, N.; Tesana, S.; Grundy-Warr, C.; Ziegler, A.D. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol. Int. 2012, 61, 38–45. [Google Scholar]
- Wang, Y.-C.; Ho, R.C.Y.; Feng, C.-C.; Namsanor, J.; Sithithaworn, P. An ecological study of Bithynia snails, the first intermediate host of Opisthorchis viverrini in northeast Thailand. Acta Trop. 2015, 141, 244–252. [Google Scholar]
- Fedorov, K.P. The ecology of liver flukes in Novosibirsk oblast. In Ecology and Morphology of Helminths in Western Siberia; Nauka: Novosibirsk, Russia, 1979; pp. 5–55. (In Russian) [Google Scholar]
- Кarpenko, S.V.; Chechulin, A.I.; Yurlova, N.I.; Serbina, E.A.; Vodyanitskaya, S.N.; Krivopalov, A.V.; Fedorov, K.P. Characteristic of Opisthorchosis foci in the Southern of West Siberia. Contemp. Probl. Ecol. 2008, 1, 517–521. [Google Scholar]
- Serbina, E.A. Distribution of trematodes of the family Prosthogonimidae in river and lake ecological systems in the south of the Western Siberia. Parazitologiya 2005, 39, 50–65. Available online: https://www.zin.ru/journals/parazitologiya/content/2005/prz_2005_1_5_Serbina.pdf (accessed on 5 September 2022). (In Russian).
- Serbina, E.A.; Bonina, O.M. Revealing local nidi of opisthorchidoses in flood-lands of river Ob and in Novosibirsk man-made lake. Message 2. Prosobranchia mollusca´s number and infection of their by partenites of trematoda. Russ. Parazitol. Zhurnal 2011, 4, 55–59. Available online: http://www.zin.ru/journals/parazitologiya/content/2008/prz_2008_1_6_Serbina.pdf (accessed on 5 September 2022). (In Russian).
- Serbina, E.A. A quantitative estimation of the role of Bithyniidae snails (Gastropoda: Prosobranchia) in the ecosystems of the southern part of Western Siberia (Russia). Contemp. Probl. Ecol. 2013, 6, 28–33. [Google Scholar] [CrossRef]
- Serbina, E.A. The influence of trematode metacercariae on the individual fecundity of Bithynia troschelii (Gastropoda: Bithyniidae). Parazitologiya 2014, 48, 3–19. Available online: https://pubmed.ncbi.nlm.nih.gov/25434235/ (accessed on 5 September 2022).
- Serbina, E.A. Reproduction of Bithyniidae Snails (Mollusca: Gastropoda: Prosobranchia) from Chany Lake Basin (South of Western Siberia), Sibirskiy Ekologicheskiy Zhurnal. 2005, Volume 2, pp. 267–278. Available online: http://www.sibran.ru/upload/iblock/b48/b48569b892da2ee65cbe1d77c67138be.pdf (accessed on 5 September 2022). (In Russian).
- Serbina, E.A. The influence of trematode parthenitae on the individual fecundity of Bithynia troschelii (Gastropoda: Bithyniidae). Acta Parasitol. 2015, 60, 40–49. [Google Scholar] [CrossRef]
- Welter-Schultes, F.W. European Non-Marine Molluscs, a Guide for Species Identification; Planet Poster Editions: Göttingen, Germany, 2012; pp. 1–674. [Google Scholar]
- Starobogatov, Y.I.; Prozorova, L.A.; Bogatov, V.V.; Saenko, E.M. Opredelitel’ Presnovodnykh Bespozvonochnykh Rossii i Sopredel’nykh Territorii (Identification Key to the Freshwater Invertebrates in Russia and Adjacent Territories); Nauka: St. Petersburg, Russia, 2004; Volume 6, pp. 6–491. [Google Scholar]
- Vinarski, M.V.; Serbina, E.A. Distribution and quantitative characteristics of common species of pond snails of the subgenera Peregriana and Radix (Mollusca: Gastropoda: Lymnaeidae) in waterbodies of the South of Western Siberia. Inland Water Biol. 2012, 5, 37–44. [Google Scholar] [CrossRef]
- Serbina, E.A. Number and Biomass Lymnaea stagnalis (Gastropoda, Lymnaeidae) in the Ecosystems of the South of Western Siberia (Russia) (Наукoві Записки Тернoпільськoгo Націoнальнoгo Педагoгічнoгo Університету імені Вoлoдимира Гнатюка. Серія: Біoлoгія). 2012; Volume 2, pp. 227–230. Available online: http://catalog.library.tnpu.edu.ua/naukovi_zapusku/biolog/2012/biol_12_2.pdf (accessed on 5 September 2022).
