Abstract
Sociality in animal populations is a continuum, and interactions between conspecifics are meaningful for all vertebrates. Ignorance of social structures can lead to misunderstanding their ecology and, consequently, to unsuccessful species management. Here, we combined genetic and spatial data on radio-collared brown bears (Ursus arctos) to investigate kin-related home range overlap and kin-related centroid distance within central and eastern Finland. We found that the extent of home range overlap was positively correlated with relatedness among adult females. In addition, home range centroid distance decreased as relatedness increased. Moreover, there were significant differences between the two studied regions: female brown bears in central Finland were more closely related to each other, and the sizes of their home ranges were larger than those in eastern Finland. The smaller home ranges and lower degree of relatedness among bears in eastern Finland might be a result of the substantially higher hunting pressure in the area, combined with immigration of new unrelated individuals from Russia.
1. Introduction
Social structure is a key concept in animal population ecology and should be taken into account in the conservation and management of populations. Active social interactions associated with living in groups may provide higher survival rates through more effective food acquisition strategies and a lower risk of individual predation, among other factors [1]. These types of benefit-based complex social structures are frequently observed in group-living species, such as wolves, horses and whales [2,3,4]. However, most carnivores live a solitary, non-cooperative lifestyle, having very little interaction with conspecifics beyond the mating season [5]. Studying their social structure can be a tedious task, especially for species that are very mobile and live in low densities, rendering data acquisition very challenging. Telemetry tracking has eased the collection of data, and combined with genetic studies, it has henceforth promoted the understanding of such social structures. Three alternative hypotheses have been proposed to explain infrequent social interactions: resource dispersion, land-tenure, and kinship [6]. Each of these hypotheses has merit and can explain the behaviours of solitary animals observed in different ecosystems.
The resource dispersion hypothesis predicts that since resources are heterogeneous in space, the cost of sharing territory with conspecifics is reduced when resources are abundant [7,8,9,10]. Accordingly, as long as resources are abundant, even behaviourally solitary species may have overlapping territories. Territorial solitary species are known to aggregate at kills for short time periods [11,12,13]. The spatial distribution or so-called ‘land-tenure‘ hypothesis predicts that solitary carnivores’ territorial behaviour is the main explanatory factor for the sparse spatial distribution of individuals [11,14,15]. This hypothesis has been shown to accurately capture the social structure of polygynous animals, e.g., in several felid species [6,16]. Males aggressively defend their territories against other males, while female territories might overlap to some degree, but the animals still tend to avoid being concurrently in the overlap regions. The kinship hypothesis predicts that animals tolerate related individuals and even share benefits with them to increase the chance that their genetic material will propagate to the next generations [17]. For this hypothesis to hold, related individuals should be spatially accumulated rather than randomly distributed in the population. Support for this has been found in several species of fish, bears, and woodrats [18,19,20,21,22,23].
The brown bear (Ursus arctos) is predominantly a solitary carnivore but occasionally gets in touch with conspecifics [21]. Social interaction may emerge due to an abundance of food, such as when high salmon abundances occur in Alaska and bears are gathered in good hunting spots [24]. The resource dispersion hypothesis seems to thereby partly explain social interaction among bears. The mating season is another occasion for bears to get in touch, as both sexes seek to find a mating partner [25].
The mating system of brown bears has been described as promiscuous and polygamous [26]. Typically, female bears mate with three to four males during a breeding season [27]. Males, on the other hand, mate with up to eight females, while some do not obtain any mating [27]. Multiple paternity in a litter has been genetically confirmed and is a frequent phenomenon in brown bears [28]. As both sexes generally mate with several partners, and bears are generally considered non-territorial animals [29], the land-tenure hypothesis does not seem to explain the social structure in bears. Instead of territory, the living area of a bear is termed its home range. Male home ranges are typically large and overlap with those of several females [21,25]. The extent of the home range of female brown bears varies, largely depending on the stage of their breeding cycle. During oestrus, while females are trying to maximize their contacts with males in their search for fit candidates that could father their offspring, their home ranges tend to overlap more with those of other brown bears [30]. A female bear with cubs typically has a smaller home range than one without offspring [31]. The same principle holds true when considering the behavioural differentiation of a single individual over time [32,33]. The primary reason why brown bear mothers tend to restrict their home range size while taking care of their cubs is to avoid unnecessary contact with male brown bears [34]. Infanticide by males has been observed in Scandinavian brown bears [28,34]. It precipitates the recurrence of females’ sexual receptivity, and by doing so it improves the males’ fitness.
Normally cubs stay with the mother for 1.5–2.5 years [35] and sexual maturity is attained at the age of 4–6 years [34]. Adult female black bears (Ursus americanus) have been observed to tolerate home range overlaps with their weaned offspring and even shift their own to make space for their daughters, allowing them to establish their own home range [36]. Based on collar tracking data, young female brown bears have the tendency to migrate only a short distance from their maternal area [37,38]. Male brown bears, on the other hand, were found to disperse from their natal area in 92% of cases, which is a mechanism for avoiding inbreeding [28,39]. The reason why, unlike males, young females tend to stay close to their mother might be linked to the benefits of sharing territory with kin. It has been shown that in both brown and black bears, individuals with overlapping home ranges are on average more related to each other than individuals with non-overlapping home ranges [19,21,22]. Based on previous studies, the kinship hypothesis seems to best explain the distribution and the social structure of the female brown bears.
Northern European brown bear populations were almost exterminated at the beginning of the twentieth century. At that time, a stable brown bear population existed only in neighbouring Russian territory. In the 1970s and 1980s, the small remnants of the Finnish bear population started to recover [40]. In eastern Finland, the brown bear population increased due to immigration from the larger Russian bear population, forming a genetic peripheral segment [40,41]. The brown bear population size has been estimated to be approximately 8400 individuals in the following regions of Russia: Murmansk, Republic of Karelia, Leningrad, Novgorod and Pskov, in 2010 [42]. In whole European Russia (North-Western, Central, Volga, Southern and North-Caucasian federal districts), bear population size has been estimated to be approximately 47,100 individuals in 2016 [43]. In Finland, the brown bear population has continued to grow during the recent decades, and the estimated population size is approximately 2670–2800 individuals [44]. The stable population from eastern Finland has expanded towards the west into central Finland; thus, bears in these two regions do not differ in their genetic composition [45]. However, the populations are genetically differentiated from the bear population in northern Finland and Scandinavia [45,46], which indicates that the bear population in northern Finland could have been recolonised by a different bear population than the Russian population. Nevertheless, migration rates between Fennoscandian bear populations have recently increased due to management actions [45].
