Abstract
The aim of this research was to investigate the structure of the benthic diatom community and its relations to selected environmental parameters. We collected samples in 16 karst ponds in the alpine region of Slovenia, where the Alpine karst is found. Since the predominating substrate in these ponds was clay, the epipelic community was analyzed. Hydromorphological characteristics, and physical and chemical conditions were also measured at each site. We found 105 species of diatoms, which belonged to 32 genera. The most frequent taxa were Gomphonema parvulum (Kützing) Kützing, Navicula cryptocephala Kützing, Sellaphora pupula (Kützing) Mereschkowsky (species group) and Achnanthidium pyrenaicum (Hustedt) Kobayasi. The pond with the lowest diversity was found at the highest altitude, while, on the other hand, the most species-rich pond was found at the lowest altitude. Regarding the ecological types, the most common were motile species. We confirmed a positive correlation between the number of diatom species and the saturation of water with oxygen, while correlation between species richness and NH4-N was negative. The content of NO3-N and NH4-N explained almost 20% of the total variability of diatom community. Unlike our expectations, we calculated a negative correlation between the diversity of macroinvertebrates and diatoms, which is probably a consequence of different responses to environmental conditions.
1. Introduction
Ponds are water bodies ranging from 1 m2 to 2 hectares, of natural or anthropogenic origin, with permanent or seasonal water [1]. Researchers used to treat them as lakes, but ponds differ from lakes due to several characteristics [2]: (a) smaller surface area and depth, (b) smaller ratio between the volume of water and the shore area, and therefore more direct contact with the terrestrial environment making them more susceptible to various influences; (c) smaller drainage basin and therefore bigger isolation [1]; (d) relatively small volume and water intake, which increases the connection between the sediments and water column and a more significant impact of sediment on the nutrient content in water, (e) due to the low water depth, the surface of the entire waterbody could be covered with macrophytes [3,4]. This is also the main reason why we consider ponds as a type of wetland. It is characteristic that their conditions change faster than in larger water bodies [5], which is reflected in large daily and seasonal fluctuations [1,5].
Ponds as a habitat have been neglected in ecological studies [6]. Today, we recognize them as an important carbon sink, pollution filter, and source of biodiversity, hosting several specialized and rare species [2,6]. For organisms living in the aquatic environment, ponds are refuges in degraded and inhospitable areas [1,7].
Karst ponds were made in areas with no surface water bodies (e.g., Karst), where people had problems with water supply [8]. Although they were used to water livestock and gardens, they lost their importance when water pipelines were constructed. However, today they represent an important source of biodiversity, like all other types of ponds [1,7,9,10]. Smol and Stoermer [11] suggest that Karstic aquatic habitats are the most interesting environments in which to study algae, especially diatoms.
With their distribution, they form a network of aquatic ecosystems, which increases γ diversity [1,8]. Biodiversity and abundance of the biota in Alpine ponds significantly correlate with altitude—with it, the average air temperature decreases, the amount of local precipitation increases, and UV radiation is more intense. In addition, the organisms in these environments face high daily and annual temperature differences and have a short period suitable for growth, which gives cold stenothermic species a better chance of survival [12,13,14].
The substrate consisting of clay and silt mostly covers the entire bottom of these ponds. On such a substrate, an epipelic biofilm develops, which is dominated by diatoms constituting the basal trophic levels for extensive food webs [15]. Diatoms are present in different aquatic environments and their sensitivity to various environmental factors, makes them a good bioindicator of water quality [16]. Recent studies have highlighted the high level of cryptic diversity of diatoms [17]. The diatom community is influenced by several factors such as water chemistry (pH, nutrient concentration, and organic load), physical (electrical conductivity, temperature, light) hydromorphological characteristics (substrate, water regime), and biotic pressures such as grazing, competition, and parasitism [17,18,19,20].
Benthic diatoms are important primary producers in shallow waters where light penetrates to the bottom [21]. On a fine substrate, a specific epipelic diatom community usually forms, which is adapted to low light conditions, consisting mainly of motile taxa that can move through interstitial waters to avoid newly deposited sediments [22]. Due to their location between substrate and water, they play a fundamental role in various biogeochemical cycles and dynamics of aquatic ecosystems [23].
The biological characteristics of diatoms, such as cell size class and ecological types, give us information about the structure of the community [17,24], as well as environmental conditions. Low-profile diatoms are well adapted to physical disturbances and are more abundant in waters with low nutrient content [17,24,25]. For high-profile diatoms, the formation of colonies allows exploiting nutrients that are not available to other groups but are therefore more exposed to grazing [24,25]. Motile diatoms are fast-growing species. Their abundance increases with a higher concentration of nutrients and organic load. They are also well adapted to high physical disorders [24]. Planktic species are present in lentic water, where they float in the water column [25], but due to sinking they can also be abundant in phytobenthos [26].
Despite their import roles, karst ponds are disappearing due to the abandonment of their original use. In addition to natural processes such as overgrowing with plants, they are also threatened by anthropogenic factors, especially intensification of agriculture, abandonment of livestock farming, backfilling, the input of non-native species and chemical pollution [1,2,3]. Pollutants cannot be sufficiently diluted [27], and nutrients are retained and potentially recycled by internal processes, which is difficult in the affected ecosystem [4]. All this can be significantly reflected in the structure of the diatom community.
However, we have not found any published work on the epipelic diatom community in karst ponds. Even the studies of periphytic diatom communities in ponds are rare, which had been discovered by Šumberova et al. [28]. In central Europe, we have found one paper about epipelic diatoms in ponds [29], while in southern Europe there are some papers that analyze epipelic diatoms (e.g., [16,30,31,32,33]).
We measured physical, hydromorphological, and chemical factors in 16 ponds at various locations in the Alpine region and sampled the epipelon. In this paper, we focused primarily on their response to various environmental characteristics. The study aimed to determine the species composition of the benthic diatom community in the Alpine karst ponds, determine the relationships between the structure of diatom community and the studied parameters, and find out the significant correlation between them.
We hypothesized that: (a) the diatom’s species diversity correlates with the diversity of macroinvertebrates; (b) the diversity of species will decline with altitude and declining of ponds size; (c) the species composition will be significantly affected by the pH and electrical conductivity of the water and the land use in the drainage basin.
2. Materials and Methods
2.1. Study Sites and Sampling
We chose 16 karst ponds in the alpine region of Slovenia, which is a part of the South—Eastern calcareous Alps. Since limestone and dolomite are predominating rocks in this area, the Alpine karst is found there [34]. These water bodies are found in the area of the Julian Alps (Pokljuka, Jelovica, Ratitovec) and the Kamnik Alps (Krvavec, Velika planina, and Menina) (Figure 1). During the sample preparation we realized, that there were almost no frustules in samples from four ponds.
