Abstract
Current trends in the global climate facilitate the displacement of numerous marine species from their native distribution ranges to higher latitudes when facing warming conditions. In this work, we analyzed occurrences of a circumtropical reef fish, the spotfin burrfish, Chilomycterus reticulatus (Linnaeus, 1958), in the Madeira Archipelago (NE Atlantic) between 1898 and 2021. In addition to available data sources, we performed an online survey to assess the distribution and presence of this species in the Madeira Archipelago, along with other relevant information, such as size class and year of the first sighting. In total, 28 valid participants responded to the online survey, georeferencing 119 C. reticulatus sightings and confirming its presence in all archipelago islands. The invasiveness of the species was screened using the Aquatic Species Invasiveness Screening Kit. Five assessments rated the fish as being of medium risk of establishing a local population and becoming invasive. Current temperature trends might have facilitated multiple sightings of this thermophilic species in the Madeira Archipelago. The present study indicates an increase in C. reticulatus sightings in the region. This underlines the need for updated comprehensive information on species diversity and distribution to support informed management and decisions. The spread of yet another thermophilic species in Madeiran waters provides further evidence of an ongoing tropicalization, emphasizing the need for monitoring programs and the potential of citizen science in complementing such programs.
Keywords:
climate change; range expansion; Macaronesia; non-indigenous species; NIS; AS-ISK; citizen science 1. Introduction
Numerous human-mediated actions, like climate change and biological invasions, trigger environmental changes which impact on global biodiversity and ecosystems [1,2]. Ongoing climate change encompasses many ocean modifications, such as temperature changes, ocean acidification, sea-level rise, and consequent variations in ocean stratification, upwellings, currents, and weather patterns [2,3]. All of these can have determinant roles in many ecological and biological mechanisms and functioning [4,5]. One of climate change’s most direct ecological effects is species distribution shifts [6], often increasing the proportion of warm-water species in temperate or subtropical regions. This phenomenon, commonly referred to as tropicalization [7], has been observed globally and across several marine areas and taxa [7,8,9,10,11]. In European waters, including the Mediterranean Sea, there are multiple examples of such events across a variety of taxa (e.g., algae, crustaceans, molluscs, and fishes) [12,13,14,15]. Evidence from across the globe shows that these shifts of tropical species can promote changes in community structures, often resulting in biodiversity loss [6] and even inverting the net trophic state and carbon balance on some occasions [16].
The Macaronesian Islands (composed of the Azores, Madeira, the Canary Islands, and Cabo Verde Archipelagos) constitute a suitable example of oceanic islands hosting marine flora and fauna influenced by the arrival of new species and impacted by climate change. Over recent decades, there has been an increase in detecting new species in these archipelagos from various taxa ([17] and references within). This increase over the last 30 years in Madeira is related to the arrival of fishes, macroalgae, arthropods, tunicates, and echinoderms [17]. These new additions are the combined consequence of an increase in sampling efforts, an intensification of maritime traffic (with a consequent rise in propagule pressure), and the range expansion of some species, likely facilitated by temperature increases (e.g., [11,17,18,19,20,21]).
In early October 2020, one specimen of Chilomycterus reticulatus (Linnaeus, 1758) (Tetraodontiformes, Diodontidae) was observed and photographed at −20 m depth during a scientific campaign at Porto Santo Island (Madeira Archipelago). The sighting generated some curiosity since the presence of this species in Madeira is considered rare. Further research revealed that the species had also been observed a couple of months earlier in Madeira Island, with images posted on social media platforms. These recent sightings of C. reticulatus in the area sparked (scientific) attention and, ultimately, triggered this study. The first record of C. reticulatus in Madeira dates to 1898, with a specimen deposited in the Natural History Museum of Funchal (Museu Municipal do Funchal, MMF). Subsequently, new records and specimens from Madeira have been sporadically reported and deposited in the collections of MMF and the British Natural History Museum London [21].
With a circumtropical distribution, C. reticulatus can be found in tropical to subtropical seas [22]. Adult individuals are around 50 cm long, on average, with a maximum of 75 cm [23,24]. This species is associated with a wide variety of habitats, including rocky reefs, coral reefs, and sandy bottoms, with preferred depths between −20 to −100 m, usually deeper in the tropics [25,26]. The preferred temperature for C. reticulatus is, on average, 25.5 °C, but it has been observed in areas with a temperature range between 12.7 °C to 29.5 °C [27]. Regarding food preferences, the spotfin burrfish elects hard-shell invertebrates but also feeds on large-sized sea urchins [28]. The eggs, larvae, and juveniles are pelagic until reaching about 20 cm of standard total length [25,29]. In Madeira Island, C. reticulatus is the only validated representative of the Diodontidae family [21]. However, other representatives from this family have been recorded, but their identification remains doubtful between the species Diodon hystrix Linnaeus, 1758, and Diodon eydouxii Brisout de Barneville, 1846 [21].
Prevention is primarily recognized as an effective method to avoid or mitigate the impacts associated with the proliferation of non-indigenous species (NIS) [30]. Preventing and/or avoiding the arrival of range expansion species induced by tropicalization is quite challenging. Therefore, detecting and monitoring non-indigenous and potentially invasive species in a particular location provides a crucial baseline for advice to politicians, decision-makers, and other stakeholders regarding management options in coping with those species [31,32,33,34,35,36,37]. Risk screening and hazard identification are other crucial steps in assessing the risks associated with NIS proliferations [38] by identifying and ranking NIS that likely could cause a threat to native species and ecosystems in a particular region [33]. Risk assessment protocols for NIS and pathways/vectors are crucial for implementing best practice procedures for risk reduction in NIS introduction by different pathways [31,32,33], assuming high importance in biosecurity [39].
One method to identify potentially invasive aquatic species is by using risk assessment tools such as the Aquatic Invasiveness Screening Kit (AS-ISK) [33]. This software measures the risk associated with introducing a given taxon based on its biological traits, ecological characteristics, and the similarities between its native range and the risk assessment area [37,40]. Usually, aquatic species inherit specific life-history characteristics, including high fecundity, large body size, long life span, opportunistic feeding behaviour, amongst others [41,42], showing a higher risk of becoming invasive. In addition, invasive species often tolerate higher salinity, broader environmental temperatures, and higher levels of organic pollution than native species [41,42].
Nevertheless, some invasive species can be elusive or difficult to detect [43] as data related to biological traits, and other relevant information can be costly or challenging to obtain [44]. This is particularly true for the routine collection of data on the occurrence, abundance, and local marine taxa distribution to monitor new species’ arrival and proliferation. In this context, citizen science initiatives present an opportunity to address some of these challenges, fill knowledge gaps and simultaneously promote awareness and increase efforts in marine conservation around the globe [45,46]. Correctly executed, citizen science can provide robust, high-quality and accurate data that can be used for policy and decision-making [47,48,49], complementing datasets that are collected with traditional scientific methods and surveys at a fraction of their typical costs [50,51]. Worldwide, citizen science programs and initiatives are increasing and have demonstrated effectiveness in providing valuable data, tackling NIS with meagre costs (e.g., [51,52,53,54]).
In this context, the present work combines different approaches designed to: (i) map and assess the presence of C. reticulatus in the Madeira Archipelago by using data from monitoring surveys, citizen science, and other sources (i.e., museum records); (ii) assess if a rise in seawater temperatures in the region may facilitate the establishment of this fish species in the region; and (iii) verify the invasion risk of C. reticulatus regarding Madeira Archipelago as the assessment area.
2. Materials and Methods
2.1. Study Area
The Madeira Archipelago (Portugal) is located in the Northeast Atlantic Ocean (30°00′ N to 33°12′ N and 15°50′ W to 17°20′ W, Figure 1). It is situated in the Macaronesia region, with the Azores, the Canary Islands, and Cabo Verde Archipelagos. The Madeira Archipelago comprises two populated islands, Madeira Island (resident population of 262,302) and Porto Santo (resident population of 5483) [55] and two smaller uninhabited groups of islands (Desertas and Selvagens) that are functioning as Marine Protected Areas (MPA). Madeira Island falls under the Temperate Northern Atlantic ecoregion [56], being surrounded by oligotrophic waters [57]. Its coastline and shallow waters are dominated by rocky reefs (platforms and boulders) and sand [58]. The average yearly seawater temperature is around 20.4 °C, with seasonal variations of 16 to 26 °C [59]. The north of Madeira is under the influence of the Azores current, with a pattern of water displacement from west to east, feeding the Canary Current. The latter passes Madeira on the east and goes from north to south [60].
