Genetic Divergence between Two Sympatric Ecotypes of Phalaenopsis pulcherrima on Hainan Island
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Sampling
2.2. Leaf Color Reflectance
2.3. Molecular Markers
2.3.1. DNA Extraction
2.3.2. ISSR–PCR Amplification
2.3.3. SRAP–PCR Amplification
2.4. Data Analysis
3. Results
3.1. Leaf Color Reflectance
3.2. Genetic Diversity
3.3. Inferred Genetic Groups
3.4. Genetic Similarity among Individuals
3.5. Proportion of Genetic Variance
3.6. Genetic Differentiation and Gene Flow among Populations
3.7. Genetic Relationships among Populations and Individuals
4. Discussion
4.1. Genetic Divergence between Two Ecotypes
4.2. Genetic Divergence within Ecotypes
4.3. Implications for Conservation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galen, C.; Shore, J.S.; Deyoe, H. Ecotypic divergence in alpine Polemonium viscosum: Genetic structure, quantitative variation, and local adaptation. Evolution 1991, 45, 1218–1228. [Google Scholar] [CrossRef]
- Kruckeberg, A.R. An essay: The stimulus of unusual geologies for plant speciation. Syst. Bot. 1986, 11, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Saint-Laurent, R.; Legault, M.; Bernatchez, L. Divergent selection maintains adaptive differentiation despite high gene flow between sympatric rainbow smelt ecotypes (Osmerus mordax Mitchill). Mol. Ecol. 2003, 12, 315–330. [Google Scholar] [CrossRef] [Green Version]
- Foote, A.D.; Newton, J.; Piertney, S.B.; Willerslev, E.; Thomas, M.; Gilbert, P. Ecological, morphological and genetic divergence of sympatric North Atlantic killer whale populations. Mol. Ecol. 2009, 18, 5207–5217. [Google Scholar] [CrossRef]
- Paun, O.; Turner, B.; Trucchi, E.; Munzinger, J.; Chase, M.W.; Samuel, R. Processes driving the adaptive radiation of a tropical tree (Diospyros, Ebenaceae) in New Caledonia, a biodiversity hotspot. Syst. Biol. 2015, 65, 212–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Z.N.; Meng, Q.W.; Ren, M.X. Pollination ecotypes and herkogamy variation of Hiptage benghalensis (Malpighiaceae) with mirror-image flowers. Biodivers. Sci. 2016, 24, 1364–1372. [Google Scholar] [CrossRef] [Green Version]
- Clausen, J. Stages in the Evolution of Plant Species, 6th ed.; Cornell University Press: New York, NY, USA, 1951. [Google Scholar]
- Hendry, A.P.; Taylor, E.B.; McPhail, J.D. Adaptive divergence and the balance between selection and gene flow: Lake and stream stickleback in the Misty system. Evolution 2002, 56, 1199–1216. [Google Scholar] [CrossRef]