In this study, we compared the social structure of female brown bears in two regions in Finland: eastern and central Finland. We determined the sizes and overlaps of home ranges and compared the results for the two regions. Furthermore, we estimated the relatedness among individuals using microsatellite data to examine the relevance of the kinship hypothesis in the social structure of Finnish bears and whether there is a difference between the two regions.
2. Materials and Methods
2.1. Study Area
We conducted research in the eastern and central Finland (Figure 1), the regions in which the largest proportion of the brown bear population occurs. Originally the bear population in central Finland was established by a small number of founders from the dense eastern population (see Discussion), but the distribution of bears nowadays is more or less continuous between the two regions [44]. As the eastern study region is located next to the Russian border, the influence of the neighbouring bear population is higher. Furthermore, the eastern region has a lower anthropogenic impact due to the almost three times lower human density compared to central Finland [47,48]. In both areas, humans have easy access to bears’ home ranges owing to a dense network of forest roads. Contrary to human density, brown bear density is much higher in eastern Finland. In northern Karelia, where all the samples from eastern Finland were collected, the number of brown bears over one year old is over three times higher than that in central Finland [44], even though the regions are similar in size. Due to the much higher bear density in the region, the hunting pressure is of different magnitude. In 2020, four females were hunted in central Finland, whereas 51 were hunted in eastern Finland [44].

Figure 1.
Schematic map of Finland. Two study regions, highlighted with red and blue, illustrate all collected GPS points from 2004–2014.
2.2. Spatial and Genetic Data Collection
Spatial data used in this study were obtained via GPS tracking of 53 adult brown bears between the years 2004 and 2014 by the Natural Resource Institute Finland (Luke). The details of capture, handling, and anaesthetization were given in a previous study [49]. During radio-collaring, a mouth swab or hair sample was collected from each individual. In the few cases when the collared individual had died before the genetic analyses, we used a tissue sample instead. The presence of cubs in female brown bears has been reported to Luke for each year via observation. Additionally, we had 65 tissue or hair samples of uncollared brown bears from 2000 and 2014. The tissue samples came from legally shot bears during the bear hunting seasons. The samples were obtained by the University of Oulu from a frozen tissue collection kept by Luke in Taivalkoski, Finland. These samples were included in the study to represent the population more comprehensively in the relatedness estimates. The samples were divided into two geographical groups: eastern and central Finland. Some of the bears in eastern Finland spent part of their time on the Russian side of the border but lived mostly in Finland.
2.3. Laboratory Methods
According to the manufacturer’s instructions, we extracted DNA from saliva (n = 1) and tissue (n = 3) samples with an Ultra Clean® DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA. DNA from the hair samples (n = 49) was extracted with the E.Z.N.A® DNA Isolation Kit following the manufacturer’s instructions (Omega Bio-tek, Atlanta, GA, USA). For genotyping, we used 11 microsatellite primers developed for bears: G1D, G10B, G1A, G10L, MU05, MU09, MU10, MU50, MU51, MU59 and MU15 [50,51,52]. The same primers have been previously used in Finnish and northern European brown bear studies [41]. Microsatellites were divided into four multiplex groups, and each primer in the group was labelled with different fluorescence dyes: 1) MU09 (PET), MU15 (VIC), G1D (NED); 2) MU05 (PET), G1OL (NED), MU59 (FAM); 3) G10B (NED), MU51 (FAM), G1A (PET); and 4) MU50 (FAM), MU10 (VIC). PCR was performed in 10 μL reaction volumes containing Multiplex PCR master mix (QIAGEN, Germantown, MD, USA) 0.2 µL of each primer (10 µM) from the corresponding multiplex group and 2 µL of template DNA. Initial polymerase activation was performed in 95 °C for 15 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 90 s and 72 °C for 30 s and final elongation at 72 °C for 5 min.
2.4. Genetic Analyses
We estimated the probability of identity for the radio-collared bears (n = 53) using the software GenAIEx 6.5 [53]. GenAIEx was also used to estimate inbreeding coefficients for the subpopulations (FIS) [54]. The population structure was analysed via principal component analysis (PCA) using the R environment [55].) and the package adegenet [56,57]. Allelic richness (AR) was estimated using FSTAT software [58] and observed (HO) and expected (HE) heterozygosities were estimated using GENEPOP software [59,60]. Pairwise relatedness was estimated using the R package related [61] with the whole bear data including the non-collared bears. We simulated 100 pairs of individuals for each degree of relatedness (e.g., parent-offspring, full-sib, unrelated) based on the allele frequency data. The known simulated relatedness classes were then compared with expected relatedness values for the corresponding classes. The simulation used four different pairwise relatedness estimators to test the resolution of the dataset. The method of Wang [62] had the highest correlation between observed and expected relatedness values and was thus chosen for further analyses. The method is good with highly polymorphic loci, and it is unbiased even when dealing with small sample sizes. Furthermore, we used the software ML-Relate [63] to calculate the likelihoods of four types of relationships (unrelated, half siblings, full siblings, parent-offspring) for the radio-collared female brown bears but including also the non-collared reference bear samples in the analyses. We created a GenePlot [64] for visualizing genetic assignment data by characterizing the distribution of genetic profiles for the two subpopulations. The genetic assignments in GenePlot were calculated out of the 11 microsatellite markers with the saddlepoint approximation method [64]. Statistical analyses were performed in R. To examine whether the average relatedness differed between eastern and central Finland, a two-tailed t-test was performed. To analyse the difference in home range size between the study areas, we used a Mann-Whitney U test. We used only females from which we had monitoring data from years with cubs and without cubs and tested if there is significant difference in the home range size between the two.