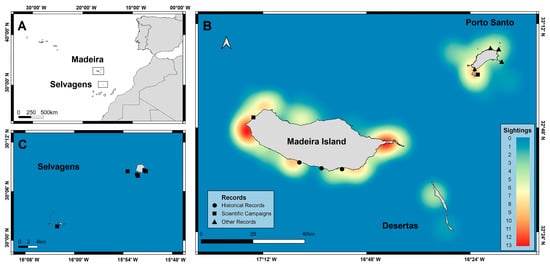
Figure 1.
Map of the Madeira Archipelago showing (A) the location of the study area in the Northeast Atlantic, including (B) Madeira Island, Porto Santo, Desertas, and (C) the Selvagens Islands and locations of Chilomycterus reticulatus sightings around the Archipelago of Madeira between the years 1898 to 2021. Heatmap colors display hotspots of first sightings obtained by the online survey. Records from museum records (●), scientific campaigns (■), and other sources (▲) are also displayed.
2.2. Chilomycterus reticulatus Sightings
2.2.1. Annual Monitoring Surveys
Since 2017, a yearly monitoring program was developed to assess the abundance and distribution of fishes, macroinvertebrates, and sessile organisms on the south coast of Madeira and Porto Santo. Since then, more than 500 dives have been performed. These surveys were based on an underwater visual census (UVC), conducted at depths of −5, −10, and −20 m along a 50 m transect parallel to the coastline, with each dive ranging from 60 to 90 min duration.
2.2.2. Citizen Science
In early 2021, a question-based online survey targeting fishing, spearfishing, and SCUBA diving practitioners was developed to assess the occurrence and distribution of C. reticulatus in Madeira Archipelago (Supplementary Material S1—Figure S1). Using a customized online interface (Supplementary Material S1, available at http://wave-labs.org/studies (accessed on 1 May 2021), selecting “Markers” and then “Chilomycterus reticulatus”), participants were asked if they recognized and could identify C. reticulatus from a photograph and were prompted to report all sightings they recalled while SCUBA diving, snorkelling/spearfishing, and/or fishing.
The survey was structured in two parts. The first part was designed to assess the participant’s familiarity with C. reticulatus and tropicalization, using a mix of open-ended questions, Likert-scale, and fixed choice questions. The second part aimed to collect information about the type of activity practised by the respondent and obtain georeferenced data about C. reticulatus sightings. The interface permitted public participation GIS (PPGIS) to report georeferenced information by allowing users to pinpoint C. reticulatus sightings on a map of the Madeira Archipelago (excluding the Selvagens). Prototype versions of the questionnaire were previously piloted on a random group of people (n = 10). This preliminary pilot allowed minor modifications to prevent misunderstandings and confirm that the online PPGIS worked effectively.
Portuguese and English survey versions were widely disseminated in late April 2021 on social media platforms (i.e., Facebook and Twitter) and by email to maximize participation. The survey was active for one month, with weekly dissemination and posts to enhance participation and reach a wider audience. Participants were informed about the objective of the study and were prompted for their consent to participate. For this research, all data were collected, stored, and analyzed anonymously. Responded surveys were examined and validated for completeness and consistency of responses before analysis.
2.2.3. Complementary Records
A comprehensive literature search was conducted on scientific papers, books, book chapters, theses, and reports for data regarding sightings of this species in the study area. This search included literature published between 1880 and May 2020 in English, Portuguese, and Spanish. Web of Science database (www.webofscience.com (accessed on 3 January 2021)), Scopus (www.scopus.com (accessed on 7 January 2021)), and Google Scholar (https://scholar.google.com (accessed on 12 January 2021)) were examined using the following relevant keywords (and/or): “alien”, “invasive”, “introduced”, “NIS”, “non-indigenous species’’, “invasion”, “non-native”, “exotic”, “Madeira’’, “Fishes”, “Fish”, “Chilomycterus reticulatus”, “Chilomycterus atringa”, and “Diodon atringa”. Moreover, museum records of the species were also scrutinized, namely records from the Museu Municipal do Funchal (MMF) and the Natural History Museum of London. Scientific cruise data provided by EMEPC/M@rBis regarding sightings of C. reticulatus in the region were also included.
2.2.4. Data Analysis
After concluding the online survey, spatial data (icon/marker location) and non-spatial data (survey responses) were downloaded from the webserver for analysis in QGIS (version 3.10; [61]) and IBM SPSS (version 27; [62]). A buffer of 1 km was used around the markers placed ashore to include tags that were likely intended to be placed in seawater that is close to the coast. Data points placed in the terrestrial part of Madeira Archipelago (inland) were removed from the consecutive analyses. Furthermore, interviewed citizens who could not correctly identify a photograph of C. reticulatus by providing its scientific or common name (in English or Portuguese) were excluded from the analyses. To summarize the survey responses, descriptive statistics were conducted using IBM SPSS Statistic with a significance level of 5%. Shapiro–Wilk tests were performed to check the normal distribution of data. Potential differences among the three activities (i.e., scuba diving, spearfishing, and fishing) and the possible relationship between respondent expertise and the number of dropped data points were analyzed using the Kruskal-Wallis test. A map with all georeferenced data points was generated in QGIS using the heatmap function.
2.3. Seawater Temperatures and Analysis
The daily sea surface temperatures (SST), between 1982 and 2020, were extracted from NOAA OI SST V2 High Resolution Dataset [63] for the 24 grid cells around Madeira Island (32°15′ N to 33°15′ N and 16°15′ W to 17°45′ W). Collected values were summarized across the 24 selected raster cells to obtain a dataset describing the daily average values, considering the mean, minimum, and maximum values for the study region per year.
The temperature dataset of the daily average SST of the last 38 years was used to analyze temperature changes. A time-series was created from the dataset and was then decomposed into its trend, seasonal and irregular components [64]. After subtracting the seasonal component from the dataset, a linear regression was fitted to the seasonally adjusted dataset. The slope was extrapolated to obtain the rate of change in the area [65].
Furthermore, the past (1966–1970) and current (2016–2020) SST ranges at Madeira were compared to the recorded and preferred temperature range of C. reticulatus [27]. Seawater temperature data were obtained from the Portuguese Institute for Sea and Atmosphere (IPMA) [66], which was measured daily in the port of Funchal (Madeira Island) since 1961. The data were summarized for the periods 1966–1970 and 2016–2020. The SST preference of C. reticulatus was extracted from Aquamaps [27]. The dataset provides information on SST where C. reticulatus has been recorded and indicates the species preferred temperature range between the 10th and 90th percentile of all recorded temperatures.
Temperature analyses were performed using R (version R-3.6.3; [67]) and RStudio (version 1.2.5033; [68]) with the packages “Ggpubr” [69], “ncdf4” [70], and “dplyr” [71].
2.4. Aquatic Species Invasiveness Screening Kit (AS-ISK)
To determine the invasion risk of C. reticulatus, the AS-ISK v2.3 was used [33]. AS-ISK is a free software that consists of 55 questions divided into three main subjects: (1) biogeography and history of the species; (2) species’ biological and ecological characteristics; and (3) climate change. The package produces an invasion score that expresses the species’ potential to spread in a new environment and alter the environment directly or indirectly [37]. Within the package, a confidence value is assigned to each of the 55 responses and scores.
AS-ISK provides a Basic Risk Assessment (BRA) comprising the first 49 questions related to the biogeographical and biological aspects of the species being screened. The last six questions address a Climate Change Assessment (CCA), which requires the assessor to evaluate how predicted future climate conditions are likely to affect the species and the respective BRA score regarding introduction, establishment, dispersal, and impact risks. To achieve a proper screening, the assessor must provide a response, a level of confidence in the answer, and a justification for each question. Upon completing the screening, the species receive a BRA and a BRA + CCA (composite) scores (−20 to 68 and −32 to 80, respectively). Scores 1 suggest that the species is unlikely to become invasive and is classified as ‘low risk’ [72]. The tropical threshold of 12.5 was used to distinguish between medium and high risk for the BRA score, and the threshold of 23.4 (12.5 + 10.9) was used to differentiate between medium and high risk for the BRA + CCA score [37]. Since there is no specific threshold for marine fishes in the Madeira Archipelago and considering that C. reticulatus has more tropical affinities [27], the tropical threshold was chosen since it provides a more conservative score, being, therefore, the most appropriate (G. Copp, personal communication; [37]). In this study, five independent screenings were used to account for variation in assessors’ opinions (in each of the 55 questions) and better assess the potential invasiveness risk of C. reticulatus in Madeira waters. A risk matrix was developed using BRA and BRA + CCA for each axis using thresholds that were previously defined.