- Lowry, D.B. Ecotypes and the controversy over stages in the formation of new species. Biol. J. Linn. Soc. 2012, 106, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Nosil, P. Ecological Speciation; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Lamichhaney, S.; Berglund, J.; Almén, M.S.; Maqbool, K.; Grabherr, M.; Martinez-Barrio, A.; Promerová, M.; Rubin, C.J.; Wang, C.; Zamani, N.; et al. Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature 2015, 518, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Macnair, M.R.; Gardner, M. The evolution of edaphic endemics. In Endless Forms: Species and Speciation, 1st ed.; Howard, D.J., Berlocher, S.H., Eds.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- O’Dell, R.E.; Rajakaruna, N. Intraspecific variation, adaptation, and evolution. In Serpentine: Evolution and Ecology in a Model System; Harrison, S., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011. [Google Scholar]
- Antonovics, J.; Bradshaw, A.D.; Turner, R.G. Heavy metal tolerance in plants. Adv. Ecol. Res. 1971, 7, 1–85. [Google Scholar]
- Reznick, D.N.; Ghalambor, C.K. The population ecology of contemporary adaptations: What empirical studies reveal about the conditions that promote adaptive evolution. Genetica 2001, 112, 183–198. [Google Scholar] [CrossRef]
- Hall, M.C.; Basten, C.J.; Willis, J.H. Pleiotropic quantitative trait loci contribute to population divergence in traits associated with life-history variation in Mimulus guttatus. Genetics 2006, 172, 1829–1844. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.C.; Willis, J.H. Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 2006, 60, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.C.; Lowry, D.B.; Willis, J.H. Is local adaptation in Mimulus guttatus caused by trade-offs at individual loci? Mol. Ecol. 2010, 19, 2739–2753. [Google Scholar] [CrossRef] [PubMed]
- Lowry, D.B.; Rockwood, R.C.; Willis, J.H. Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evol. Int. J. Org. Evol. 2008, 62, 2196–2214. [Google Scholar] [CrossRef]
- Lowry, D.B.; Willis, J.H. A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biol. 2010, 8, e1000500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, R.L.; Diggle, P.K. Node-specific branching and heterochronic changes underlie population-level differences in Mimulus guttatus (Phrymaceae) shoot architecture. Am. J. Bot. 2011, 98, 1924–1934. [Google Scholar] [CrossRef]
- Baker, R.L.; Hileman, L.C.; Diggle, P.K. Patterns of shoot architecture in locally adapted populations are linked to intraspecific differences in gene regulation. New Phytol. 2012, 196, 271–281. [Google Scholar] [CrossRef]