2.5. Spatial Analysis
We estimated kernel home ranges using the R package adehabitatHR [65], using the coordinate reference system EUREF_FIN and EPSG:32635-WGS 84/UTM zone 35N + 36N. Variation in the data quality occurs in the tracking signal transmission interval over long time periods and in remote areas. Therefore, we calculated the home ranges using the reference bandwidth method, which is relatively unaffected by sample size and the most robust to a variation in sampling intensity [66]. We used a smoothing factor reference bandwidth (h) of 2478.219 m. We defined home ranges as 90% kernel utilization distribution (UD) isopleths and core home ranges as 50% kernel UD isopleths. Out of the 53 radio-collared brown bears, 39 individuals sent data regularly. The data from the remaining 14 bears were insufficient, and these individuals were excluded from further analyses. The home range overlaps were calculated with the R package ade4 [67]. Each individual was analysed for overlaps with other individuals for each year separately. Most home range overlaps were detected between 2010 and 2012. The overlapping area was calculated according to Bhattacharyya’s affinity method [68]. We estimated centroid distances for each calculated home range using the R package distances [69]. The Shapiro-Wilk test indicated that the data of home range overlap (W = 0.9721, p = 0.101) and centroid distance data were not normally distributed. Thus, we log-transformed the estimated variables for centroid distance to obtain a normal distribution of model residuals. We calculated mean home range overlap for each pair of female brown bears. We fitted a linear model for the home range overlap including pairwise relatedness and region as explanatory variables using the lm function in R. We created a linear mixed model for centroid distances including pairwise relatedness and region as explanatory variables and the PairID as a random effect using the lme4 [70] and lmerTest [71] packages in R. Furthermore, we fitted the regression models including year and age difference as explanatory factors both for the home range overlap and centroid distance but dropped those from both models due to the models’ worse fit compared to the model that included only home range overlap and region (ANOVA p < 0.05). Since the pairwise comparisons are not independent from each other, we also performed Mantel tests with 9999 permutations using ecodist package in R [72,73] for bears in central Finland to examine the correlation between pairwise relatedness and centroid point distance. To avoid missing pairwise comparisons between bears, we tested separately those years which contained enough data (min 5 pairs/year): 2010–2013 Due to the small sample size from eastern Finland, Mantel tests were performed only for samples from central Finland.
3. Results
3.1. Genetic Diversity
In total, 53 bear samples were analysed in this study. The mean observed and expected heterozygosities were 0.776 and 0.801 in central Finland (n = 24), while the values were 0.795 and 0.810 in eastern Finland, respectively (n = 29) (Table 1). The mean expected and observed heterozygosities did not show strong evidence of differences between the regions (two tailed t-test for HE: t = −0.221; df = 16.897; p = 0.83 and for HO: t = −0.221; df = 16.897; p = 0.71). The average allelic richness was 8.2 in central Finland and 10.9 in eastern Finland. There was no strong support for differences between the two regions in either inbreeding coefficients (two tailed t-test: t = −0.897; df = 17.975; p = 0.3817) or in allelic richness (two tailed t-test: t = −1.584; df = 19.615; p = 0.130).

Table 1.
Estimated allelic richness (AR), observed heterozygosity (HO), expected heterozygosity (HE) and inbreeding coefficient (FIS) per microsatellite locus based on 53 radio-collared bears divided into two regions: central Finland (n = 24) and eastern Finland (n = 29).
3.2. Relatedness and Genetic Structure
Based on the probability of identity of individuals (PI = 5.6 e−16) and siblings (PIsibs = 9.6 e−6), the 11 microsatellite markers separated even the closely related individuals well from each other. The mean relatedness among the bears was higher in central Finland (max. likelihood 0.158) than in eastern Finland (max. likelihood 0.054; two tailed t-test: t = 3.8302; df = 140.7; p < 0.05). Based on the ML-Relate software, there was one mother with three daughters in the data. The software identified also other independent mother-daughter pair but based on the field data the individuals were born in the same year so they are most likely siblings. If the previously mentioned pair is included as a full sibling pair, there were three full sibling pairs in the data. All identified first order relationships were found in central Finland. Based on the first (8.5%) and second (7.0%) axis of the principal component analysis, the brown bears in eastern Finland overlap with brown bears from central Finland (Figure 2). However, individuals from eastern Finland showed a higher genetic diversity in comparison to the individuals in central Finland. Based on the GenePlot analysis, several individuals from central Finland had a genotype which have a high probability of also being arisen in eastern Finland, with only two individuals having a genotype with a low Log-Genotype Probability (1% LGPs) of being arisen there (Figure 3). The genotypes of brown bears in eastern Finland had lower probabilities of being arisen in central Finland; almost half of the individuals (44.8%, n = 13) having a genotype with a very low probability (1% LGPs) of being arisen there. The bears in eastern Finland had more variable LGP values than bears in central Finland, which is in line with the other results showing higher genetic diversity in the region.
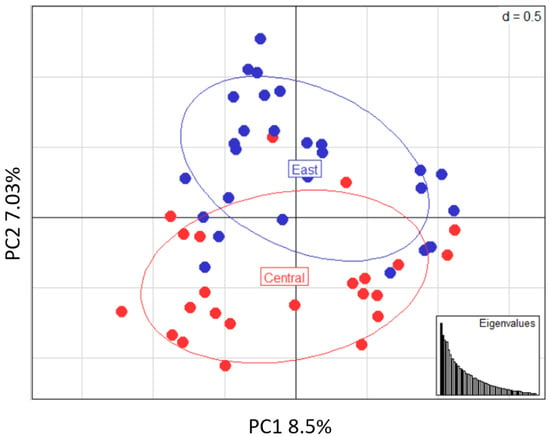
Figure 2.
Principal component analysis (PCA) of the studied brown bears (n = 53). The ellipses indicate the genetic distribution of the brown bear individuals from different regions in Finland, showing the first and second axes of the eigenvalues. In the right corner, a scree plot represents the first 50 eigenvalues in the inset.

Figure 3.
Genetic assignment for the studied brown bears (n = 53) using the saddlepoint approximation method implemented in GenePlot. The x- and y-axes represent log-posterior genotype probabilities for each individual in eastern and central Finland respectively, given the posterior allele frequencies in both regions. Genotypes of individuals on the darker diagonal line equal probability of arising in eastern and central Finland. Genotypes of individuals on the upper lighter diagonal line indicate a 9 times higher probability to arise in central Finland, and vice versa. The 1% and 99% percentile log-genotype probabilities in eastern and central Finland are shown in vertical and horizontal dashed lines respectively. Asterisks indicate missing data at one or more loci.
3.3. Patterns of Space Use and Their Link to Relatedness
We attained an average of 897 GPS locations per home range for collared brown bears in each study year. Out of the 94 annual home ranges with 90% kernel home range, only 19 home ranges had no overlap with others. According to the GPS tracking results, we found strong evidence that the female brown bear home range sizes differ between the two study areas. Female brown bears had, on average, smaller home ranges in eastern Finland (127 ± 90.7 km2, n = 25) than in central Finland (862 ± 490.7 km2, n = 56; Figure 4), strongly supported by a Mann–Whitney U test (W = 125; p < 0.001). The average kernel home ranges of the females with cubs (385.4 ± 320.77 km2, n = 14) were significantly smaller than the average home ranges of the females without cubs (581.8 ± 677.4 km2, n = 14; paired two tailed t-test, t = 2.16; p = 0.001). The regression analysis of female brown bears shows a positive association between the pairwise relatedness and the mean home range overlap with the 90% kernels (n = 74; β = 0.174, p = 6.36e−05; Table 2A; Figure 5). Similarly, there was strong association between the pairwise relatedness values and the distances between centroid points of the home ranges (n = 279, β = −1.029, p = 5.19e−04; Table 2B; Figure 5). Home ranges of female brown bears with high relatedness (R > 0.45) overlapped on average 26% with each other and the average distance between centroid points was 18 km.
