A New Signal of Tropicalization in the Northeast Atlantic: The Spread of the Spotfin Burrfish Chilomycterus reticulatus in Madeira Archipelago and Its Invasion Risk
Abstract
1. Introduction
2. Materials and Methods
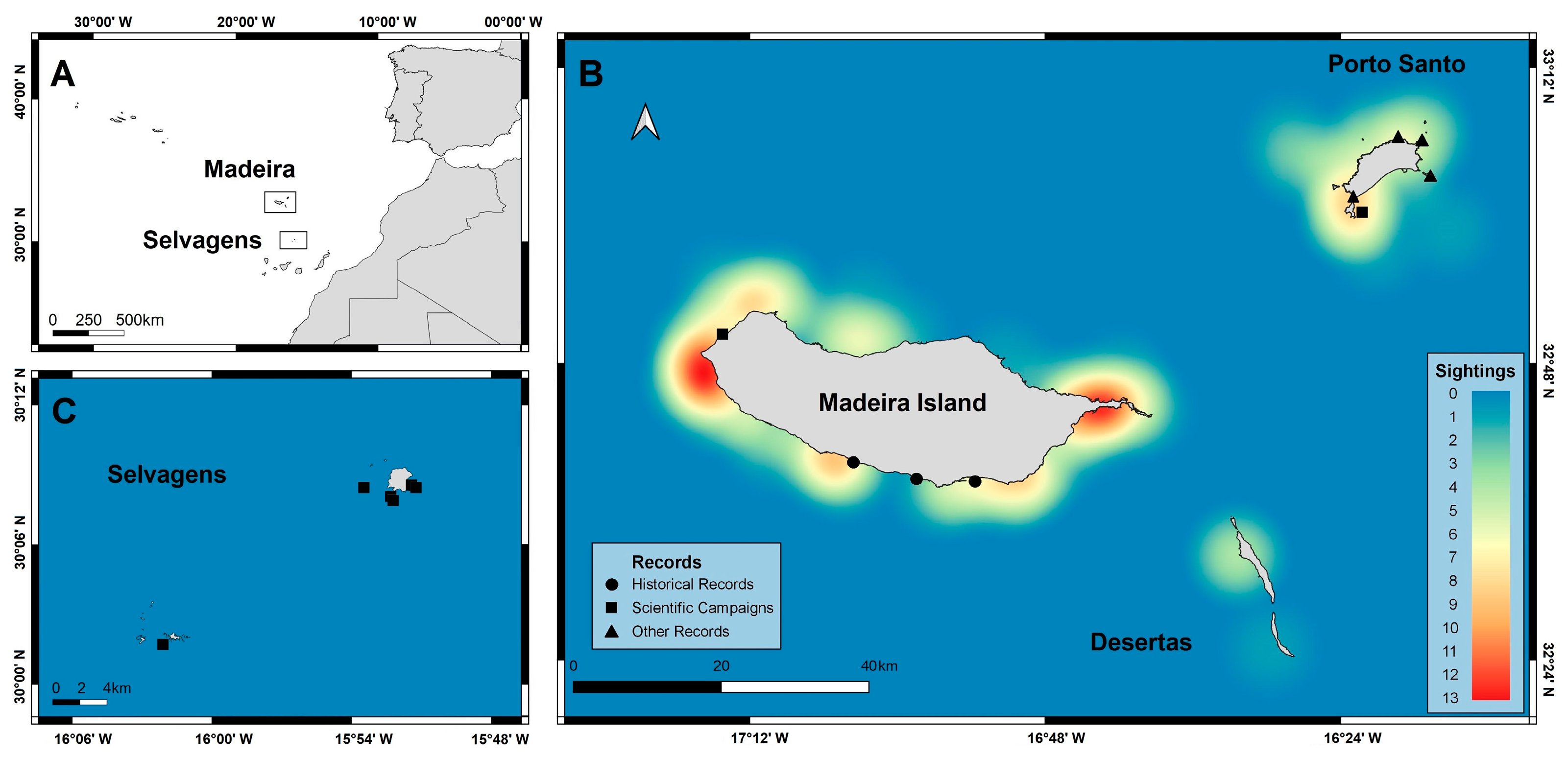
2.1. Study Area
2.2. Chilomycterus reticulatus Sightings
2.2.1. Annual Monitoring Surveys
2.2.2. Citizen Science
2.2.3. Complementary Records
2.2.4. Data Analysis
2.3. Seawater Temperatures and Analysis
2.4. Aquatic Species Invasiveness Screening Kit (AS-ISK)
3. Results
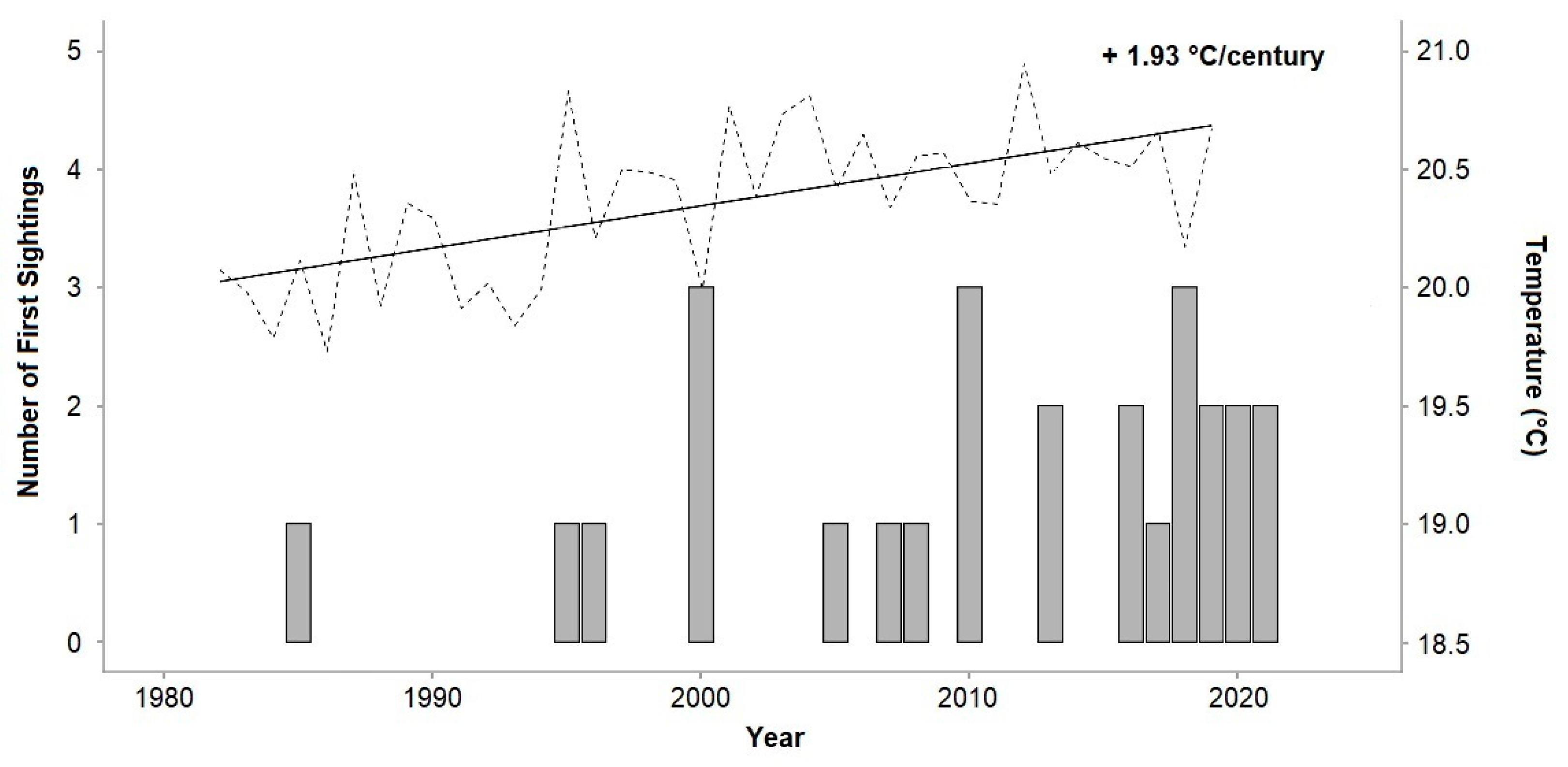
3.1. Chilomycterus reticulatus Sightings
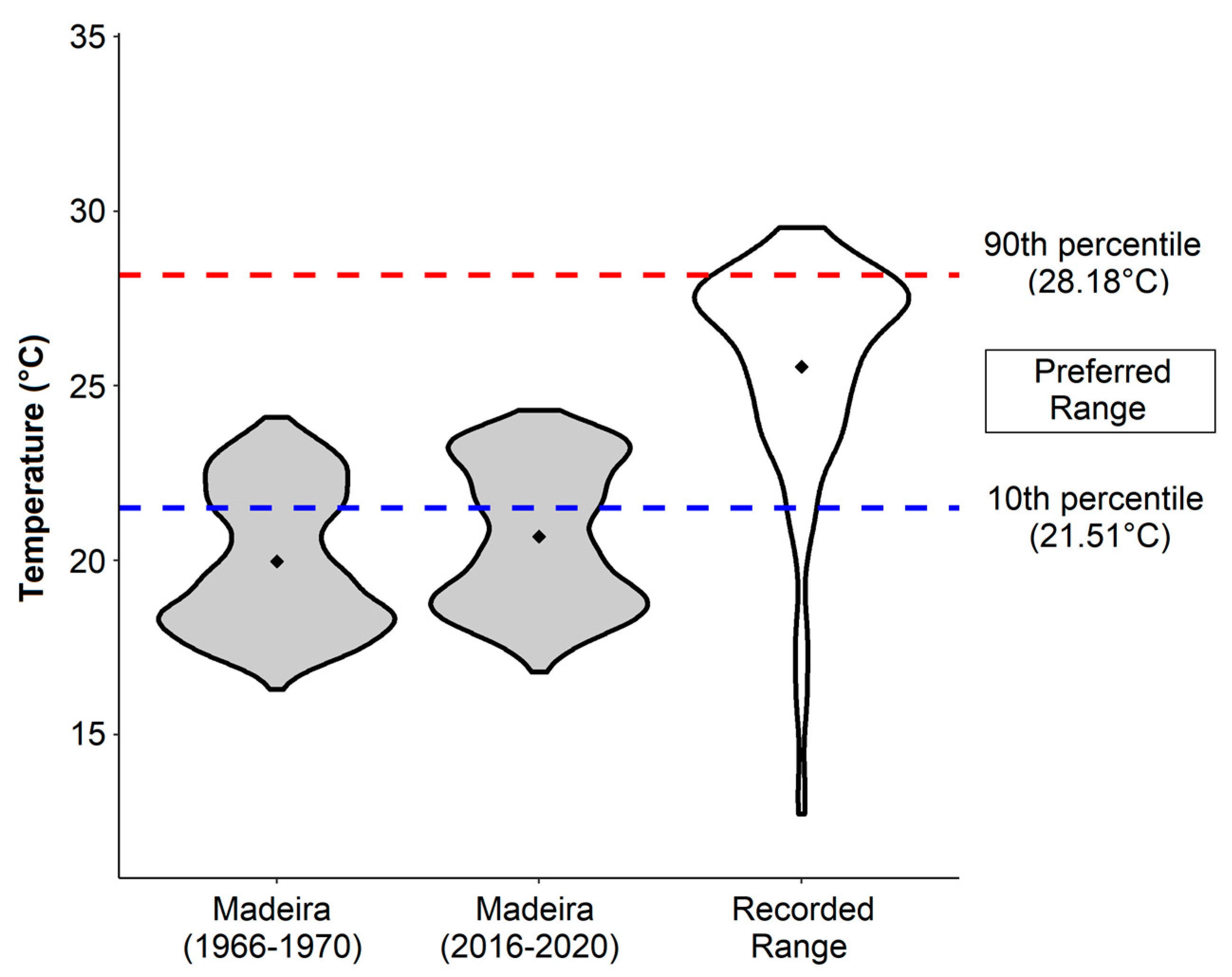
3.2. Seawater Temperatures and Analysis
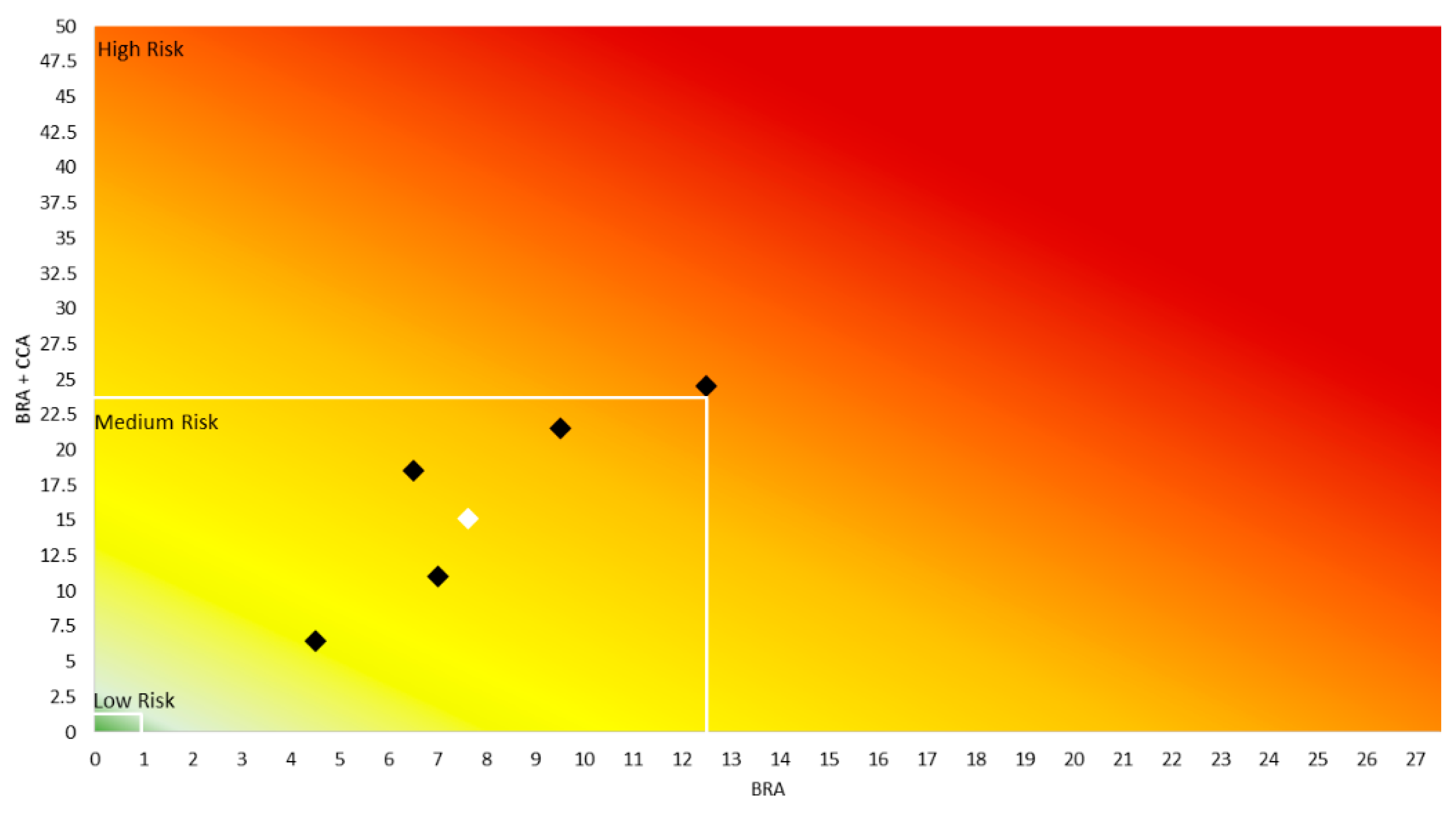
3.3. AS-ISK
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costello, M.J.; Coll, M.; Danovaro, R.; Halpin, P.; Ojaveer, H.; Miloslavich, P. A Census of Marine Biodiversity Knowledge, Resources, and Future Challenges. PLoS ONE 2010, 5, e12110. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Global Sea warming and “tropicalization” of the Mediterranean Sea: Biogeographic and ecological aspects. Biogeogr. J. Integr. Biogeogr. 2003, 24, 319–327. [Google Scholar] [CrossRef]
- Jansen, J.M.; Pronker, A.E.; Bonga, S.W.; Hummel, H. Macoma balthica in Spain, a few decades back in climate history. J. Exp. Mar. Biol. Ecol. 2007, 344, 161–169. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Williams, S.L.; Carlton, J.T. Marine range shifts and species introductions: Comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 2010, 19, 303–316. [Google Scholar] [CrossRef]
- Canning-Clode, J.; Fowler, A.E.; Byers, J.E.; Carlton, J.T.; Ruiz, G.M. ‘Caribbean Creep’ Chills Out: Climate Change and Marine Invasive Species. PLoS ONE 2011, 6, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Canning-Clode, J.; Carlton, J.T. Refining and expanding global climate change scenarios in the sea: Poleward creep complexities, range termini, and setbacks and surges. Divers. Distrib. 2017, 23, 463–473. [Google Scholar] [CrossRef]
- Ribeiro, C.; Neto, A.I.; Moreu, I.; Haroun, R.; Neves, P. A new signal of marine tropicalization in the Macaronesia region: First record of the mesophotic macroalga Avrainvillea canariensis A. Gepp E.S. Gepp in the Madeira archipelago. Aquat. Bot. 2019, 153, 40–43. [Google Scholar] [CrossRef]
- González, J.A.; Triay-Portella, R.; Escribano, A.; Cuesta, J.A. Northernmost record of the pantropical portunid crab Cronius ruber in the eastern Atlantic (Canary Islands): Natural range extension or human-mediated introduction? Sci. Mar. 2017, 81, 81–89. [Google Scholar] [CrossRef]