- Oneal, E.; Lowry, D.B.; Wright, K.M.; Zhu, Z.; Willis, J.H. Divergent population structure and climate associations of a chromosomal inversion polymorphism across the Mimulus guttatus species complex. Mol. Ecol. 2014, 23, 2844–2860. [Google Scholar] [CrossRef] [Green Version]
- Twyford, A.D.; Friedman, J. Adaptive divergence in the monkey flower Mimulus guttatus is maintained by a chromosomal inversion. Evolution 2015, 69, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Gould, B.A.; Chen, Y.; Lowry, D.B. Pooled ecotype sequencing reveals candidate genetic mechanisms for adaptive differentiation and reproductive isolation. Mol. Ecol. 2017, 26, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Horie, K.; Ishikawa, N.; Nishio, S.; Worth, J.R.; Fukushima, K.; Yamasaki, M.; Ito, M. Maintenance of soil ecotypes of Solidago virgaurea in close parapatry via divergent flowering time and selection against immigrants. J. Ecol. 2019, 107, 418–435. [Google Scholar] [CrossRef] [Green Version]
- Savolainen, V.; Anstett, M.C.; Lexer, C.; Hutton, I.; Clarkson, J.J.; Norup, M.V.; Powell, M.P.; Springate, D.; Salamin, N.; Baker, W.J. Sympatric speciation in palms on an oceanic island. Nature 2006, 441, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Vekemans, X.; Lefebvre, C. On the evolution of heavy-metal tolerant populations in Armeria maritima: Evidence from allozyme variation and reproductive barriers. J. Evol. Biol. 1997, 10, 175–191. [Google Scholar] [CrossRef]
- Mengoni, A.; Barabesi, C.; Gonnelli, C.; Galardi, F.; Gabbrielli, R.; Bazzicalupo, M. Genetic diversity of heavy metal-tolerant populations in Silene paradoxa L. (Caryophyllaceae): A chloroplast microsatellite analysis. Mol. Ecol. 2001, 10, 1909–1916. [Google Scholar] [CrossRef]
- Pauwels, M.; Saumitou-Laprade, P.; Holl, A.C.; Petit, D.; Bonnin, I. Multiple origins of metallicolous populations of the pseudometallophyte Arabidopsis halleri (Brassicaceae) in central Europe: The cpDNA testimony. Mol. Ecol. 2005, 14, 4403–4414. [Google Scholar] [CrossRef]
- Jiménez-Ambriz, G.; Petit, C.; Bourrié, I.; Dubois, S.; Olivieri, I.; Ronce, O. Life history variation in the heavy metal tolerant plant Thlaspi caerulescens growing in a network of contaminated and noncontaminated sites in southern France: Role of gene flow, selection and phenotypic plasticity. New Phytol. 2007, 173, 199–215. [Google Scholar] [CrossRef]
- Avise, J.C. Molecular Markers, Natural History and Evolution, 2nd ed.; Springer Science & Business Media: Sunderland, MA, USA, 2012. [Google Scholar]