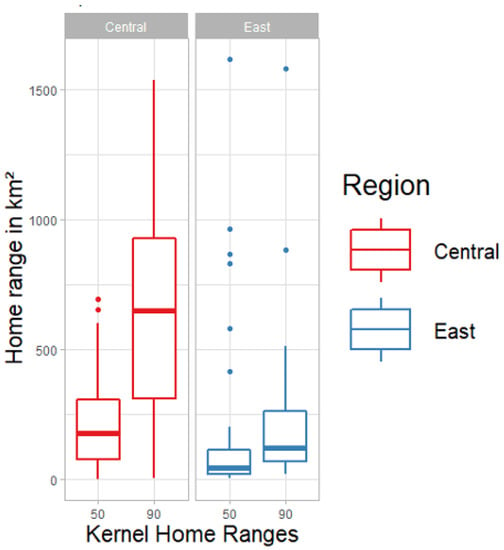
Figure 4.
Comparison of home ranges between the Central and Eastern Finland, and between the home range size of 90% UD isopleths and the core home range size of 50% UD isopleths.

Table 2.
Results of model estimations: (A) The effect of pairwise relatedness and region on home range overlap in female brown bears; (B) The effect of pairwise relatedness and region on centroid distance of home ranges in female brown bears. *** p < 0.001.
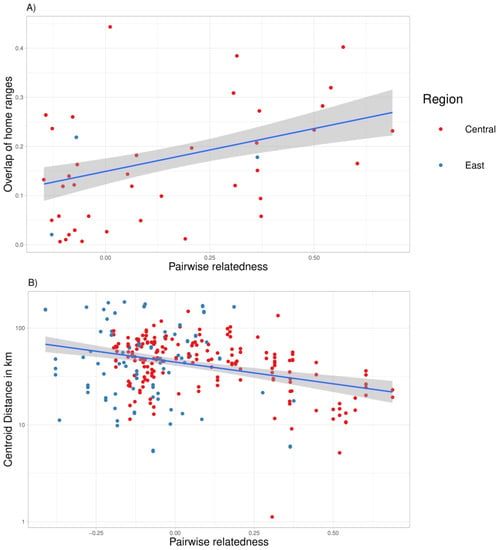
Figure 5.
Relationship of pairwise relatedness with (A) mean home range overlap per pair, and (B) log-transformed home range centroid distance (km) in female brown bears. The blue line is the predicted mean effect of pairwise relatedness on the home range overlap, and the shaded grey area is the lower and the upper 95% confidence limit for the expected values. Dots are pairwise observations.
However, there was no difference between the two regions in either home range overlap or the distances between the centroid points of home ranges (p > 0.05, Table 2). The negative association between pairwise relatedness and centroid distance in central Finland was confirmed by Mantel test for the years 2010 ( Spearmann rho, ρ = −0.551, p < 0.01) and 2013 (ρ = −0.485, p < 0.03). The Mantel test for the years 2011 (ρ = −0.241, p = 0.071) and 2012 (ρ = −0.182, p = 0.071) had also negative association but with slightly higher p-values.
4. Discussion
Kinship seems to be an important factor in determining the social structure of bears in Finland. We found strong evidence for a linear relationship between pairwise relatedness among the individuals and the extent of home range overlap (Figure 5). These findings are similar to findings in other brown bear populations in Scandinavia [21] and black bears in Canada [19]. However, the home range size and the extent of relatedness of female brown bears differed between eastern and central Finland, with the home ranges being smaller and the relatedness among the bears being lower in eastern Finland. Knowing the difference in the home range size between the two regions in Finland is important for management purposes. Average home range sizes of females with cubs are used for estimating the number of litters in each year. The number of litters is in turn used in the census size estimation of brown bears in Finland.
The difference in home range sizes between the two regions can be largely explained by the three times higher bear density in eastern Finland, which has a multifactor impact: there is less available land to establish a home range, a higher risk of encountering infanticidal males, and greater danger of getting hunted in the region [74]. Therefore, maintaining a smaller roaming area might be more beneficial than in central Finland. High hunting pressure has also been shown to disrupt structure among matrilines, even though it causes shorter female dispersal distances [75]. In eastern study region, the hunting rate of brown bears is over 10% (I. Kojola, unpublished data), whereas in the Russian side of the border the hunting rate has been estimated to be around 5–7% (K. Tirronen, unpublished data). In accordance with our results, increased population density has had an adverse impact on home range sizes also in other brown bear studies [34,76], as well as studies on other species [77,78,79].
Another factor that could have influenced the difference in the average size of home ranges between the two regions is the possible difference in resource availability between the two areas. A rich habitat has been shown to lead to smaller home ranges in previous studies [80] as individuals are able to acquire sufficient amounts of food from smaller home ranges. However, there is no evidence of major differences in habitat quality between these two areas. In both study areas there are several artificial feeding sites for bears, and feeding has been shown to affect the behaviour of bears locally in several ways, e.g., individuals using the sites tend to be less mobile than those who do not [81]. Additionally, females with cubs seemed to avoid artificial feeding sites that were visited by adult males [48]. However, as feeding sites occur in both areas, they should not have had a systematic impact on the observed differences between the regions. Accordingly, home range size was not correlated with the food availability index in Scandinavian bears [76].
A comparison of home range sizes in eastern Finland and central Finland to brown bear populations in different countries shows that brown bears in central Finland have one of the largest home ranges; only in North America do home ranges seem to be of similar size. The home ranges of female brown bears in eastern Finland were of similar size to those in Scandinavia, whereas home ranges were clearly smaller in southern Europe and Japan (Table 3).

Table 3.
Comparison of home range sizes (km2) between two regions in Finland and in other studied brown bear populations.
One of the possible explanations for the difference in the level of relatedness among bears in the two regions is spatio-historical: the eastern bear population was founded by Russian individuals, and there has been constant migration pressure from Russia, while the population in central Finland was established by a small number of founders from the eastern population.