3. Results
3.1. Chilomycterus reticulatus Sightings
During the one-month active period of the online survey, a total of 30 participants completed the questionnaire. Overall, 28 interviews (93.3%) were valid, as respondents had unambiguously identified the species, and 119 data points were validated on the virtual map. The oldest record of C. reticulatus obtained with this online survey was registered in 1985, while 2000, 2010, and 2018 had the highest number of first sightings (n = 3). Three-quarters of respondents confirmed to have seen the fish repeatedly and marked more than one sighting. Chilomycterus reticulatus was observed frequently with a body size smaller than 30 cm (46.4%), and only 14.3% of the respondents declared that they had observed larger individuals regularly (50 cm). Fish lengths between 30 and 50 cm were rarely witnessed (Supplementary Material S2—Table S1). Spearfishing was the dominant activity performed by the participants during the sightings (n = 13; 46.4%). This category was also responsible for the majority of positioned data points on the map (n = 66). A Kruskal–Wallis test showed no statistically significant differences among the three activities and the number of data points placed on the map (p = 0.555). The activity’s detailed online survey results are presented in Supplementary Material S2 (Supplementary Material S2—Tables S1 and S2).
In addition to the online survey observations (119), georeferenced data points were obtained by museum records (6), scientific campaigns (7), authors’ personal observations (4), and scientific literature (1) resulting in a total of 137 observations of C. reticulatus in the Madeira Archipelago (Figure 1, Supplementary Material S2—Table S3).
Over the years, C. reticulatus was detected on all islands of the archipelago: Madeira Island (96), Porto Santo (30), Desertas (5), and Selvagens (6) (Figure 1). Most observations were recorded for the populated islands: Madeira Island and Porto Santo (Figure 1). At Madeira Island, most sightings occurred on the south coast and were primarily distributed in the western and eastern tip of the island. At Porto Santo, most C. reticulatus sightings were near Ilhéu da Cal. Records of C. reticulatus in Madeira span more than 120 years, with records from 1898 (Supplementary Material S2—Table S3) until today (Figure 2).
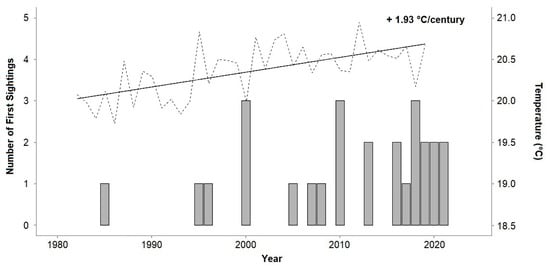
Figure 2.
The number of first sightings of Chilomycterus reticulatus in Madeira per year, reported on the online survey. Sea surface temperatures in Madeira are displayed as yearly averages between 1982 and 2020 (dashed line). The extrapolated temperature change over a century (solid line) was calculated as a linear regression based on the seasonally adjusted dataset of daily means. Data: NOAA OI SST V2 High Resolution Dataset [63].
3.2. Seawater Temperatures and Analysis
The temperature trend analysis for Madeira revealed a warming trend of 1.93 °C per century based on data from the last 38 years (Figure 2). Half a decade ago, the average yearly temperature at Madeira was 20.0 °C (minimum of 16.3 and maximum of 24.1 °C; Figure 3). In the past five years, seawater temperature reached an average of 20.7 °C with a maximum and minimum of 24.3 and 16.8 °C, respectively (Figure 3). The current temperature regime at Madeira Island shows a 38.8% overlap with the preferred temperature window of C. reticulatus, while 50 years ago, the overlap was only 28.4% (Figure 3). However, Madeira’s average and maximal values are still lower than the optimal average temperature (25.5 °C) of C. reticulatus (Figure 3).
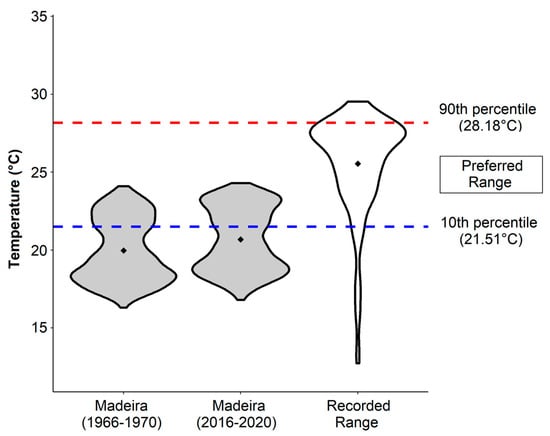
Figure 3.
The sea surface temperatures range in Madeira in the past and present (daily SST from 1966–1970 and 2016–2020, data source: [66]) compared to the recorded and preferred temperature range for Chilomycterus reticulatus (based on Aquamaps, [27]). Means are indicated as points.
3.3. AS-ISK
Four out of the five AS-ISK assessments, and the overall average, were on the Medium Risk mark, based on the BRA scores (threshold of 12.5 for tropical fishes) (Figure 4). Only one assessment fell in the “high risk” category (Figure 4). Considering the potential effects of climate change on the risk screening (BRA + CCA), the results were similar, with only one assessment being considered to be “high risk” (BRA + CCA = 25.5) (Figure 4). All the other four, plus the average value, were considered to be medium risk (Figure 4) (BRA + CCA 23.4).
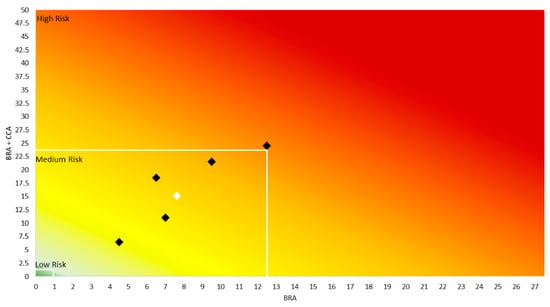
Figure 4.
The risk matrix of BRA values against BRA + CCA values displays a gradient of “low risk” (green), “medium risk” (yellow), and “high risk” (red). Observations of the AS-ISK screening of Chilomycterus reticulatus are represented by black and white squares: individual assessments in black (n = 5) and the overall average result in white. The thresholds for low, medium and high risk are indicated in the figure with white lines (tropical fish threshold of 12.5 for BRA and 23.4 for BRA + CCA was used).
The main characteristics that reflected the invasiveness of C. reticulatus are climate, distribution, introduction risk, and history of being invasive elsewhere (Supplementary Material S2—Figure S2). Overall, with the current climate change predictions, the species risk of invasiveness appears to increase (Supplementary Material S2—Figure S1). The confidence factors varied according to the assessor, and average values were 0.52 (±0.10) for BRA, 0.62 (±0.03) for CCA and 0.59 (±0.04) for BRA + CCA scores. These were influenced by the lack of information regarding tolerance attributes and reproduction (Supplementary Material S3).
4. Discussion
The present study suggests that C. reticulatus is at a medium risk of establishing self-sustained populations and becoming invasive. The reported numbers of first sightings and the wide spatial distribution suggest the expansion of this thermophilic species in the Madeira Archipelago, which may be facilitated by the current temperature trends influenced by climate change.
Many of the featured recreational activities in oceanic islands, such as Madeira, focus on the sea, namely scuba diving, spearfishing, whale watching, and recreational fishing [73,74,75]. Involving stakeholders and their clients can complement and improve scientific data at various spatial and temporal scales [76,77]. Citizen science may contribute to ecological conservation by collecting environmental and biological data or by targeting particular species [77,78,79,80,81]. In the last decade, marine citizen science was widely employed in identifying different threats of marine ecosystems (e.g., marine litter or NIS [81,82,83]) and monitoring of/detecting iconic species [82,83]. In this study, citizen science was used to assess the range expansion of C. reticulatus in the Madeira Archipelago by providing valid information on its presence in the study area. There are potential challenges when involving volunteers in data collection, including their limited experience in species identification. However, the spotfin burrfish is a distinctive reef fish species, easy to identify, and is one of the few Tetradontidae species recorded in Madeira see [21] for more details. However, the possibility that in some cases, misidentification with individuals of the brown pufferfish, Sphoeroides marmoratus (Lowe, 1838), as juvenile burrfish (size category 30 cm) occurred, cannot be completely excluded.
Due to spatial or temporal differences in sampling efforts, the distribution data obtained from citizen science and other sources (e.g., scientific campaigns, personal observations, and museum collections) can be biased [84]. Therefore, some caution needs to be exercised in interpreting these results since no measure of sampling effort was integrated. Abundance data are often spatially skewed towards areas of higher population density or more marine activity [85]. However, occurrences (i.e., sightings) can be used to delineate the general spatial range and extent of the species.
In this study, C. reticulatus records were expectedly higher in areas of higher population density and/or locations of increased marine activities (e.g., marked diving spots). However, the results showed that C. reticulatus is widely distributed in the region and occurs on all islands (populated and unpopulated) of the Madeira Archipelago.