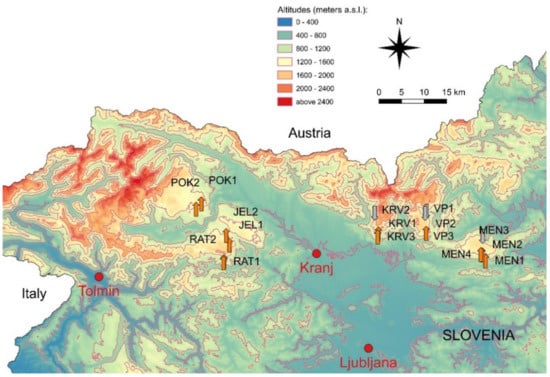
Figure 1.
Map of sampled karst ponds. The arrow-tips indicate the localities of the studied ponds. Gray arrows represent ponds where samples contained low number of frustules. POK1, POK2—Pokljuka; JEL1, JEL2—Jelovica; RAT1, RAT2—Ratitovec; KRV1, KRV2, KRV3—Krvavec; VP1, VP2, VP3—Velika planina; MEN1, MEN2, MEN3, MEN4—Menina.
Mountain climate prevails in the area, where the average temperature of the coldest month is lower than −3 °C, and the average temperature of the warmest month depends on the altitude and location [35]. Macrophyte and macroinvertebrate communities were studied before in the same ponds and results were published in Zelnik et al. [10].
Sampling took place in August of 2016, during the peak pasture season. Argilal and clay, respectively, was the only type of substrate present in all sites, so we decided to sample epipelon. Since we experienced difficulties with cleaning the samples from four ponds as well as very poor presence of diatom frustules in them, the samples from 12 sites were studied only (Table 1).

Table 1.
Information about sampling sites.
Basic physical and chemical factors were measured with a portable multimeter (EUTECH, PCD 650). For each pond, we measured the pH and T of water (°C), electrical conductivity (µS/cm), total dissolved solids (mg/L), saturation with O2 (%), and O2 concentration (mg/L). For laboratory analyzes, a water sample (1 L) was taken at each site.
In the laboratory, the concentrations of NO3-N (LCK 339), NH4-N (LCK 304), TN (LCK 138), and orthophosphates (LCK 349) were determined using HACH Lange cuvette tests. Values were measured in individual samples with a HACH Lange LT 200 spectrophotometer. Dry mass and total suspended solids content (TSS) were determined by filtration and drying at 105 °C.
2.2. Biotic Analyses
Due to the absence of a firm substrate, diatom samples were taken from the surface of the loamy substrate. We scraped the top layer of argilal with an area of approximately 2 cm2, with a spoon, at a 20–25 cm water depth. The samples were placed into bottles and 37% formaldehyde was added for fixation, in a ratio of 1:9.
Each sample was first homogenized with magnetic stirrer at a rate of 1200 rpm. We put 2 mL of the sample into a test tube and added 2.5 mL of 65% nitric (V) acid (HNO3). The samples were heated over a fire until the smoke turned white to remove organic matter from the sample. After cooling the tube contents were centrifuged with a SIGMA 2-16PK centrifuge, 4 min at 4000 rpm, and the supernatant was discarded. The sample was further washed with distilled water. The resulting pellet was added to 2 mL of distilled water and mixed. We put single drops onto slides, dried them, and fixed them with Naphrax® mountant.
The prepared preparations were examined with an Olympus CX41 microscope under 1000× magnification, and the first 400 frustules of each sample were determined. Identification was performed using the keys of Hoffman et al. [36], Lange-Bertalot et al. [37], and in some cases Krammer and Lange-Bertalot [38,39,40,41].
2.3. Data Analysis
Correlation analysis was performed with PAST program [42]. Some data (land use, number of habitat types, turbidity) were of the interval type and thus not normally distributed, so we used Kendall correlation coefficients (tau).
Similarity in taxonomic composition of diatom community between the ponds was calculated using Sørensen similarity index. Diversity was calculated as Shannon-Wiener diversity index (S-WI) and Margalef diversity index. The trophic index (TI) was calculated according to Rott et al. [43].
The influence of individual factors on the composition of the diatom community was checked by direct gradient analyzes. First, we performed a detrended correspondence analysis (DCA) to determine whether the distribution of the diatom species along potential gradients is unimodal or linear. We found that the mentioned distribution was unimodal (Length of gradient: 9.7 S.D.), so we used Canonical Correspondence Analysis (CCA). All analyzes were performed with the Canoco 4.5 software package [44].
Environmental parameters were grouped into spatial variables (coordinates, altitude, annual precipitation, a distance from the next pond or road), substrate (inorganic and organic), chemical and physical variables, hydromorphological data, drainage basin etc. We used the method of forward selection to check the effect of individual environmental factors on the taxonomic composition. The program made 999 permutations in each round, three rounds were performed. In each next round, we considered only factors with p less than 0.1. In the last round, we considered the two most statistically significant factors, that were in fact marginally significant (p = 0.06 and 0.07). Based on two factors that had a marginally statistically significant effect on the structure of the diatom community, we also created an ordination diagram in which the ponds are distributed along gradients of environmental factors.
3. Results
3.1. Structure of the Benthic Diatom Community
A total of 105 species of diatoms were identified in 12 ponds (Table A1). Of these, most species-rich was JEL1 (43 species) and POK1 (30 species) (Figure 2). The pond with the lowest number of species was KRV1 (14 species). Dominant species and their proportions vary significantly between ponds (Table 2). Navicula cryptocephala Kützing was the most dominant in four ponds (POK1, JEL2 MEN2 and MEN4), and it was also present in a large proportion in RAT1. The pioneer complex Achnanthidium minutissimum (Kützing) Czarnecki was the most common taxon in three ponds (JEL1, RAT2 and MEN2). The highest dominance index is in POK2 and KRV1, where two dominant taxa represent 77% of the identified species (Table 2).
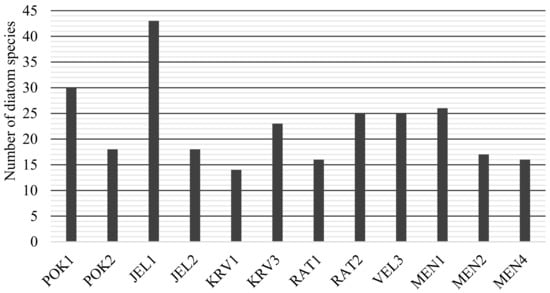
Figure 2.
Number of diatom species in individual karst ponds.

Table 2.
Dominance index (proportion in %) of the two most common species (highlighted in gray) in studied ponds. Diatoms that are not dominant in the sample but have a proportion ≥10% are also shown.
Ponds with the highest similarity of diatom community are POK1 and VEL3, although the huge distance between them (see Figure 1). On the other side there was POK2, which stood out the most in rare species—with four ponds (KRV1, KRV3, MEN2, and MEN4) had no species in common (Table 3).

