Abstract
Since the early 1900s, researchers have attempted to unravel the origin and evolution of tetrapod limb muscles using a combination of comparative anatomy, phylogeny, and development. The methods for reconstructing soft tissues in extinct animals have been refined over time as our ability to determine muscle homology and phylogenetic relationships between tetrapods has improved. Since many muscles do not leave osteological correlates, muscle reconstruction in extinct animals is largely based on anatomy and development in extant animals. While muscle anatomy in extant tetrapods is quite conservative, the homologies of certain muscles between taxonomic groups are still uncertain. Comparative developmental studies can help to resolve these controversies, as well as revealing general patterns of muscle morphogenesis across tetrapod groups. We review the methods, results, and controversies in the muscle reconstructions of early members of the amniote, mammalian, and lissamphibian lineages, including recent attempts to reconstruct limb muscles in members of the tetrapod stem group. We also review the contribution of recent comparative developmental studies toward understanding the evolution of tetrapod limb muscles, including morphogenic gradients, the origin of paired fins, and the evolution of morphological complexity. Finally, we discuss the role of broad, comparative myological studies as part of an integrative research program on vertebrate evolutionary biology.
1. Introduction
The study of muscles has too often been neglected in recent decades, especially in a broad phylogenetic context that spans all major tetrapod groups. Exceptional comparative myological studies were conducted in the late 1800s and early 1900s by authors such as M. Fürbringer, H. Gadow, J. P. McMurrich, W. K. Gregory, A. S. Romer, F. H., Edgeworth, and G. M. Humphry. However, these authors lacked crucial information about the phylogenetic relationships of tetrapods and developmental patterning of muscles provided by modern molecular, genetic, and developmental studies.
One research area that integrates comparative anatomy, evolution, and development is the reconstruction of soft tissues in extinct animals. These reconstructions formed the basis for influential hypotheses about animal ecology, performance, and adaptation to new environments. Although fossils enjoyed a fair amount of attention from comparative anatomists in the early 1900s, scientifically based muscle reconstructions (e.g., [1,2,3,4,5,6]) are relatively rare. Recent decades have seen a renewed interest in muscles and in developing new tools and methods to reconstruct them more accurately in fossil taxa [7,8]. These include recent studies of animals close to the fin-limb and water-land transitions (e.g., [9,10,11,12]), which employed a modern phylogenetic framework and understanding of the developmental relationship between muscles and bones.
The recent interest in fossil muscle reconstructions can be seen as part of a resurgence of the study of comparative anatomy, largely a by-product of the rise of Evo-Devo [13]. In fact, over the past several decades, the advances in developmental biology have probably contributed more to our understanding of tetrapod limb evolution than any other research area. Yet, comparative studies of muscle development lag far behind similar studies of bones, and muscle development in non-model organisms is poorly understood. Recently developed embryological approaches and techniques hold great promise for advancing our understanding of the evolution of tetrapod muscle anatomy and musculoskeletal function.
2. Limb Muscle Reconstructions in Extinct Tetrapods and Persistent Controversies
This first section reviews the literature on limb muscle reconstructions in fossil tetrapods and their implications for the evolution of tetrapod limb function. It also addresses some controversial aspects of tetrapod muscle evolution in light of modern tetrapod phylogeny and muscle development. We cover the mammalian, amniote, lissamphibian, reptilian, and tetrapod stem groups (see [8] for human ancestors, see [14] for dinosaurs).
2.1. Evolution of Reconstruction Methodology
Reconstruction of soft tissues in extinct animals is important for inferring functional characteristics and thus their ecology and performance, bridging the gaps between extant taxa and ultimately understanding how animals adapt to different environments [7]. Muscle reconstruction has a long history, but only recently has it been formalized using explicit phylogenetically based hypothesis-testing methods, such as the extant phylogenetic bracket (EPB) [7,15]. These methods can provide evidence of muscle attachment areas but cannot reliably predict muscle sizes or architecture [16].
“Extant phylogenetic bracket” describes the relationship between a fossil taxon and its two closest extant sister groups, and the EPB approach is a method of phylogenetic inference based on this relationship [7]. For example, an EPB of the stem mammal Dimetrodon must include at least a crown reptile and a mammal (Figure 1). The EPB approach involves three steps: first, determine a causal relationship between a preserved and an unpreserved attribute; for example, the cnemial crest in extant amniotes is produced by the quadriceps tendon that attaches to it. Second, form a hypothesis that this association is homologous among the extant taxa of interest; for example, that a common ancestor of extant amniotes had a cnemial crest produced by a quadriceps tendon. Finally, test this hypothesis by looking for the osteological correlate in the fossil ancestors of extant amniotes (e.g., Dimetrodon, Cynognathus, Captorhinus). If the causal relationship is valid and at least the first two extant sister groups (bracket taxa) of a fossil taxon with a cnemial crest possess both the soft tissue and the osteological correlate, then the soft tissue can be confidently reconstructed in the fossil that also possesses the osteological correlate [7]. Phylogenetically based reconstruction is most straightforward in clades with a good fossil record and at least two morphologically similar bracket taxa, such as human ancestors. It is much more challenging to reconstruct muscles in animals with no morphologically similar extant analogue and/or highly divergent bracket taxa, such as stem tetrapods and non-avian dinosaurs.
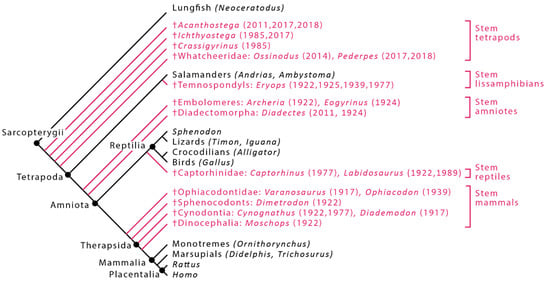
Figure 1.
Cladogram showing hypothesized phylogenetic relationships of taxa and the dates of published reconstructions [1,3,4,6,9,10,11,19,20,21,22]. Extant animals are shown in black and extinct animals (†) are shown in red. An extant phylogenetic bracket (EPB) consists of minimally the two closest extant sister groups; for example, the EPB of the stem mammal Dimetrodon would include a crown reptile (e.g., Iguana) and a crown mammal (e.g., Didelphis).
Muscle reconstructions based on comparative anatomy flourished in the early 1900s, driven by a desire to understand locomotor function in extinct tetrapods [17]. Although the methods and approaches varied widely among researchers, the basic principles of what would later be formalized as the extant phylogenetic bracket method arose during this period. They include the use of a broad phylogenetic range of extant taxa [6,18], tracing of the osteological correlates of soft tissue throughout tetrapod lineages over time [1,6,19], and hypotheses of a causal relationship between soft and hard tissues (e.g., [3]). The 20th century saw muscle reconstructions of forelimb anatomy in the amniote, lissamphibian, mammalian, reptilian, and archosaurian lineages (Figure 1). Only recently have researchers attempted detailed reconstruction of limb musculature in the tetrapod stem group.
2.2. Stem Mammalia: “Mammal-Like Reptiles” and the Evolution of Mammalian Locomotion
The earliest muscle reconstructions in extinct animals focused on stem mammals (“mammal-like reptiles” more closely related to mammals than to reptiles but outside Mammalia). These reconstructions were largely based on the anatomy of extant lepidosaurs and, to a lesser extent, on monotremes. Thus, from the beginning an attempt was made to deduce the ancestral state of limb musculature from characters shared by its extant relatives. In contrast to modern methods that use a wide range of extant taxa to infer an ancestral condition, these studies often identified a few “primitive” extant taxa, or “types,” that bore a general osteological resemblance to the fossils in question. Watson [23] created muscle maps of the humeri of two stem mammals based on comparisons with an extant lepidosaurs (the tuatara Sphenodon) and an extant monotreme (the anteater Echidna). As members of an early diverging reptilian lineage, tuataras have often been used for comparison because they were considered “primitive” reptiles whose musculature presumably represented the ancestral reptilian state. Watson [23] was interested in the origin of the mammalian shoulder girdle and believed that the best way to approach it was to study the most basal members of the mammalian lineage, specifically the Early Permian pelycosaur Varanosaurus and the Middle Triassic cynodont Diademodon. A muscle map of the proximal end of the humerus of Varanosaurus was constructed based on comparison with tuataras, with the justification that tuatara limb musculature “must be very similar to that of the Pelycosaurs [23], p. 14.” A muscle map of the humerus of Diademodon was based mainly on the pelycosaur humerus, but dissections of tuataras, anteaters, and an embryonic opossum were also consulted. Following the hierarchical concepts common in early 19th century comparative anatomy (which were based on the ancient concept of scala naturae, or ‘ladder-of-life’, also designated the ‘chain of being’), the author concluded that changes in the orientation of the humerus between pelycosaurs and cynodonts would have resulted in changes in the leverage of the shoulder muscles, producing a “mechanically more superior animal” [23], p. 62.
Almost simultaneously, Gregory and Camp [6] released a similar study on the proximal fore- and hindlimb musculature of another stem mammal, the cynodont Cynognathus (Mid-Triassic) (as well as the saurischian dinosaur Ornitholestes). Their stated aim was to demonstrate how comparative anatomy could bring new interest to paleontology. Like Watson [23], they based their reconstructions primarily on tuataras and a monotreme (the platypus Ornithorhynchus), but Gregory and Camp [6] also considered crocodilians, marsupials, and birds. Their reconstructions were based on the homology hypotheses of Gadow [18] and Fürbringer [24], which emphasized the primacy of innervation in determining homology and are extremely similar to the modern consensus. Notably, this manuscript explicitly traced the osteological correlates of muscle attachment (femoral trochanters) through the fossil record. Similar to Watson [23], the authors concluded that the cynodont shoulder girdle and humerus had both reptilian and mammalian characteristics, but they interpreted the pelvic girdle as being more similar to that of the platypus because of shared osteological characteristics, including the forward expansion of the ilium, the shape of the puboischiadic plate, and the large external (third) trochanter of the femur. In addition, Gregory and Camp [6] proposed functional hypotheses explaining the differences in musculoskeletal structure between tuataras, Cynognathus, monotremes, and humans.