- Linde, M.; Diel, S.; Neuffer, B. Flowering ecotypes of Capsella bursa-pastoris (L.) Medik. (Brassicaceae) analysed by a cosegregation of phenotypic characters (QTL) and molecular markers. Ann. Bot. 2001, 87, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Knapp, E.E.; Rice, K.J. Genetic structure and gene flow in Elymus glaucus (blue wildrye): Implications for native grassland restoration. Restor. Ecol. 1996, 4, 110. [Google Scholar] [CrossRef]
- Hu, X.Y. Population Genetic Structure of Phalaenopsis pulcherrima. Master’s Thesis, Hainan University, Haikou, China, 2014. [Google Scholar]
- Zhang, Z.; Gale, S.W.; Li, J.H.; Fischer, G.A.; Ren, M.X.; Song, X.Q. Pollen-mediated gene flow ensures connectivity among spatially discrete sub-populations of Phalaenopsis pulcherrima, a tropical food-deceptive orchid. BMC Plant Biol. 2019, 19, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ke, H.L.; Song, X.Q.; Tan, Z.Q.; Liu, H.X.; Luo, Y.B. Endophytic fungi diversity in root of Doritis pulcherrima (Orchidaceae). Biodivers. Sci. 2007, 15, 456–462. [Google Scholar]
- Yang, Q.; Song, X.Q.; Hu, X.W.; Zhu, G.P. Soil Physical and Chemical Properties in Phalaenopsis pulcherrima (Orchidaceae) Habitat in Bawangling Natural Reserve, Hainan Island. Chin. J. Trop. Agric. 2013, 33, 7–12. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple-sequence repeat (SSR) anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Quiros, C.F. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Bassam, B.J.; Caetanoanolles, G.; Gresshoff, P.M. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 1991, 196, 80–83. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T. Popgene Version 1.32: Microsoft Windows-Based Freeware for Population Genetic Analysis; University of Alberta: Edmonton, AB, Canada, 1999. [Google Scholar]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. 2007, 7, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Baumel, A.; Médail, F.; Juin, M.; Paquier, T.; Clares, M.; Laffargue, P.; Lutard, H.; Dixon, L.; Pires, M. Population genetic structure and management perspectives for Armeria belgenciencis, a narrow endemic plant from Provence (France). Plant Ecol. Evol. 2020, 153, 219–228. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 117. [Google Scholar] [CrossRef] [Green Version]
- Hedrick, P. Genetics of Populations, 4th ed.; Jones & Bartlett Learning: Sudbury, MA, USA, 2011. [Google Scholar]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 4th ed.; Sinauer Associates: Sunderland, UK, 2007. [Google Scholar]
- Menz, M.H.; Phillips, R.D.; Anthony, J.M.; Bohman, B.; Dixon, K.W.; Peakall, R. Ecological and genetic evidence for cryptic ecotypes in a rare sexually deceptive orchid, Drakaea elastica. Bot. J. Linn. Soc. 2015, 177, 124–140. [Google Scholar] [CrossRef] [Green Version]