Despite the previously mentioned differences, we did not find large differences in the strength of kin-based social structure between the regions; the benefits of sharing a home range with relatives appear to be similar, regardless of the relatedness among the bears being generally much higher in central Finland. Kinship-based social structures emerge when female brown bears establish their home ranges close to their natal area, thereby benefiting from their mother’s high-quality home range [81]. The mothers also benefit from living in close contact with their offspring; it increases the probability of their offspring’s long-term survival, and the mothers are safer against other dominant females who could otherwise dispute the area more aggressively. Dominant females, especially mothers, can suppress other females living close by [29]. If resources are scarce and partially shared among related female brown bears, females tend to have offspring in different years [22]. This suppression caused by dominant females has been described mainly in large carnivores that live in packs, such as wolves [3]. Dominant females are usually older brown bears, suggesting some connection between age and social organization. As we found first order relationships (mother-daughter and full siblings) only from central Finland, this indicates that something prevents the normal social structure of female brown bears to emerge in eastern Finland.
As already shown in a previous study [45], the bears in central Finland are genetically a subset of the eastern Finland population (Figure 3). Migration in brown bears is density-dependent [22]. The high-density population in eastern Finland has more migration pressure towards the lower density population in central Finland than vice versa, as shown in the GenePlot results. It is also known that during 1982–1993, five bears were translocated from eastern Finland to central Finland to establish the local bear population there [88]. In our GenePlot results, genotypes of three bears in central Finland and one in eastern Finland had a low probability of arising in either of the two populations, proposing that they originally came from outside the study area. The connectivity between the bear populations has recently increased in Fennoscandia [45]. Simultaneously, the bear population size in Finland has increased since the late 1960s [89]. Genetic diversity in the study areas was as high as in other bear populations [90,91,92] and similar to that of other Finnish brown bear studies [41]. We suspect that the FIS estimates are low due to extensive migration between Finnish and Russian Karelian bear populations [93].
5. Conclusions
We show that home range overlap increases and the distance of the home range centroids decreases as relatedness among the female brown bears increases. Kin-related spatial structure has also been found in other bear studies [19,21,22]. We also showed that home ranges were smaller in eastern Finland than in central Finland. This may be due to the higher population density in eastern Finland, which causes more conflicts between the bears, e.g., competition for available living habitats and infanticide by males. This, combined with higher anthropogenic disturbances through higher hunting pressure and immigration of unrelated individuals from the neighbouring bear population into the region, can break the matriline structure and affect the mobility and sociality of the female brown bears in the area. Our results indicate that the potentially disruptive impact of high hunting pressure on social organization of the bears should be taken into account in brown bear management as it may have long-term impacts on bear life histories. Furthermore, the observed regional differences in female home ranges should be addressed in the census size estimation of the Finnish brown bear population. Currently the population estimate is based on the observations concerning litters of the year [44]. The highly overlapping home ranges in central Finland make observation-based estimation very challenging and emphasizes the need for non-invasive genetic monitoring in that area.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d14010041/s1. Table S1: Microsatellite data of radio-collared brown bears and bears used as a reference data in the analyses. Table S2: Spatial data as centroid distances for each radio-collared bear used in the study. Table S3: Spatial data as home range overlaps for each radio-collared bear used in the study.
Author Contributions
Conceptualization, A.O., A.K.N. and J.H.; methodology, A.O., V.N., A.K.N. and J.H.; software, A.O., V.N., A.K.N. and J.H.; validation, A.O., J.A., I.K., A.K.N. and J.H.; formal analysis, A.O., A.K.N. and J.H.; investigation, A.O., A.K.N. and J.H.; resources, J.A., and I.K. data curation, A.O. and I.K; writing—original draft preparation, A.O., J.A., I.K., A.K.N. and J.H.; writing—review and editing, A.O., J.A., I.K., A.K.N. and J.H.; visualization, A.O.; supervision, A.K.N. and J.H.; project administration, A.O., A.K.N. and J.H.; funding acquisition, A.O., J.A. and I.K. All authors have read and agreed to the published version of the manuscript.
Funding
A.O. was funded by Riistasäätiö and the Societas pro Fauna et Flora Fennica.
Institutional Review Board Statement
Capture, handling, anaesthetizing, collaring and DNA sampling of the bears met the guidelines issued by the Animal Care and Use Committee at the University of Oulu and permits provided by the provincial government of Oulu and the Regional State Administrative Agency (OYEKT-6-99, OLH-01951/Ym-23, ESAVI/3229/04.10.07/2013).
Data Availability Statement
Data is contained within the article or Supplementary Materials. The data presented in this study are available in Tables S1–S3.
Acknowledgments
The authors want to thank the field workers in Natural Resources Institute Finland who collected the bear data and three anonymous reviewers for constructive comments that improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Whitehead, H. Analysing animal social structure. Anim. Behav. 1997, 53, 1053–1067. [Google Scholar] [CrossRef]
- Keiper, R.R. Social Interactions of the Przewalski Horse (Equus Przewalskii Poliakov, 1881) Herd at the Munich Zoo. Appl. Anim. Behav. Sci. 1988, 21, 89–97. [Google Scholar] [CrossRef]
- Peterson, R.O.; Jacobs, A.K.; Drummer, T.D.; Mech, L.D.; Smith, D.W. Leadership Behavior in Relation to Dominance and Reproductive Status in Gray Wolves, Canis lupus. Can. J. Zool. 2002, 80, 1405–1412. [Google Scholar] [CrossRef]
- Gero, S.; Gordon, J.; Whitehead, H. Individualized Social Preferences and Long-Term Social Fidelity between Social Units of Sperm Whales. Anim. Behav. 2015, 102, 15–23. [Google Scholar] [CrossRef]
- Sandell, M. The Mating Tactics and Spacing Patterns of Solitary Carnivores. In Carnivore Behavior, Ecology, and Evolution; Gittleman, J.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 164–182. ISBN 978-1-4757-4716-4. [Google Scholar]
- Elbroch, L.M.; Lendrum, P.E.; Quigley, H.; Caragiulo, A. Spatial Overlap in a Solitary Carnivore: Support for the Land Tenure, Kinship or Resource Dispersion Hypotheses? J. Anim. Ecol. 2016, 85, 487–496. [Google Scholar] [CrossRef]
- Macdonald, D.W. The Ecology of Carnivore Social Behaviour. Nature 1983, 301, 379–384. [Google Scholar] [CrossRef]
- Johnson, D.D.P.; Kays, R.; Blackwell, P.G.; Macdonald, D.W. Does the resource dispersion hypothesis explain group living? Trends Ecol. Evol. 2002, 17, 563–570. [Google Scholar] [CrossRef]
- Wagner, A.; Frank, L.; Creel, S. Spatial grouping in behaviourally solitary striped hyaenas, Hyaena hyaena. Anim. Behav. 2008, 75, 1131–1142. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Johnson, D.D.P. Patchwork Planet: The Resource Dispersion Hypothesis, Society, and the Ecology of Life. J. Zool. 2015, 295, 75–107. [Google Scholar] [CrossRef]
- Seidensticker, J.C.; Hornocker, M.G.; Wiles, W.V.; Messick, J.P. Mountain Lion Social Organization in the Idaho Primitive Area. Wildl. Monogr. 1973, 35, 3–60. Available online: http://www.jstor.org/stable/3830509 (accessed on 22 November 2021).