Table 3.
Similarity of diatom community between the studied ponds according to Sørenson index. The similarity indices >0.5 are in bold.
Figure 3 shows the proportion of diatoms according to their ecological type. Motile and high-profile diatoms are present in all samples. Low-profile diatoms are absent in one pond, while in four ponds (KRV3, RAT1, MEN1, and MEN4) they are very rare. Their largest proportion is in RAT2 (68%) and MEN2 (52%). Planktic diatoms are present with a negligible proportion (JEL1, KRV3, and RAT1), except for KRV1, representing half of the specimens. The most common are motile diatoms. In POK1, JEL2, KRV3, RAT1, VEL3 and MEN4, they represent the majority proportion of diatoms.
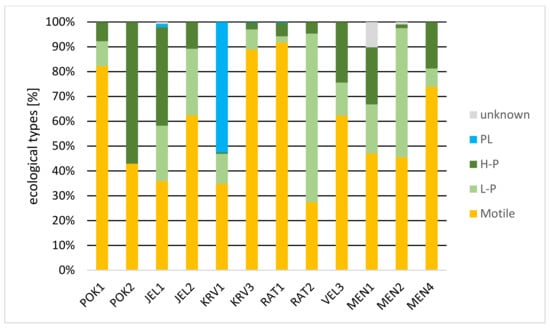
Figure 3.
Diatoms according to their ecological type [in %]. (PL—planktic, H-P—high-profile, L-P—low-profile).
Figure 4 shows the size classes of diatoms. The most common size class is 3, followed by 2 and 4. Members of size classes 1 and 5 are infrequent. Smaller diatoms (size classes 1 and 2) are dominant in RAT2, POK2, and MEN2. Data for POK2 are not representative, as 77% of specimens were not determined a size class due to lack of data in the literature. There is also a considerable proportion of unknown size classes in KRV1 and KRV3 (22% and 28%).
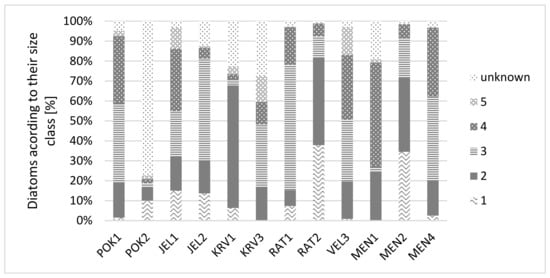
Figure 4.
Diatoms according to their size class [%].
3.2. Effects of Environmental Factors on the Diatom Community Composition
The concentration of NO3-N and NH4-N in water explains almost 20% of the total variability of the diatom community in ponds (Table 4). The concentration of NO3-N explains 10% of the variability, and the NH4-N concentration in water 9.6%. The content of these two nutrients or nitrogen species is probably mainly due to the higher load in ponds and their basin area with livestock. The same shows the ordination diagram based on CCA (Figure 5), where ponds are arranged according to the diatom taxonomic composition along the gradients of NO3-N and NH4-N concentration in water.

Table 4.
Results of Canonical correspondence analysis (CCA) and forward selection. (% TVE- proportion of the explained variability by specific variable).
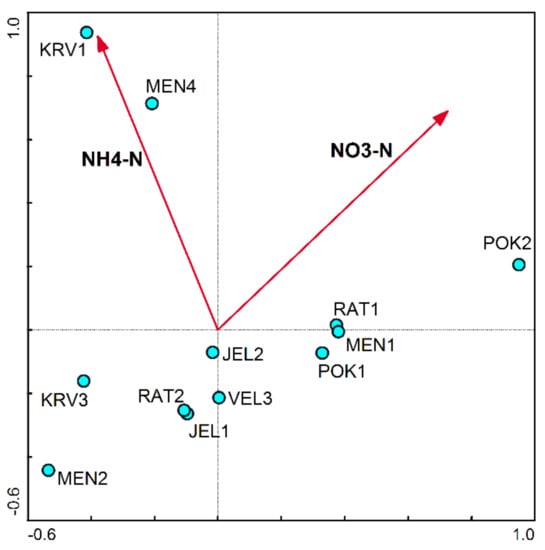
Figure 5.
A CCA-based ordination diagram in which karst ponds are distributed along environmental gradients.
According to the S-WI index (Figure 6), the highest diversity is in JEL1, VEL 3 is next. The lowest diversity is in POK2, the lower diversity is also in KRV1 and RAT1. The Margalef index (Figure 7) showed a different assessment of diversity than S-WI.
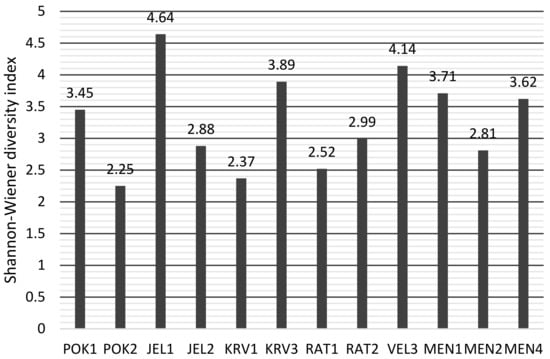
Figure 6.
Shannon-Wiener diversity index values of diatoms in karst ponds.
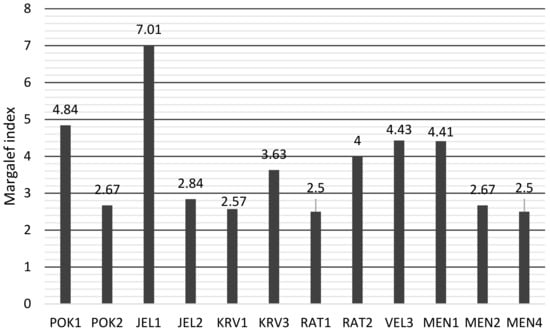
Figure 7.
Margalef index values of diatoms in karst ponds.
JEL1 still has the highest diversity value (7.01), but the ponds with the lowest diversity are RAT1 and MEN4.
3.3. Environmental Factors and Diversity of Diatom Community
Kendall correlation coefficients showed that the number of diatom species is in a statistically significant positive correlation with oxygen saturation and a negative correlation with the concentration of NH4-N (Table 5). The Margalef index was also positively correlated with oxygen saturation and negatively with NH4-N concentration. A negative statistically significant correlation (p = 0.05) was calculated between altitude and the Margalef index.

Table 5.
Kendall (tau) correlation coefficients between environmental factors and diversity parameters of diatom communities in ponds. Only statistically significant correlations (*—p < 0.05) and marginally statistically significant correlations (p = 0.05) are shown.
We also found a negative correlation between the number of diatom species and S-WI and the Margalef index calculated based on the composition of the invertebrate community, which was contrary to our expectations.