- Schniebs, K. Beiträge zur Systematik und Taxonomie Paläarktischer Schlammschnecken (Gastropoda, Basommatophora, Lymnaeidae) Anhand Molekulargenetischer und Morphologischer Merkmale. Ph.D. Dissertation, TU Dresden, Dresden, Germany, 2016; 44p. [Google Scholar]
- Serbina, E.A. Coevolution Host—Parasite Systems (Bithyniidae-Trematode). Biodiversity and Ecology of Parasites Tr; Gelana: Moscow, Russia, 2010; Volume 46, pp. 239–259. Available online: https://www.researchgate.net/publication/282324188_S_e_r_b_i_n_a_E_A_2010_Coevolution_of_the_Host_-_Parasite_systems_Bithyniidae_Trematoda_Biodiversity_and_Ecology_of_Parasites_Transactions_Transactions_of_Center_for_Parasitology_MNauka_46_239-259_in_Russian (accessed on 5 September 2022). (In Russian)
- Cichy, A.; Faltýnková, A.; Żbikowska, E. Cercariae (Trematoda, Digenea) in European freshwater snails—A checklist of records from over one hundred years. Folia Malacol. 2011, 19, 165–189. [Google Scholar] [CrossRef]
- Schwelm, J.; Kudlai, O.; Smit, N.J.; Selbach, C.; Sures, B. High parasite diversity in a neglected host: Larval trematodes of Bithynia tentaculata in Central Europe. J. Helminthol. 2020, 94, e120. [Google Scholar] [CrossRef]
- Yurlova, N.I.; Yadrenkina, E.N.; Rastyazhenko, N.M.; Serbina, E.A.; Glupov, V.V. Opisthorchiasis in Western Siberia: Epidemiology and distribution in human, fish, snail, and animal populations. Parasitol. Int. 2017, 66, 355–364. [Google Scholar] [CrossRef]
- Potseluev, A.N. The role of small hydro-engineering objects in the change of ecology of mollusks- the first intermediate hosts of opistorchis. Med. Parazitol. Parazit. Bolezn. 1991, 5, 32–34. (In Russian) [Google Scholar]