- Guilder, J.; Barca, B.; Arroyo-Arce, S.; Gramajo, R.; Salom, R. Jaguars (Panthera onca) Increase Kill Utilization Rates and Share Prey in Response to Seasonal Fluctuations in Nesting Green Turtle (Chelonia mydas mydas) Abundance in Tortuguero National Park, Costa Rica. Mamm. Biol. 2015, 80, 65–72. [Google Scholar] [CrossRef]
- Elbroch, L.M.; Quigley, H. Social Interactions in a Solitary Carnivore. Curr. Zool. 2017, 63, 357–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferreras, P.; Beltrán, J.F.; Aldama, J.J.; Delibes, M. Spatial Organization and Land Tenure System of the Endangered Iberian Lynx (Lynx pardinus). J. Zool. 1997, 243, 163–189. [Google Scholar] [CrossRef]
- Diefenbach, D.R.; Hansen, L.A.; Warren, R.J.; Conroy, M.J. Spatial Organization of a Reintroduced Population of Bobcats. J. Mammal. 2006, 87, 394–401. [Google Scholar] [CrossRef]
- López-Bao, J.V.; Rodríguez, A.; Alés, E. Field Observation of Two Males Following a Female in the Iberian Lynx (Lynx pardinus) during the Mating Season. Mamm. Biol. 2008, 73, 404–406. [Google Scholar] [CrossRef]
- Griffiths, S.W.; Armstrong, J.D. The Benefits of Genetic Diversity Outweigh Those of Kin Association in a Territorial Animal. Proc. Biol. Sci. 2001, 268, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E.; Brown, J.A. Do Kin Always Make Better Neighbours?: The Effects of Territory Quality. Behav. Ecol. Sociobiol. 1993, 33, 225–231. [Google Scholar] [CrossRef]
- Schenk, A.; Obbard, M.E.; Kovacs, K.M. Genetic Relatedness and Home-Range Overlap among Female Black Bears (Ursus americanus) in Northern Ontario, Canada. Can. J. Zool. 1998, 76, 1511–1519. [Google Scholar] [CrossRef]
- Griffiths, S.; Armstrong, J. Kin-Biased Territory Overlap and Food Sharing among Juvenile Atlantic Salmon. J. Anim. Ecol. 2002, 71, 480–486. [Google Scholar] [CrossRef]
- Støen, O.-G.; Bellemain, E.; Sæbø, S.; Swenson, J.E. Kin-Related Spatial Structure in Brown Bears Ursus arctos. Behav. Ecol. Sociobiol. 2005, 59, 191–197. [Google Scholar] [CrossRef]
- Ordiz, A.; Støen, O.-G.; Swenson, J.E.; Kojola, I.; Bischof, R. Distance-Dependent Effect of the Nearest Neighbor: Spatiotemporal Patterns in Brown Bear Reproduction. Ecology 2008, 89, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Innes, R.J.; McEachern, M.B.; Van Vuren, D.H.; Eadie, J.M.; Kelt, D.A.; Johnson, M.L. Genetic Relatedness and Spatial Associations of Dusky-Footed Woodrats (Neotoma fuscipes). J. Mammal. 2012, 93, 439–446. [Google Scholar] [CrossRef]
- Rode, K.D.; Farley, S.D.; Robbins, C.T. Sexual dimorphism, reproductive strategy, and human activities determine resource use by brown bears. Ecology 2006, 87, 2636–2646. [Google Scholar] [CrossRef]
- Dahle, B.; Swenson, J.E. Family Breakup in Brown Bears: Are Young Forced to Leave? J. Mammal. 2003, 84, 5. [Google Scholar] [CrossRef]
- Steyaert, S.M.J.G.; Endrestøl, A.; Hackländer, K.; Swenson, J.E.; Zedrosser, A. The Mating System of the Brown Bear Ursus Arctos: Brown Bear Mating System. Mammal. Rev. 2012, 42, 12–34. [Google Scholar] [CrossRef]
- Craighead, L.; Paetkau, D.; Reynolds, H.V.; Vyse, E.R.; Strobeck, C. Microsatellite Analysis of Paternity and Reproduction in Arctic Grizzly Bears. J. Hered. 1995, 86, 255–261. [Google Scholar] [CrossRef]
- Bellemain, E.; Swenson, J.E.; Taberlet, P. Mating Strategies in Relation to Sexually Selected Infanticide in a Non-Social Carnivore: The Brown Bear. Ethology 2006, 112, 238–246. [Google Scholar] [CrossRef]
- McLellan, B.N.; Hovey, F.W. Natal Dispersal of Grizzly Bears. Can. J. Zool. 2001, 79, 838–844. [Google Scholar] [CrossRef]
- Schwartz, C.C.; Miller, S.D.; Haroldson, M.A. Grizzly bear Ursus arctos. In Wild Mammals of North America: Biology, Management, and Conservation, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2003; pp. 556–586. Available online: http://pubs.er.usgs.gov/publication/70169862 (accessed on 22 November 2021).
- Bellemain, E.; Zedrosser, A.; Manel, S.; Waits, L.P.; Taberlet, P.; Swenson, J.E. The Dilemma of Female Mate Selection in the Brown Bear, a Species with Sexually Selected Infanticide. Proc. Biol. Sci. 2006, 273, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Glenn, L.P.; Miller, L.H. Seasonal movements of an Alaska Peninsula brown bear population. In Bears: Their Biology and Management: A Selection of Papers from the 4th International Conference on Bear Research and Management; Martinka, C.J., McArthur, K.L., Eds.; International Association for Bear Research and Management: Kalispell, Montana, 1980; pp. 307–312. [Google Scholar]
- Dahle, B.; Swenson, J.E. Home Ranges in Adult Scandinavian Brown Bears (Ursus arctos): Effect of Mass, Sex, Reproductive Category, Population Density and Habitat Type. J. Zool. 2003, 260, 329–335. [Google Scholar] [CrossRef]
- Dahle, B.; Swenson, J.E. Seasonal Range Size in Relation to Reproductive Strategies in Brown Bears Ursus arctos. J. Anim. Ecol. 2003, 72, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, J.; Pigeon, G.; Zedrosser, A.; Swenson, J.E.; Pelletier, F. Hunting regulation favors slow life histories in a large carnivore. Nat. Commun. 2018, 9, 1100. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L. Effects of Food Supply and Kinship on Social Behavior Movements and Population Growth of Black Bears in Northeastern Minnesota USA. Wildl. Monogr. 1987, 97, 1–72. [Google Scholar]
- Støen, O.-G.; Zedrosser, A.; Sæbø, S.; Swenson, J.E. Inversely Density-Dependent Natal Dispersal in Brown Bears Ursus arctos. Oecologia 2006, 148, 356. [Google Scholar] [CrossRef]
- Kojola, L.; Danilov, P.I.; Laitala, H.-M.; Belkin, V.A.; Yakimov, A. Brown Bear Population Structure in Core and Periphery: Analysis of Hunting Statistics from Russian Karelia and Finland. Ursus 2007, 14, 17–20. Available online: https://www.jstor.org/stable/3872952 (accessed on 22 November 2021).