Great differences in TI values were found between the ponds (Figure 8). The lowest TI value was in POK1 (ultraoligotrophic) and the highest in JEL2, KRV3, KRV1, POK1, and MEN4 (polytrophic).
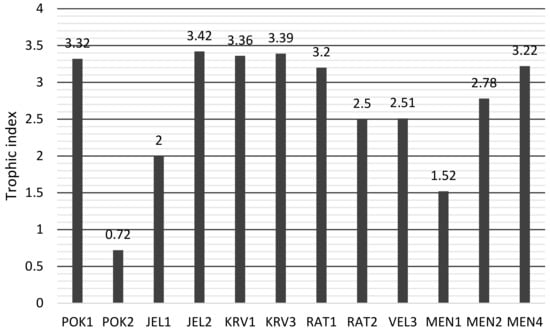
Figure 8.
Trophic index values for sampled karst ponds.
4. Discussion
4.1. Structure of the Benthic Diatom Community
In total, 105 diatom species belonging to 32 genera were identified. The most common taxa were Gomphonema parvulum (Kützing) Kützing, Navicula cryptocephala Kützing, species group Sellaphora pupula (Kützing) Mereschkowsky (present in 10 sites). Almost half of the species (52) were present in only one site, from which we can assume that the composition of diatom communities differs much between the ponds. The genera with the highest number of species were Nitzschia, Pinnularia, Navicula and Neidium. The highest number of species was identified in the JEL1, whereas in KRV1, we found the lowest number of species, of which Nitzschia acicularis (Kützing) W. Smith represented more than half of the identified frustules. We expected lower diversity as well as variability of epipelic diatom community, as karst ponds are small water bodies with frequent disturbances, which make the conditions unfavorable. The number of species varied from 14 to 43, which is much higher than 11–26 taxa from ponds in South-eastern Alps reported by Cantonati et al. [29]. However, the mentioned researchers studied different type of ponds in alpine region.
Among the ecological types, the motile diatoms were the most common. They dominated in four ponds (POK1, KRV3, RAT1, and MEN4) and were codominant in another four ponds (Figure 2). Sites where deposition occurs are advantageous for motile diatoms [45,46,47] as well as nutrient-rich sites [48,49,50]. Typical representatives from genera Navicula, Nitzschia, Sellaphora, and Surirella [24] were also present in our samples. However, we did not calculate any significant correlation between environmental factors and the share of motile species. In ponds with higher trophic index values motile species dominated, which are well adapted to higher nutrient content. We expected that high-profile (H-P) diatoms would also be present here with higher proportion. However, they were probably not present in such high proportion due to physical disturbances.
High-profile diatoms, which are also common in nutrient-rich water but with fewer disturbances [48] are less common in our samples. The proportion of H-P negatively correlated with TSS (p = 0.009), which negatively influence light conditions with turbidity and deposition. On the other hand, we calculated positive correlation between proportions of H-P diatoms and argilal (p = 0.029). The typical genera of this group, which were also present in our samples, were Eunotia, Fragilaria and Gomphonema. The proportions of H-P diatoms were lower than motile, except POK2, where H-P represent two-thirds of the community. Disturbances and grazing, made motile species more efficient than H-P ones.
Low-profile (L-P) diatoms were rare, but in two samples (RAT2 and MEN2) they were dominant. Both ponds are fenced, so with no access of the cattle. Proportions of L-P diatoms negatively correlated with NO3-N (p = 0.023) and positively with habitat diversity in the catchment area (p = 0.039), which actually means low density of the cattle. Typical representatives are from the genera Achnantes, Achnanthidium, Amphora, Cocconeis, and Meridion [24]. Achnantidium minutissiumum (Kützing) Czarnecki was the most dominant taxon in RAT2 and MEN2, as well as in JEL1. It seems that cattle cause problem for L-P diatoms due to high input of nutrients to ponds, to which L-P species are not adapted [48]. In some samples (JEL1, KRV1, KRV3, and RAT1), planktic diatoms were also present.
In ponds with higher concentrations of orthophosphates, we find mainly motile and H-P diatoms adapted to higher concentrations of nutrients [24,51,52] (Figure 3). In POK2 (0.3 mg/L of ortophosphate), MEN1 (0.92 mg/L) and VEL3 (0.23 mg/L) motile and H-P diatoms represent almost the entire sample, L-P diatoms are almost absent. However, the significant correlation between P and ecological types was not calculated. There was also no correlation between P concentration and diatom size classes, which also report Lavoie et al. [53].
The concentration of NO3-N and NH4-N in water explained almost 20% of the total variability of diatom community (Table 4). The concentration of NO3-N explains 10% of the variability of the diatom community, and the concentration of NH4-N 9.6% (Table 4, Figure 5). The ponds are arranged according to the taxonomic composition of diatom communities along the gradients of NO3-N and NH4-N concentration in water.
The results did not show statistically significant correlations between the composition of diatom community and concentrations of either orthophosphate or TP as expected, which is consistent with Soininen et al. [54]. This is probably because absorption rate for phosphorus from the water column by epipelon is lower than in other groups of primary producers [55].
Haubois et al. [56] report that large and small species do coexist within the epipelon. We found that size-class three had the highest proportion in five ponds, while size-class 2 and 4 in three ponds each (Figure 4). However, most of the identified frustules belonged to the middle-size class (3), which also report Lavoie et al. [53]. In ponds with higher biodiversity (JEL1, KRV3, VEL3, MEN1, and MEN4), size-classes 4 and 3 dominated.
4.2. Diversity of Benthic Diatom Community and Environmental Factors
In general, altitude affects biota in ponds as it affects temperature, precipitation, and radiation [12]. The results showed a negative correlation between altitude and the Margalef index, which is in line with our hypothesis and with the general rules in ecology [57]. The diatom species richness did not correlate with altitude, but pond at the highest altitude (KRV1) had the lowest number of species, while pond at the lowest altitude (JEL1) had the highest diversity. On the contrary for mountain ponds in Spain Blanco et al. [31] report positive correlation of diatom diversity with altitude.
The water depth in these shallow ponds is important mainly because of poor light conditions in turbid water. One of the dominant species was also Nitzschia perminuta (Grunow) M. Peragallo, which dominates in low light conditions [58]. Due to shallowness, there is no stratification during the summer [59].
We calculated no significant correlation between pH and diversity indices. The most extreme values were measured at POK2 (pH = 3.8) and MEN1 (pH = 9.6) (Table A2). The first is located in a coniferous forest and is a dystrophic system. Therefore, diatom species in this pond differed from others the most (Table 3). As reported in DeNicola [60] and Della Bella [16], we found there mainly species from the genera Neidium, Eunotia, Pinnularia, Stauroneis, and Sellaphora, which occurred in small numbers or were absent in other ponds. Diatom community from this pond had no species in common with four other ponds. This pond was more similar to the shallow ponds on mires presented in [29,61]. The lowest value of the electrical conductivity was also measured there (16 µS/cm), which coincides with the trophic index, which defines it as ultraoligotrophic.