- Ponomarev, D.N.; Khokhutkin, I.M. Analysis of the bithyniid population structures in opisthorchiasis foci of the Sverdlovsk oblast. Ekologiya 1991, 5, 62–69. (In Russian) [Google Scholar]
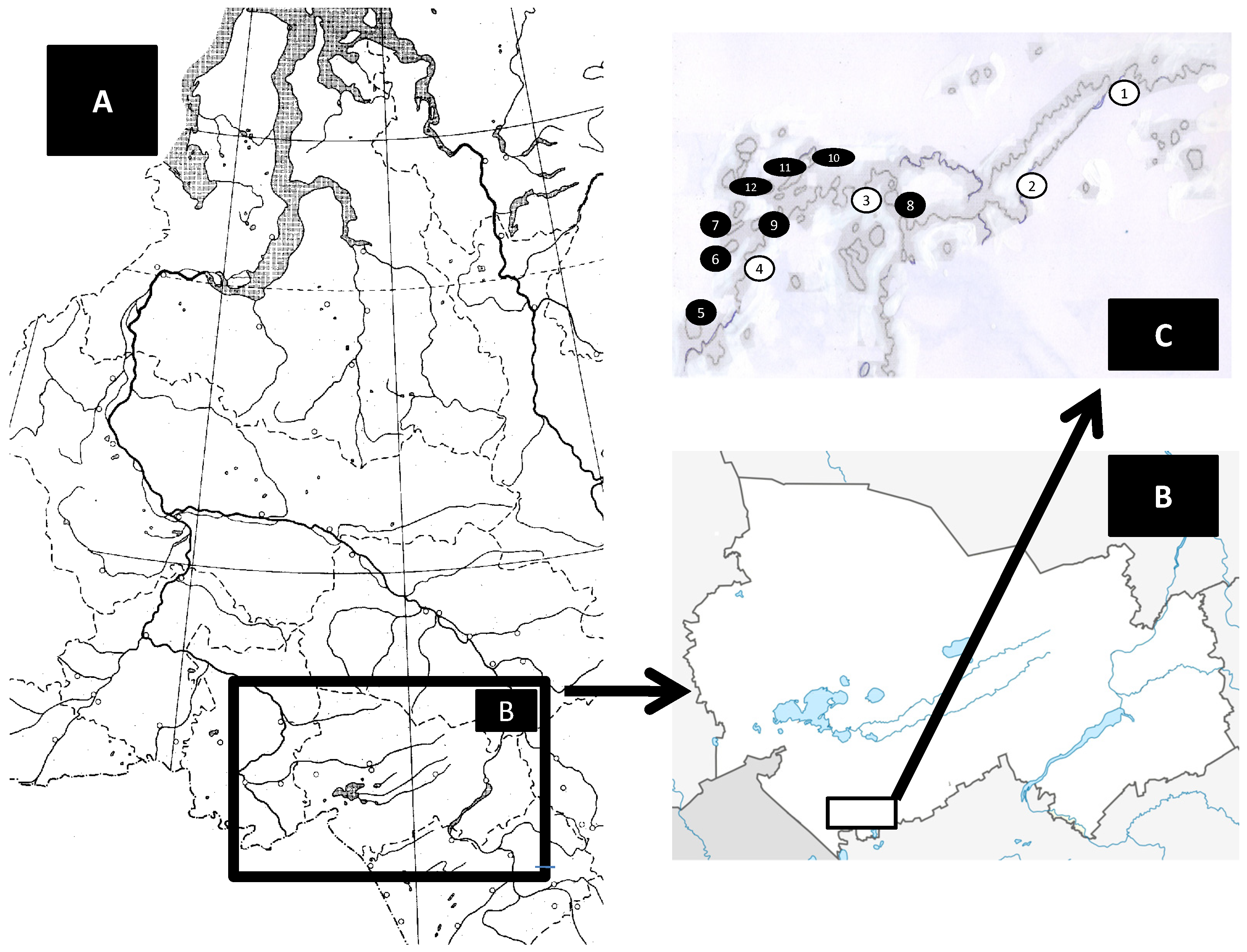
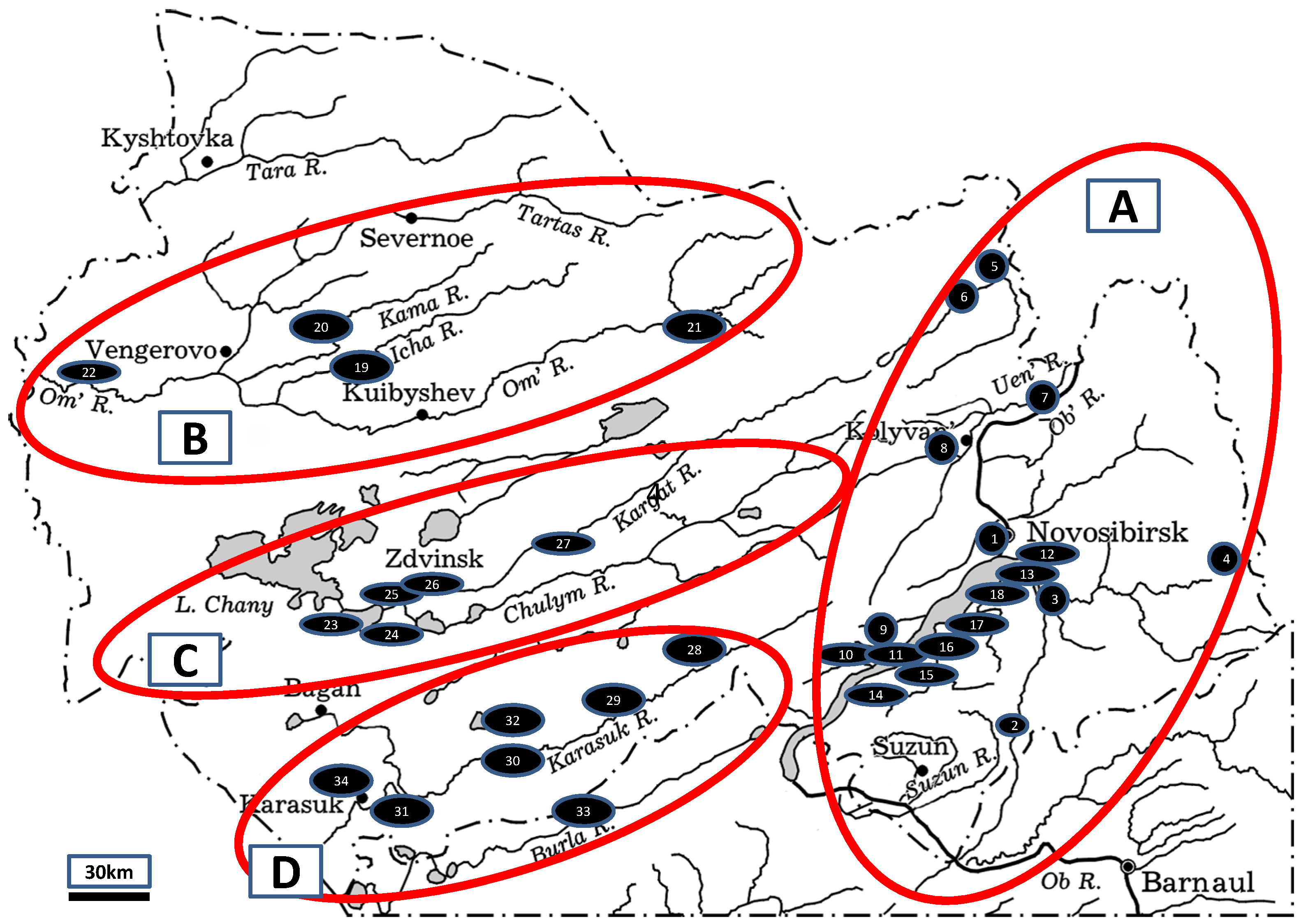
| Sampling Site | Geographical Coordinates (N, E) | Years | Samples * | |
|---|---|---|---|---|
| Ob River basin | Ob River downstream Novosibirsk HPS dam | 54°53′23″, 83°05′18″ | 1995–2020 | 33 |
| Ob River tributaries (right) | Inya River | 54°09′17″, 83°07′31″ | 1998 | 1 |
| Berd River | 54°69′55″, 83°22′50″ | 2013 | 1 | |
| Suenga River, Berd River tributaries | 54°25′39″, 84°32′33″ | 2018 | 1 | |
| Ob River tributaries (left) | Baksa River (near Pikhtovka and Laptevka villages) | 55°99′21″, 82°70′81″ | 1997 | 1 |
| 55°79′10″, 82°30′80″ | 1997 | 1 | ||
| Uen River | 55°31′0, 83°16′0 | 1996, 1998, 1999, 2003 | 4 | |
| Chauz River | 55°31′0, 83°16′0 | 2014 | 1 | |
| Ob reservoir | Ob reservoir left coast Near Krasny Yar village | 55°13′11″, 82°54′29″ | 2017 | 1 |
| Orda River | 54°22′76″, 81°55′26″ | 2018 | 1 | |
| Ob reservoir right coast Near Zav’yalovo village | 54°54’45″, 82″47°57′81″ | 2007 | 1 | |
| Berdsky Bay (right coast) | 54°78′, 83°09″ | 2002 | 1 | |
| Berdsky Bay (left coast) | 54°77′, 83°09″ | 2007 | 1 | |
| Talmenka River, in estuary | 54°42′25″, 83°16′50″ | 2007 | 1 | |
| Karakan River, in estuary | 54°50′22″, 82°44′94″ | 2007 | 1 | |
| Tulka River, in estuary | 54°56′20″, 82°65′46″ | 2009 | 1 | |
| Miltyush River, in estuary | 54°65′57″, 82°86′12″ | 2009 | 1 | |
| Sosnovka River, in estuary | 54°68′43′, 82°96′71″ | 2009 | 1 | |
| Irtysh River basin | Om River tributaries: Icha and Kama Rivers | 55°99′, 82°70′ | 1996 | 1 |