2.3. Stem Amniota: “Bridging the Gap”
It is impossible to overstate the importance of A. S. Romer’s contributions to comparative anatomy, functional morphology, and paleontology, as many writers have pointed out (e.g., [17,25]). Romer’s [1] manuscript on the appendicular muscles of stem amniotes and stem mammals built upon the work of Watson [23] and Gregory and Camp [6], and it aimed to “bridge the gap” in limb structure between reptiles and mammals [1], p. 519. Notably, this manuscript described the tetrahedral structure underlying the tetrapod humerus, a great achievement that enabled the identification of homologous regions, processes, and foramina across amniotes and lissamphibians. Muscle maps and muscle reconstructions were produced for a dizzying array of taxa, including the stem amniotes Diadectes and Archeria and the non-mammalian synapsid Dimetrodon (all of which lived in the Early Permian) and the Middle Permian therapsid Moschops. The manuscript even included partial muscle maps of the humeri of a stem lissamphibian (Eryops) and a stem reptile (Labidosaurus). An extant lizard (Iguana) and an extant marsupial (the opossum Didelphys) were used for comparison. The interpretation of the pectoral musculature in Cynognathus agreed with that of Gregory and Camp [6] in all but a few details (see Controversies). New muscle homology hypotheses were proposed based on attachments, anatomical relationships, and innervation. In addition to venturing outside of crown Amniota, this manuscript was also the first to propose homology between the limb muscles of tetrapods and the fin muscles of fish [1]. The author followed Sewertzoff [26] in dividing tetrapod limb muscles into dorsal and ventral groups, which are largely the same as the ones recovered in modern developmental studies. However, unlike Sewertzoff [26] and modern studies, Romer [1] classed the deltoid and procoracohumeralis and their derivatives as ventral muscles.
Romer [19] further developed the idea of homologizing structures between the fish fin and the tetrapod limb in a subsequent manuscript. The forelimbs of the stem amniotes Eogyrinus (also known as Pholiderpeton) and Diadectes were compared with the fins of various fishes, including sharks, lungfish, bichirs, bowfins, and sturgeons, thereby laying the groundwork for future phylogenetically based reconstructions of stem tetrapods. At the time, it was thought that tetrapods were most closely related to bichirs, but the author noted that lungfish had the most tetrapod-like distal fin musculature: “Only in [the Australian lungfish] Ceratodus is there a series of muscles extending down most of the length of the fin which is comparable with the tetrapod limb musculature” [19], p. 133. Now considered the closest extant relatives of tetrapods based on comparisons of their DNA [27,28], lungfish have been used as bracket taxa to help reconstruct limb muscles in early tetrapods [10,11].
2.4. Stem Lissamphibia
Although many aspects of what would become known as the extant phylogenetic bracket method were already in use, Miner [3] was the first to clearly articulate its central principles: the use of at least two extant outgroups, and explicit hypotheses of biological homology [7]. Miner [3] reconstructed the muscles of the forelimb (including the wrist and manus) of the stem lissamphibian Eryops by tracing osteological correlates of muscle attachment in extant amphibians and reptiles (giant salamanders and tuataras) “because of the established ancestral relationship of the Stegocephalia as a group to both the amphibian and the reptilian stems” [3], p. 151. Further, the author hypothesized a causal relationship between soft and hard tissues: “The vertebrate endoskeleton may, therefore, be said to have been extensively molded by muscle activity” [3], p. 149. Although his methods did not differ greatly from those of earlier researchers, Miner’s articulation of this causal hypothesis in the context of muscle reconstruction deserves recognition. Future studies would rely on hypotheses of causal relationships between preserved and unpreserved attributes to reconstruct other soft tissues, such as joints and vasculature, and even behaviors (e.g., [29,30,31,32,33]). To establish the homology hypotheses, Miner [3] considered not only the anatomical relationships, attachments, and innervation of muscles, but also their embryological origins. Like Romer [1,19], he divided the musculature into dorsal and ventral groups, but, emphasizing innervation patterns over relative position, he (correctly) placed the deltoid group with the dorsal musculature. Nevertheless, the muscle maps of the humerus of Eryops produced by the two authors are extremely similar.
Whereas most previous reconstructions were mainly concerned with the proximal limb regions, Haines [4] focused exclusively on the forearm and manus. He reconstructed the extensor musculature of the distal forelimb in the stem lissamphibian Eryops and the stem mammal Ophiacodon using a broad comparative sample including turtles, “lizards,” crocodylians, salamanders, frogs, monotremes, and marsupials. The reconstructions were based primarily on turtles, tuataras, and the terrestrial fire salamander (Salamandra), which he considered to be “primitive types,” arguing that the giant salamander used by Miner [3] is more specialized because it is secondarily adapted to a fully aquatic mode of life. The choice of extant taxa was especially important for this manuscript because osteological correlates of muscle attachment on the carpals, metacarpals, and phalanges are extremely difficult to identify, meaning that most of the muscle anatomy had to be deduced from that of extant animals. The author argued that the anatomy of extant turtles is most like the ancestral tetrapod condition, and his reconstruction of muscle attachments in the stem lissamphibian and the stem mammal were almost identical. Haines’s [4] interpretation of Eryops, which relied heavily on the osteological similarity between fossils and the bones of extant taxa, differs in some respects from that of Miner [3], which emphasized commonality in muscle anatomy among most extant tetrapod groups (see Controversies).
2.5. Stem Reptilia
Although Romer [1] had previously published partial muscle maps of the pectoral girdle and humerus of the stem reptile Labidosaurus, Holmes [5] was the first to produce a detailed reconstruction of a member of the reptilian stem group. Using a broad range of extant non-avian reptiles for comparison (“lizards,” tuataras, turtles, and crocodilians), the author reconstructed forelimb musculature in captorhinids, a group of stem reptiles from the Early Permian. Where osteological correlates were lacking and living forms diverged in morphology, he used evidence such as osteological and ecological similarity, resulting in a reconstruction extremely similar to the musculature of modern tuataras and iguanas. With a few exceptions (see Controversies), these reconstructions closely match those of Romer [1]. Sumida [22] continued the work of Holmes [5] by reconstructing the hindlimb musculature (including the tarsus and pes as well as the hip and thigh) of the captorhinid Labidosaurus based on extant “lizards” and crocodiles. Though he had access to much more complete material, Sumida’s [22] reconstructions of the thigh region mainly agree with the muscle maps drawn by Romer [1].
2.6. Stem Tetrapoda: The Fish Fin Meets the Tetrapod Limb
Stem tetrapods present a problem for phylogenetically based muscle reconstruction because of the vast morphological gulf between the appendages of tetrapods and those of fish. This gap makes it difficult to establish hypotheses of homology between appendicular muscles in the extant bracket taxa. Andrews and Westoll [34] reconstructed the pectoral fin musculature of Eusthenopteron, a tetrapodomorph fish thought to be closely related to tetrapods. This reconstruction was mainly based on Romer’s [1,19] interpretation of ancestral crown tetrapod musculature and referred only generally to the musculature of extant fishes. Panchen [21] was the first to reconstruct attachments of appendicular muscles in stem tetrapods with limbs (as opposed to finned tetrapodomorphs like Eusthenopteron that are nonetheless more closely related to crown tetrapods than to lungfish). However, Panchen’s [21] muscle maps of the humeri of the Devonian stem tetrapods Crassigyrinus and Ichthyostega did not refer to fish at all, but were based on descriptions of the stem amniotes Archeria [2] and Proterogyrinus [35]. Drawing upon the much expanded literature of the early 21st century on humeral morphology in tetrapodomorph fish and Devonian stem tetrapods, Ahlberg [20] produced partial muscle maps of the humeri of two additional stem tetrapods, Acanthostega and the “Catskill humerus,” and a new map of Ichthyostega. The “Catskill humerus” is an isolated element from the Late Devonian period that may represent the most basal known tetrapod humerus [20,36]. Although they depicted only a small part of the limb musculature, the muscle maps provided evidence that the earliest Devonian tetrapods possessed a habitually flexed elbow, possibly allowing locomotion like that of extant sprawling tetrapods.
The first reasonably complete muscle reconstruction of stem tetrapods with limbs was produced by Bishop [9] for the Early Carboniferous whatcheeriid Ossinodus. In the absence of detailed muscle homology hypotheses, the author was able to reconstruct all the forelimb muscles in this fossil because of its extraordinary preservation. Scars of what appear to be individual muscle fascicles cover distinct areas of the bones and seem to indicate direct fleshy attachments, which seldom leave recognizable osteological correlates [9]. This extensive scarring allowed the author to use Ossinodus as a bracket taxon, similar to the Lagerstätten that preserve soft tissues [7]. The identification of muscles was based on the comparative anatomies of giant salamanders and tuataras, considered to be the “most primitive” extant members of the amphibian and amniote lineages, respectively [9], p. 227. Although only a humerus, ulna, and partial girdle were preserved, this approach permitted reconstruction of the very early history of tetrapod forelimb musculature and shed additional light on the complicated, contentious evolution of the deltoid muscle group (see Controversies). Most importantly, as an “extant” bracket taxon, this specimen revealed many changes in forelimb musculature that presumably occurred before the amphibian-amniote split, and therefore would not be possible to reconstruct based solely on comparative anatomy of extant tetrapods. These changes include the separation of coracobrachialis longus and brevis and of the scapular and clavicular heads of deltoideus, acquisition of biceps brachii and a scapular head of triceps, and much of the differentiation of the forearm extensor musculature.
Shortly thereafter, Molnar and colleagues [10,11] attempted the first detailed muscle reconstructions in stem tetrapods using the extant phylogenetic bracket method. Building upon the work of Romer [1,19], they reconstructed forelimb and hindlimb muscles in the stem tetrapods Acanthostega (Devonian) and Pederpes (Early Carboniferous), as well as a Devonian tetrapodomorph fish (Eusthenopteron), based on extant coelacanths, lungfish, salamanders, and “lizards” [10,11]. They posited that the dorsal and ventral muscle masses of the fish fin underwent both proximo-distal and anterior-posterior segmentation over the fin-limb transition, passing through an intermediate stage that somewhat resembled the fins of extant Australian lungfish and coelacanths. The reconstructed anatomy of Ossinodus was used to resolve ambiguous character states. Unsurprisingly, Molnar et al.’s [10] reconstruction of the forelimb musculature of the whatcheeriid Pederpes was extremely similar to Bishop’s [9] reconstruction of Ossinodus except for the attachments of the supracoracoideus, which lacked osteological correlates in either taxa. In Pederpes the attachments were based on those in extant lobe-finned fish and in other fossil tetrapod reconstructions, whereas in Ossinodus they were based on extant tetrapods. In addition, in Pederpes the origin of forearm extensors was confined to the ventral aspect of the entepicondyle, as in most extant tetrapods, rather than extending to its dorsal aspect, as reconstructed for Ossinodus [9]. Musculoskeletal models of the three stem tetrapods (including Eusthenopteron) based on these reconstructions revealed increased muscle moment arms for humeral retractor muscles across the fin-limb transition, implying that the earliest steps in tetrapod forelimb evolution were related to aquatic limb-substrate interactions rather than body support [12].