- Sexton, J.P.; Hangartner, S.B.; Hoffmann, A.A. Genetic isolation by environment or distance: Which pattern of gene flow is most common? Evolution 2014, 68, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Ge, S. Genetic variation and conservation of Changnienia amoena, an endangered orchid endemic to China. Plant Syst. Evol. 2006, 258, 251–260. [Google Scholar] [CrossRef]
- Fay, M.F.; Bone, R.; Cook, P.; Kahandawala, I.; Greensmith, J.; Harris, S.; Pedersen, H.; Ingrouille, M.J.; Lexer, C. Genetic diversity in Cypripedium calceolus (Orchidaceae) with a focus on north-western Europe, as revealed by plastid DNA length polymorphisms. Ann. Bot. 2009, 104, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.; Warner, J.; Albertazzi, F.J. Genetic diversity analysis of the endangered slipper orchid Phragmipedium longifolium in Costa Rica. Plant Syst. Evol. 2010, 290, 217–223. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; van Staden, J. Molecular insights into genetic diversity and population dynamics of five medicinal Eulophia species: A threatened orchid taxa of Africa. Physiol. Mol. Biol. Plants 2018, 24, 631–641. [Google Scholar] [CrossRef]
- Huang, Y.; Li, F.; Chen, K. Analysis of diversity and relationships among Chinese orchid cultivars using EST-SSR markers. Biochem. Syst. Ecol. 2010, 38, 93–102. [Google Scholar] [CrossRef]
- Macnair, M.R. Heavy-metal tolerance in plants: A model evolutionary system. Trends Ecol. Evol. 1987, 2, 354–359. [Google Scholar] [CrossRef]
- Martin, N.H.; Willis, J.H. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 2007, 61, 68–82. [Google Scholar] [CrossRef]
- Hirao, A.; Kudo, G. The effect of segregation of flowering time on fine-scale spatial genetic structure in an alpine-snowbed herb Primula cuneifolia. Heredity 2008, 100, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, Y.; Kudo, G. Local differentiation of flowering phenology in an alpine-snowbed herb Gentiana nipponica. Botany 2011, 89, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Wadgymar, S.M.; Weis, A.E. Phenological mismatch and the effectiveness of assisted gene flow. Conserv. Biol. 2017, 31, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Schemske, D.W.; Bradshaw, H. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc. Natl. Acad. Sci. USA 1999, 96, 11910–11915. [Google Scholar] [CrossRef] [Green Version]
- Streisfeld, M.A.; Kohn, J.R. Contrasting patterns of floral and molecular variation across a cline in Mimulus aurantiacus. Evolution 2005, 59, 2548–2559. [Google Scholar] [CrossRef]