- Arias, A.; Crocetta, F. Umbraculum umbraculum (Gastropoda: Heterobranchia) spreading northwards: Additional evidence to the “tropicalization” of the Bay of Biscay. Cah. Biol. Mar. 2016, 57, 285–286. [Google Scholar]
- Encarnação, J.; Morais, P.; Baptista, V.; Cruz, J.; Teodósio, M.A. New evidence of marine fauna tropicalization off the Southwestern Iberian Peninsula (Southwest Europe). Diversity 2019, 11, 48. [Google Scholar] [CrossRef]
- Peleg, O.; Rilov, G.; Guy-Haim, T.; Yeruham, E.; Silverman, J.; Rilov, G. Tropicalization may invert trophic state and carbon budget of shallow temperate rocky reefs. J. Ecol. 2020, 108, 844–854. [Google Scholar] [CrossRef]
- Castro, N.; Carlton, J.T.; Costa, A.C.; Marques, C.; Hewitt, C.L.; Cacabelos, E.; Gizzi, F.; Gestoso, I.; Monteiro, J.G.; Costa, J.L.; et al. Archipelagos of invasions: Diversity and patterns of marine introduced species of Macaronesia. Divers. Distrib. 2021, in press. [Google Scholar]
- Canning-Clode, J.; Fofonoff, P.; McCann, L.; Carlton, J.T.; Ruiz, G. Marine invasions on a subtropical island: Fouling studies and new records in a recent marina on Madeira Island (Eastern Atlantic Ocean). Aquat. Invasions 2013, 8, 261–270. [Google Scholar] [CrossRef]
- Castro, N.; Ramalhosa, P.; Jiménez, J.; Costa, J.L.; Gestoso, I.; Canning-Clode, J. Exploring marine invasions connectivity in a NE Atlantic Island through the lens of historical maritime traffic patterns. Reg. Stud. Mar. Sci. 2020, 37, 101333. [Google Scholar] [CrossRef]
- Ramalhosa, P.; Gestoso, I.; Rocha, R.M.; Lambert, G.; Canning-Clode, J. Ascidian biodiversity in the shallow waters of the Madeira Archipelago: Fouling studies on artificial substrates and new records. Reg. Stud. Mar. Sci. 2021, 43, 101672. [Google Scholar] [CrossRef]
- Wirtz, P.; Fricke, R.; Biscoito, M.J. The coastal fishes of Madeira Island—New records and an annotated check-list. Zootaxa 2008, 1715, 1–26. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 2 June 2021).
- Leis, J.M. Family Diodontidae. In The Living Marine Resources of the Western Central Pacific; Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 2001; Volume 3, pp. 3958–3965. [Google Scholar]
- Leis, J.M. Family Diodontidae. In The Living Marine Resources of the Western Central Atlantic; Carpenter, K., Ed.; FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists, Special Publication Nº 5, Bony Fishes Part 2 (Opisthognathidae to Molidae), Sea Turtles and Marine Mammals; FAO: Rome, Italy, 2002; Volume 3, pp. 2007–2013. [Google Scholar]