2.7. Controversies in Tetrapod Muscle Reconstruction
Controversies about the homologies of limb muscles between extant amphibians, reptiles, and mammals abound in the paleontological literature. These disputed muscles include the mammalian supraspinatus, infraspinatus, teres major, teres minor, brachioradialis, pectoralis minor, dorsoepitrochlearis, gluteal muscles, and hamstrings; the reptilian humeroradialis, scapulohumeralis anterior and posterior, pubotibialis, and the amphibian coracoradialis and femorofibularis (see [37] and references therein). However, many of these disputes involve hypothetical “transitional” forms that are not represented in the fossil record. Here we discuss only those controversies directly related to published muscle reconstructions in extinct tetrapods discussed in the previous section. Almost all of these muscles are in the forelimb because so few hindlimb reconstructions have been published that there is little room for disagreement.
2.7.1. Evolution of the Deltoid Group
The deltoideus and scapulohumeralis and their derivatives comprise the deltoid group of muscles [3,9,38], whose evolutionary relationships are controversial. Many authors contend that the deltoideus first split into scapular and clavicular portions (probably in stem tetrapods since both extant amphibians and amniotes possess a scapular deltoid), and that the scapular deltoid gave rise to the scapulohumeralis, which in turn gave rise to the scapulohumeralis anterior and posterior within amniotes [3,9]. Therefore, the stem lissamphibian Eryops was reconstructed with two deltoid muscles and a single scapulohumeralis [3,5] (Figure 2C), and the stem tetrapod Ossinodus with a single deltoid and a single scapulohumeralis [9] (Figure 2A). Holmes [5] argued that the separation took place later, within Diapsida, and he reconstructed a single “scapulohumeralis” in the stem reptile Captorhinus (Figure 2E). In contrast, Romer [1] contended that the reptilian scapulohumeralis posterior is a derivative of the subscapularis, and therefore, in his reconstructions of stem mammals, the single muscle arising anterior and superior to the glenoid was identified as “scapulohumeralis anterior” (homologous with the mammalian teres minor) (Figure 2F). Developmental studies of squamate reptiles have shown that the scapulohumeralis posterior is derived from the embryonic subcoracoscapularis muscle mass, supporting the latter view [38,39]. However, in support of the former view, the two muscles do not share a common innervation: the scapulohumeralis posterior is innervated by the axillary nerve, whereas the subcoracoscapularis is innervated by a branch of the radial nerve [3]. We agree with Romer [1] and Russell and Bauer [39] that in this case innervation patterns, while usually a good indication of homology (especially in combination with topology), are overridden by the shared developmental origin of the two muscles. Diogo et al. [40] expanded on Romer’s [1] idea, proposing that the amniote muscles scapulohumeralis anterior and deltoideus clavicularis both are derived from an equivalent of the lissamphibian muscle procoracohumeralis. Notably, all three muscles are innervated by branches of the axillary nerve [3]. Following Diogo and Tanaka’s [41] scenario, Molnar et al. [10] reconstructed the stem tetrapods Acanthostega and Pederpes with a single deltoid muscle and a single scapulohumeralis (“procoracohumeralis”) muscle (Figure 2B). Thus, the two different evolutionary scenarios produced identical muscle reconstructions in stem tetrapods and stem mammals, and only the names of these muscles were changed. Although mechanically these results are equivalent, resolving the evolutionary and developmental histories of these muscles is important for understanding the selective pressures and developmental processes that have shaped them.
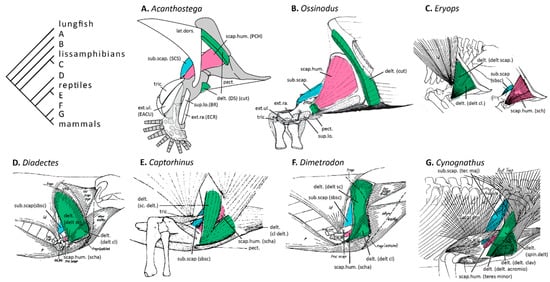
Figure 2.
Evolution of the deltoid group of muscles and subcoracoscapularis in tetrapods. Reconstructions by various authors of the deltoideus (green), scapulohumeralis (pink), and subcoracoscapularis (blue) muscles. (A) The stem tetrapod Acanthostega, modified from Molnar et al. [10,12] (the scapular head of triceps is not shown as the authors considered its presence in stem tetrapods to be uncertain); (B) The stem tetrapod Ossinodus, modified from Bishop [9]; (C) The stem lissamphibian Eryops, modified from Miner [3]; (D) The stem amniote Diadectes, modified from Romer [1]; (E) The stem reptile Captorhinus, modified from Holmes; (F) The stem mammal Dimetrodon, modified from Romer [1]; and (G) The stem mammal Cynognathus, modified from Gregory and Camp [6]. A simplified version of the phylogeny in Figure 1 is shown on the top left. Despite different evolutionary hypotheses and different naming conventions, the muscle reconstructions are almost identical. Muscle names follow Bishop [9], with the names given in the original publications in parentheses.
2.7.2. Was a True Subcoracoscapularis Present in Stem Mammals? In Stem Tetrapods?
A subcoracoscapularis consistently is present in “lizards” (e.g., [38,39]), but in turtles, crocodylians, and mammals, the muscle lacks a coracoid portion and is designated “subscapularis” [25]. Whether a coracoid head was present in stem mammals is equivocal. Watson [23] inferred a subcoracoid portion of this muscle in Dimetrodon, but Romer [1] argued that the construction of the pectoral girdle in this animal made it impossible for such a muscle to be mechanically effective, and he reconstructed a scapular portion only. Holmes [5] countered that the Dimetrodon fossil was taphonomically compressed and that the ancestral presence of a subcoracoid muscle in amniotes was possible, and he reconstructed both subcoracoid and subscapular heads in the stem reptile Captorhinus. Less controversially, stem tetrapods probably lacked a true subcoracoscapularis. Miner [3] reconstructed a “subcoracoscapularis” in the stem lissamphibian Eryops (Figure 2C) because the origin in giant salamanders is partially from the coracoid (a partial origin from the coracoid also is described in fire salamanders [42]). Bishop [9] disagreed, stating that extant amphibians lack “any significant coracoid part to this muscle” and that it would be more correct to call the muscle “subscapularis” in stem tetrapods [9], p. 233. Similarly, Diogo and Tanaka [41] described the muscle in axolotls as originating solely from the scapula.
2.7.3. Was a Teres Major Present Ancestrally in Amniotes?
The homology and origins of the teres major are still in doubt: A ‘teres major’ is present in some reptiles, such as turtles and crocodiles [19,25,43], but some authors dispute that it is homologous to the mammalian teres major. In addition, whether the mammalian teres major is derived from the latissimus dorsi or the subcoracoscapularis is controversial. Following the idea that a homologue of the teres major is present ancestrally in both reptiles and mammals, Gregory and Camp [6] reconstructed a teres major in the stem mammal Cynognathus. However, Romer [1] did not reconstruct this muscle in stem amniotes, stem mammals, or stem reptiles, concluding that it probably differentiated from the latissimus dorsi independently within reptiles and placental mammals. Likewise, Holmes [5] did not reconstruct a teres major muscle in stem reptiles. If teres major is present in some lepidosaurs, as reported by Dilkes [25], it would be more parsimonious to assume that the muscle was present ancestrally in amniotes [37], but only if the two muscles are homologous.
The question of homology rests upon the evolutionary origin of the mammalian teres major. Romer [19] argued that it is a derivative of the latissimus dorsi because the two muscles often blend together at their attachment to the humerus, although the teres major is innervated by the subscapular nerve and the latissimus dorsi by the thoracodorsal nerve. However, we think it is more likely that the teres major is instead derived from the subcoracoscapularis because the two muscles develop from the same anlage (e.g., [44]) and both are innervated by subscapular nerves. In contrast, the reptilian “teres major” is most likely derived from the latissimus dorsi, as Romer [1] believed. In turtles, the latissimus dorsi and “teres major” develop from a single muscle mass and are both innervated by the same branch of the deltoid nerve [45]. Therefore, it is unlikely that the reptilian “teres major” is homologous with the mammalian teres major, suggesting that the teres major was not present ancestrally in amniotes.
2.7.4. How many Radial Extensors Were Present Ancestrally in Tetrapods?
Most amphibians and reptiles have a radial extensor muscle (“extensor carpi radialis” or “extensor antebrachii et carpi radialis”) subdivided into three bundles: superficialis, intermedius, and profundus (e.g., [5,25,45,46,47]). This muscle is homologous with the extensors carpi radialis longus and brevis and the supinator (brevis) of mammals. Whether the bundles of the amphibian and reptilian extensor carpi radialis correspond to individual mammalian muscles, or even to each other, is a subject of controversy. In addition to the extensor carpi radialis, reptiles usually have a supinator longus, or “tractor radii” muscle, thought by some researchers to be homologous with the mammalian brachioradialis (e.g., [1]). However, in at least some lizards and turtles, the supinator longus is innervated by a ventral nerve (inferior brachial nerve) [4,39,43], whereas the brachioradialis is innervated by a dorsal nerve (radial nerve). Most authors agree that the supinator longus is not present in extant amphibians [4], but some maintain that the “intermedius” bundle of the extensor carpi radialis is homologous with the supinator longus of reptiles and the brachioradialis of mammals, and not with the bundle of the same name in reptiles [46,48]. Haines [4] reconstructed four radial extensors in Eryops, arguing that the presence of a supinator crest in Eryops indicated that a supinator longus (“tractor radii”) was present in stem lissamphibians and thus also most likely in stem tetrapods. If the three bundles of extensor carpi radialis (superficialis, intermedius, and profundus) truly are homologous between reptiles and amphibians, then this interpretation, though phylogenetically equivocal, could be justified by the “argument of compelling morphological evidence” [7]. However, if the intermedius portion is not homologous between amphibians and reptiles, then there is little evidence that it was present in stem lissamphibians like Eryops. For instance, Miner [3] (not recognizing the “intermedius” portion in Sphenodon), reconstructed only three radial extensor muscles in Eryops: extensors carpi radialis superficialis and profundus, and supinator longus. Since the three bundles of the extensor carpi radialis are often indistinguishable in lizards [37,39] and their homologies are in doubt, we consider Miner’s [3] reconstruction to be more likely.