- Sobel, J.M.; Streisfeld, M.A. Strong premating reproductive isolation drives incipient speciation in Mimulus aurantiacus. Evolution 2015, 69, 447–461. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, Q.; Li, S.L.; Li, Z.Y.; Yang, L.Y. Study on mycorrhizal fungi in some species of tropical orchids in Xishuangbanna, Yunnan. J. Yunnan Univ. 2003, 25, 161–163. [Google Scholar]
- Yin, J.M.; Yang, G.S.; Tong, H.L.; Peng, H.C. A comparative analysis of the karyotypes between doirtis puleherirma Lindley with two colors on leaf dorsal side. Chin. J. Trop. Plants 2008, 29, 762–766. [Google Scholar]
- Field, T.S.; Lee, D.W.; Holbrook, N.M. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol. 2001, 127, 566–574. [Google Scholar] [CrossRef]
- Phillips, R.D.; Dixon, K.W.; Peakall, R. Low population genetic differentiation in the Orchidaceae: Implications for the diversification of the family. Mol. Ecol. 2012, 21, 5208–5220. [Google Scholar] [CrossRef] [PubMed]
- Baus, E.; Darrock, D.J.; Bruford, M.W. Gene-flow patterns in Atlantic and Mediterranean populations of the Lusitanian sea star Asterina gibbosa. Mol. Ecol. 2005, 14, 3373–3382. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Plant species diversity in old-field successional ecosystems in Southern Illinois. Ecology 1975, 56, 485–488. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Henle, K.; Davies, K.F.; Kleyer, M.; Margules, C.; Settele, J. Predictors of species sensitivity to fragmentation. Biodivers. Conserv. 2004, 13, 207–251. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liang, Q.L.; Hao, G.Q.; Chen, C.L.; Liu, J.Q. Population genetic analyses of the endangered alpine Sinadoxa corydalifolia (Adoxaceae) provide insights into future conservation. Biodivers. Conserv. 2018, 27, 2275–2291. [Google Scholar] [CrossRef]
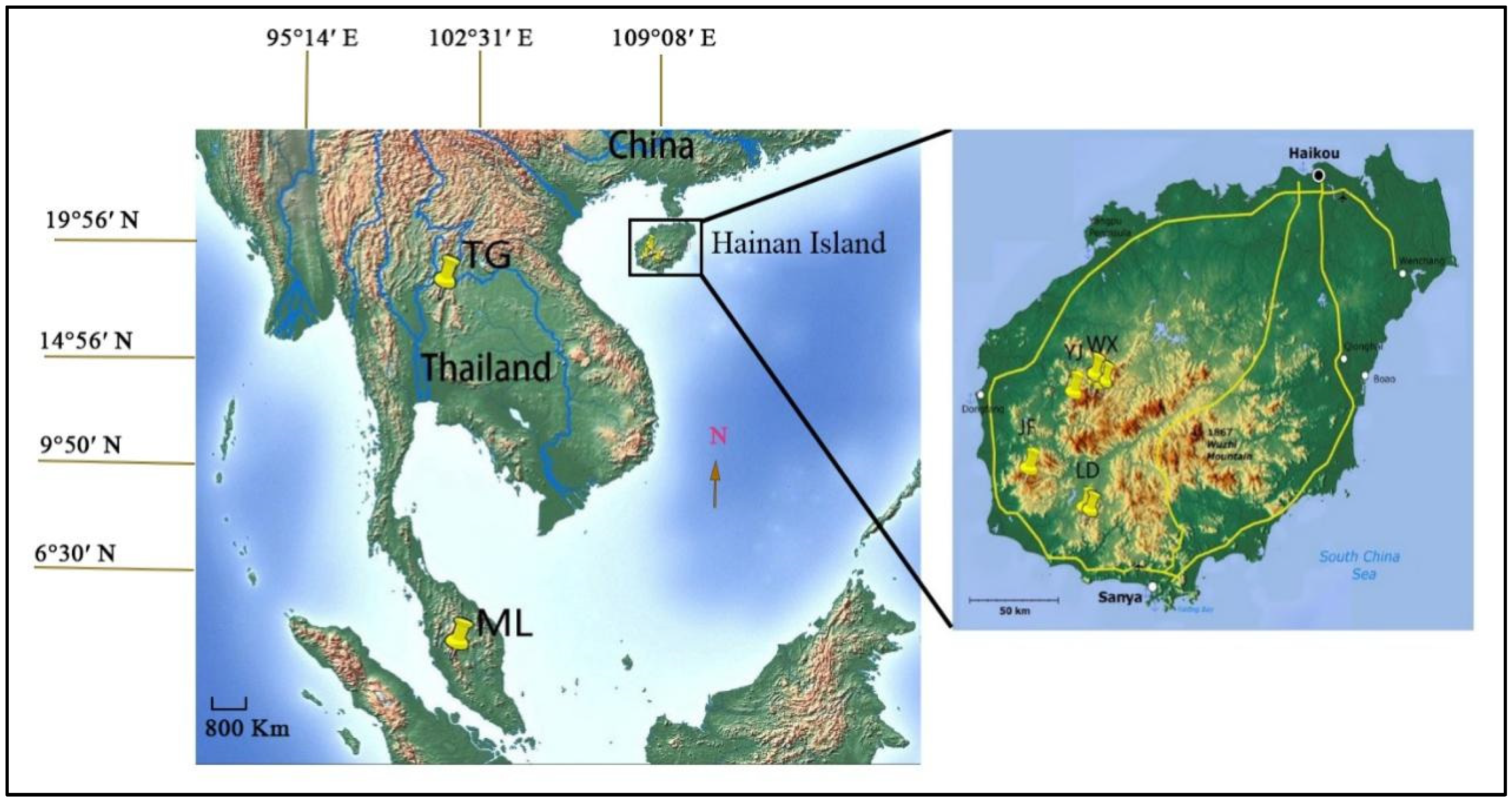
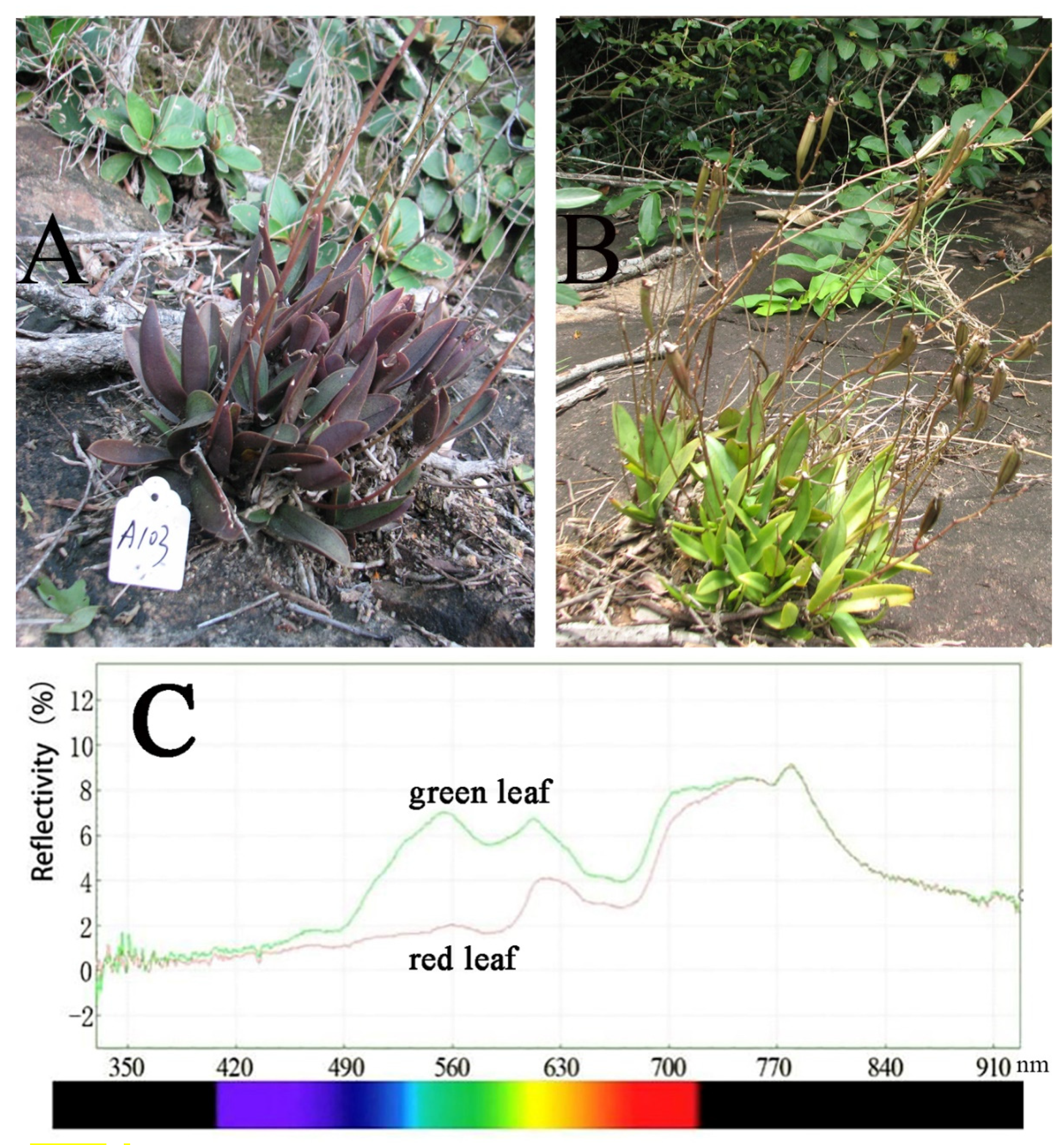
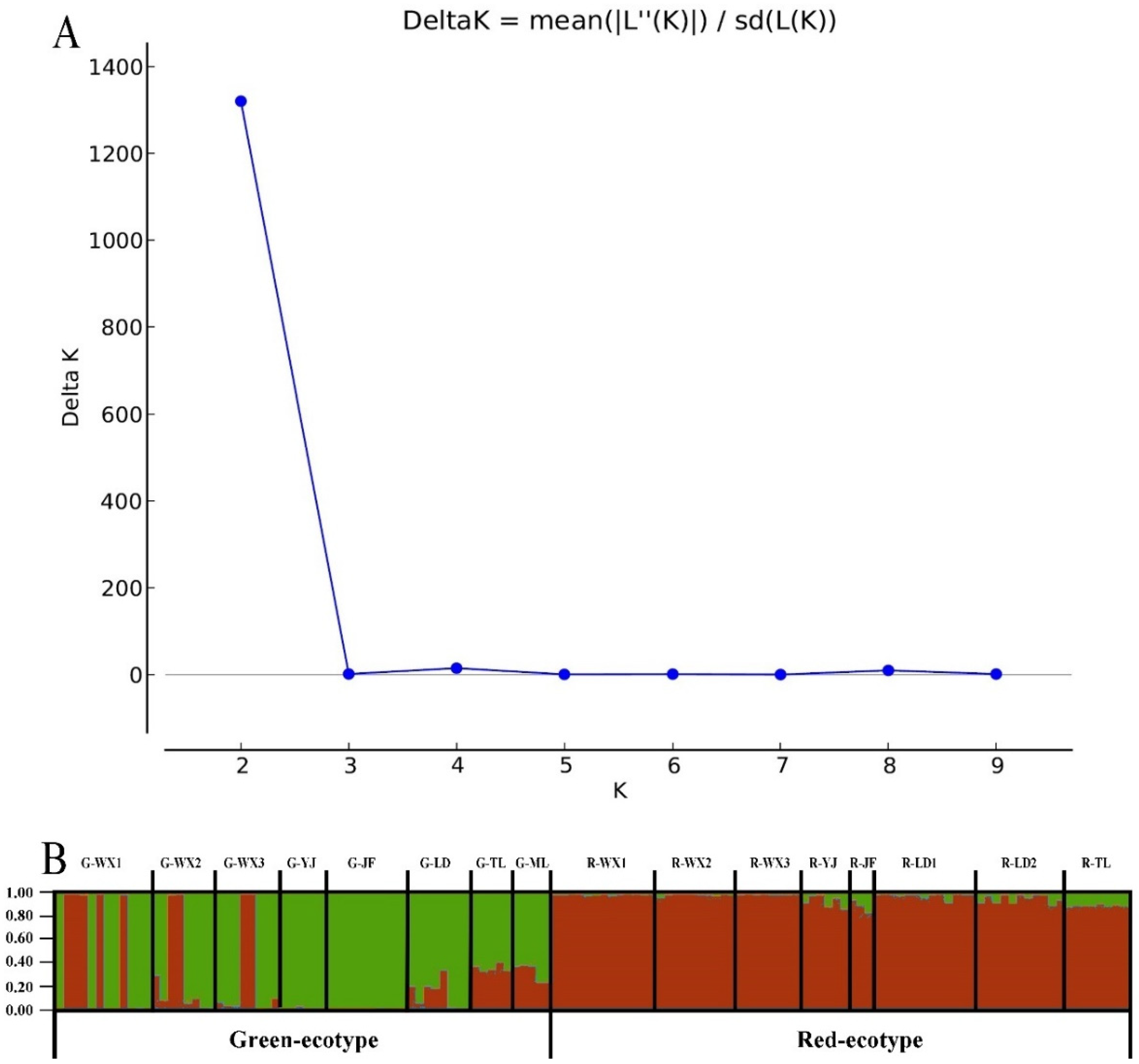
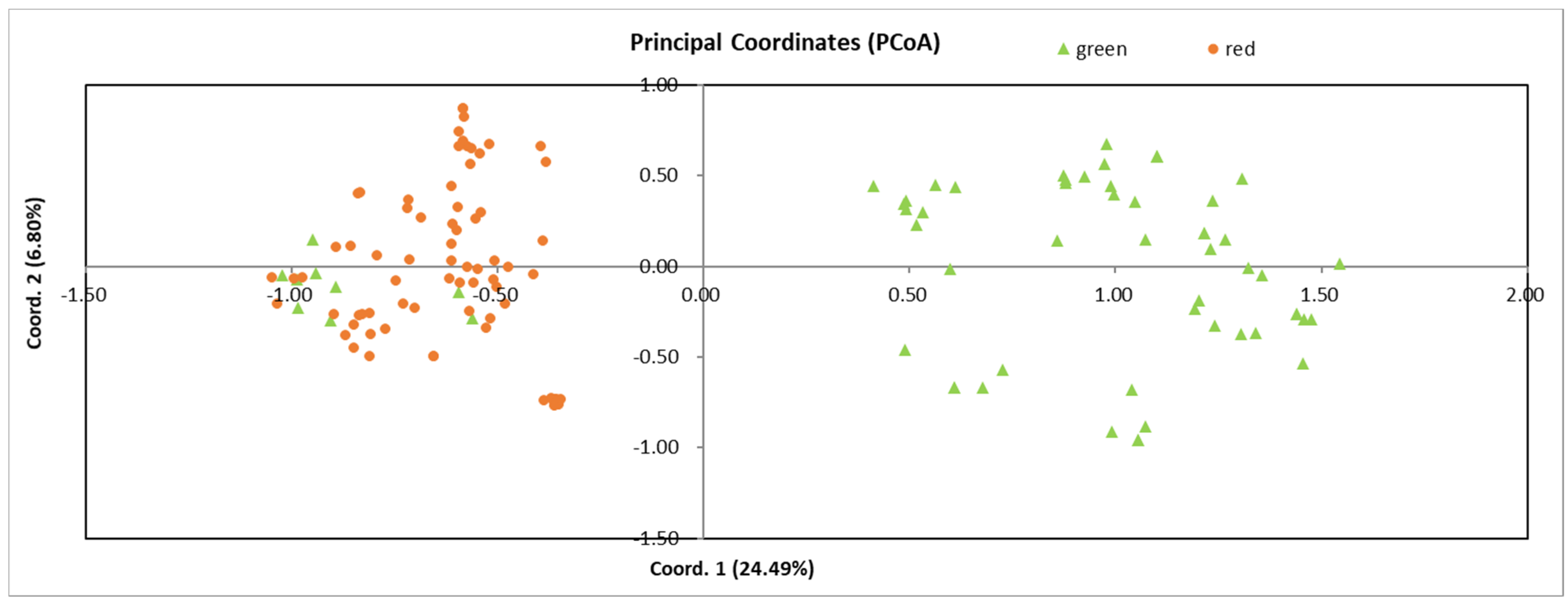


| Pop. | Location | Altitude (m) | Sample Size for Molecular Marker | |
|---|---|---|---|---|
| Red-Leaf | Green-Leaf | |||
| WX1 | Wangxia | 520–760 | 13 | 12 |
| WX2 | Wangxia | 200–280 | 10 | 8 |
| WX3 | Wangxia | 200–260 | 8 | 8 |
| YJ | Yajia | 530–740 | 6 | 6 |
| JF | Jianfengling | 430–500 | 3 | 10 |
| LD1 | Ledong | 430–460 | 13 | 4 |
| LD2 | Ledong | 410–420 | 11 | 4 |
| TL | Thailand | 350–410 | 8 | 5 |
| ML | Malaysia | 450–530 | 0 | 5 |
| ISSR | SRAP | ||
|---|---|---|---|
| Primer (5′-3′) | T (℃) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
| ISSR-1: (CAAC)4 | 55 | ME1: TGAGTCCAAACCGGATA | EM1: GACTGCGTACGAATTAAT |
| ISSR-2: A(GCT)5G | 65 | ME2: TGAGTCCAAACCGGAGC | EM2: GACTGCGTACGAATTTGC |