- Sommer, C.; Schneider, W.; Poutiers, J.M. The Living Marine Resources of Somalia; Sommer, C., Schneider, W., Poutiers, J.-M., Eds.; FAO Species Identification Guide for Fishery Purposes; FAO: Rome, Italy, 1996; p. 337. [Google Scholar]
- Leis, J.M. Nomenclature and distribution of the species of the porcupine fish family Diodontidae (Pisces, Teleostei). Mem. Mus. Vic. 2006, 63, 77–90. [Google Scholar] [CrossRef]
- Kaschner, K.; Kesner-Reyes, K.; Garilao, C.; Segschneider, J.; Rius-Barile, J.; Rees, T.; Froese, R. Aquamaps. 2019. Available online: www.aquamaps.org. (accessed on 10 June 2021).
- Brito, A.; Falcón, J.M. Contribution to the knowledge of the distribution and ecology of Chilomycterus atringa (Pisces, Diodontidae) in the Canary Islands. Vieraea 1990, 19, 271–275. [Google Scholar]
- Espino, F.; Tuya, F.; Rosario, A.; Bosch, N.E.; Coca, J.; González-Ramos, A.J.; del Rosario, F.; Otero-Ferrer, F.J.; Moreno, Á.C.; Haroun, R. Geographical Range Extension of the Spotfin burrfish, Chilomycterus reticulatus (L. 1758), in the Canary Islands: A Response to Ocean Warming? Diversity 2019, 11, 230. [Google Scholar] [CrossRef]
- Pearce, F.; Peeler, E.; Stebbing, P. Modelling the Risk of the Introduction and Spread of Non-Indigenous Species in the UK and Ireland; Project Report for E5405W; CEFAS: Weymouth, UK, 2012. [Google Scholar]
- Copp, G.H.; Godard, M.J.; Russell, I.C.; Peeler, E.J.; Gherardi, F.; Tricarico, E.; Miossec, L.; Goulletquer, P.; Almeida, D.; Britton, J.R.; et al. A preliminary evaluation of the European Non-native Species in Aquaculture Risk Assessment Scheme applied to species listed on Annex IV of the EU Alien Species Regulation. Fish. Manag. Ecol. 2016, 23, 12–20. [Google Scholar] [CrossRef]
- Copp, G.H.; Garthwaite, R.; Gozlan, R.E. Risk identification and assessment of non-native freshwater fishes: A summary of concepts and perspectives on protocols for the UK. J. Appl. Ichthyol. 2005, 21, 371–373. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Tidbury, H.; Stebbing, P.D.; Tarkan, A.S.; Miossec, L.; Goulletquer, P. Development of a generic decision-support tool for identifying potentially invasive aquatic taxa: AS-ISK. Manag. Biol. Invasions 2016, 7, 343–350. [Google Scholar] [CrossRef]
- Mumford, J.D.; Booy, O.; Baker, R.H.A.; Rees, M.; Copp, G.H.; Black, K.; Holt, J.; Leach, A.W.; Hartley, M. Invasive non-native species risk assessment in Great Britain. Asp. Appl. Biol. 2010, 104, 49–54. [Google Scholar]