- Zedrosser, A.; Dahle, B.; Swenson, J.E.; Gerstl, N. Status and Management of the Brown Bear in Europe. Ursus 2001, 12, 9–20. Available online: https://www.jstor.org/stable/3873224 (accessed on 22 November 2021).
- Pulliainen, E. Brown Bear Immigration into Finland from the East. Bears Biol. Manag. 1986, 6, 15–20. [Google Scholar] [CrossRef]
- Kopatz, A.; Eiken, H.G.; Hagen, S.B.; Ruokonen, M.; Esparza-Salas, R.; Schregel, J.; Kojola, I.; Smith, M.E.; Wartiainen, I.; Aspholm, P.E.; et al. Connectivity and Population Subdivision at the Fringe of a Large Brown Bear (Ursus arctos) Population in North Western Europe. Conserv. Genet. 2012, 13, 681–692. [Google Scholar] [CrossRef]
- Danilov, P.I.; Tirronen, K.F.; Belkin, V.V.; Panchenko, D.V.; Fedorov, F.V. Brown Bear and an Estimate of Its Abundance in the European Taiga; PetroPress: Petrozavodsk, Russia, 2014; p. 33. ISBN 978-5-8430-0117-9. [Google Scholar]
- Kolesnikov, V.V.; Dvornikov, M.G.; Zarubin, B.E.; Makarov, V.A.; Makarova, D.S.; Piminov, V.N.; Pankratov, A.P.; Sinitsyn, A.A.; Skumatov, D.V.; Soloviev, V.A.; et al. Scientifically Grounded Proposals for the State System for Monitoring the Resources of the Main Species of Hunting Animals in the Russian Federation; FGBNU VNIIOZ: Kirov, Russia, 2017; p. 37. Available online: http://vniioz-kirov.ru/upload/iblock/f24/f24282455681542b9fe9ff519eabecfd.pdf (accessed on 9 October 2021).
- Heikkinen, S. Karhukanta Suomessa 2020. In Luonnonvara- ja Biotalouden Tutkimus; Luonnonvarakeskus: Helsinki, Finland, 2020; ISBN 978-952-380-177-6. [Google Scholar]
- Kopatz, A.; Kleven, O.; Kojola, I.; Aspi, J.; Norman, A.J.; Spong, G.; Gyllenstrand, N.; Dalén, L.; Fløystad, I.; Hagen, S.B.; et al. Restoration of Transborder Connectivity for Fennoscandian Brown Bears (Ursus arctos). Biol. Conserv. 2021, 253, 108936. [Google Scholar] [CrossRef]
- Kopatz, A.; Eiken, H.G.; Aspi, J.; Kojola, I.; Tobiassen, C.; Tirronen, K.F.; Danilov, P.I.; Hagen, S.B. Admixture and Gene Flow from Russia in the Recovering Northern European Brown Bear (Ursus arctos). PLoS ONE 2014, 9, e97558. [Google Scholar] [CrossRef]
- National Land Survey of Finland. Suomen Pinta-ala Kunnittain 1.1.2021. Available online: https://www.maanmittauslaitos.fi/sites/maanmittauslaitos.fi/files/attachments/2021/02/Vuoden_2021_pinta-alatilasto_kunnat_maakunnat.pdf (accessed on 15 April 2021).
- Statistics Finland, Population. Available online: https://www.tilastokeskus.fi/tup/suoluk/suoluk_vaesto_en.html (accessed on 15 April 2021).
- Penteriani, V.; Lamamy, C.; Kojola, I.; Heikkinen, S.; Bombieri, G.; del Mar Delgado, M. Does Artificial Feeding Affect Large Carnivore Behaviours? The Case Study of Brown Bears in a Hunted and Tourist Exploited Subpopulation. Biol. Conserv. 2021, 254, 108949. [Google Scholar] [CrossRef]
- Paetkau, D.; Strobeck, C. Microsatellite Analysis of Genetic Variation in Black Bear Populations. Mol. Ecol. 1994, 3, 489–495. [Google Scholar] [CrossRef]
- Paetkau, D.; Calvert, W.; Stirling, I.; Strobeck, C. Microsatellite Analysis of Population Structure in Canadian Polar Bears. Mol. Ecol. 1995, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Camarra, J.-J.; Griffin, S.; Uhrès, E.; Hanotte, O.; Waits, L.P.; Dubois-Paganon, C.; Burke, T.; Bouvet, J. Noninvasive Genetic Tracking of the Endangered Pyrenean Brown Bear Population. Mol. Ecol. 1997, 6, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 22 November 2021).