Besides spatial biases, temporal biases should also be considered. Observations over time could be skewed towards recent years because of multiple factors. For example, the number of citizens engaging in recreational marine activities has been increasing over the last few years, and portable waterproof cameras are now less expensive and are commonly used to promote more digital evidence. Similarly, participant demographics, experience, and frequency of practice are likely to constrain the report in time (e.g., practitioners can only report for the period they have practised an activity). Another consideration contemplates the increase in monitoring, sampling, and studies focused on invasion ecology over the last decades [18,29]. Moreover, the growing exchange of information and advancements to generalized access and the exchange of information over the internet and across social media platforms have improved. Although the recent increase in the report of C. reticulatus first sightings may be partly due to the previously mentioned factors, the results of the present study indicate an increasing trend in the number of sightings and in seawater temperatures (Figure 2). Evidence that rising temperatures due to climate change can cause poleward range shifts and/or expansions in species distribution is widely acknowledged (e.g., [59,86,87]). Moreover, the wide spatial distribution of sightings since 1985 (Figure 2) suggests that C. reticulatus may, in fact, be expanding its range.
Madeira seawater temperatures are becoming increasingly closer to the optimal range of the species in question (Figure 3; [27]), similar to the neighbouring Canary Islands where this species increased its abundance and formed self-sustained populations [28,29]. Changes in global temperature regimes are predicted to shift tropical temperatures to values outside the tolerance thresholds of long-lasting resident species, causing range retractions on the southern distribution edge (e.g., [88]). On the other hand, temperature regimes of higher-latitude regions will shift closer to values that are tolerated by species that have been so far restricted to warmer, lower-latitude areas (e.g., [89]). At current rates, Madeira’s increase of almost 2 °C per century aligns well with the IPCC predictions for the end of this century (+1.9 °C; CMIP6—SST change in °C—long term 2081–2100 under SSP2 4.5 relative to 1986–2005 at Long: −17.07 Lat: −32.75; [90]). This increase in water temperatures supports the northward spread of tropical and subtropical species, such as C. reticulatus, in the Madeira Archipelago. Considering the current temperature trend, Madeiran waters will continue to warm, creating even more favourable temperature conditions for C. reticulatus individuals.
The spotfin burrfish has been recorded as a vagrant species in the Mediterranean Sea [91] and is also present in the Canary Islands [29]. Since its first detection (1981) in the Canary Islands, the abundance of C. reticulatus has increased over the last three decades [29,92]. Similar trends were detected in the Madeira Archipelago, where over previous decades, several species extended their distributional range as part of a tropicalization process, increasing the number of taxa detected [11,21,59]. For example, the crab Cronius ruber (Lamarck, 1818) is a recent case of the ongoing tropicalization in Macaronesia [13,17,59,93]. In fact, after its first record in 2016 at the Canary Islands [13,93], it continued to advance poleward until it was first registered in 2018 at Madeira Island and repeatedly observed in the following months and years ([59]; Schäfer, personal observation). Furthermore, several other fishes with tropical affinities are being detected in Madeira (e.g., Canthidermis sufflamen (Mitchill, 1815) and Caranx crysos (Mitchill, 1815); [21,94]), illustrating the same poleward shift from the Canary Islands [95,96]. Additionally, not only motile species, such as fish and macroinvertebrates, are included in this trend. For example, the alga Avrainvillea canariensis A. Gepp E.S. Gepp was recently detected in Madeira [12]. According to the authors, this finding represents a northern expansion by nearly 500 km since the species was previously limited to the Canary Islands [97], and was considered neoendemic. The seaweed Caulerpa chemnitzia (Esper) J.V.Lamouroux is another example. It was identified as C. racemosa var. peltata (a later heterotypic synonym) in 2000 at Funchal [98] and 2015 in Porto Santo, forming large beds (Patricio Ramalhosa, personal observation). Chilomycterus reticulatus is a polyvectic species [99], having two or more potential vectors associated with its introduction. The spotfin burrfish may have entered the Madeira Archipelago by currents and ballast water in its pelagic phase (eggs, larvae, and juveniles) [100] or by rafting underneath floating debris or Sargassum spp. [101]. The arrival vector of such species to Madeira remains, in most cases, unknown. Many range expansions are cryptovectic, which describes species unknown to the introduction vector [102]. In Madeira, it is improbable that species moving poleward are doing so naturally since they are moving against significant ocean currents [103]. Therefore, the natural transport of larvae from the Canary Islands to Madeira Island by ocean currents is not impossible but remains quite unlikely. However, on some occasions, oceanographic phenomena might revert their normal functions (e.g., the inverse of the Canary current or the North Atlantic Oscillation (NAO)), pushing seawater poleward [104,105] and facilitating newcomers.
Although most introductions have a negligible impact on the environment, some may threaten ecosystems, resulting in reduced biodiversity [1,2] and structural changes in communities [6]. Many aquatic ecosystems have been seriously affected by invasive species, which can displace native organisms (i.e., predation and competition), modify the genetic characteristics of the populations through hybridization, or introduce exotic diseases [106]. In terms of undesirable traits, C. reticulatus hosts different parasites (e.g., [107,108]), including species that have not been recorded in the risk assessment area yet. In addition, some puffers and porcupine fishes, including the fish in question, have tetrodotoxin present in their liver [109] that in some cases causes fatalities [110]. Moreover, C. reticulatus when inflated, due to their numerous spines, can cause injury to humans (e.g., for fishers or curious scuba divers).
The impacts caused by NIS may be irreversible, particularly in the marine environment, where these species can be tough to eradicate once they have established self-sustaining populations [111]. For example, structural changes in temperate reefs were observed due to the increased abundance of tropical herbivorous fishes leading to a decline in habitat-forming kelp [112]. The spotfin burrfish is likely to maintain a viable population even if currently occurring only in low densities (e.g., [28,29]). For these reasons, biological invasions can incur high socioeconomic costs through direct and indirect adverse impacts on ecosystem function and services [113], including remediation and/or mitigation actions [114]. The burrfish preys on benthic invertebrates (e.g., echinoderms, crustaceans, molluscs), with the sea urchin Diadema africanum described by Rodríguez, Hernández, Clemente and Coppard, 2013, as being one of the burrfish’s main prey items at the Canary Islands [28]. Years after the first detection of C. reticulatus in the Canary Islands, their importance was highlighted in controlling the sea urchin populations [115]. Bacallado and colleagues proposed protecting the species from fisheries to preserve algae-populated areas from urchin grazing [116]. In Madeira, sea urchin densities have also influenced local ecosystems [77,117], and the present increasing numbers of C. reticulatus could potentially positively affect this unbalance.
Risk assessment has been a valuable tool to identify, prioritize and manage invasion scenarios [37,118,119]. Results from the AS-ISK have shown that C. reticulatus presents, conservatively, medium risk of becoming invasive in the Madeira Archipelago under present and future climate scenarios. Despite its circumtropical distribution [22], there is a lack of information regarding the biology and ecology of this species [29,120]. Moreover, the absence of knowledge about its cultivation, domestication, invasiveness, or direct fisheries has likely influenced the confidence values and made the AS-ISK assessments challenging for the assessors. The strategy of using multiple independent assessors with different marine ecology/biology backgrounds produced a consolidated risk assessment, providing an average and a range of BRA and BRA + CCA scores instead of a single assessment (Figure S2 in Supplementary Material S2 and Supplementary Material S3). In addition, using the marine fishes’ threshold for tropical regions is more conservative, as it considers a taxon to pose a medium to a higher risk of invasiveness at lower scores [37].
The evidence provided by the present study suggests that C. reticulatus has passed from a vagrant species since the XIX and XX centuries [21] to a range expansion in recent years (also named winner species see [121]). Vagrant species appear from time to time beyond their normal distribution range [122], while range expansions occur when species expand their distribution beyond their historically known range [10,17,59]. The relatively short periods between the sightings of C. reticulatus at different locations around the Madeira Archipelago suggest this species has already spread, and its sightings are no longer random or sporadic. The present work adds more evidence to the ongoing tropicalization of the Madeira Archipelago, with an increase in sightings of C. reticulatus over time. Different tools have proven valuable in gaining information on the current distribution and for evaluating the species status of invasiveness around the Madeira Archipelago. This kind of synergy is helpful to register new incomers in this tropicalization phase. Recent temperature developments in the area are slowly moving the local climate towards the optimal temperature range of C. reticulatus and might support its future spread and establishment in the Madeira Archipelago. The average of the five AS-ISK assessments verified that C. reticulatus has a medium risk of becoming invasive. Therefore, its abundance and distribution need to be monitored, preventing uncontrolled outbursts and potential adverse effects on native species and ecosystems.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13120639/s1, Supplementary Material S1 contains Figure S1: Online custom web-based user interface used for citizen science during the study. Users complete the form (left) and proceed to pinpoint the location of spotted C. reticulatus during SCUBA diving, fishing, or spearfishing (right). Supplementary Material S2 contains Table S1: Frequency of observation (%) based on the online survey for each size class of C. reticulatus (30 cm; 30 to 50 cm and 50 cm); Table S2: Characteristics of respondents to the online survey by conducted activity in number and percentage; Table S3: Dataset on additional C. reticulatus sightings based on scientific campaigns, museum records, scientific literature, and personal observations; and Figure S2: Radar plots showing the scores C. reticulatus achieved in the different AS-ISK categories for all five individual assessments (#1–#5) and their average. Supplementary Material S3 contains the tables with the total scoring of the five individual AS-ISK assessments.