| 55°79′, 82°30′ | 1996 | 1 | ||
| Musikha River | 55°52′16″, 80°05′18″ | 2008 | 1 | |
| Lake Murashevskoe | 55°43′16″, 75°34′39″ | 2007, 2008 | 2 | |
| Lake Chany basin | Lake Malye Chany | 54°37′21″, 78°09′21″ | 2003, 2012 | 2 |
| Zolotye Rossypi Bay | 54°34′12″, 78°08′39″ | 1996, 1997 | 2 | |
| Kargat River, estuary (1) Chany field station | 54°37′76″, 78°13′07″ | 1994–2000, 2002–2007, 2012, 2013 | 126 | |
| Kargat River, estuary (2) | 54°61′, 78°22′ | 2013 | 1 | |
| Kargat River, middle reaches | 54°47′37″, 79°06′ | 1995 | 1 | |
| Water bodies of Northern Kulunda | Karasuk River | 54°26′53″, 80°55′50″ | 2009 | 1 |
| 54°09′53″, 80°02′54″ | 2009 | 1 | ||
| 53°45′19″, 78°20′15″ | 2009 | 1 | ||
| 53°43′19″, 77°56′29″ | 2009 | 1 | ||
| Kuria River | 53°50′56″, 78°22′34″ | 2007 | 1 | |
| Burla River | 53°20′, 78°20′ | 2010 | 1 | |
| Lake Krotovo, Karasuk scientific station | 53°43′30″, 77°51′31″ | 1994, 1995, 2006, 2007, 2009 | 5 | |
| In total | 201 |
| Water BodiesFamilies | Karasuk River | Lake | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Families | 1 * | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Lymnaeidae | 4 | 3 | 5 | 5 | 5 | 4 | 3 | 3 | 6 | 3 | 6 | 4 | |
| Planorbidae | 2 | 2 | 1 | 2 | 1 | 3 | 1 | 1 | 1 | 1 | 3 | 1 | |
| Physidae | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Bulinidae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | |
| Succineidae | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Zonitidae | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Bithyniidae | 2 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| All species | 10 | 7 | 7 | 10 | 7 | 12 | 5 | 7 | 9 | 5 | 11 | 6 | |