- David, M.; Gollasch, S.; Leppäkoski, E. Risk assessment for exemptions from ballast water management—The Baltic Sea case study. Mar. Pollut. Bull. 2013, 75, 205–217. [Google Scholar] [CrossRef]
- David, M.; Gollasch, S. Risk assessment for ballast water management—Learning from the Adriatic Sea case study. Mar. Pollut. Bull. 2019, 147, 36–46. [Google Scholar] [CrossRef]
- Vilizzi, L.; Copp, G.H.; Hill, J.E.; Adamovich, B.; Aislabie, L.; Akin, D.; Al-Faisal, A.J.; Almeida, D.; Azmai, M.N.A.; Bakiu, R.; et al. A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Sci. Total Environ. 2021, 788, 147868. [Google Scholar] [CrossRef]
- Copp, G.H.; Russell, I.C.; Peeler, E.J.; Gherardi, F.; Tricarico, E.; MacLeod, A.; Cowx, I.G.; Nunn, A.D.; Occhipinti-Ambrogi, A.; Savini, D.; et al. European Non-native Species in Aquaculture Risk Analysis Scheme—A summary of assessment protocols and decision support tools for use of alien species in aquaculture. Fish. Manag. Ecol. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Cook, E.J.; Payne, R.D.; Macleod, A.K.; Brown, S.F. Marine biosecurity: Protecting indigenous marine species. Res. Rep. Biodivers. Stud. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Copp, G.; Vilizzi, L.; Wei, H.; Li, S.; Piria, M.; Al-faisal, A.J.; Almeida, D.; Al-Wazzan, Z.; Bakiu, R.; Bašić, T.; et al. Speaking their language—Development of a multilingual decision-support tool for communicating invasive species risks to decision makers and stakeholders. Environ. Model. Softw. 2021, 135, 104900. [Google Scholar] [CrossRef]
- Statzner, B.; Bonada, N.; Doledec, S. Biological attributes discriminating invasive from native European stream macroinvertebrates. Biol. Invasions 2008, 10, 517–530. [Google Scholar] [CrossRef]
- Chan, J.; Zeng, Y.; Yeo, D.C.J. Invasive species trait-based risk assessment for non-native freshwater fishes in a tropical city basin in Southeast Asia. PLoS ONE 2021, 16, e0248480. [Google Scholar] [CrossRef]
- Piaggio, A.J.; Engeman, R.M.; Hopken, M.W.; Humphrey, J.S.; Keacher, K.L.; Bruce, W.E.; Avery, M.L. Detecting an elusive invasive species: A diagnostic PCR to detect Burmese python in Florida waters and an assessment of persistence of environmental DNA. Mol. Ecol. Resour. 2014, 14, 374–380. [Google Scholar] [CrossRef]
- Pendleton, L.H.; Beyer, H.; Estradivari; Grose, S.O.; Hoegh-Guldberg, O.; Karcher, D.B.; Kennedy, E.; Llewellyn, L.; Nys, C.; Shapiro, A.; et al. Disrupting data sharing for a healthier ocean. ICES Mar. Sci. 2019, 76, 1415–1423. [Google Scholar] [CrossRef]