2.7.5. Was Caudifemoralis Reduced in Stem Reptiles?
Muscle attachments on the femur of the stem reptile Captorhinus were reconstructed by both Romer [1] and Sumida [22], enabling one of the few direct comparisons that can be made between hindlimb muscle reconstructions by various authors. Though the two are largely in agreement, Romer [1] described the caudifemoralis (“coccygeo-femoralis”) insertion in captorhinids as “reduced” compared to the ancestral amniote condition represented by the stem amniote Diadectes. Sumida [22], in his reconstruction of Labidosaurus, described “a substantial attachment of M. caudifemoralis at the proximal end of the adductor ridge … contrary to the interpretation of Romer, 1922.” Whereas Romer [1] relied on composite specimens, Sumida [22] based his reconstruction on a more recently discovered, extremely well-preserved specimen (University of California Los Angeles, UCLA VP 3167). Therefore, it seems likely that captorhinids had a large caudifemoralis insertion. The extent to which attachment size reflects muscle development is uncertain [16], but the combination of a large tail and a large, well-developed insertion area supports the idea that the caudifemoralis in Labidosaurus was “probably a principal retractor of the femur” [22] as in many extant non-avian reptiles, such as lizards and crocodylians (e.g., [49]).
As these examples illustrate, determining muscle homology is crucial for evidence-based reconstruction of muscles in extinct tetrapods. This necessity formed part of the motivation for many early comparative muscle development studies, and some more recent ones as well (e.g., [38,44,45,50,51,52,53,54,55]). As embryological techniques continue to improve (including methods such as in situ hybridization and antibody staining for visualizing soft tissues in embryos; see the next section), many of these remaining controversies may be resolved.
3. Development of Limb Muscles in Tetrapods
In addition to informing reconstructions of muscles in extinct taxa, comparative developmental studies can reveal general patterns of development across tetrapod groups, supporting or undermining evolutionary hypotheses. Despite overall similarities in muscle patterning, recent developmental studies have demonstrated taxonomic differences in the tempo and mode of tetrapod limb muscle morphogenesis, implying a degree of developmental plasticity. In addition, developmental evidence has been used to challenge–and sometimes support–evolutionary hypotheses such as serial homology between the forelimb and hindlimb, evolutionary trend toward increased complexity, and parallels between ontogeny and phylogeny.
3.1. Morphogenesis and Developmental Patterns
Recent comparative development studies by our lab and others, building on the work of 20th century anatomists (e.g., [26,38,45,50,51,56,57]), have revealed general developmental patterns. For instance, our work on the development of fore- and hindlimb muscles in salamanders (Figure 3A–C) [58], frogs [59], reptilians (e.g., chameleons, in review), and mammals (humans; [60]) support the ‘in-out’ mechanistic hypothesis, sensu Valasek et al. [61]. A similar idea was proposed more than a century ago, by Sewertzoff [26]. According to this hypothesis, the myogenic cells that form the superficial girdle muscles that attach to the humerus first migrate “in” from the somites into the limb bud and subsequently extend “out” from the limb bud toward the body wall [61]. In contrast, the deep girdle muscles (‘axial pectoral muscles’) are induced by the forelimb field that promotes myotomal extension directly from the somites. Our studies of tetrapod limb development support this ‘in-out’ mechanism because, at earlier ontogenetic stages, the pectoral appendicular muscles begin to develop far from the midline, at the level of the proximal region of the arm, and only later extend medially to cover a substantial part of the ventral and dorsal surfaces of the thoracic region (Figure 3A–D). The in-out mechanism has implications for tetrapod limb evolution, including the origin of limbs and girdles [61] and the potential constraints surrounding limb loss [62]. In addition, this mechanism allows for evolutionary and developmental flexibility in muscle morphology, exemplified by the modification of the latissimus dorsi muscle in turtles (in which the ribs are external to the pectoral girdle). As an appendicular muscle, the latissimus dorsi in turtles initially follows the conserved tetrapod pattern: it develops in the limb bud and attaches normally to the humerus. Only as it extends medially later in development does it divert from the conserved pattern and pass ventral to the carapace, attaching to the nuchal plate in a “turtle-specific” manner [63].
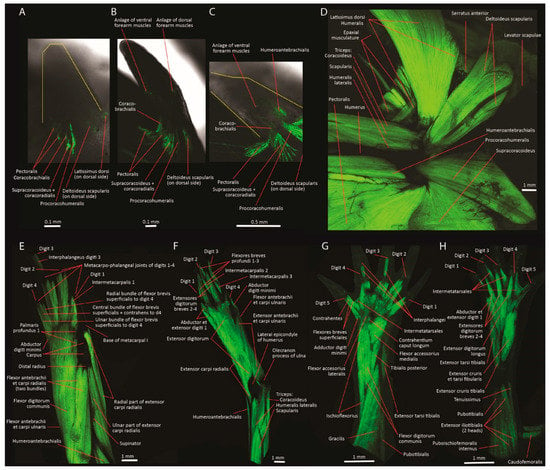
Figure 3.
Transgenic axolotl (Ambystoma mexicanum) specimens that express GFP in muscle fibers. (A–C) show embryos; (D–H) show adults. Ventral views of right forelimb at stages mid-46 (A), early 47 (B), and mid-47 (C) (yellow lines show limits of the developing limbs); left lateral view of adult right forelimb after removal of the protractor pectoralis (D); adult left forelimb and hand in ventral (E) and dorsal (F) views; adult right hindlimb in dorsal (G) and ventral views. Modified from Diogo and Tanaka [58].
Although muscle patterning is remarkably conservative among tetrapods [50,64], the tempo and mode of muscle morphogenesis vary among taxa. In regeneration studies of the salamander forelimb, the formation and differentiation of the muscles follow proximo-distal and preaxial-postaxial gradients (e.g., [65,66,67]). However, Diogo et al.’s [67] study using transgenetic animals that express green fluorescent protein also found a marked ventro-dorsal gradient during the regeneration of at least some axolotl forearm muscles. In a similar study of hindlimb regeneration, only proximo-distal and preaxial-postaxial morphogenetic gradients were present [68]. The ontogeny of axolotl limb muscles follows the same pattern as regeneration [58]. The presence of a ventro-dorsal gradient in the regeneration and ontogeny of the muscles of the forelimb, but not of the hindlimb, might represent a genuine difference between tetrapod forelimbs and hindlimbs. However, dorso-ventral ontogenetic gradients have been reported in the hindlimbs of other tetrapods, such as chickens [69] and frogs [59]. Adding to the variation among taxa, in the frog the polarity of the anterior-posterior gradients in both limbs is reversed from that of the salamander (postaxial-preaxial versus preaxial-postaxial). A postaxial-preaxial morphogenetic pattern is also found in the limb skeletons of other non-urodele tetrapod groups and in the limb muscles in at least some of these groups.
The remarkable plasticity implied by the existence of different morphogenetic gradients among tetrapod taxa and limbs makes it difficult to discern plesiomorphic states and general morphogenetic patterns [37]. If there were a general anterior-posterior (preaxial-postaxial) gradient in tetrapod limb muscle morphogenesis, it might explain why most tetrapod taxa possess more radial/tibial muscles than ulnar/fibular muscles [58]. That is, the ulnar musculature never reaches an equivalent developmental stage to that of the radial musculature in the adult. In support of this hypothesis, in the dorsum of the axolotl hindlimb (which has a preaxial-postaxial morphogenetic gradient) there are two long extensors on the tibial side (extensor cruris tibialis and extensor tarsi tibialis) and only one on the fibular side (extensor cruris et tarsi tibialis) (Figure 3G,H). A similar pattern is found in adult axolotls, chickens, and humans (Figure 3E,F), in which more muscles are associated with the most radial digit than with the most ulnar digit [37,58]. However, as noted in the previous paragraph, some tetrapod limb structures develop across a postaxial-preaxial morphogenetic gradient; in fact, this seems to be the most common pattern in tetrapod skeletal development (e.g., [70]). It is possible that preaxial-postaxial and proximo-distal gradients in both limbs represent the plesiomorphic condition for tetrapods, and that this condition is explained by the dependence of muscle patterning upon the patterning of connective tissue. Many markers and patterning genes have been extensively implicated in the patterning of limb connective tissue and are upregulated in proximo-distal (e.g., Hox, FGFs, RA) and preaxial-postaxial fashion (e.g., Shh) (e.g., [71,72]). Therefore, these molecules may either influence muscle patterning directly or via patterning of connective tissue, which might explain, at least in part, the general presence of more radial/tibial than ulnar/fibular muscles in tetrapods. In fact, some early amphibian fossils (thought to be phylogenetically more basal than the last common ancestor of extant urodeles and anurans) seem to show a preaxial-postaxial sequence of digit development [73,74]. This evidence also raises questions about whether the highly conserved postaxial-preaxial sequence of digit development seen in extant anurans and amniotes truly represents the plesiomorphic condition for tetrapods (e.g., [75]). How broadly generalized the preaxial-postaxial developmental pattern is across tetrapods, and whether there is a causal relationship with the number of adult limb muscles, remains to be tested. If the relationship exists, then morphogenetic gradients of limb muscle formation might be a target of selective pressure for changing limb function.
3.2. The Fore-Hindlimb Enigma and the Origin of Pectoral and Pelvic Appendages
Developmental evidence has made great contributions to our understanding of the origin of paired fins and the processes by which fins were transformed into limbs (e.g., [17,76,77,78,79]). The idea that the pectoral and pelvic appendages, like vertebrae, arose by duplication of an ancestral structure (serial homology hypothesis) has recently been called into question. These challenges rest on comparative anatomical studies of adult appendicular muscles among numerous vertebrate taxa and on a review of other lines of evidence, including paleontology, functional morphology, Evo-Devo, and genetics [80,81,82,83,84]. Diogo et al. [81] concluded that the enigmatic similarity between many forelimb and hindlimb structures (‘fore-hindlimb enigma’), including muscles, was acquired during the ‘fins-limbs transition’ through the derived cooption of some similar genes for the development of the more distal parts of both the forelimb and the hindlimb. That is, while it is possible that the fore- and hindlimbs display “deep homology” in the developmental sense (i.e., shared gene regulatory circuits [85]), they are not homologous in the morphological or phylogenetic sense.
Evidence against serial homology between the fore- and hindlimbs has also been gathered from comparative developmental studies. If the two appendages were homologous, one might predict that they are more alike early in development. In fact, both in the larvae and the froglets/adults of Eleutherodactylus coqui, there is a marked similarity between many forearm/hand and leg/foot muscles [59]. The similarity is even more noticeable in axolotls, in which all the leg/foot and forearm/hand muscles have a clear ‘topological equivalent’ in the other limb, with the exception of the flexor antebrachii et carpi radialis and flexor antebrachii et carpi ulnaris [58]. However, the serial homology hypothesis is undermined by the lack of clear similarity or correspondence between any pelvic/thigh and any pectoral/arm muscles in anatomically plesiomorphic tetrapods such as salamanders (Figure 3E,F vs. Figure 3G,H), even during early development [58]. This dissimilarity is likely due to a phylogenetic constraint; i.e., the musculature of the girdles is phylogenetically extremely ancient (unlike the autopodial musculature, which was only acquired in the tetrapod stem group) [86]. The muscles of the pectoral girdle are extremely different from the muscles of the pelvic girdle not only in tetrapods, but also in non-tetrapod gnathostomes, reinforcing the idea that the pelvic and pectoral appendages are not serial homologues.