We found a positive correlation between the number of diatom species and water saturation with oxygen and the Margalef index and water saturation with oxygen. The highest oxygen saturation was in MEN1 (almost 250%) due to intense photosynthetic activity of the phytoplankton, making the water very turbid.
In KRV1 and MEN4, a large proportion of N is in the form of NH4-N, which can be explained by the high density of cattle in their catchments. Correlation coefficients showed a negative correlation between the Margalef index and the NH4-N concentration. In ponds with a higher concentration (KRV1 and MEN4), the diversity was lower, while it was higher in ponds with lower NH4-N concentrations (POK1, JEL1, RAT1, and VEL3). In contrast to NH4-N concentrations, NO3-N concentrations did not differ much between ponds. Values were 0.2–0.5 mg/L. NO3-N and NH4-N concentrations classify our ponds as eutrophic (POK2, JEL2, KRV3, RAT1), mesotrophic (POK1 and MEN1), or oligotrophic (JEL1, RAT2, and MEN2) [54]. In KRV1 and MEN4, the values of NH4-H and NO3-N were so high that they can be classified as hypereutrophic.
Cattle can have a substantial negative effect on the diversity of communities in ponds [62]. Trampling the bottom and the shore presents physical disturbances. In ponds with moderate intensity of trampling, the diatom diversity was higher than in those without trampling, which is consistent with the intermediate-disturbance hypothesis [63]. More important is the influence of the cattle as the source of nutrients and organic matter from their excrements. Smaller water bodies in the agricultural landscape are highly exposed to influences from nearby agricultural areas, since they can be strongly affected by nutrient accumulation [4].
Based on the trophic index (TI), ponds vary from ultraoligotrophic to polytrophic. Della Bella et al. [30] report that trophic diatom index highly correlated with nutrient content, especially orthophosphate and NO3-N in wetlands in central Italy. However, in our case orthophosphate concentrations were the highest where the TI values were low (POK2 and MEN1). According to TP concentrations and nutrient estimates for lakes [58], both ponds were hypertrophic, but TI classified them as ultraoligotrophic (POK2) and mesotrophic (MEN1). Due to the pH = 3.8, there were probably not enough basic ions in POK2, despite the high concentration of TP and NO3−. Insufficient amount of HCO3− was present at pH = 9.6, which reduced primary production and thus nutrient uptake, which was probably the explanation for the condition in the MEN1.
4.3. Correlations between Diatoms and Macroinvertebrates
We found a negative correlation between the diatom species richness and the S-WI, and Margalef index calculated on the base of the macroinvertebrate community, which was contrary to our expectations. Similar findings report also Gascón et al. [64], which found out that different aquatic communities respond differently to the environmental factors, so we could not generalize relations between parameters and diversity patterns. Due to the larger size of macroinvertebrates, they might be more susceptible to physical destruction of the littoral zone, and loss of mesohabitats due to trampling of the bottom compared to diatoms, whereas diatoms, as primary producers, are particularly sensitive to water chemistry and light conditions [16,65]. Another reason is probably grazing [66]. We should not neglect the fact that on the same substrate on which diatoms thrive, Chironomidae dominate, which graze on epipelon.
5. Conclusions
We found a negative correlation between species-richness and diversity of the diatom community and diversity of the macroinvertebrate community (S-WI, Margalef index).
Despite relatively small differences in altitude, the results showed a marginal statistical correlation between altitude and Margalef Index. No effect of the pond size on the diversity of diatom community was observed.
We did not calculate significant correlations between pH and diversity. Half of the species in most acidic pond POK2 were present only in this pond. Correlations between electrical conductivity, land use, and diversity of diatom community were not significant.
Motile diatoms were most common. They are adapted to high nutrient concentrations and disturbances and can migrate to the site with sufficient light or nutrients when the re-suspended substrate is depositing.
We found a positive correlation between the number of diatom species and O2 saturation and the Margalef index and O2 saturation. The pond with the lowest oxygen saturation value (KRV1) had the lowest species diversity.
The results also showed a negative correlation between the number of diatoms and NH4-N concentration and the Margalef index and NH4-N concentration. NH4-N is probably present in the ponds due to the cattle grazing in the area in the summer. The concentrations of NO3-N and NH4-N explain almost 20% of the total variability of the diatom community.
Author Contributions
Conceptualization, I.Z.; methodology, I.Z. and K.N.; validation, K.N.; formal analysis, I.Z. and K.N.; investigation, K.N.; data curation, I.Z. and K.N.; writing—original draft preparation, K.N.; writing—review and editing, I.Z. and K.N.; visualization, I.Z. and K.N.; supervision, I.Z.; funding acquisition, I.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were partly funded by the Slovenian Research Agency, Research program Biology of plants, grant number P1-0212.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are stored within the documentation of Master Study program theses and P1-0212 Research program.
Acknowledgments
Authors thank to Matej Holcar for creation of the Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The list of the names of diatom taxa found in studied karst ponds.
Table A1.
The list of the names of diatom taxa found in studied karst ponds.