Author Contributions
Conceptualization: N.C., S.S. and J.G.M.; formal analysis: N.C., S.S. and P.P.; investigation: N.C., S.S., P.P., J.G.M., F.G., S.C., M.R., R.F., E.A., F.H., M.F., N.V.-R. and R.S.; resources, J.C.-C.; writing—original draft preparation, N.C. and S.S.; writing—review and editing, all the authors; visualization, N.C. and S.S.; supervision, J.C.-C.; funding acquisition, J.C.-C. All authors have read and agreed to the published version of the manuscript.
Funding
N.C. was funded by a doctoral grant (SFRH/BD/146881/2019) awarded by Fundação para a Ciência e Tecnologia (FCT). S.S. was supported by Agência Regional para o Desenvolvimento da Investigação, Tecnologia e Inovação (ARDITI) research fellowship in the scope of the H2020 project GoJelly. P.P. was funded by a PhD grant ref. M3.1.a/F/065/2015 by Fundo Regional de Ciência e Tecnologia (FRCT) and the program AÇORES 2020. A post-doctoral research fellowship by ARDITI (ARDITI–M1420-09-5369-FSE-000002) supported J.G.M. F.G. was endorsed by a post-doctoral research fellowship granted by ARDITI in the framework of project RAGES (ARDITI-RAGES-2019-001). R.F. was supported by the funding from project INTERWHALE (PTDC/CCI-COM/0450/2020) by FCT. Doctoral fellowships were financially supported S.C. by ARDITI (ARDITI-M1420-09-5369-FSE-000002). R.S. was supported by a research fellowship in the framework of project PLASMAR+ (MAC2/1.1a/347). J.C.-C. is funded by national funds through FCT under the Scientific Employment Stimulus—Institutional Call—[CEECINST/00098/2018]. This work was partially funded by MIMAR+ (MAC2/4.6.d/249) in the INTERREG MAC 2014–2020 Programme framework. This study also had the support of Fundação para a Ciência e Tecnologia (FCT), Portugal, through the strategic project (UIDB/04292/2020) granted to MARE UIIUIDB/04292/2020. Finally, the present paper benefited from underwater surveys during the expedition ‘MARE@Porto Santo 2020′ conducted by MARE-Madeira.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sea surface temperature data provided by NOAA/OAR/ESRL PSD, Boulder, CO, USA, is available from their Web site https://www.esrl.noaa.gov/psd/(accessed on 10 March 2021). The SST data on the preferred temperature range of Chilomycterus reticulatus from Marine AquaMaps is available and can be accessed directly through www.aquamaps.org. All remaining datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request due to privacy restrictions.
Acknowledgments
We like to thank MarBIS (Sistema de Informação para a Biodiversidade Marinha) for providing information obtained in the scientific campaign EMEPC/M@rBis/Selvagens2010. The authors thank Victor Prior (IPMA), I.P. for the seawater temperature data provided for this work. We also acknowledge Manuel Biscoito (Museu Municipal do Funchal (MMF) for the data on Chilomycterus reticulatus.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Costello, M.J.; Coll, M.; Danovaro, R.; Halpin, P.; Ojaveer, H.; Miloslavich, P. A Census of Marine Biodiversity Knowledge, Resources, and Future Challenges. PLoS ONE 2010, 5, e12110. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Global Sea warming and “tropicalization” of the Mediterranean Sea: Biogeographic and ecological aspects. Biogeogr. J. Integr. Biogeogr. 2003, 24, 319–327. [Google Scholar] [CrossRef]
- Jansen, J.M.; Pronker, A.E.; Bonga, S.W.; Hummel, H. Macoma balthica in Spain, a few decades back in climate history. J. Exp. Mar. Biol. Ecol. 2007, 344, 161–169. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Williams, S.L.; Carlton, J.T. Marine range shifts and species introductions: Comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 2010, 19, 303–316. [Google Scholar] [CrossRef]
- Canning-Clode, J.; Fowler, A.E.; Byers, J.E.; Carlton, J.T.; Ruiz, G.M. ‘Caribbean Creep’ Chills Out: Climate Change and Marine Invasive Species. PLoS ONE 2011, 6, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Canning-Clode, J.; Carlton, J.T. Refining and expanding global climate change scenarios in the sea: Poleward creep complexities, range termini, and setbacks and surges. Divers. Distrib. 2017, 23, 463–473. [Google Scholar] [CrossRef]
- Ribeiro, C.; Neto, A.I.; Moreu, I.; Haroun, R.; Neves, P. A new signal of marine tropicalization in the Macaronesia region: First record of the mesophotic macroalga Avrainvillea canariensis A. Gepp E.S. Gepp in the Madeira archipelago. Aquat. Bot. 2019, 153, 40–43. [Google Scholar] [CrossRef]
- González, J.A.; Triay-Portella, R.; Escribano, A.; Cuesta, J.A. Northernmost record of the pantropical portunid crab Cronius ruber in the eastern Atlantic (Canary Islands): Natural range extension or human-mediated introduction? Sci. Mar. 2017, 81, 81–89. [Google Scholar] [CrossRef]
- Arias, A.; Crocetta, F. Umbraculum umbraculum (Gastropoda: Heterobranchia) spreading northwards: Additional evidence to the “tropicalization” of the Bay of Biscay. Cah. Biol. Mar. 2016, 57, 285–286. [Google Scholar]
- Encarnação, J.; Morais, P.; Baptista, V.; Cruz, J.; Teodósio, M.A. New evidence of marine fauna tropicalization off the Southwestern Iberian Peninsula (Southwest Europe). Diversity 2019, 11, 48. [Google Scholar] [CrossRef]
- Peleg, O.; Rilov, G.; Guy-Haim, T.; Yeruham, E.; Silverman, J.; Rilov, G. Tropicalization may invert trophic state and carbon budget of shallow temperate rocky reefs. J. Ecol. 2020, 108, 844–854. [Google Scholar] [CrossRef]
- Castro, N.; Carlton, J.T.; Costa, A.C.; Marques, C.; Hewitt, C.L.; Cacabelos, E.; Gizzi, F.; Gestoso, I.; Monteiro, J.G.; Costa, J.L.; et al. Archipelagos of invasions: Diversity and patterns of marine introduced species of Macaronesia. Divers. Distrib. 2021, in press. [Google Scholar]
- Canning-Clode, J.; Fofonoff, P.; McCann, L.; Carlton, J.T.; Ruiz, G. Marine invasions on a subtropical island: Fouling studies and new records in a recent marina on Madeira Island (Eastern Atlantic Ocean). Aquat. Invasions 2013, 8, 261–270. [Google Scholar] [CrossRef]
- Castro, N.; Ramalhosa, P.; Jiménez, J.; Costa, J.L.; Gestoso, I.; Canning-Clode, J. Exploring marine invasions connectivity in a NE Atlantic Island through the lens of historical maritime traffic patterns. Reg. Stud. Mar. Sci. 2020, 37, 101333. [Google Scholar] [CrossRef]
- Ramalhosa, P.; Gestoso, I.; Rocha, R.M.; Lambert, G.; Canning-Clode, J. Ascidian biodiversity in the shallow waters of the Madeira Archipelago: Fouling studies on artificial substrates and new records. Reg. Stud. Mar. Sci. 2021, 43, 101672. [Google Scholar] [CrossRef]
- Wirtz, P.; Fricke, R.; Biscoito, M.J. The coastal fishes of Madeira Island—New records and an annotated check-list. Zootaxa 2008, 1715, 1–26. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 2 June 2021).