Recently, Diogo [87] reviewed the question of the origin of the paired appendages in face of the huge amount of gross anatomical and developmental data about the fore and hindlimb muscles accumulated over the past quarter century. Two main theories have been used to explain the origin of pectoral and pelvic appendages: the “fin-fold” theory and the “gill-arch” theory. According to the “fin-fold theory,” the paired appendages evolved from a bilateral fin fold on the trunk [88,89,90], whereas according to the “gill-arch” theory, they are derived from the branchial arches of the head [91]. However, neither of these theories is strongly supported by paleontological data. The former has been supported by similarities in gene expression between paired and median fins [92,93], but recent ontogenetic studies have revived the gill arch theory by revealing common mechanisms underlying the patterning of branchial arches and the paired appendages [94,95]. These apparent contradictions could be explained by a dual origin of the pectoral appendage: that the pectoral girdle originates mainly from the head and the fin/limb mainly from the trunk. If this hypothesis is correct, the pectoral and pelvic girdles cannot be serial homologues, and the term “developmental serial homologues” could only potentially be applied to the pectoral and pelvic fins/limbs.
3.3. Atavisms, Variations, Anomalies, and Links between Ontogeny and Phylogeny
Counterintuitively, the evolution of human musculature is marked by a pattern of reduction in the overall number of muscles and the complexity of musculoskeletal connections. Recent studies using antibody staining to visualize early human limb muscle development have confirmed the presence of numerous atavistic muscles [60,96]. These muscles, which appear and disappear or become fused with other muscles early in human development, are thought to have been part of the normal adult anatomy of our non-human ancestors. They include the shoulder and arm muscles epitrochleoanconeus and dorsoepitrochlearis, and the hand and foot muscles contrahentes digitorum and dorsometacarpales (Figure 4), all which are part of the normal adult phenotype in some tetrapod taxa (Table 1, Table A1 and Table A2). These atavistic muscles are remarkable both for their number and for their persistence over time. Of the 30 muscles present in the human hand and foot at seven weeks gestation, only two thirds are still present at 13 weeks (Table 1). Some, such as the dorsometacarpales, disappeared from our adult ancestors more than 250 million years ago. These muscles are part of a pattern of parallel reduction in the number of muscles and the complexity of musculoskeletal connections over evolution and development that goes against the ancient concept of a “scala naturae,” as well as the modern idea that evolutionary systems tend toward increases in pure morphological complexity [97].
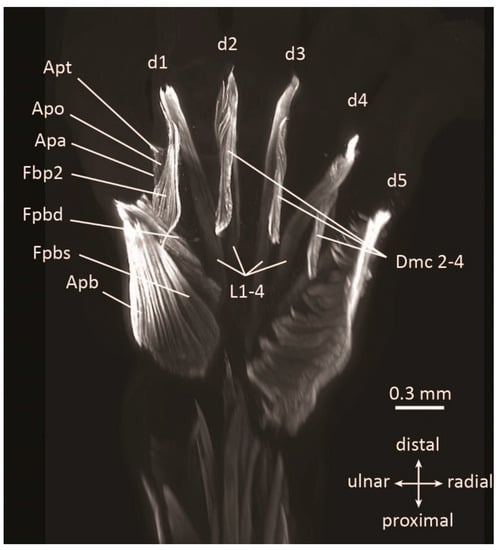
Figure 4.
Ventral view of hand of CR51 mm (11 GW) fetus stained with anti-MHC antibody showing muscle anatomy and atavistic dorsometacarpales muscles (Dmc 2-4). Other muscle abbreviations: Apa, adductor pollicis accessorius; Apb, abductor pollicis brevis; Apo, adductor pollicis oblique head; Apt, adductor pollicis transverse head; Fbp2, flexor brevis profundus 2; Fpbd, flexor pollicis brevis deep head; Fpbs, flexor pollicis brevis superficial head; and L, lumbricales. Modified from Diogo et al. [60].

Table 1.
Order of appearance of forelimb and hindlimb muscles in human ontogeny and phylogeny. First column shows crown-rump length (CR) when the muscle is visibly distinct from other muscles. Second column shows most inclusive clade in which the muscle is present: Tetrapoda (also present in salamanders); Amniota (also present in “lizards”); Mammalia (also present in monotremes); Theria (also present in opossums); Euarchontoglires (also present in rats); and Homo (only present in modern humans). With some exceptions, the muscles that appear earlier in embryonic development also appear in successively larger taxonomic groups including humans. Modified from Diogo et al. [60].
The embryonic presence, and subsequent loss in humans, of muscles possessed by human ancestors is an example of ‘phylo-devo’ parallelism. Diogo et al. [98] defined phylo-devo parallelism as the idea that the order of developmental changes in a certain taxon is similar to the order of changes that occurred during the evolutionary history of that taxon. This concept is related to Haeckelian recapitulation (ontogeny recapitulates phylogeny), but it can apply to features found only in the non-adult stages. It is less rigid than von Baer’s notion of embryonic similarity [99] because it does not presuppose a trend from a general condition toward a specialized condition. Thus, phylo-devo parallelism is more similar to De Beer’s [100] “repetition” but with a focus on specific traits rather than on the organism as a whole. Although lacking in most human adults, the aforementioned atavistic muscles may be found as rare variations in karyotypically normal adults and are often found in individuals with chromosomal abnormalities, such as trisomies, reinforcing the idea that their persistence in adults is related to delayed or arrested development [101,102,103,104]. Phylo-devo parallelism supports an ‘ontogenetically constrained’ (internalist) view of evolution, as proposed by authors such as Gould [105,106], rather than the ‘adaptationist’ (externalist) view of Darwin [107], because it implies that many aspects of development are phylogenetically constrained. Other examples of phylo-devo parallelism concern the order in which muscles appear in evolution and development. For example, five of the last six forearm muscles to differentiate in human ontogeny are only found in therian mammals (Table 1) [60]. Similarly, the hindlimb muscle sartorius was acquired in amniotes and differentiates at extremely early stages of human development, whereas the gemellus inferior and superior and the obturator internus (only acquired in the last common ancestor of marsupial and placental mammals) appear much later in human ontogeny. However, counterexamples exist as well; the rhomboid complex evolved later than the serratus anterior and levator scapulae, but in human ontogeny it differentiates before the split between these two muscles.
4. General Remarks and Future Work
Our knowledge of the anatomy, evolution, and development of tetrapod limb muscles has exploded over the past century, from the flurry of muscle evolution studies in the early 1900s to the resurgence of interest in comparative anatomy brought about by the rise of Evo-Devo. However, much remains to be done, and it is striking that some essential aspects of tetrapod limb morphogenesis, such as those discussed above, are only now being addressed. We hope that our work will stimulate other researchers to investigate the comparative anatomy, evolution, and development of muscles as part of a multidisciplinary, collaborative research program. Comparative developmental studies of limb anatomy are part of this program, as are mechanistic developmental studies in new model organisms.
Similarly, a challenge facing muscle reconstruction in fossils is how to move beyond the extant phylogenetic bracket and appreciate the diversity of muscle anatomy in taxa that have no analogous bracketing relatives. New ways of analyzing fossils may reveal direct evidence of soft tissue anatomy, such as evidence of muscle attachments in the microstructure of the humerus of Eusthenopteron revealed by synchrotron scanning [108]. Generally, as we learn more about the developmental and biomechanical interactions between hard tissues and soft tissues, we will be able to tease more information out of the fossil record. For example, recent studies have explored the role of muscle contractions during embryonic development in producing different musculoskeletal phenotypes both in extant species [109] and in fossils [110]. Thus, indirect evidence of soft tissue anatomy can be found in ontogenetic series of fossilized animals. Exploiting new techniques and concepts in molecular biology, embryology, and visualization will allow us to appreciate more fully the diversity in limb structure and function that exists today, as well as the far greater diversity of extinct tetrapods we can learn about from the fossil record.
Author Contributions
J.L.M. and R.D. wrote and edited the manuscript. J.L.M. prepared the new figures. Both authors approved the final version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Thanks to Raul Diaz for inviting us to contribute to this special issue. Thanks to the two anonymous reviewers for their time and attention.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Muscle Homology Tables

Table A1.
Evolution and homologies of tetrapod forelimb muscles. Muscles listed in each column are usually plesiomorphically present in that taxonomic group (bold), and they are present in that particular genus (italics). Muscles are organized by developmental group (first column). Muscles in the same row with different names indicate partial homology resulting from muscle splits or fusions. Synonyms in parentheses. * Indicates controversial or uncertain homologies. Modified from Diogo et al. [37].
Table A1.
Evolution and homologies of tetrapod forelimb muscles. Muscles listed in each column are usually plesiomorphically present in that taxonomic group (bold), and they are present in that particular genus (italics). Muscles are organized by developmental group (first column). Muscles in the same row with different names indicate partial homology resulting from muscle splits or fusions. Synonyms in parentheses. * Indicates controversial or uncertain homologies. Modified from Diogo et al. [37].