| Achnanthidium pyrenaicum (Hustedt) Kobayasi |
| Achnanthidium minutissimum (Kützing) Czarnecki |
| Adlafia minuscula (Grunow) Lange-Bertalot var. minuscula |
| Amphora copulata (Kützing) Schoeman et Archibald |
| Amphora pediculus (Kützing) Grunow |
| Brachysira neoexilis Lange-Bertalot |
| Caloneis tenuis (Gregory) Krammer |
| Chamaepinnularia mediocris (Krasske) Lange-Bertalot |
| Chamaepinnularia muscicola (Petersen) Kulikovskiy, Lange-Beralot et Witkowski |
| Chamaepinnularia soehrensis (Krasske) Lange-Bertalot et Krammer |
| Cocconeis pediculus Ehrenberg |
| Craticula accomoda (Hustedt) D.G. Mann |
| Craticula ambigua (Ehrenberg) D.G. Mann |
| Craticula halophila (Grunow) D.G. Mann |
| Craticula molestiformis (Hustedt) Lange-Bertalot |
| Cyclotella stelligera Cleve & Grunow |
| Cymbopleura amphicephala (Nägeli) Krammer |
| Cymbopleura naviculiformis (Auerswald) Krammer |
| Diploneis krammeri Lange-Bertalot et Reichardt |
| Encyonema hebridicum Grunow ex Cleve |
| Encyonema minutum (Hilse) D.G. Mann |
| Encyonema silesiacum (Bleisch) D.G. Mann |
| Eucocconeis alpestris (Brun) Lange-Bertalot |
| Eunotia arcus Ehrenberg |
| Eunotia bilunaris (Ehrenberg) Schaarschmidt |
| Eunotia exigua (Brébisson) Rabenhorst |
| Eunotia minor (Kützing) Grunow |
| Eunotia paludosa Grunow |
| Eunotia pseudogroenlandica Lange-Bertalot et Tagliaventi |
| Eunotia subarcuatoides Alles, Nörpel et Lange-Bertalot |
| Eunotia tenella (Grunow) Hustedt |
| Fragilaria radians (Kützing) Williams et Round |
| Fragilaria tenera (W. Smith) Lange-Bertalot |
| Frustulia crassinervia (Brébisson) Lange-Bertalot et Krammer |
| Gomphonema acuminatum Ehrenberg |
| Gomphonema angustum (Kützing) Rabenhorst |
| Gomphonema calcifugum Lange-Bertalot et Reichardt |
| Gomphonema exilissimum (Grunow) Lange-Bertalot et Reichardt |
| Gomphonema occultum Reichardt et Lange-Bertalot |
| Gomphonema parvulum (Kützing) Kützing |
| Gomphonema sarcophagus Gregory |
| Hantzschia abundans Lange-Bertalot |
| Luticola nivalis (Ehrenberg) D.G. Mann |
| Luticola mutica (Kützing) D.G. Mann |
| Meridion circulare (Gréville) C. Agardh |
| Navicula antonii Lange-Bertalot |
| Navicula cryptocephala Kützing |
| Navicula cryptotenella Lange-Bertalot |
| Navicula exilis Kützing |
| Navicula menisculus Schumann |
| Navicula reichardtiana Lange-Bertalot |
| Navicula trivialis Lange-Bertalot |
| Navicula veneta Kützing |
| Navicula wildii Lange-Bertalot |
| Neidium affine (Ehrenberg) Pfitzer |
| Neidium alpinum Hustedt |
| Neidium ampliatum (Ehrenberg) Krammer |
| Neidium bergii (Cleve-Euler) Krammer |
| Neidium binodeforme Krammer |
| Neidium bisulcatum (Lagerstedt) Cleve var. bisulcatum |
| Neidium dubium (Ehrenberg) Cleve |
| Neidium iridis (Ehrenberg) Cleve |
| Neidium productum (W. Smith) Cleve |
| Nitzschia acicularis (Kützing) W. Smith |
| Nitzschia adamata Hustedt |
| Nitzschia angustata (W. Smith) Grunow |
| Nitzschia communis Rabenhorst |
| Nitzschia dissipata (Kützing) Grunow ssp. dissipata |
| Nitzschia fonticola Grunow |
| Nitzschia gisela Lange-Bertalot |
| Nitzschia palea (Kützing) W. Smith |
| Nitzschia perminuta (Grunow) M. Peragallo |
| Nitzschia pura Hustedt |
| Nitzschia pusilla Grunow |
| Nitzschia supralitorea Lange-Bertalot |
| Nitzschia umbonata (Ehrenberg) Lange-Bertalot |
| Pinnularia borealis Ehrenberg |
| Pinnularia gibba Ehrenberg |
| Pinnularia grunowii Krammer |
| Pinnularia interupta W. Smith |
| Pinnularia marchica I. Schönfelder ex Krammer |
| Pinnularia microstauron (Ehrenberg) Cleve |
| Pinnularia rupestris Hantzsch |
| Pinnularia sinistra Krammer |
| Pinnularia subcapitata Gregory var. subcapitata |
| Pinnularia viridiformis Krammer |
| Placoneis ignorata (Schimanski) Lange-Bertalot |
| Placoneis paraelginensis Lange-Bertalot |
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot |
| Psammothidium grischunum (Wunthrich) Bukhtiyarova et Round |
| Psammothidium helveticum (Hustedt) Bukhtiyarova & Round |
| Sellaphora pseudopupula (Krasske) Lange-Bertalot |
| Sellaphora pupula (Kützing) Mereschkowsky (species group) |
| Sellaphora stroemii (Hustedt) D.G.Mann |
| Sellaphora verecundiae Lange-Bertalot |
| Stauroneis acidoclinata Lang-Bertalot et Werum |
| Stauroneis anceps Ehrenberg |
| Stauroneis gracilis Ehrenberg |
| Stauroneis kriegeri Patrick |
| Stauroneis smithii Grunow |
| Stauroneis thermicola (Petersen) Lund |
| Stephanodiscus alpinus Hustedt |
| Surirella angusta Kützing |
| Surirella minuta Brébisson ex Kützing |
| Tabellaria flocculosa (Roth) Kützing |

Table A2.
Characteristics of karst ponds in the year 2016. * Secchi depth in most transparent ponds is the same as water depth; the bottom of the pond MEN2 was covered with plastic layer on which fine substrate deposited. + represents presence of substrate, cover <5%.
Table A2.
Characteristics of karst ponds in the year 2016. * Secchi depth in most transparent ponds is the same as water depth; the bottom of the pond MEN2 was covered with plastic layer on which fine substrate deposited. + represents presence of substrate, cover <5%.