- Leis, J.M. Family Diodontidae. In The Living Marine Resources of the Western Central Pacific; Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 2001; Volume 3, pp. 3958–3965. [Google Scholar]
- Leis, J.M. Family Diodontidae. In The Living Marine Resources of the Western Central Atlantic; Carpenter, K., Ed.; FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists, Special Publication Nº 5, Bony Fishes Part 2 (Opisthognathidae to Molidae), Sea Turtles and Marine Mammals; FAO: Rome, Italy, 2002; Volume 3, pp. 2007–2013. [Google Scholar]
- Sommer, C.; Schneider, W.; Poutiers, J.M. The Living Marine Resources of Somalia; Sommer, C., Schneider, W., Poutiers, J.-M., Eds.; FAO Species Identification Guide for Fishery Purposes; FAO: Rome, Italy, 1996; p. 337. [Google Scholar]
- Leis, J.M. Nomenclature and distribution of the species of the porcupine fish family Diodontidae (Pisces, Teleostei). Mem. Mus. Vic. 2006, 63, 77–90. [Google Scholar] [CrossRef]
- Kaschner, K.; Kesner-Reyes, K.; Garilao, C.; Segschneider, J.; Rius-Barile, J.; Rees, T.; Froese, R. Aquamaps. 2019. Available online: www.aquamaps.org. (accessed on 10 June 2021).
- Brito, A.; Falcón, J.M. Contribution to the knowledge of the distribution and ecology of Chilomycterus atringa (Pisces, Diodontidae) in the Canary Islands. Vieraea 1990, 19, 271–275. [Google Scholar]
- Espino, F.; Tuya, F.; Rosario, A.; Bosch, N.E.; Coca, J.; González-Ramos, A.J.; del Rosario, F.; Otero-Ferrer, F.J.; Moreno, Á.C.; Haroun, R. Geographical Range Extension of the Spotfin burrfish, Chilomycterus reticulatus (L. 1758), in the Canary Islands: A Response to Ocean Warming? Diversity 2019, 11, 230. [Google Scholar] [CrossRef]
- Pearce, F.; Peeler, E.; Stebbing, P. Modelling the Risk of the Introduction and Spread of Non-Indigenous Species in the UK and Ireland; Project Report for E5405W; CEFAS: Weymouth, UK, 2012. [Google Scholar]
- Copp, G.H.; Godard, M.J.; Russell, I.C.; Peeler, E.J.; Gherardi, F.; Tricarico, E.; Miossec, L.; Goulletquer, P.; Almeida, D.; Britton, J.R.; et al. A preliminary evaluation of the European Non-native Species in Aquaculture Risk Assessment Scheme applied to species listed on Annex IV of the EU Alien Species Regulation. Fish. Manag. Ecol. 2016, 23, 12–20. [Google Scholar] [CrossRef]
- Copp, G.H.; Garthwaite, R.; Gozlan, R.E. Risk identification and assessment of non-native freshwater fishes: A summary of concepts and perspectives on protocols for the UK. J. Appl. Ichthyol. 2005, 21, 371–373. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Tidbury, H.; Stebbing, P.D.; Tarkan, A.S.; Miossec, L.; Goulletquer, P. Development of a generic decision-support tool for identifying potentially invasive aquatic taxa: AS-ISK. Manag. Biol. Invasions 2016, 7, 343–350. [Google Scholar] [CrossRef]
- Mumford, J.D.; Booy, O.; Baker, R.H.A.; Rees, M.; Copp, G.H.; Black, K.; Holt, J.; Leach, A.W.; Hartley, M. Invasive non-native species risk assessment in Great Britain. Asp. Appl. Biol. 2010, 104, 49–54. [Google Scholar]
- David, M.; Gollasch, S.; Leppäkoski, E. Risk assessment for exemptions from ballast water management—The Baltic Sea case study. Mar. Pollut. Bull. 2013, 75, 205–217. [Google Scholar] [CrossRef]
- David, M.; Gollasch, S. Risk assessment for ballast water management—Learning from the Adriatic Sea case study. Mar. Pollut. Bull. 2019, 147, 36–46. [Google Scholar] [CrossRef]
- Vilizzi, L.; Copp, G.H.; Hill, J.E.; Adamovich, B.; Aislabie, L.; Akin, D.; Al-Faisal, A.J.; Almeida, D.; Azmai, M.N.A.; Bakiu, R.; et al. A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Sci. Total Environ. 2021, 788, 147868. [Google Scholar] [CrossRef]
- Copp, G.H.; Russell, I.C.; Peeler, E.J.; Gherardi, F.; Tricarico, E.; MacLeod, A.; Cowx, I.G.; Nunn, A.D.; Occhipinti-Ambrogi, A.; Savini, D.; et al. European Non-native Species in Aquaculture Risk Analysis Scheme—A summary of assessment protocols and decision support tools for use of alien species in aquaculture. Fish. Manag. Ecol. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Cook, E.J.; Payne, R.D.; Macleod, A.K.; Brown, S.F. Marine biosecurity: Protecting indigenous marine species. Res. Rep. Biodivers. Stud. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Copp, G.; Vilizzi, L.; Wei, H.; Li, S.; Piria, M.; Al-faisal, A.J.; Almeida, D.; Al-Wazzan, Z.; Bakiu, R.; Bašić, T.; et al. Speaking their language—Development of a multilingual decision-support tool for communicating invasive species risks to decision makers and stakeholders. Environ. Model. Softw. 2021, 135, 104900. [Google Scholar] [CrossRef]
- Statzner, B.; Bonada, N.; Doledec, S. Biological attributes discriminating invasive from native European stream macroinvertebrates. Biol. Invasions 2008, 10, 517–530. [Google Scholar] [CrossRef]
- Chan, J.; Zeng, Y.; Yeo, D.C.J. Invasive species trait-based risk assessment for non-native freshwater fishes in a tropical city basin in Southeast Asia. PLoS ONE 2021, 16, e0248480. [Google Scholar] [CrossRef]
- Piaggio, A.J.; Engeman, R.M.; Hopken, M.W.; Humphrey, J.S.; Keacher, K.L.; Bruce, W.E.; Avery, M.L. Detecting an elusive invasive species: A diagnostic PCR to detect Burmese python in Florida waters and an assessment of persistence of environmental DNA. Mol. Ecol. Resour. 2014, 14, 374–380. [Google Scholar] [CrossRef]
- Pendleton, L.H.; Beyer, H.; Estradivari; Grose, S.O.; Hoegh-Guldberg, O.; Karcher, D.B.; Kennedy, E.; Llewellyn, L.; Nys, C.; Shapiro, A.; et al. Disrupting data sharing for a healthier ocean. ICES Mar. Sci. 2019, 76, 1415–1423. [Google Scholar] [CrossRef]
- Cigliano, J.A.; Meyer, R.; Ballard, H.L.; Freitag, A.; Phillips, T.B.; Wasser, A. Making marine and coastal citizen science matter. Ocean Coast. Manag. 2015, 115, 77–87. [Google Scholar] [CrossRef]
- Kelly, R.; Fleming, A.; Pecl, G.T.; Von Gönner, J.; Bonn, A. Citizen science and marine conservation: A global review. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190461. [Google Scholar] [CrossRef]
- McKinley, D.C.; Miller-Rushing, A.J.; Ballard, H.L.; Bonney, R.; Brown, H.; Cook-Patton, S.; Evans, D.M.; French, R.A.; Parrish, J.K.; Phillips, T.B.; et al. Citizen science can improve conservation science, natural resource management, and environmental protection. Biol. Conserv. 2017, 208, 15–28. [Google Scholar] [CrossRef]
- Warner, K.A.; Lowell, B.; Timme, W.; Shaftel, E.; Hanner, R.H. Seafood sleuthing: How citizen science contributed to the largest market study of seafood mislabeling in the U.S. and informed policy. Mar. Policy 2019, 99, 304–311. [Google Scholar] [CrossRef]
- Hyder, K.; Townhill, B.; Anderson, L.G.; Delany, J.; Pinnegar, J.K. Can citizen science contribute to the evidence-base that underpins marine policy? Mar. Policy 2015, 59, 112–120. [Google Scholar] [CrossRef]
- Fritz, S.; See, L.; Carlson, T.; Haklay, M.; Oliver, J.; Fraisl, D.; Mondardini, R.; Brocklehurst, M.; Shanley, L.; Schade, S.; et al. Citizen science and the United Nations Sustainable Development Goals. Nat. Sustain. 2019, 2, 922–930. [Google Scholar] [CrossRef]
- Bodilis, P.; Louisy, P.; Draman, M.; Arceo, H.O.; Francour, P. Can Citizen Science Survey Non-indigenous Fish Species in the Eastern Mediterranean Sea? Environ. Manag. 2014, 53, 172–180. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Outinen, O.; Puntila-Dodd, R. Citizen science provides added value in the monitoring for coastal non-indigenous species. J. Environ. Manag. 2020, 267, 110608. [Google Scholar] [CrossRef]
- De Sherbinin, A.; Bowser, A.; Chuang, T.-R.; Cooper, C.; Danielsen, F.; Edmunds, R.; Elias, P.; Faustman, E.; Hultquist, C.; Mondardini, R.; et al. The Critical Importance of Citizen Science Data. Front. Clim. 2021, 3, 650760. [Google Scholar] [CrossRef]
- Garcia-Soto, C.; Seys, J.J.C.; Zielinski, O.; Busch, J.A.; Luna, S.I.; Baez, J.C.; Domegan, C.; Dubsky, K.; Kotynska-Zielinska, I.; Loubat, P.; et al. Marine Citizen Science: Current State in Europe and New Technological Developments. Front. Mar. Sci. 2021, 8, 621472. [Google Scholar] [CrossRef]
- INE Instituto Nacional de Estatística (INE); Direção Regional de Estatística da Madeira (DREM). Census 2021. 2021. Available online: www.estatistica.Madeira.gov.pt (accessed on 13 June 2021).