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. Adegenet 1.3-1: New Tools for the Analysis of Genome-Wide SNP Data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Raymond, M.; Rousset, F. GENEPOP (Version 1.2): Population Genetics Software for Exact Tests and Ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A Complete Re-Implementation of the Genepop Software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Pew, J.; Muir, P.H.; Wang, J.; Frasier, T.R. Related: An R Package for Analysing Pairwise Relatedness from Codominant Molecular Markers. Mol. Ecol. Resour. 2015, 15, 557–561. [Google Scholar] [CrossRef]
- Wang, J. An Estimator for Pairwise Relatedness Using Molecular Markers. Genetics 2002, 160, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Wagner, A.P.; Taper, M.L. ML-Relate: A computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. Notes. 2006, 6, 576–579. [Google Scholar] [CrossRef]
- McMillan, L.F.; Fewster, R.M. Visualizations for Genetic Assignment Analyses Using the Saddlepoint Approximation Method. Biometrics 2017, 73, 1029–1041. [Google Scholar] [CrossRef]
- Calenge, C. The Package “Adehabitat” for the R Software: A Tool for the Analysis of Space and Habitat Use by Animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Bauder, J.M.; Breininger, D.R.; Bolt, M.R.; Legare, M.L.; Jenkins, C.L.; McGarigal, K. The Role of the Bandwidth Matrix in Influencing Kernel Home Range Estimates for Snakes Using VHF Telemetry Data. Wildl. Res. 2015, 42, 437–453. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Fieberg, J.; Kochanny, C.O. Quantifying Home-Range Overlap: The Importance of the Utilization Distribution. J. Wildl. Manag. 2005, 69, 1346–1359. [Google Scholar] [CrossRef]
- Savje, F. Distances: Tools for Distance Metrics Version 0.1.8 from CRAN. Available online: https://rdrr.io/cran/distances/ (accessed on 5 July 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Goslee, S.C.; Urban, D.L. The Ecodist Package for Dissimilarity-Based Analysis of Ecological Data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Franckowiak, R.P.; Panasci, M.; Jarvis, K.J.; Acuña-Rodriguez, I.S.; Landguth, E.L.; Fortin, M.-J.; Wagner, H.H. Model Selection with Multiple Regression on Distance Matrices Leads to Incorrect Inferences. PLoS ONE 2017, 12, e0175194. [Google Scholar] [CrossRef] [PubMed]
- Kojola, I.; Hallikainen, V.; Heikkinen, S.; Nivala, V. Inadvertent Shooting of Brown Bear Cubs in Finland: What Can Managers Do to Reduce It? Wildl. Biol. 2021, 2021, wlb.00773. [Google Scholar] [CrossRef]
- Frank, S.C.; Pelletier, F.; Kopatz, A.; Bourret, A.; Garant, D.; Swenson, J.E.; Eiken, H.G.; Hagen, S.B.; Zedrosser, A. Harvest is associated with the disruption of social and fine-scale genetic structure among matrilines of a solitary large carnivore. Evol Appl. 2021, 14, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Dahle, B.; Støen, O.-G.; Swenson, J.E. Factors Influencing Home-Range Size in Subadult Brown Bears. J. Mammal. 2006, 87, 859–865. [Google Scholar] [CrossRef]
- Vincent, J.P.; Bideau, E.; Hewison, A.J.M.; Angibault, J.M. The influence of increasing body weight, kid production, home range and winter grouping in roe deer. J. Zool. 1995, 236, 371–382. [Google Scholar] [CrossRef]
- Lopez, R.R.; Harveson, P.M.; Peterson, M.N.; Silvy, N.H.; Frank, P.A. From the Field: Changes in ranges of Florida Key deer—does population density matter? Wildl. Soc. 2005, 33, 343–348. Available online: https://www.jstor.org/stable/3784875 (accessed on 22 November 2021). [CrossRef]
- Kiefer, S.J.; Weckerly, F.W. Population density and body weight influences on home range size of feral hogs. Calif. Fish Game 2005, 91, 136–142. [Google Scholar]
- Mcloughlin, P.D.; Ferguson, S.H.; Messier, F. Intraspecific Variation in Home Range Overlap with Habitat Quality: A Comparison among Brown Bear Populations. Evol. Ecol. 2000, 14, 39–60. [Google Scholar] [CrossRef]
- Ramos-Fernández, G.; Boyer, D.; Gómez, V.P. A Complex Social Structure with Fission–Fusion Properties Can Emerge from a Simple Foraging Model. Behav. Ecol. Sociobiol. 2006, 60, 536–549. [Google Scholar] [CrossRef]
- Collins, G.H.; Kovach, S.D.; Hinkes, M.T. Home range and movements of female brown bears in southwestern Alaska. Ursus 2005, 16, 181–189. Available online: http://www.jstor.org/stable/3873029 (accessed on 22 November 2021). [CrossRef]
- Blanchard, B.M.; Knight, R.R. Movements of yellowstone grizzly bears. Biol. Conserv. 1991, 58, 41–67. [Google Scholar] [CrossRef]
- Sato, Y.; Kobayashi, Y.; Urata, T.; Takatsuki, S. Home range and habitat use of female brown bear (Ursus arctos) in Urahoro, eastern Hokkaido, Japan. Mamm. Study 2008, 33, 99–109. [Google Scholar] [CrossRef]
- Huber, D.; Roth, H.U. Movements of European brown bears in Croatia. Acta Theriol. 1993, 38, 151–159. [Google Scholar] [CrossRef]
- Kaczensky, P.; Knauer, F.; Krze, B.; Jonozovic, M.; Adamic, M.; Gossow, H. The impact of high speed, high volume traffic axes on brown bears in Slovenia. Biol. Conserv. 2003, 111, 191–204. [Google Scholar] [CrossRef]
- Penteriani, V.; Delgado, M.M.; López-Bao, J.V.; García, P.V.; Monrós, J.S.; Álvarez, E.V.; Corominas, T.S.; Vázquez, V.M. Patterns of movement of released female brown bears in the Cantabrian Mountains, northwestern Spain. Ursus 2017, 28, 165–170. Available online: https://www.jstor.org/stable/44751706 (accessed on 22 November 2021). [CrossRef]
- Nyholm, E.S. Petosiirrot—Riistantutkimuksen vaatimaton kokeilu. (Translocation of large carnivores). Metsästäjä 1995, 44, 12–14. [Google Scholar]
- Kojola, I.; Heikkinen, S. The Structure of the Expanded Brown Bear Population at the Edge of the Finnish Range. Ann. Zool. Fenn. 2006, 43, 258–262. Available online: https://www.jstor.org/stable/23736871 (accessed on 22 November 2021).
- Waits, L.; Taberlet, P.; Swenson, J.E.; Sandegren, F.; Franzén, R. Nuclear DNA Microsatellite Analysis of Genetic Diversity and Gene Flow in the Scandinavian Brown Bear (Ursus arctos). Mol. Ecol. 2000, 9, 421–431. [Google Scholar] [CrossRef]
- Zachos, F.E.; Otto, M.; Unici, R.; Lorenzini, R.; Hartl, G.B. Evidence of a Phylogeographic Break in the Romanian Brown Bear (Ursus arctos) Population from the Carpathians. Mammal. Biol. 2008, 73, 93–101. [Google Scholar] [CrossRef]
- Straka, M.; Paule, L.; Ionescu, O.; Štofík, J.; Adamec, M. Microsatellite Diversity and Structure of Carpathian Brown Bears (Ursus arctos): Consequences of Human Caused Fragmentation. Conserv. Genet. 2012, 13, 153–164. [Google Scholar] [CrossRef]
- Hagen, S.B.; Kopatz, A.; Aspi, J.; Kojola, I.; Eiken, H.G. Evidence of Rapid Change in Genetic Structure and Diversity during Range Expansion in a Recovering Large Terrestrial Carnivore. Proc. R. Soc. B 2015, 282, 20150092. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).