| ISSR-3: A(TGC)6 | 65 | ME3: TGAGTCCAAACCGGAAT | EM3: GACTGCGTACGAATTGAC |
| ISSR-4: A(GCAC)4G | 58 | ME4: TGAGTCCAAACCGGACC | EM4: GACTGCGTACGAATTTGA |
| ISSR-5: (AC)8T | 58 | ME5: TGAGTCCAAACCGGAAG | EM5: GACTGCGTACGAATTAAC |
| ISSR-6: (AC)8G | 54 | ME6: TGAGTCCAAACCGGTAA | EM6: GACTGCGTACGAATTGCA |
| ISSR-7: (AC)8CTT | 48 | ME7: TGAGTCCAAACCGGTCC | EM7: GACTGCGTACGAATTGAG |
| ISSR-8: (AC)8CTA | 58 | ME8: TGAGTCCAAACCGGTGC | EM8: GACTGCGTACGAATTCTG |
| ISSR-9: (ATG)6 | 55 | ME9: TGAGTCCAAACCGGTAG | EM9: GACTGCGTACGAATTGTC |
| ISSR-10: (GACA)4 | 51 | ME10: TGAGTCCAAACCGGTTG | EM10: GACTGCGTACGAATTCAG |
| ISSR-11: (CA)8G | 59 | ME11: TGAGTCCAAACCGGTGT | EM11: GACTGCGTACGAATTCCA |
| ISSR-12: (CA)8AGC | 50 | ME12: TGAGTCCAAACCGGTCA | EM12: GACTGCGTACGAATTCAC |
| ME13: TGAGTCCAAACCGGGAC | EM13: GACTGCGTACGAATTGCC | ||
| ME14: TGAGTCCAAACCGGGTA | EM14: GACTGCGTACGAATTGGT | ||
| ME15: TGAGTCCAAACCGGGGT | EM15: GACTGCGTACGAATTCGG | ||
| ME16: TGAGTCCAAACCGGCAG | EM16: GACTGCGTACGAATTATG | ||
| ME17: TGAGTCCAAACCGGCTA | EM17: GACTGCGTACGAATTAGC | ||
| EM18: GACTGCGTACGAATTACG | |||
| EM19: GACTGCGTACGAATTTAG | |||
| EM20: GACTGCGTACGAATTTCG | |||
| Morphotype | Number of Loci | Polymorphic Loci | na | ne | h | I |
|---|---|---|---|---|---|---|
| Green | 164 | 163 | 1.9879 | 1.7666 | 0.4209 | 0.6061 |
| Red | 164 | 162 | 1.9818 | 1.5571 | 0.3296 | 0.4959 |
| Source of Variation | Degree of Freedom | Sum of Squares | Variance Components | Percentage Variation (%) | Fixation Indices |
|---|---|---|---|---|---|
| Between ecotypes | 1 | 726.860 | 9.66015 | 24.54707 | FCT = 0.245 *** |
| Among populations within ecotypes | 14 | 1075.642 | 6.50733 | 16.53553 | FSC = 0.219 *** |
| Among individuals within populations | 118 | 2735.961 | 23.18611 | 58.91740 | FST = 0.411 *** |
| Total | 133 | 4538.463 | 39.35358 |
| G-WX1 | G-WX2 | G-WX3 | G-YJ | G-JF | G-LD | G-TL | G-ML | R-WX1 | R-WX2 | R-WX3 | R-YJ | R-JF | R-LD1 | R-LD2 | R-TL | |
| G-WX1 | - | - | 1.703 | 0.606 | 0.669 | 0.994 | 1.783 | 0.946 | 0.861 | 1.026 | 1.943 | 4.558 | 0.800 | 1.154 | 0.420 | |
| G-WX2 | 0.000 | - | 2.497 | 0.861 | 0.924 | 1.342 | 1.869 | 0.646 | 0.659 | 0.823 | 1.406 | 2.997 | 0.704 | 0.940 | 0.373 | |
| G-WX3 | 0.000 | 0.000 | 3.426 | 0.750 | 0.519 | 0.792 | 1.373 | 0.549 | 0.564 | 0.653 | 1.059 | 1.630 | 0.544 | 0.779 | 0.297 | |
| G-YJ | 0.128 | 0.091 * | 0.068 | 0.940 | 0.318 | 0.452 | 0.814 | 0.223 | 0.218 | 0.240 | 0.342 | 0.370 | 0.241 | 0.321 | 0.147 | |
| G-JF | 0.292 * | 0.225 * | 0.250 * | 0.210 * | 0.301 | 0.249 | 0.413 | 0.164 | 0.155 | 0.161 | 0.202 | 0.197 | 0.162 | 0.200 | 0.109 | |