- Cigliano, J.A.; Meyer, R.; Ballard, H.L.; Freitag, A.; Phillips, T.B.; Wasser, A. Making marine and coastal citizen science matter. Ocean Coast. Manag. 2015, 115, 77–87. [Google Scholar] [CrossRef]
- Kelly, R.; Fleming, A.; Pecl, G.T.; Von Gönner, J.; Bonn, A. Citizen science and marine conservation: A global review. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190461. [Google Scholar] [CrossRef]
- McKinley, D.C.; Miller-Rushing, A.J.; Ballard, H.L.; Bonney, R.; Brown, H.; Cook-Patton, S.; Evans, D.M.; French, R.A.; Parrish, J.K.; Phillips, T.B.; et al. Citizen science can improve conservation science, natural resource management, and environmental protection. Biol. Conserv. 2017, 208, 15–28. [Google Scholar] [CrossRef]
- Warner, K.A.; Lowell, B.; Timme, W.; Shaftel, E.; Hanner, R.H. Seafood sleuthing: How citizen science contributed to the largest market study of seafood mislabeling in the U.S. and informed policy. Mar. Policy 2019, 99, 304–311. [Google Scholar] [CrossRef]
- Hyder, K.; Townhill, B.; Anderson, L.G.; Delany, J.; Pinnegar, J.K. Can citizen science contribute to the evidence-base that underpins marine policy? Mar. Policy 2015, 59, 112–120. [Google Scholar] [CrossRef]
- Fritz, S.; See, L.; Carlson, T.; Haklay, M.; Oliver, J.; Fraisl, D.; Mondardini, R.; Brocklehurst, M.; Shanley, L.; Schade, S.; et al. Citizen science and the United Nations Sustainable Development Goals. Nat. Sustain. 2019, 2, 922–930. [Google Scholar] [CrossRef]
- Bodilis, P.; Louisy, P.; Draman, M.; Arceo, H.O.; Francour, P. Can Citizen Science Survey Non-indigenous Fish Species in the Eastern Mediterranean Sea? Environ. Manag. 2014, 53, 172–180. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Outinen, O.; Puntila-Dodd, R. Citizen science provides added value in the monitoring for coastal non-indigenous species. J. Environ. Manag. 2020, 267, 110608. [Google Scholar] [CrossRef]
- De Sherbinin, A.; Bowser, A.; Chuang, T.-R.; Cooper, C.; Danielsen, F.; Edmunds, R.; Elias, P.; Faustman, E.; Hultquist, C.; Mondardini, R.; et al. The Critical Importance of Citizen Science Data. Front. Clim. 2021, 3, 650760. [Google Scholar] [CrossRef]
- Garcia-Soto, C.; Seys, J.J.C.; Zielinski, O.; Busch, J.A.; Luna, S.I.; Baez, J.C.; Domegan, C.; Dubsky, K.; Kotynska-Zielinska, I.; Loubat, P.; et al. Marine Citizen Science: Current State in Europe and New Technological Developments. Front. Mar. Sci. 2021, 8, 621472. [Google Scholar] [CrossRef]
- INE Instituto Nacional de Estatística (INE); Direção Regional de Estatística da Madeira (DREM). Census 2021. 2021. Available online: www.estatistica.Madeira.gov.pt (accessed on 13 June 2021).