| Sample | POK1 | POK2 | JEL1 | JEL2 | KRV1 | KRV3 | RAT1 | RAT2 | VEL3 | MEN1 | MEN2 | MEN4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| date | 23.8. | 23.8. | 23.8. | 23.8. | 19.8. | 19.8. | 23.8. | 23.8. | 19.8. | 18.8. | 18.8. | 18.8. |
| pH | 5.9 | 3.8 | 6.5 | 6.4 | 6.7 | 8.3 | 7.4 | 6.5 | 5.9 | 9.6 | 7.2 | 6.2 |
| T [°C] | 17.5 | 12.2 | 14.1 | 9.8 | 14.9 | 15.3 | 7.7 | 10.2 | 17.3 | 17.7 | 17.9 | 16.0 |
| Conductivity [μS/cm] | 37 | 16 | 149 | 47 | 242 | 92 | 95 | 256 | 36 | 158 | 55 | 90 |
| O2 saturation [%] | 75 | 53 | 56 | 62 | 10 | 69 | 56 | 74 | 100 | 244 | 90 | 25 |
| O2 [mg/L] | 6.6 | 4.7 | 5.0 | 6.0 | 0.9 | 5.9 | 4.9 | 7.5 | 8.1 | 19.4 | 7.3 | 2.0 |
| Secchi depth [cm] | 25 * | 30 * | 60 * | 55 * | 30 * | 13.0 | 20 * | 30 * | 35 | 10 | 56 | 36 |
| depth [cm] | 25 | 30 | 60 | 55 | 30 | 100 | 20 | 30 | 40 | 20 | 100 | 48 |
| Turbidity [1,2,3] | 1 | 1 | 1 | 3 | 3 | 3 | 1 | 1 | 3 | 3 | 1 | 3 |
| Clay, silt [%] | 100 | 100 | 100 | 90 | 80 | 5 | 100 | 100 | 100 | 95 | - | 100 |
| Sand, gravel [%] | 0 | 0 | 0 | 10 | 20 | 65 | 0 | 0 | 0 | 0 | - | 0 |
| Pebbles [%] | 0 | 0 | 0 | + | 0 | 30 | 0 | 0 | 0 | 5 | - | 0 |
| Stones [%] | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 |
| CPOM [%] | 0 | 20 | 0 | 0 | + | 5 | + | + | + | 0 | 1 | 0 |
| FPOM [%] | 0 | 80 | 0 | 0 | 0 | 1 | 100 | 80 | 100 | 100 | 80 | 0 |
| [%] of trampled shore | 1 | 1 | 0 | 45 | 70 | 70 | 20 | 0 | 50 | 100 | 0 | 80 |
| Intensity of trampled shores (0–5) | 1 | 1 | 0 | 3 | 5 | 2 | 3 | 0 | 4 | 5 | 0 | 4 |
| TP [mg/L] | 0.17 | 0.34 | 0.03 | 0.05 | 0.28 | 0.07 | 0.07 | 0.06 | 0.23 | 0.92 | 0.08 | 0.15 |
| PO43- [mg/L] | 0.17 | 0.30 | 0.02 | 0.02 | 0.07 | 0.03 | 0.01 | 0.001 | 0.23 | 0.92 | 0.05 | 0.02 |
| TN [mg/L] | 1.35 | 0.82 | 0.59 | 0.84 | 5.91 | 1.21 | 1.62 | 0.56 | 1.53 | 6.56 | 0.95 | 16.0 |
| NO3-N [mg/L] | 0.39 | 0.52 | 0.30 | 0.34 | 0.42 | 0.26 | 0.41 | 0.30 | 0.32 | 0.40 | 0.21 | 0.42 |
| NH4-N [mg/L] | 0.08 | 0.14 | 0.03 | 0.51 | 4.0 | 0.73 | 0.28 | 0.07 | 0.06 | 0.21 | 0.03 | 3.08 |
| TDS [mg/l] | 72 | 70 | 96 | 50 | 120 | 78 | 94 | 58 | 80 | 226 | 74 | 92 |
| TSS [mg/L] | 3 | 8 | 17 | 58 | 151 | 98 | 49 | 93 | 30 | 201 | 257 | 39 |
References
- Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Weatherby, A. 15 years of pond assessment in Britain: Results and lessons learned from the work of Pond Conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 6, 693–714. [Google Scholar] [CrossRef]
- Oertli, B.; Biggs, J.; Céréghino, R.; Grillas, P.; Joly, P.; Lachavanne, J.-B. Conservation and monitoring of pond biodiversity: Introduction. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 6, 535–540. [Google Scholar] [CrossRef]
- Declerck, S.; De Bie, T.; Ercken, D.; Hampel, H.; Schrijvers, S.; Van Wichelen, J.; Gillard, V.; Mandiki, R.; Losson, B.; Bau-wens, D.; et al. Ecological characteristics of small farmland ponds: Associations with land use practices at multiple spatial scales. Biol. Conserv. 2006, 131, 523–532. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P. Pond or lake: Does it make any difference? Fundam. Appl. Limnol. 2005, 162, 143–165. [Google Scholar] [CrossRef]
- Davies, B.; Biggs, J.; Williams, P.; Whitfield, M.; Nicolet, P.; Sear, D.; Bray, S.; Maund, S. Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agric. Ecosyst. Environ. 2008, 125, 1–8. [Google Scholar] [CrossRef]
- Hassall, C.; Hollinshead, J.; Hull, A. Environmental correlates of plant and invertebrate species richness in ponds. Biodivers. Conserv. 2011, 20, 3189–3222. [Google Scholar] [CrossRef]
- Céréghino, R.; Biggs, J.; Oertli, B.; Declerck, S. The ecology of European ponds: Defining the characteristics of a neglected freshwater habitat. Hydrobiologia 2008, 597, 1–6. [Google Scholar] [CrossRef]
- Čelik, T.; Zelnik, I.; Babij, V.; Vreš, B.; Pirnat, A.; Seliškar, A.; Drovenik, B. Inventory of karstic ponds and their importance for biotic diversity. In Kras: Water and Life in a Rocky Landscape; Mihevc, A., Ed.; ZRC: Ljubljana, Slovenia, 2005; pp. 72–82. [Google Scholar]
- Zelnik, I.; Potisek, M.; Gaberščik, A. Environmental Conditions and Macrophytes of Karst Ponds. Pol. J. Environ. Stud. 2012, 21, 1911–1920. [Google Scholar]
- Zelnik, I.; Gregorič, N.; Tratnik, A. Diversity of macroinvertebrates positively correlates with diversity of macrophytes in karst ponds. Ecol. Eng. 2018, 117, 96–103. [Google Scholar] [CrossRef]
- Smol, J.P.; Stoermer, E.F. (Eds.) The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-1-107-56496-1. [Google Scholar]
- Hinden, H.; Oertli, B.; Menetrey, N.; Sager, L.; Lachavanne, J.-B. Alpine pond biodiversity: What are the related environmental variables? Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 613–624. [Google Scholar] [CrossRef]
- Ilg, C.; Oertli, B. How can we conserve cold stenotherm communities in warming Alpine ponds? Hydrobiologia 2014, 723, 53–62. [Google Scholar] [CrossRef]
- Frisbie, M.P.; Lee, R.E. Inoculative Freezing and the Problem of Winter Survival for Freshwater Macroinvertebrates. J. N. Am. Benthol. Soc. 1997, 16, 635–650. [Google Scholar] [CrossRef]
- Ocón, C.S.; López van Oosterom, M.V.; Munoz, M.I.; Rodrigues-Capítulo, A. Macroinvertebrate trophic responses to nutrient addition in a temperate stream in South America. Arch. Hydrobiol. 2013, 182, 17–30. [Google Scholar] [CrossRef]
- Bella, V.D.; Mancini, L. Freshwater diatom and macroinvertebrate diversity of coastal permanent ponds along a gradient of human impact in a Mediterranean eco-region. Hydrobiologia 2009, 634, 25–41. [Google Scholar] [CrossRef]
- Berthon, V.; Bouchez, A.; Rimet, F. Using diatom life-forms and ecological guilds to assess organic pollution and trophic level in rivers: A case study of rivers in south-eastern France. Hydrobiologia 2011, 673, 259–271. [Google Scholar] [CrossRef]
- Sayer, C.D. Problems with the application of diatom-total phosphorus transfer functions: Examples from a shal-low English lake. Freshw. Biol. 2001, 46, 743–757. [Google Scholar] [CrossRef]