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Canning-Clode, J.; Kaufmann, M.; Molis, M.; Wahl, M.; Lenz, M. Influence of disturbance and nutrient enrichment on early successional fouling communities in an oligotrophic marine system. Mar. Ecol. 2008, 29, 115–124. [Google Scholar] [CrossRef]
- Monteiro, J.G.; Jiménez, J.L.; Gizzi, F.; Přikryl, P.; Lefcheck, J.S.; Santos, R.S.; Canning-Clode, J. Novel approach to enhance coastal habitat and biotope mapping with drone aerial imagery analysis. Sci. Rep. 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Monteiro, J.; Castro, N.; Rilov, G.; Canning-Clode, J. Cronius ruber (Lamarck, 1818) arrives to Madeira Island: A new indication of the ongoing tropicalization of the northeastern Atlantic. Mar. Biodivers. 2019, 49, 2699–2707. [Google Scholar] [CrossRef]
- Mason, E.; Colas, F.; Molemaker, J.; Shchepetkin, A.F.; Troupin, C.; McWilliams, J.C.; Sangrà, P. Seasonal variability of the Canary Current: A numerical study. J. Geophys. Res. 2011, C06001, 1–20. [Google Scholar] [CrossRef]
- QGIS Association. QGIS Geographic Information System. 2021. Available online: QGIS.org (accessed on 22 June 2021).
- IBM Corp. IBM SPSS Statistics for Windows, Version 27.0; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]
- Reynolds, R.D.; Smith, T.M.; Liu, C.; Chelton, D.B.; Casey, K.S.; Schlax, M.G. Daily High-Resolution-Blended Analyses for Sea Surface Temperature. J. Clim. 2007, 20, 5473–5496. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Newnham, G.; Culvenor, D. Detecting Trend and Seasonal Changes in Satellite Image Time Series. Remote Sens. Environ. 2010, 114, 106–115. [Google Scholar] [CrossRef]
- Goela, P.C.; Cordeiro, C.; Danchenko, S.; Icely, J.; Cristina, S.; Newton, A. Time series analysis of data for sea surface temperature and upwelling components from the southwest coast of Portugal. J. Mar. Syst. 2016, 163, 12–22. [Google Scholar] [CrossRef]
- IPMA. Sea Water Temperatures Measured at the Station 522—Observatório Meteorológico do Funchal between 1961–2020; Instituto Português do Mar e da Atmosfera: Lisbon, Portugal, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Kassambara, A. “ggplot2” Based Publication Ready Plots, R Package. 2020. Available online: https://Cran.r-Project.org/web/packages/ggpubr (accessed on 26 June 2021).
- Pierce, D. ncdf4: Interface to Unidata netCDF (Version 4 or Earlier) Format Data Files, R Package. 2019. Available online: https://CRAN.R-Project.org/package=ncdf4 (accessed on 2 July 2021).
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation, R Package. 2020. Available online: https://CRAN.R-Project.org/package=dplyr (accessed on 4 July 2021).
- Pheloung, P.C.; Williams, P.A.; Halloy, S.R. A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. J. Environ. Manag. 1999, 57, 239–251. [Google Scholar] [CrossRef]
- Oliveira, P.; Pereira, P.T. Who values what in a tourism destination? The case of Madeira Island. Tour. Econ. 2008, 14, 155–168. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Vieira, C.; Gouveia, L.; Gouveia, N.; Hermida, M. Characterization and evolution of spearfishing in Madeira archipelago, Eastern Atlantic. Aquat. Living Resour. 2020, 33, 15. [Google Scholar] [CrossRef]
- Martínez-Escauriaza, R.; Hermida, M.; Villasante, S.; Gouveia, L.; Gouveia, N.; Pita, P. Importance of recreational shore angling in the archipelago of Madeira, Portugal (northeast Atlantic). Sci. Mar. 2020, 84, 331–341. [Google Scholar] [CrossRef]
- Azzurro, E.; Cerri, J. Participatory mapping of invasive species: A demonstration in a coastal lagoon. Mar. Policy 2021, 126, 104412. [Google Scholar] [CrossRef]
- Gizzi, F.; Jiménez, J.; Schäfer, S.; Castro, N.; Costa, S.; Lourenço, S.; José, R.; Canning-Clode, J.; Monteiro, J. Before and after a disease outbreak: Tracking a keystone species recovery from a mass mortality event. Mar. Environ. Res. 2020, 156, 104905. [Google Scholar] [CrossRef]
- Cerrano, C.; Milanese, M.; Ponti, M. Diving for science—Science for diving: Volunteer scuba divers support science and conservation in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 27, 303–323. [Google Scholar] [CrossRef]
- Crall, A.W.; Jordan, R.; Holfelder, K.; Newman, G.J.; Graham, J.; Waller, D.M. The impacts of an invasive species citizen science training program on participant attitudes, behavior, and science literacy. Public Underst. Sci. 2012, 22, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.K.M.; Tuya, F.; Barker, J.; Alvarado, D.J.; Castro-Hernández, J.J.; Haroun, R.; Rödder, D. Population structure, distribution and habitat use of the Critically Endangered Angelshark, Squatina squatina, in the Canary Islands. Aquat. Conserv. 2017, 27, 1133–1144. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Thiel, M. The Contribution of Citizen Scientists to the Monitoring of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 429–447. [Google Scholar] [CrossRef]
- Casale, P.; Ciccocioppo, A.; Vagnoli, G.; Rigoli, A.; Freggi, D.; Tolve, L.; Luschi, P. Citizen science helps assessing spatio-temporal distribution of sea turtles in foraging areas. Aquat. Conserv. 2020, 30, 123–130. [Google Scholar] [CrossRef]
- Donnelly-Greenan, E.L.; Nevins, H.M.; Harvey, J.T. Entangled seabird and marine mammal reports from citizen science surveys from coastal California (1997–2017). Mar. Pollut. Bull. 2019, 149, 110557. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen Science as an Ecological Research Tool: Challenges and Benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Geldmann, J.; Heilmann-Clausen, J.; Holm, T.E.; Levinsky, I.; Markussen, B.; Olsen, K.; Rahbek, C.; Tøttrup, A.P. What determines spatial bias in citizen science? Exploring four recording schemes with different proficiency requirements. Divers. Distrib. 2016, 22, 1139–1149. [Google Scholar] [CrossRef]
- Zeidberg, L.D.; Robison, B.H. Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc. Natl. Acad. Sci. USA 2007, 104, 12948–12950. [Google Scholar] [CrossRef]
- Neumann, H.; de Boois, I.; Kröncke, I.; Reiss, H. Climate change facilitated range expansion of the non-native angular crab Goneplax rhomboides into the North Sea. Mar. Ecol. Prog. Ser. 2013, 484, 143–153. [Google Scholar] [CrossRef][Green Version]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-driven regime shift of a temperate marine ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Caroli, F.; Guidetti, P.; Morri, C. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. J. Mar. Biol. Assoc. UK 2018, 98, 1–12. [Google Scholar] [CrossRef]
- Iturbide, M.; Fernández, J.; Gutiérrez, J.M.; Bedia, J.; Cimadevilla, E.; Díez-Sierra, J.; Manzanas, R.; Casanueva, A.; Baño-Medina, J.; Milovac, J.; et al. Repository Supporting the Implementation of FAIR Principles in the IPCC-WG1 Atlas. Zenodo. 2021. Available online: https://github.com/IPCC-WG1/Atlas (accessed on 17 May 2021).