| Lissamphibia (Salamandra) | Squamata (Iguana) | Archosauria (Alligator) | Mammalia (Didelphis) | Eutheria (Homo) | |
|---|---|---|---|---|---|
| Superficial dorsomesial | Latissimus dorsi | Latissimus dorsi | |||
| Dorsoepitrochlearis | - | ||||
| Extensor digitorum (extensor digitorum communis, humerodorsalis, extensor digitorum longus) | |||||
| Extensor antebrachii et carpi radialis (supinator longus; tractor radii) | Extensor carpi radialis brevis | ||||
| Extensor carpi radialis longus + abductor radialis | Extensor carpi radialis longus | ||||
| Brachioradialis | |||||
| Supinator (supinator brevis) | |||||
| Triceps scapularis (triceps longus) | |||||
| Triceps humeralis lateralis | Triceps humeralis lateralis | Triceps humeralis lateralis | |||
| triceps humeralis posticum | |||||
| Triceps humeralis medialis | |||||
| Extensor antebrachii et carpi ulnaris (flexor ulnaris) | Extensor carpi ulnaris | ||||
| Anconeus | |||||
| Triceps coracoideus | - | ||||
| Deltoideus scapularis | Deltoideus | ||||
| Deep dorsomesial | Procoraco-humeralis | Deltoideus acromialis et clavicularis | Deltoideus acromialis et clavicularis | ||
| humeroradialis | - | ||||
| Scapulohumeralis anterior | - | Teres minor | |||
| Subcoraco-scapularis | Subcoracoscapularis | Subcoracoscapularis | Subscapularis | ||
| Teres major | |||||
| Scapulohumeralis posterior | - | ||||
| Abductor et extensor digit 1 (supinator manus) | Abductor pollicis longus * | Abductor pollicis longus | |||
| Extensor pollicis brevis | |||||
| Extensores digitorum breves | Extensores digitorum breves | Extensor digiti minimi | |||
| Extensor indicis | |||||
| Extensor pollicis longus | |||||
| Dorsometacarpales | - | ||||
| Superficial ventrolateral | Pectoralis | Pectoralis major | |||
| Pectoralis minor | |||||
| Panniculus carnosus | - | ||||
| Flexor antebrachii et carpi ulnaris | Flexor carpi ulnaris | ||||
| Epitrochleo-anconeus | - | Epitrochleo-anconeus | - | ||
| Coracobrachialis | Coracobrachialis longus | - | Coracobrachialis | ||
| Coracobrachialis brevis | |||||
| Flexor antebrachii et carpi radialis | Flexor carpi radialis | ||||
| Pronator teres | |||||
| Humero-antebrachialis | Brachialis * | ||||
| Flexor digitorum communis | Flexor digitorum longus (palmaris communis) | Flexor digitorum superficialis* | |||
| Deep ventrolateral | Flexor accessorius medialis | palmaris longus | Palmaris longus | ||
| Flexor accessorius lateralis | Palmaris longus internus | ||||
| Contrahentium caput longum | Flexor digitorum profundus | Flexor digitorum profundus | |||
| Flexor pollicis longus | |||||
| Supracoracoideus | Infraspinatus | ||||
| Supraspinatus | Supraspinatus | ||||
| Cleidoacromialis | |||||
| Coracoradialis | Biceps brachii * | ||||
| Palmaris profundus 1 | - | ||||
| Pronator quadratus (flexor palmaris profundus) | Pronator quadratus | - | Pronator quadratus | ||
| Pronator accessorius | - | ||||
| Intrinsic autopodial | Flexores digitorum minimi | - | |||
| Interphalangeaus | - | ||||
| Abductor digiti minimi | - | Abductor digiti minimi | |||
| - | Abductor pollicis brevis (abductor metacarpi 1) | ||||
| Flexores breves superficiales | Flexores breves superficiales (flexor digitorum brevis superficialis) | Flexor brevis digitorum manus | - | ||
| - | Palmaris brevis | ||||
| Lumbricales | |||||
| Contrahentes digitorum | Transversus palmaris + flexor digiti quinti? | Contrahentes digitorum | - | ||
| - | Adductor pollicis | ||||
| - | Adductor pollicis accessorius | ||||
| Intermetacarpales (“interossei”) | Interossei | ||||
| Flexores breves profundii | Flexores breves profundii | ||||
| Flexor pollicis brevis | Flexor pollicis brevis | ||||
| Opponens pollicis | |||||
| Flexor digiti minimi brevis | Flexor digiti minimi brevis | ||||
| Opponens digiti minimi | |||||

Table A2.
Evolution and homologies of tetrapod hindlimb muscles. Columns as in A1. Muscles in the same row with different names indicate partial homology resulting from muscle splits or fusions. Synonyms in parentheses. * Indicates controversial or uncertain homologies. Modified from Diogo et al. [37].
Table A2.
Evolution and homologies of tetrapod hindlimb muscles. Columns as in A1. Muscles in the same row with different names indicate partial homology resulting from muscle splits or fusions. Synonyms in parentheses. * Indicates controversial or uncertain homologies. Modified from Diogo et al. [37].
| Lissamphibia (Ambystoma) | Squamata (Timon) | Archosauria (Alligator) | Mammalia (Didelphis) | Eutheria (Homo) | |
|---|---|---|---|---|---|
| Axial | Caudofemoralis | Caudofemoralis longus | - | ||
| Caudofemoralis brevis | |||||
| Superficial dorsomesial | Iliotibialis | Extensor iliotibialis | Femorococcygeus | - | |
| Gluteus maximus | |||||
| Rectus femoris | |||||
| Femorotibialis | Vastus lateralis | ||||
| - | Vastus intermedius | ||||
| Vastus medialis | |||||
| Ambiens (sartorius) | |||||
| Extensor cruris tibialis | Tibialis anterior | ||||
| Extensor tarsi tibialis | |||||
| Extensor digitorum longus | Extensor hallucis longus | ||||
| Extensor digitorum longus | Extensor digitorum longus | ||||
| Fibularis tertius | |||||
| Iliofibularis (tenuissimus) | Biceps femoris (part) | ||||
| Extensor cruris et tarsi fibularis | Fibularis longus | ||||
| Fibularis brevis | |||||
| Deep dorsomesial | Puboischiofemoralis internus | Iliopsoas (iliacus + psoas major) | |||
| pectineus | |||||
| Iliofemoralis | Gluteus medius + minimus | ||||
| Piriformis | |||||
| Scansorius | - | ||||
| - | Tensor fasciae latae | ||||
| Abductor et extensor digit 1 (adductor et extensor hallucis et indicis; adductor hallucis dorsalis) | Extensor hallucis brevis * | ||||
| Extensores digitorum breves | Extensor digitorum brevis + adductor et extensor hallucis et indicus + abductor digiti 4 | Extensor digitorum brevis | Extensor digitorum brevis | ||
| Fibularis digiti quinti | |||||
| Superficial ventrolateral | Puboischiotibialis (gracilis) | ||||
| Pubotibialis (?) | Pubotibialis (?) | - | Adductor longus | ||
| Femorofibularis (?) | |||||
| Ischioflexorius | Flexor tibialis internus | Semimembranosus | |||
| Biceps femoris (part) | |||||
| Flexor tibialis externus | Semitendinosus * | ||||
| Flexor digitorum communis | Gastrocnemius internus | Gastrocnemius | |||
| Gastrocnemius externus | Gastrocnemius externus | ||||
| Soleus | |||||
| Plantaris | |||||
| Deep ventrolateral | Flexor accessorius medialis | Flexor digitorum longus | Flexor digitorum longus | Flexor digitorum longus | |
| Flexor accessorius lateralis | Flexor hallucis longus | ||||
| Contrahentium caput longum | Flexor accessorius (“flexor hallucis longus”) | Quadratus plantae | |||
| Pubofemoralis (adductor femoris) | Adductor magnus | ||||
| Adductor brevis | |||||
| Puboischiofemoralis externus | Quadratus femoris | ||||
| Obturator externus | |||||
| Ischiotrochantericus (ischiofemoralis) | Obturator internus | ||||
| Gemellus superior | |||||
| Gemellus inferior | |||||
| Tibialis posterior (pronator profundus) | |||||
| Interosseous cruris | Popliteus | - | Popliteus | ||
| Interosseous cruris | - | ||||
| Intrinsic autopodial | Flexores breves superficiales (flexores digitores breves) | Flexor digitorum brevis superficialis | Flexor digitorum brevis | ||
| Fibulocalcaneus | |||||
| - | Lumbricales | ||||
| - | Abductor hallucis (flexor hallucis) | - | Abductor hallucis | ||
| Contrahentes pedis | Flexores digiti 2,3,4 | Contrahentes pedis | Adductor hallucis | ||
| Adductor hallucis accessorius | |||||
| Flexores breves profundi | - | Abductor hallucis brevis | |||
| Flexores breves profundi (interossei plantares) | Flexor digiti minimi brevis | ||||
| Flexor hallucis brevis | |||||
| Flexor brevis profundus 2 | |||||
| Flexores breves profundi | Interossei plantares | ||||
| Intermetatarsales | Interossei dorsales | ||||
| Flexores digitorum minimi | - | ||||
| Interphalangeii | - | ||||
| Abductor digiti minimi | |||||
References
- Romer, A.S. The Locomotor Apparatus of Certain Primitive and Mammal-Like Reptiles; Columbia University Press: New York, NY, USA, 1922. [Google Scholar]
- Romer, A.S. The appendicular skeleton of the Permian embolomerous amphibian Archeria. Contrib. Mus. Pale-Ontol. Univ. Mich. 1957, 8, 103–159. [Google Scholar]
- Miner, R.W. The pectoral limb of Eryops and other primitive tetrapods. Bull. Am. Mus. Nat. Hist. 1925, L1, 148–309. [Google Scholar]
- Haines, R.W. A revision of the extensor muscles of the forearm in tetrapods. J. Anat. 1939, 73, 211–233. [Google Scholar] [PubMed]
- Holmes, R. The osteology and musculature of the pectoral limb of small captorhinids. J. Morphol. 1977, 152, 101–140. [Google Scholar] [CrossRef]
- Gregory, W.K.; Camp, C.L. Studies in Comparative Myology and Osteology; American Museum of Natural History: New York, NY, USA, 1918. [Google Scholar]
- Witmer, L.M. Functional Morphology in Vertebrate Paleontology; Cambridge University Press: Cambridge, MA, USA, 1995; Volume 1, pp. 19–33. [Google Scholar]
- Campbell, R.M.; Vinas, G.; Henneberg, M.; Diogo, R. Visual Depictions of Our Evolutionary Past: A Broad Case Study Concerning the Need for Quantitative Methods of Soft Tissue Reconstruction and Art-Science Collaborations. Front. Ecol. Evol. 2021, 9, 60. [Google Scholar] [CrossRef]
- Bishop, P.J. The humerus of Ossinodus pueri, a stem tetrapod from the Carboniferous of Gondwana, and the early evolution of the tetrapod forelimb. Alcheringa Australas. J. Palaeontol. 2013, 38, 209–238. [Google Scholar] [CrossRef]
- Molnar, J.L.; Diogo, R.; Hutchinson, J.R.; Pierce, S.E. Reconstructing pectoral appendicular muscle anatomy in fossil fish and tetrapods over the fins-to-limbs transition. Biol. Rev. 2017, 93, 1077–1107. [Google Scholar] [CrossRef]