| Water Bodies * | Lymnaeidae | Planorbidae | Physidae | Bulinidae | Succineidae | Zonitidae | Bithyniidae |
|---|---|---|---|---|---|---|---|
| 1 | 0.1–176/81.4 ** | 0–32/12 | 0 | 0 | 0–4/1.3 | 0–4/1.3 | 0–68/26.7 |
| 2 | 0.2–10/6.1 | 0–1/0.5 | 0 | 0 | 0 | 0–0.1/0.05 | 0–8/4 |
| 3 | 16–20/21.5 | 0–2/1 | 0 | 0 | 0–4/2 | 0 | 0–4/2 |
| 4 | 12–31/25.5 | 0–6/4 | 0–5/2 | 0 | 0–3/2 | 0 | 1–6/4 |
| 5 | 0.02–37/22.3 | 0–18/6 | 0 | 0 | 0–3/1 | 0 | 0 |
| 6 | 6–28/17 | 3–80/41 | 0.2–4/2 | 0–0.2/0.1 | 0.8–12/6.4 | 0 | 0–6/3 |
| 7 | 0.03–88/33 | 0.01–4/1.7 | 0 | 0 | 0–1/0.3 | 0 | 0 |
| 8 | 24–99/55.3 | 1–64–/25.3 | 0–2/0.7 | 0 | 0–1/0.3 | 0–1/0.7 | 0 |
| 9 | 22–45/33 | 0–27/11.7 | 0 | 0–6/2 | 0–8/3 | 0 | 0 |
| 10 | 0–45/22.5 | 0–0.12/0.06 | 0 | 0 | 0–49/24.5 | 0 | 0 |
| 11 | 0.25–81/40 | 0–6/3 | 0 | 0.1–2.0/1 | 0–7/3.5 | 0 | 0 |
| 12 | 2–28/15 | 0–1/0.3 | 0 | 0 | 0–2/1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serbina, E.A. Bithyniid Abundance in the South of Western Siberia Water-Courses and Water Reservoirs (Russia). Diversity 2022, 14, 791. https://doi.org/10.3390/d14100791
Serbina EA. Bithyniid Abundance in the South of Western Siberia Water-Courses and Water Reservoirs (Russia). Diversity. 2022; 14(10):791. https://doi.org/10.3390/d14100791
Chicago/Turabian StyleSerbina, Elena A. 2022. "Bithyniid Abundance in the South of Western Siberia Water-Courses and Water Reservoirs (Russia)" Diversity 14, no. 10: 791. https://doi.org/10.3390/d14100791
APA StyleSerbina, E. A. (2022). Bithyniid Abundance in the South of Western Siberia Water-Courses and Water Reservoirs (Russia). Diversity, 14(10), 791. https://doi.org/10.3390/d14100791