- Follesa, M.C.; Mulas, A.; Porcu, C.; Cau, A. First record of Chilomycterus reticulatus (Osteichthyes: Diodontidae) in the Mediterranean Sea. J. Fish Biol. 2009, 74, 1677–1681. [Google Scholar] [CrossRef]
- Brito, A.; Lozano, G. Consideraciones Zoogeográficas Sobre la Fauna Ictiológica Bentónica y Epibentónica de las Islas Canarias; Jornadas de Ictiología Ibérica: León, Spain, 1981. [Google Scholar]
- COINVA Project. Conocer al Invasor (COINVA). 2018. Available online: http://www.proyectocoinva.com (accessed on 20 August 2021).
- Freitas, M.; Canning-Clode, J. Non-Indigenous Fish in the Fresh and Marine Waters of the Madeira Archipelago. In Proceedings of the SIBIC2014—V Jornadas Lbérica de Lctiologia, Lisbon, Portugal, 24–27 June 2014. [Google Scholar]
- Brito, A.; Falcón, J.M.; Herrera, R. Sobre la tropicalización reciente de la ictiofauna litoral de las islas Canarias y su relación con cambios ambientales y actividades antrópicas. Vieraea 2005, 33, 515–526. [Google Scholar]
- Triay-Portella, R.; Pajuelo, J.G.; Manent, P.; Espino, F.; Ruiz-Díaz, R.; Lorenzo, J.M.; González, J.A. New records of non-indigenous fishes (Perciformes and Tetraodontiformes) from the Canary Islands (north-eastern Atlantic). Cybium 2015, 39, 163–174. [Google Scholar] [CrossRef]
- Gallardo, T.; Bárbara, I.; Afonso-Carrillo, J.; Bermejo, R.; Altamirano, M.; Garreta, A.G.; Martí, M.C.B.; Lluch, J.R.; Ballesteros, E.; La Rosa, J.D. A new checklist of benthic marine algae of Spain. Algas. Boletín Informativo Sociedad Española Ficología 2016, 51, 7–52. [Google Scholar]
- Verlaque, M.; Durand, C.; Huisman, J.M.; Boudouresque, C.-F.; Le Parco, Y. On the identity and origin of the Mediterranean invasive Caulerpa racemosa (Caulerpales, Chlorophyta). Eur. J. Phycol. 2003, 38, 325–339. [Google Scholar] [CrossRef]
- Cohen, A. Have claw, will travel. Aquatic Nuisance Species (ANS) Digest. Aquatic 1997, 2, 16–17. [Google Scholar]
- Leis, J.M. Diodontidae. Porcupine fishes (burrfishes, spiny puffers). In The Living Marine Resources ofthe Eastern Central Atlantic; FAO Species Identification Guide for Fishery Purposes, Bony Fishes, Part 2 (Perciformes to Tetraodontiformes) and Sea Turtles; Carpenter, K.E., De Angelis, N., Eds.; FAO: Rome, Italy, 2016; Volume 4, pp. 3074–3079. [Google Scholar]
- Afonso, P.; Porteiro, F.M.; Fontes, J.; Tempera, F.; Morato, T.; Cardigos, F.; Santos, R.S. New and rare coastal fishes in the Azores islands: Occasional events or tropicalization process? J. Fish Biol. 2013, 83, 272–294. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.T.; Ruiz, G.M. Vector science and integrated vector management in bioinvasion ecology: Conceptual frameworks. In Invasive Alien Species; Mooney, H.A., Mack, R.N., McNeely, J.A., Neville, L.E., Schei, P.J., Waage, J.K., Eds.; Island Press: Washington, DC, USA, 2005; pp. 36–58. [Google Scholar]
- Freitas, R.; Romeiras, M.; Silva, L.; Cordeiro, R.; Madeira, P.; González, J.A.; Wirtz, P.; Falcón, J.M.; Brito, A.; Floeter, S.R.; et al. Restructuring of the ‘Macaronesia’ biogeographic unit: A marine multi-taxon biogeographical approach. Sci. Rep. 2019, 9, 15792. [Google Scholar] [CrossRef]
- Henriques, M.; Gonçalves, E.J.; Almada, V.C. Rapid shifts in a marine fish assemblage follow fluctuations in winter sea conditions. Mar. Ecol. Prog. Ser. 2007, 340, 259–270. [Google Scholar] [CrossRef]
- Hernández-Guerra, A.; Espino-Falcón, E.; Vélez-Belchí, P.; Dolores Pérez-Hernández, M.; Martínez-Marrero, A.; Cana, L. Recirculation of the Canary Current in fall 2014. J. Mar. Syst. 2017, 174, 25–39. [Google Scholar] [CrossRef]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Nagasawa, K.; Uyeno, D. Geographical distribution affected by the Kuroshio of the fish parasite Cymothoa pulchra (Isopoda: Cymothoidae) in Japanese waters. Biogeography 2012, 14, 151–153. [Google Scholar] [CrossRef]
- Martin, S.B.; Ribu, D.; Cutmore, S.C.; Cribb, T.H. Opistholobetines (Digenea: Opecoelidae) in Australian tetraodontiform fishes. Syst. Parasitol. 2018, 95, 743–781. [Google Scholar] [CrossRef]
- Nagashima, Y.; Ohta, A.; Yin, X.; Ishizaki, S.; Matsumoto, T.; Doi, H.; Ishibashi, T. Difference in Uptake of Tetrodotoxin and Saxitoxins into Liver Tissue Slices among Pufferfish, Boxfish and Porcupinefish. Mar. Drugs 2018, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Evaluation of the Safety of Fishery Products. Naturally Occurring Fish and Shellfish Poisons; Ahmed, F.E., Ed.; Seafood Safety; National Academies Press: Washington, DC, USA, 1991; p. 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK235731 (accessed on 10 October 2021).
- Green, S.J.; Akins, J.L.; Maljkovic, A.; Cote, I.M. Invasive Lionfish Drive Atlantic Coral Reef Fish Declines. PLoS ONE 2012, 7, e32596. [Google Scholar] [CrossRef]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Rotter, A.; Klun, K.; Francé, J.; Mozetič, P.; Orlando-Bonaca, M. Non-indigenous Species in the Mediterranean Sea: Turning From Pest to Source by Developing the 8Rs Model, a New Paradigm in Pollution Mitigation. Front. Mar. Sci. 2020, 7, 178. [Google Scholar] [CrossRef]
- Bacallado, J.J.; Brito, A.; Cruz, T.; Carrillo, M.; Barquín, J. Proyecto Bentos II. Anexo: Estudio de la Biología del Erizo de Lima (Diadema antillarum); Informes de La Consejería de Agricultura y Pesca Del Gobierno de Canarias: Sevilla, Spain, 1987. [Google Scholar]
- Bacallado, J.J.; Cruz, T.; Brito, A.; Barquín, J.; Carrillo, M. Reservas Marinas de Canarias; Consejería de Agricultura y Pesca: Sevilla, Spain, 1989. [Google Scholar]
- Gizzi, F.; Monteiro, J.G.; Silva, R.; Schäfer, S.; Castro, N.; Almeida, S.; Chebaane, S.; Bernal-Ibáñez, A.; Henriques, F.; Gestoso, I.; et al. Disease Outbreak in a Keystone Grazer Population Brings Hope to the Recovery of Macroalgal Forests in a Barren Dominated Island. Front. Mar. Sci. 2021, 8, 645578. [Google Scholar] [CrossRef]
- Hewitt, C.L.; Hayes, K.R. Risk assessment of marine biological invasions. In Invasive Aquatic Species of Europe, Distribution, Impacts and Management; Leppakoski, E., Gollasch, S., Olenin, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 456–466. [Google Scholar] [CrossRef]
- Forrest, B.M.; Taylor, M.D.; Sinner, J. Setting priorities for the management of marine pests using a risk-based decision support framework. In Biological Invasions in New Zealand; Springer: Berlin/Heidelberg, Germany, 2006; pp. 389–405. [Google Scholar] [CrossRef]
- Leis, J.L.; Matsuura, K.; Shao, K.-T.; Hardy, G.; Zapfe, G.; Liu, M.; Jing, L.; Robertson, R.; Tyler, J. Chilomycterus reticulatus (errata version published in 2017). IUCN Red List Threat. Species 2015, e.T193752A115331266. [Google Scholar] [CrossRef]
- Castro, N.; Ramalhosa, P.; Cacabelos, E.; Costa, J.L.; Canning-Clode, J.; Gestoso, I. Winners and losers: Prevalence of non-indigenous species under simulated marine heatwaves and high propagule pressure. Mar. Ecol. Prog. Ser. 2021, 668, 21–38. [Google Scholar] [CrossRef]
- Minchin, D. Exotic Species, Introduction of. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Cambridge, UK, 2009; pp. 289–300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).