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Canning-Clode, J.; Kaufmann, M.; Molis, M.; Wahl, M.; Lenz, M. Influence of disturbance and nutrient enrichment on early successional fouling communities in an oligotrophic marine system. Mar. Ecol. 2008, 29, 115–124. [Google Scholar] [CrossRef]
- Monteiro, J.G.; Jiménez, J.L.; Gizzi, F.; Přikryl, P.; Lefcheck, J.S.; Santos, R.S.; Canning-Clode, J. Novel approach to enhance coastal habitat and biotope mapping with drone aerial imagery analysis. Sci. Rep. 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Monteiro, J.; Castro, N.; Rilov, G.; Canning-Clode, J. Cronius ruber (Lamarck, 1818) arrives to Madeira Island: A new indication of the ongoing tropicalization of the northeastern Atlantic. Mar. Biodivers. 2019, 49, 2699–2707. [Google Scholar] [CrossRef]
- Mason, E.; Colas, F.; Molemaker, J.; Shchepetkin, A.F.; Troupin, C.; McWilliams, J.C.; Sangrà, P. Seasonal variability of the Canary Current: A numerical study. J. Geophys. Res. 2011, C06001, 1–20. [Google Scholar] [CrossRef]
- QGIS Association. QGIS Geographic Information System. 2021. Available online: QGIS.org (accessed on 22 June 2021).
- IBM Corp. IBM SPSS Statistics for Windows, Version 27.0; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]
- Reynolds, R.D.; Smith, T.M.; Liu, C.; Chelton, D.B.; Casey, K.S.; Schlax, M.G. Daily High-Resolution-Blended Analyses for Sea Surface Temperature. J. Clim. 2007, 20, 5473–5496. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Newnham, G.; Culvenor, D. Detecting Trend and Seasonal Changes in Satellite Image Time Series. Remote Sens. Environ. 2010, 114, 106–115. [Google Scholar] [CrossRef]
- Goela, P.C.; Cordeiro, C.; Danchenko, S.; Icely, J.; Cristina, S.; Newton, A. Time series analysis of data for sea surface temperature and upwelling components from the southwest coast of Portugal. J. Mar. Syst. 2016, 163, 12–22. [Google Scholar] [CrossRef]
- IPMA. Sea Water Temperatures Measured at the Station 522—Observatório Meteorológico do Funchal between 1961–2020; Instituto Português do Mar e da Atmosfera: Lisbon, Portugal, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Kassambara, A. “ggplot2” Based Publication Ready Plots, R Package. 2020. Available online: https://Cran.r-Project.org/web/packages/ggpubr (accessed on 26 June 2021).
- Pierce, D. ncdf4: Interface to Unidata netCDF (Version 4 or Earlier) Format Data Files, R Package. 2019. Available online: https://CRAN.R-Project.org/package=ncdf4 (accessed on 2 July 2021).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation, R Package. 2020. Available online: https://CRAN.R-Project.org/package=dplyr (accessed on 4 July 2021).
- Pheloung, P.C.; Williams, P.A.; Halloy, S.R. A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. J. Environ. Manag. 1999, 57, 239–251. [Google Scholar] [CrossRef]
- Oliveira, P.; Pereira, P.T. Who values what in a tourism destination? The case of Madeira Island. Tour. Econ. 2008, 14, 155–168. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Vieira, C.; Gouveia, L.; Gouveia, N.; Hermida, M. Characterization and evolution of spearfishing in Madeira archipelago, Eastern Atlantic. Aquat. Living Resour. 2020, 33, 15. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Hermida, M.; Villasante, S.; Gouveia, L.; Gouveia, N.; Pita, P. Importance of recreational shore angling in the archipelago of Madeira, Portugal (northeast Atlantic). Sci. Mar. 2020, 84, 331–341. [Google Scholar] [CrossRef]
- Azzurro, E.; Cerri, J. Participatory mapping of invasive species: A demonstration in a coastal lagoon. Mar. Policy 2021, 126, 104412. [Google Scholar] [CrossRef]
- Gizzi, F.; Jiménez, J.; Schäfer, S.; Castro, N.; Costa, S.; Lourenço, S.; José, R.; Canning-Clode, J.; Monteiro, J. Before and after a disease outbreak: Tracking a keystone species recovery from a mass mortality event. Mar. Environ. Res. 2020, 156, 104905. [Google Scholar] [CrossRef]
- Cerrano, C.; Milanese, M.; Ponti, M. Diving for science—Science for diving: Volunteer scuba divers support science and conservation in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 27, 303–323. [Google Scholar] [CrossRef]
- Crall, A.W.; Jordan, R.; Holfelder, K.; Newman, G.J.; Graham, J.; Waller, D.M. The impacts of an invasive species citizen science training program on participant attitudes, behavior, and science literacy. Public Underst. Sci. 2012, 22, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.K.M.; Tuya, F.; Barker, J.; Alvarado, D.J.; Castro-Hernández, J.J.; Haroun, R.; Rödder, D. Population structure, distribution and habitat use of the Critically Endangered Angelshark, Squatina squatina, in the Canary Islands. Aquat. Conserv. 2017, 27, 1133–1144. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Thiel, M. The Contribution of Citizen Scientists to the Monitoring of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 429–447. [Google Scholar] [CrossRef]
- Casale, P.; Ciccocioppo, A.; Vagnoli, G.; Rigoli, A.; Freggi, D.; Tolve, L.; Luschi, P. Citizen science helps assessing spatio-temporal distribution of sea turtles in foraging areas. Aquat. Conserv. 2020, 30, 123–130. [Google Scholar] [CrossRef]
- Donnelly-Greenan, E.L.; Nevins, H.M.; Harvey, J.T. Entangled seabird and marine mammal reports from citizen science surveys from coastal California (1997–2017). Mar. Pollut. Bull. 2019, 149, 110557. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen Science as an Ecological Research Tool: Challenges and Benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Geldmann, J.; Heilmann-Clausen, J.; Holm, T.E.; Levinsky, I.; Markussen, B.; Olsen, K.; Rahbek, C.; Tøttrup, A.P. What determines spatial bias in citizen science? Exploring four recording schemes with different proficiency requirements. Divers. Distrib. 2016, 22, 1139–1149. [Google Scholar] [CrossRef]
- Zeidberg, L.D.; Robison, B.H. Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc. Natl. Acad. Sci. USA 2007, 104, 12948–12950. [Google Scholar] [CrossRef]
- Neumann, H.; de Boois, I.; Kröncke, I.; Reiss, H. Climate change facilitated range expansion of the non-native angular crab Goneplax rhomboides into the North Sea. Mar. Ecol. Prog. Ser. 2013, 484, 143–153. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Caroli, F.; Guidetti, P.; Morri, C. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J. Mar. Biol. Assoc. UK 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Iturbide, M.; Fernández, J.; Gutiérrez, J.M.; Bedia, J.; Cimadevilla, E.; Díez-Sierra, J.; Manzanas, R.; Casanueva, A.; Baño-Medina, J.; Milovac, J.; et al. Repository Supporting the Implementation of FAIR Principles in the IPCC-WG1 Atlas. Zenodo. 2021. Available online: https://github.com/IPCC-WG1/Atlas (accessed on 17 May 2021).