- Molnar, J.L.; Diogo, R.; Hutchinson, J.R.; Pierce, S.E. Evolution of hindlimb muscle anatomy across the tetrapod water-to-land transition, including comparisons with forelimb anatomy. Anat. Rec. 2020, 303, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.L.; Hutchinson, J.R.; Diogo, R.; Clack, J.A.; Pierce, S.E. Evolution of forelimb musculoskeletal function across the fish-to-tetrapod transition. Sci. Adv. 2021, 7, eabd7457. [Google Scholar] [CrossRef]
- Diogo, R.; Wood, B. The broader evolutionary lessons to be learned from a comparative and phylogenetic analy-sis of primate muscle morphology. Biol. Rev. 2013, 88, 988–1001. [Google Scholar] [CrossRef]
- Dilkes, D.W.; Hutchinson, J.R.; Holliday, C.M.; Witmer, L.M. Reconstructing the Musculature of Dinosaurs. Complete Dinosaur; Indiana University Press: Bloomington, IN, USA, 2012; pp. 151–190. [Google Scholar]
- Bryant, H.N.; Russell, A.P. The role of phylogenetic analysis in the inference of unpreserved attributes of ex-tinct taxa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1992, 337, 405–418. [Google Scholar]
- Bryant, H.N.; Seymour, K.L. Observations and comments on the reliability of muscle reconstruction in fossil vertebrates. J. Morphol. 1990, 206, 109–117. [Google Scholar] [CrossRef]
- Hall, B.K. Fins into Limbs: Evolution, Development, and Transformation; University of Chicago Press: Chicago, IL, USA, 2008. [Google Scholar]
- Gadow, H. Observations in Comparative Myology. J. Anat. Physiol. 1882, 16, 493–514. [Google Scholar]
- Romer, A.S. Pectoral limb musculature and shoulder girdle structure in fish and tetrapods. Anat. Rec. 1924, 27, 119–143. [Google Scholar] [CrossRef]
- Ahlberg, P.E. Humeral homology and the origin of the tetrapod elbow: A reinterpretation of the enigmatic specimens ANSP 21350 and GSM 104536. Stud. Foss. Tetrapods. 2011, 86, 17–29. [Google Scholar]
- Panchen, A.L. On the amphibian Crassigyrinus scoticus Watson from the Carboniferous of Scotland. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 309, 505–568. [Google Scholar]
- Sumida, S.S. The appendicular skeleton of the Early Permian genus Labidosaurus (Reptilia, Captorhinomorpha, Captorhinidae) and the hind limb musculature of captorhinid reptiles. J. Vertebr. Paleontol. 1989, 9, 295–313. [Google Scholar] [CrossRef]
- Watson, D.M.S. The Evolution of the Tetrapod Shoulder Girdle and Fore-limb. J. Anat. 1917, 52, 1–63. [Google Scholar] [PubMed]
- Fürbringer, M. Die Knochen und Muskeln der Extremitäten bei den Schlangenähnlichen Sauriern: Vergleichend-Anatomische Abhandlung; Wilhelm Engelmann: Leipzeg, Germany, 1870. [Google Scholar]
- Dilkes, D.W. Appendicular myology of the hadrosaurian dinosaur Maiasaura peeblesorum from the Late Creta-ceous (Campanian) of Montana. Earth Environ. Sci. Trans. R. Soc. Edinb. 1999, 90, 87–125. [Google Scholar] [CrossRef]
- Sewertzoff, A.N. Studien über die Entwickelung der Muskeln, Nerven und des Skeletts der Extremitäten der niederen Tetrapoda: Beiträge zu einer Theorie der pentadactylen Extremität der Wirbeltiere; Typo-lithogr. de la Société JN Kouchnéreff: Moscow, Russia, 1908. [Google Scholar]
- Brinkmann, H.; Venkatesh, B.; Brenner, S.; Meyer, A. Nuclear protein-coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc. Natl. Acad. Sci. USA 2004, 101, 4900–4905. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, C.T.; Alföldi, J.; Lee, A.P.; Fan, S.; Philippe, H.; MacCallum, I.; Braasch, I.; Manousaki, T.; Schneider, I.; Rohner, N.; et al. The African coelacanth genome provides insights into tetrapod evolu-tion. Nature 2013, 496, 311–316. [Google Scholar] [CrossRef]
- Hutson, J.; Hutson, K. A test of the validity of range of motion studies of fossil archosaur elbow mobility using repeated-measures analysis and the extant phylogenetic bracket. J. Exp. Biol. 2012, 215, 2030–2038. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Norton, J.M. Use of the Extant Phylogenetic Bracket (Epb) Concept in Reconstructing the Dinosaur Respiratory System. Paléontol. Soc. Spéc. Publ. 1996, 8, 293. [Google Scholar] [CrossRef][Green Version]
- Witmer, L.M. Homology of facial structures in extant archosaurs (birds and crocodilians), with special reference to paranasal pneumaticity and nasal conchae. J. Morphol. 1995, 225, 269–327. [Google Scholar] [CrossRef]
- Benton, M.J. Studying Function and Behavior in the Fossil Record. PLoS Biol. 2010, 8, e1000321. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.J.C.J.; Eberth, D.A.E.A. On gregarious behavior in AlbertosaurusThis article is one of a series of papers published in this Special Issue on the theme Albertosaurus. Can. J. Earth Sci. 2010, 47, 1277–1289. [Google Scholar] [CrossRef]
- Andrews, S.M.; Westoll, T.S. The Postcranial Skeleton of Eusthenopteron foordi Whiteaves. Trans. R. Soc. Edinb. 1970, 68, 207–329. [Google Scholar] [CrossRef]
- Holmes, R. The Carboniferous amphibian Proterogyrinus Scheelei Romer, and the early evolution of tetrapods. Philos. Trans. R. Soc. B Biol. Sci. 1984, 306, 431–524. [Google Scholar] [CrossRef]
- Shubin, N.H.; Daeschler, E.B.; Coates, M.I. The Early Evolution of the Tetrapod Humerus. Science 2004, 304, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Ziermann, J.M.; Molnar, J.L.; Siomova, N.; Abdala, V. Muscles of Chordates: Development, Homologies, and Evolution; Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar]
- Romer, A.S. The development of tetrapod limb musculature? The shoulder region of Lacerta. J. Morphol. 1944, 74, 1–41. [Google Scholar] [CrossRef]
- Russell, A.P.; Bauer, A.M. The appendicular locomotor apparatus of Sphenodon and normal-limbed squamates. Biol. Reptil. 2008, 21, 1–465. [Google Scholar]
- Diogo, R.; Abdala, V.; Aziz, M.; Lonergan, N.; Wood, B. From fish to modern humans–comparative anatomy, ho-mologies and evolution of the pectoral and forelimb musculature. J. Anat. 2009, 214, 694–716. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Tanaka, E.M. Anatomy of the pectoral and forelimb muscles of wildtype and green fluorescent pro-tein-transgenic axolotls and comparison with other tetrapods including humans: A basis for regenerative, evo-lutionary and developmental studies. J. Anat. 2012, 221, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Francis, E. The Anatomy of the Salamander; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Walker, W.F. Biology of the Reptilia; Academic Press: London, UK, 1974; Volume 4. [Google Scholar]
- Cheng, C.-C. The development of the shoulder region of the opossum, Didelphys virginiana, with special reference to the musculature. J. Morphol. 1955, 97, 415–471. [Google Scholar] [CrossRef]
- Walker, W.F. The development of the shoulder region of the turtle, Chrysemys picta marginata, with special reference to the primary musculature. J. Morphol. 1947, 80, 195–249. [Google Scholar] [CrossRef] [PubMed]
- Humphry, G.M. Art. XXIX.—Observations in Myology, including the Myology of Cryptobranch, Lepidosiren, Dog-Fish, Ceratodus, and Pseudopus Pallasii, with the nerves of Cryptobranch and Lepidosiren, and the disposition of muscles in Vertebrate Animals. Am. J. Med. Sci. 1873, 130, 520. [Google Scholar] [CrossRef]
- Abdala, V.; Manzano, A.S.; Herrel, A. The distal forelimb musculature in aquatic and terrestrial turtles: Phylog-eny or environmental constraints? J. Anat. 2008, 213, 159–172. [Google Scholar] [CrossRef]
- Lewis, O.J. Functional Morphology of the Evolving Hand and Foot; Oxford University Press: Oxford, MS, USA, 1989. [Google Scholar]
- Gatesy, S.M. Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology 1990, 16, 170–186. [Google Scholar] [CrossRef]
- Romer, A.S. The development of the thigh musculature of the chick twelve figures. J. Morphol. 1927, 43, 347–385. [Google Scholar] [CrossRef]
- Romer, A.S. The development of tetrapod limb musculature? The thigh of Lacerta. J. Morphol. 1942, 71, 251–298. [Google Scholar] [CrossRef]
- Chen, H.-K. The development of the pectoral limb of Necturus maculosus; Illinois Biological Monographs; Urbana, Ill.,University of Illinois: Champaign County, IL, USA; p. 1935. [CrossRef][Green Version]
- Sullivan, G. Anatomy and embryology of the Wing Musculature of the domestic fowl (gallus). Aust. J. Zool. 1962, 10, 458–518. [Google Scholar] [CrossRef]
- Jones, C.L. The morphogenesis of the thigh of the mouse with special reference to tetrapod muscle homologies. J. Morphol. 1979, 162, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, S.; Tosney, K.W. Spatial and temporal patterns of muscle cleavage in the chick thigh and their value as criteria for homology. Am. J. Anat. 1991, 191, 325–350. [Google Scholar] [CrossRef]
- Bardeen, C.R. Development and variation of the nerves and the musculature of the inferior extremity and of the neighboring regions of the trunk in man. Am. J. Anat. 1906, 6, 259–390. [Google Scholar] [CrossRef]
- Čihák, R. Ontogenesis of the Skeleton and Intrinsic Muscles of the Human Hand and Foot; Springer: Berlin/Heidelberg, Germany, 1972; pp. 12–59. [Google Scholar]
- Diogo, R.; Tanaka, E.M. Development of fore-and hindlimb muscles in GFP-transgenic axolotls: Morphogenesis, the tetrapod bauplan, and new insights on the Forelimb-Hindlimb Enigma. J. Exp. Zoolog. B Mol. Dev. Evol. 2014, 322, 106–127. [Google Scholar] [CrossRef]
- Diogo, R.; Ziermann, J.M. Development of fore-and hindlimb muscles in frogs: Morphogenesis, homeotic trans-formations, digit reduction, and the forelimb–hindlimb enigma. J. Exp. Zoolog. B Mol. Dev. Evol. 2014, 322, 86–105. [Google Scholar] [CrossRef]
- Diogo, R.; Siomava, N.; Gitton, Y. Development of human limb muscles based on whole-mount immunostaining and the links between ontogeny and evolution. Development 2019, 146, dev180349. [Google Scholar] [CrossRef]