| G-LD | 0.272 * | 0.213 * | 0.325 * | 0.440 * | 0.454 * | 0.253 | 0.273 | 0.209 | 0.199 | 0.204 | 0.248 | 0.261 | 0.190 | 0.240 | 0.121 | |
| G-TL | 0.201 * | 0.157 * | 0.240 * | 0.356 * | 0.501 * | 0.497 * | 0.876 | 0.273 | 0.268 | 0.269 | 0.369 | 0.426 | 0.347 | 0.483 | 0.132 | |
| G-ML | 0.123 | 0.118 * | 0.154 * | 0.235 * | 0.377 * | 0.478 * | 0.222 * | 0.318 | 0.335 | 0.334 | 0.494 | 0.621 | 0.370 | 0.544 | 0.167 | |
| R-WX1 | 0.209 * | 0.279 * | 0.313 * | 0.529 * | 0.604 * | 0.545 * | 0.478 * | 0.440 * | 2.997 | 3.373 | 2.836 | 3.656 | 0.918 | 1.511 | 0.354 | |
| R-WX2 | 0.225 * | 0.275 * | 0.307 * | 0.534 * | 0.617 * | 0.557 * | 0.483 * | 0.427 * | 0.077 * | 35.464 | 3.426 | 2.131 | 0.727 | 1.523 | 0.277 | |
| R-WX3 | 0.196 * | 0.233 * | 0.277 * | 0.510 * | 0.608 * | 0.551 * | 0.482 * | 0.428 * | 0.069 * | 0.007 * | 3.083 | 3.222 | 0.876 | 1.498 | 0.293 | |
| R-YJ | 0.114 | 0.151 * | 0.191 * | 0.422 * | 0.553 * | 0.502 * | 0.404 * | 0.336 * | 0.081 * | 0.068 * | 0.075 * | 83.083 | 0.856 | 1.799 | 0.325 | |
| R-JF | 0.052 | 0.077 | 0.133 | 0.403 * | 0.559 * | 0.489 * | 0.370 * | 0.287 * | 0.064 | 0.105 * | 0.072 | 0.003 | 1.322 | 2.275 | 0.274 | |
| R-LD1 | 0.238 * | 0.262 * | 0.315 * | 0.509 * | 0.607 * | 0.568 * | 0.419 * | 0.403 * | 0.214 * | 0.256 * | 0.222 * | 0.226 * | 0.159 * | 4.558 | 0.264 | |
| R-LD2 | 0.178 * | 0.210 * | 0.243 * | 0.438 * | 0.556 * | 0.510 * | 0.341 * | 0.315 * | 0.142 * | 0.141 * | 0.143 * | 0.122 * | 0.099 * | 0.052 * | 0.289 | |
| R-TL | 0.373 * | 0.401 * | 0.457 * | 0.629 * | 0.696 * | 0.674 * | 0.655 * | 0.599 * | 0.414 * | 0.474 * | 0.460 * | 0.435 * | 0.477 * | 0.486 * | 0.464 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Lan, S.; Song, X.; Yang, F.; Zhang, Z.; Peng, D.; Ren, M. Genetic Divergence between Two Sympatric Ecotypes of Phalaenopsis pulcherrima on Hainan Island. Diversity 2021, 13, 446. https://doi.org/10.3390/d13090446
Hu X, Lan S, Song X, Yang F, Zhang Z, Peng D, Ren M. Genetic Divergence between Two Sympatric Ecotypes of Phalaenopsis pulcherrima on Hainan Island. Diversity. 2021; 13(9):446. https://doi.org/10.3390/d13090446
Chicago/Turabian StyleHu, Xiangyu, Siren Lan, Xiqiang Song, Fusun Yang, Zhe Zhang, Donghui Peng, and Mingxun Ren. 2021. "Genetic Divergence between Two Sympatric Ecotypes of Phalaenopsis pulcherrima on Hainan Island" Diversity 13, no. 9: 446. https://doi.org/10.3390/d13090446
APA StyleHu, X., Lan, S., Song, X., Yang, F., Zhang, Z., Peng, D., & Ren, M. (2021). Genetic Divergence between Two Sympatric Ecotypes of Phalaenopsis pulcherrima on Hainan Island. Diversity, 13(9), 446. https://doi.org/10.3390/d13090446