- Follesa, M.C.; Mulas, A.; Porcu, C.; Cau, A. First record of Chilomycterus reticulatus (Osteichthyes: Diodontidae) in the Mediterranean Sea. J. Fish Biol. 2009, 74, 1677–1681. [Google Scholar] [CrossRef]
- Brito, A.; Lozano, G. Consideraciones Zoogeográficas Sobre la Fauna Ictiológica Bentónica y Epibentónica de las Islas Canarias; Jornadas de Ictiología Ibérica: León, Spain, 1981. [Google Scholar]
- COINVA Project. Conocer al Invasor (COINVA). 2018. Available online: http://www.proyectocoinva.com (accessed on 20 August 2021).
- Freitas, M.; Canning-Clode, J. Non-Indigenous Fish in the Fresh and Marine Waters of the Madeira Archipelago. In Proceedings of the SIBIC2014—V Jornadas Lbérica de Lctiologia, Lisbon, Portugal, 24–27 June 2014. [Google Scholar]
- Brito, A.; Falcón, J.M.; Herrera, R. Sobre la tropicalización reciente de la ictiofauna litoral de las islas Canarias y su relación con cambios ambientales y actividades antrópicas. Vieraea 2005, 33, 515–526. [Google Scholar]
- Triay-Portella, R.; Pajuelo, J.G.; Manent, P.; Espino, F.; Ruiz-Díaz, R.; Lorenzo, J.M.; González, J.A. New records of non-indigenous fishes (Perciformes and Tetraodontiformes) from the Canary Islands (north-eastern Atlantic). Cybium 2015, 39, 163–174. [Google Scholar] [CrossRef]
- Gallardo, T.; Bárbara, I.; Afonso-Carrillo, J.; Bermejo, R.; Altamirano, M.; Garreta, A.G.; Martí, M.C.B.; Lluch, J.R.; Ballesteros, E.; La Rosa, J.D. A new checklist of benthic marine algae of Spain. Algas. Boletín Informativo Sociedad Española Ficología 2016, 51, 7–52. [Google Scholar]
- Verlaque, M.; Durand, C.; Huisman, J.M.; Boudouresque, C.-F.; Le Parco, Y. On the identity and origin of the Mediterranean invasive Caulerpa racemosa (Caulerpales, Chlorophyta). Eur. J. Phycol. 2003, 38, 325–339. [Google Scholar] [CrossRef]
- Cohen, A. Have claw, will travel. Aquatic Nuisance Species (ANS) Digest. Aquatic 1997, 2, 16–17. [Google Scholar]
- Leis, J.M. Diodontidae. Porcupine fishes (burrfishes, spiny puffers). In The Living Marine Resources ofthe Eastern Central Atlantic; FAO Species Identification Guide for Fishery Purposes, Bony Fishes, Part 2 (Perciformes to Tetraodontiformes) and Sea Turtles; Carpenter, K.E., De Angelis, N., Eds.; FAO: Rome, Italy, 2016; Volume 4, pp. 3074–3079. [Google Scholar]
- Afonso, P.; Porteiro, F.M.; Fontes, J.; Tempera, F.; Morato, T.; Cardigos, F.; Santos, R.S. New and rare coastal fishes in the Azores islands: Occasional events or tropicalization process? J. Fish Biol. 2013, 83, 272–294. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.T.; Ruiz, G.M. Vector science and integrated vector management in bioinvasion ecology: Conceptual frameworks. In Invasive Alien Species; Mooney, H.A., Mack, R.N., McNeely, J.A., Neville, L.E., Schei, P.J., Waage, J.K., Eds.; Island Press: Washington, DC, USA, 2005; pp. 36–58. [Google Scholar]
- Freitas, R.; Romeiras, M.; Silva, L.; Cordeiro, R.; Madeira, P.; González, J.A.; Wirtz, P.; Falcón, J.M.; Brito, A.; Floeter, S.R.; et al. Restructuring of the ‘Macaronesia’ biogeographic unit: A marine multi-taxon biogeographical approach. Sci. Rep. 2019, 9, 15792. [Google Scholar] [CrossRef]
- Henriques, M.; Gonçalves, E.J.; Almada, V.C. Rapid shifts in a marine fish assemblage follow fluctuations in winter sea conditions. Mar. Ecol. Prog. Ser. 2007, 340, 259–270. [Google Scholar] [CrossRef]
- Hernández-Guerra, A.; Espino-Falcón, E.; Vélez-Belchí, P.; Dolores Pérez-Hernández, M.; Martínez-Marrero, A.; Cana, L. Recirculation of the Canary Current in fall 2014. J. Mar. Syst. 2017, 174, 25–39. [Google Scholar] [CrossRef]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Nagasawa, K.; Uyeno, D. Geographical distribution affected by the Kuroshio of the fish parasite Cymothoa pulchra (Isopoda: Cymothoidae) in Japanese waters. Biogeography 2012, 14, 151–153. [Google Scholar] [CrossRef]
- Martin, S.B.; Ribu, D.; Cutmore, S.C.; Cribb, T.H. Opistholobetines (Digenea: Opecoelidae) in Australian tetraodontiform fishes. Syst. Parasitol. 2018, 95, 743–781. [Google Scholar] [CrossRef]
- Nagashima, Y.; Ohta, A.; Yin, X.; Ishizaki, S.; Matsumoto, T.; Doi, H.; Ishibashi, T. Difference in Uptake of Tetrodotoxin and Saxitoxins into Liver Tissue Slices among Pufferfish, Boxfish and Porcupinefish. Mar. Drugs 2018, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Evaluation of the Safety of Fishery Products. Naturally Occurring Fish and Shellfish Poisons; Ahmed, F.E., Ed.; Seafood Safety; National Academies Press: Washington, DC, USA, 1991; p. 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK235731 (accessed on 10 October 2021).