- Valasek, P.; Theis, S.; DeLaurier, A.; Hinits, Y.; Luke, G.N.; Otto, A.M.; Minchin, J.; He, L.; Christ, B.; Brooks, G. Cel-lular and molecular investigations into the development of the pectoral girdle. Dev. Biol. 2011, 357, 108–116. [Google Scholar] [CrossRef]
- Leal, F.; Cohn, M.J. Developmental, genetic, and genomic insights into the evolutionary loss of limbs in snakes. Genesis 2017, 56, e23077. [Google Scholar] [CrossRef]
- Nagashima, H.; Sugahara, F.; Takechi, M.; Ericsson, R.; Kawashima-Ohya, Y.; Narita, Y.; Kuratani, S. Evolution of the Turtle Body Plan by the Folding and Creation of New Muscle Connections. Science 2009, 325, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Kuratani, S. Evolution of the muscular system in tetrapod limbs. Zool. Lett. 2018, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Grim, M.; Carlson, B.M. A comparison of morphogenesis of muscles of the forearm and hand during ontogene-sis and regeneration in the axolotl (Ambystoma mexicanum). Z. Für Anat. Entwicklungsgesch. 1974, 145, 149–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carlson, B.M. Muscle regeneration in amphibians and mammals: Passing the torch. Dev. Dyn. 2003, 226, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Nacu, E.; Tanaka, E.M. Is salamander limb regeneration really perfect? Anatomical and morphoge-netic analysis of forelimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative, develop-mental, and evolutionary studies: Limb regeneration in salamanders. Anat. Rec. 2014, 297, 1076–1089. [Google Scholar]
- Diogo, R.; Murawala, P.; Tanaka, E.M. Is salamander hindlimb regeneration similar to that of the forelimb? An-atomical and morphogenetic analysis of hindlimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative and developmental studies. J. Anat. 2014, 224, 459–468. [Google Scholar]
- Kardon, G. Muscle and tendon morphogenesis in the avian hind limb. Development 1998, 125, 4019–4032. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Gauthier, J.A. 1,2,3 = 2,3,4: A solution to the problem of the homology of the digits in the avian hand. Proc. Natl. Acad. Sci. USA 1999, 96, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, 8th ed.; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Carlson, B.M. Tissue Engineering and Regeneration; Academic Press: London, UK, 2007; pp. 259–278. [Google Scholar] [CrossRef]
- Fröbisch, N.B.; Carroll, R.L.; Schoch, R.R. Limb ossification in the Paleozoic branchiosaurid Apateon (Temno-spondyli) and the early evolution of preaxial dominance in tetrapod limb development. Evol. Dev. 2007, 9, 69–75. [Google Scholar] [CrossRef]
- Fröbisch, N.B. Ossification patterns in the tetrapod limb—Conservation and divergence from morphogenetic events. Biol. Rev. 2008, 83, 571–600. [Google Scholar] [CrossRef] [PubMed]
- Fröbisch, N.B.; Shubin, N.H. Salamander limb development: Integrating genes, morphology, and fossils. Dev. Dyn. 2011, 240, 1087–1099. [Google Scholar] [CrossRef]
- Shubin, N.; Tabin, C.; Carroll, S. Fossils, genes and the evolution of animal limbs. Nature 1997, 388, 639–648. [Google Scholar] [CrossRef]
- Johanson, Z.; Joss, J.; Boisvert, C.A.; Ericsson, R.; Sutija, M.; Ahlberg, P.E. Fish fingers: Digit homologues in sar-copterygian fish fins. J. Exp. Zoolog. B Mol. Dev. Evol. 2007, 308B, 757–768. [Google Scholar] [CrossRef]
- Johanson, Z. Evolution of paired fins and the lateral somitic frontier. J. Exp. Zool. Part B Mol. Dev. Evol. 2010, 314, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.I. The origin of vertebrate limbs. Development 1994, 1994, 169–180. [Google Scholar] [CrossRef]
- Coates, M.I.; Cohn, M.J. Vertebrate axial and appendicular patterning: The early development of paired ap-pendages. Am. Zool. 1999, 39, 676–685. [Google Scholar] [CrossRef]
- Diogo, R.; Linde-Medina, M.; Abdala, V.; Ashley-Ross, M.A. New, puzzling insights from comparative myologi-cal studies on the old and unsolved forelimb/hindlimb enigma. Biol. Rev. 2013, 88, 196–214. [Google Scholar] [CrossRef]
- Sears, K.E.; Capellini, T.D.; Diogo, R. On the serial homology of the pectoral and pelvic girdles of tetrapods. Evolution 2015, 69, 2543–2555. [Google Scholar] [CrossRef]
- Miyashita, T.; Diogo, R. Evolution of serial patterns in the vertebrate pharyngeal apparatus and paired append-ages via assimilation of dissimilar units. Front. Ecol. Evol. 2016, 4, 71. [Google Scholar] [CrossRef]
- Esteve-Altava, B.; Pierce, S.E.; Molnar, J.L.; Johnston, P.; Diogo, R.; Hutchinson, J.R. Evolutionary parallelisms of pectoral and pelvic network-anatomy from fins to limbs. Sci. Adv. 2019, 5, eaau7459. [Google Scholar] [CrossRef]
- Shubin, N.; Tabin, C.; Carroll, S. Deep homology and the origins of evolutionary novelty. Nature 2009, 457, 818–823. [Google Scholar] [CrossRef]
- Diogo, R.; Molnar, J. Comparative Anatomy, Evolution, and Homologies of Tetrapod Hindlimb Muscles, Comparison with Forelimb Muscles, and Deconstruction of the Forelimb-Hindlimb Serial Homology Hypothesis. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2014, 297, 1047–1075. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R. Cranial or postcranial—Dual origin of the pectoral appendage of vertebrates combining the fin-fold and gill-arch theories? Dev. Dyn. 2020, 249, 1182–1200. [Google Scholar] [CrossRef] [PubMed]
- Thacher, J.K. Median and Paired Fins, a Contribution to the History of Vertebrate Limbs; Connecticut Academy of Arts and Sciences: New Haven, CT, USA, 1874; Volume 3. [Google Scholar]
- Mivart, S.G. Notes on the fins of Elasmobranchs, with Considerations on the Nature and Homologues of Ver-tebrate Limbs. Trans. Zool. Soc. Lond. 1879, 10, 439–484. [Google Scholar] [CrossRef]
- Balfour, F.M. On the development of the skeleton of the paired fins of elasmobranchii, considered in relation to its bearings on the nature of the limbs of the vertebrata. In Proceedings of the Zoological Society of London; Blackwell Publishing Ltd: Oxford, UK, 1881; Volume 49. [Google Scholar]
- Gegenbaur, C. Grundzüge der vergleichenden Anatomie. Wilhelm Engelmann: Leipzig, Germany, 1870. [Google Scholar]
- Freitas, R.; Zhang, G.; Cohn, M.J. Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature 2006, 442, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Dahn, R.D.; Davis, M.C.; Pappano, W.N.; Shubin, N.H. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature 2006, 445, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.; Dahn, R.D.; Shubin, N.H. Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc. Natl. Acad. Sci. USA 2009, 106, 5720–5724. [Google Scholar] [CrossRef]
- Gillis, J.A.; Hall, B.K. A shared role for sonic hedgehog signalling in patterning chondrichthyan gill arch ap-pendages and tetrapod limbs. Development 2016, 143, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.K.; Mahon, V.; Diogo, R. Muscles Lost in Our Adult Primate Ancestors Still Imprint in Us: On Muscle Evolution, Development, Variations, and Pathologies. Curr. Mol. Biol. Rep. 2020, 6, 32–50. [Google Scholar] [CrossRef]
- Diogo, R.; Ziermann, J.M.; Linde-Medina, M. Is evolutionary biology becoming too politically correct? A reflec-tion on the scala naturae, phylogenetically basal clades, anatomically plesiomorphic taxa, and ‘lower’ animals: Is evolutionary biology too politically correct? Biol. Rev. 2015, 90, 502–521. [Google Scholar]
- Diogo, R.; Smith, C.M.; Ziermann, J. Evolutionary developmental pathology and anthropology: A new field linking development, comparative anatomy, human evolution, morphological variations and defects, and medicine. Dev. Dyn. 2015, 244, 1357–1374. [Google Scholar] [CrossRef] [PubMed]
- Løvtrup, S. On von Baerian and Haeckelian Recapitulation. Syst. Zool. 1978, 27, 348–352. [Google Scholar] [CrossRef]
- De Beer, G.R. Embryos and Ancestors; Clarendon Press: Oxford, UK, 1940; 108p. [Google Scholar]
- Wood, J. On Human Muscular Variations and their Relation to Comparative Anatomy. J. Anat. Physiol. 1867, 1, 44–59. [Google Scholar]
- Wood, J., VII. On a group of varieties of the muscles of the human neck, shoulder, and chest, with their transi-tional forms and homologies in the mammalia. Philos. Trans. R. Soc. Lond. 1870, 83–116. [Google Scholar]
- Macalister, A. Additional observations on muscular anomalies in human anatomy (3rd series), with a cata-logue of the principal muscular variations hitherto published. Trans Roy Ir. Acad Sci. 1871, 25, 1–134. [Google Scholar]
- Smith, C.; Ziermann, J.; Diogo, R. Muscular and Skeletal Anomalies in Human Trisomy in an Evo-Devo Context Using 3-D Imaging and Anatomical Dissections, with Notes on Down Syndrome, Cyclopia and Medical Impli-cations. FASEB J. 2015, 29, 870–871. [Google Scholar] [CrossRef]
- Gould, S.J. Ontogeny and Phylogeny; The Belknap Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Gould, S.J. The Structure of Evolutionary Theory; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Darwin, G. The Origin of Species by Natural Selection: Or the Preservation of Favored Races in the Struggle for Life, 1st ed.; Bantam Books: New York, NY, USA, 1859. [Google Scholar]
- Sanchez, S.; Dupret, V.; Tafforeau, P.; Trinajstic, K.M.; Ryll, B.; Gouttenoire, P.-J.; Wretman, L.; Zylberberg, L.; Peyrin, F.; Ahlberg, P.E. 3D Microstructural Architecture of Muscle Attachments in Extant and Fossil Vertebrates Revealed by Synchrotron Microtomography. PLoS ONE 2013, 8, e56992. [Google Scholar] [CrossRef] [PubMed]
- Woronowicz, K.C.; Gline, S.E.; Herfat, S.T.; Fields, A.J.; Schneider, R.A. Developmental Mechanisms Linking Form and Function During Jaw Evolution. bioRxiv 2018, 264556. [Google Scholar] [CrossRef]
- Botelho, J.F.; Smith-Paredes, D.; Soto-Acuña, S.; Mpodozis, J.; Palma, V.; Vargas, A.O. Skeletal plasticity in response to embryonic muscular activity underlies the development and evolution of the perching digit of birds. Sci. Rep. 2015, 5, 9840. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).