- Green, S.J.; Akins, J.L.; Maljkovic, A.; Cote, I.M. Invasive Lionfish Drive Atlantic Coral Reef Fish Declines. PLoS ONE 2012, 7, e32596. [Google Scholar] [CrossRef]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Rotter, A.; Klun, K.; Francé, J.; Mozetič, P.; Orlando-Bonaca, M. Non-indigenous Species in the Mediterranean Sea: Turning From Pest to Source by Developing the 8Rs Model, a New Paradigm in Pollution Mitigation. Front. Mar. Sci. 2020, 7, 178. [Google Scholar] [CrossRef]
- Bacallado, J.J.; Brito, A.; Cruz, T.; Carrillo, M.; Barquín, J. Proyecto Bentos II. Anexo: Estudio de la Biología del Erizo de Lima (Diadema antillarum); Informes de La Consejería de Agricultura y Pesca Del Gobierno de Canarias: Sevilla, Spain, 1987. [Google Scholar]
- Bacallado, J.J.; Cruz, T.; Brito, A.; Barquín, J.; Carrillo, M. Reservas Marinas de Canarias; Consejería de Agricultura y Pesca: Sevilla, Spain, 1989. [Google Scholar]
- Gizzi, F.; Monteiro, J.G.; Silva, R.; Schäfer, S.; Castro, N.; Almeida, S.; Chebaane, S.; Bernal-Ibáñez, A.; Henriques, F.; Gestoso, I.; et al. Disease Outbreak in a Keystone Grazer Population Brings Hope to the Recovery of Macroalgal Forests in a Barren Dominated Island. Front. Mar. Sci. 2021, 8, 645578. [Google Scholar] [CrossRef]
- Hewitt, C.L.; Hayes, K.R. Risk assessment of marine biological invasions. In Invasive Aquatic Species of Europe, Distribution, Impacts and Management; Leppakoski, E., Gollasch, S., Olenin, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 456–466. [Google Scholar] [CrossRef]
- Forrest, B.M.; Taylor, M.D.; Sinner, J. Setting priorities for the management of marine pests using a risk-based decision support framework. In Biological Invasions in New Zealand; Springer: Berlin/Heidelberg, Germany, 2006; pp. 389–405. [Google Scholar] [CrossRef]
- Leis, J.L.; Matsuura, K.; Shao, K.-T.; Hardy, G.; Zapfe, G.; Liu, M.; Jing, L.; Robertson, R.; Tyler, J. Chilomycterus reticulatus (errata version published in 2017). IUCN Red List Threat. Species 2015, e.T193752A115331266. [Google Scholar] [CrossRef]
- Castro, N.; Ramalhosa, P.; Cacabelos, E.; Costa, J.L.; Canning-Clode, J.; Gestoso, I. Winners and losers: Prevalence of non-indigenous species under simulated marine heatwaves and high propagule pressure. Mar. Ecol. Prog. Ser. 2021, 668, 21–38. [Google Scholar] [CrossRef]
- Minchin, D. Exotic Species, Introduction of. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Cambridge, UK, 2009; pp. 289–300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, N.; Schäfer, S.; Parretti, P.; Monteiro, J.G.; Gizzi, F.; Chebaane, S.; Almada, E.; Henriques, F.; Freitas, M.; Vasco-Rodrigues, N.; et al. A New Signal of Tropicalization in the Northeast Atlantic: The Spread of the Spotfin Burrfish Chilomycterus reticulatus in Madeira Archipelago and Its Invasion Risk. Diversity 2021, 13, 639. https://doi.org/10.3390/d13120639
Castro N, Schäfer S, Parretti P, Monteiro JG, Gizzi F, Chebaane S, Almada E, Henriques F, Freitas M, Vasco-Rodrigues N, et al. A New Signal of Tropicalization in the Northeast Atlantic: The Spread of the Spotfin Burrfish Chilomycterus reticulatus in Madeira Archipelago and Its Invasion Risk. Diversity. 2021; 13(12):639. https://doi.org/10.3390/d13120639
Chicago/Turabian StyleCastro, Nuno, Susanne Schäfer, Paola Parretti, João Gama Monteiro, Francesca Gizzi, Sahar Chebaane, Emanuel Almada, Filipe Henriques, Mafalda Freitas, Nuno Vasco-Rodrigues, and et al. 2021. "A New Signal of Tropicalization in the Northeast Atlantic: The Spread of the Spotfin Burrfish Chilomycterus reticulatus in Madeira Archipelago and Its Invasion Risk" Diversity 13, no. 12: 639. https://doi.org/10.3390/d13120639
APA StyleCastro, N., Schäfer, S., Parretti, P., Monteiro, J. G., Gizzi, F., Chebaane, S., Almada, E., Henriques, F., Freitas, M., Vasco-Rodrigues, N., Silva, R., Radeta, M., Freitas, R., & Canning-Clode, J. (2021). A New Signal of Tropicalization in the Northeast Atlantic: The Spread of the Spotfin Burrfish Chilomycterus reticulatus in Madeira Archipelago and Its Invasion Risk. Diversity, 13(12), 639. https://doi.org/10.3390/d13120639