- Wu, N.; Faber, C.; Sun, X.; Qu, Y.; Wang, C.; Ivetic, S.; Riis, T.; Ulrich, U.; Fohrer, N. Importance of sampling fre-quency when collecting diatoms. Sci. Rep. 2016, 6, 36950. [Google Scholar] [CrossRef] [PubMed]
- Vis, C.; Hudon, C.; Cattaneo, A.; Pinel-Alloul, B. Periphyton as an indicator of water quality in the St Lawrence River (Québec, Canada). Environ. Pollut. 1998, 101, 13–24. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Nielsen, J.; Hansen, P.K. Benthic Primary Production and Oxygen Profiles. In Nitrogen Cycling in Coastal Marine Environments; Blackburn, T.H., Sørensen, J., Eds.; John Wiley & Sons Ltd.: Hoboken, NY, USA, 1988; pp. 69–81. [Google Scholar]
- Battarbee, R.W.; Jones, V.J.; Flower, R.J.; Cameron, N.G.; Bennion, H.; Carvalho, L.; Juggins, S. Diatoms. In Tracking Environmental Change Using Lake Sediments: Terrestrial, Algal, and Siliceous Indicators, Developments in Paleoenvironmental Research; Smol, J.P., Birks, H.J., Last, W.M., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 155–202. [Google Scholar]
- Kröpfl, K.; Vladár, P.; Szabó, K.; Ács, É.; Borsodi, A.K.; Szikora, S.; Caroli, S.; Záray, G. Chemical and biological characterisation of biofilms formed on different substrata in Tisza river. Environ. Pollut. 2006, 144, 626–631. [Google Scholar] [CrossRef]
- Rimet, F.; Bouchez, A. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl. Manag. Aquat. Ecosyst. 2012, 406, 01. [Google Scholar] [CrossRef]
- Rimet, F.; Berthon, V.; Bouchez, A. Formes de vie, Guildes Écologiques et Classes de Tailles des Diatomées d’eau Douce; INRA, Station d’hydrobiologie lacustre: Thonon, France, 2010; p. 10. [Google Scholar]
- Zelnik, I.; Sušin, T. Epilithic Diatom Community Shows a Higher Vulnerability of the River Sava to Pollution during the Winter. Diversity 2020, 12, 465. [Google Scholar] [CrossRef]
- De Marco, P.; Nogueira, D.S.; Correa, C.C.; Vieira, T.B.; Silva, K.D.; Pinto, N.S.; Bichsel, D.; Hirota, A.S.V.; Vieira, R.R.S.; Carneiro, F.M.; et al. Patterns in the organization of Cerrado pond biodiversity in Brazilian pasture landscapes. Hydrobiologia 2014, 723, 87–101. [Google Scholar] [CrossRef]
- Šumberová, K.; Vild, O.; Ducháček, M.; Fabšičová, M.; Potužák, J.; Fránková, M. Drivers of Macrophyte and Diatom Diversity in a Shallow Hypertrophic Lake. Water 2021, 13, 1569. [Google Scholar] [CrossRef]
- Cantonati, M.; Lange-Bertalot, H.; Decet, F.; Gabrieli, J. Diatoms in very-shallow pools of the site of community importance Danta di Cadore Mires (south-eastern Alps), and the potential contribution of these habitats to diatom biodiversity conservation. Nova Hedwig. 2011, 93, 475–507. [Google Scholar] [CrossRef]
- Bella, D.V.; Puccinelli, C.; Marcheggiani, S.; Mancini, L. Benthic diatom communities and their relationship to water chemistry in wetlands of central Italy. J. Limnol. 2007, 43, 89–99. [Google Scholar] [CrossRef][Green Version]
- Blanco, S.; Olenici, A.; Ortega, F.; Jiménez-Gómez, F.; Guerrero, F. Identifying environmental drivers of benthic diatom diversity: The case of Mediterranean mountain ponds. PeerJ 2020, 8, e8825. [Google Scholar] [CrossRef]
- Kochoska, H.; Zaova, D.; Videska, A.; Mitic-Kopanja, D.; Naumovska, H.; Wetzel, C.E.; Ector, L.; Levkov, Z. Sellaphora pelagonica (Bacillariophyceae), a new species from dystrophic ponds in the Republic of North Macedonia. Phytotaxa 2021, 496, 2. [Google Scholar] [CrossRef]
- Vidaković, D.; Levkov, Z.; Hamilton, P.B. Neidiopsis borealis sp. nov., a new diatom species from the mountain Shar Planina, Republic of North Macedonia. Phytotaxa 2019, 402, 21. [Google Scholar] [CrossRef]
- Mihevc, A.; Gabrovšek, F.; Knez, M.; Kozel, P.; Mulec, J.; Otoničar, B.; Petrič, M.; Pipan, T.; Prelovšek, M.; Slabe, T.; et al. Karst in Slovenia. Boletín Geológico y Minero 2016, 127, 79–97. [Google Scholar]
- Ogrin, D. Podnebni tipi v Sloveniji. Acta Geogr. K 1996, 68, 39–56. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die Ökologische Praxis; Koeltz Scientific Books: Königstein, Germany, 2013; p. 908. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe. Over 800 Common Species Used in Ecological Assessment; English Edition with Updated Taxonomy and Added Species; Koeltz Scientific Books: Oberreifenberg, Germany, 2017; p. 942. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süβwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Fischer: Jena, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Epithemiaceae, Surirellaceae. In Süβwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Fischer: Jena, Germany, 1988; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae—Teil 3: Centrales, Fragilariaceae, Eunotiaceae. In Süβwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Fischer: Jena, Germany, 1991; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis. In Süβwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Fischer: Jena, Germany, 1991; p. 437. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Rott, E.; Pipp, E.; Pfister, P.; Van Dahm, H.; Ortler, K.; Binder, N.; Pall, K. Indikationslisten fur Aufwuchsalgen in Östereichen Fließgevessern, Teil 2: Trophienindikation so vie geochemische Präferenz, Taxonomische und toxicologische Anmerkungen; Bundesministerium für Land und Forstwirtschaft: Wien, Austria, 1999; p. 248. [Google Scholar]
- Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination; Microcomputer Power: Ithaca, NY, USA, 2002; p. 500. [Google Scholar]
- Pan, Y.; Hughes, R.; Herlihy, A.; Kaufmann, P. Non-wadeable river bioassessment: Spatial variation of benthic diatom assemblages in Pacific Northwest rivers, USA. Hydrobiologia 2012, 684, 241–260. [Google Scholar] [CrossRef]
- Heine-Fuster, I.; López-Allendes, C.; Aránguiz-Acuña, A.; Véliz, D. Differentiation of Diatom Guilds in Extreme Environments in the Andean Altiplano. Front. Environ. Sci. 2021, 9, 266. [Google Scholar] [CrossRef]
- Licursi, M.; Gómez, N.; Sabater, S. Effects of nutrient enrichment on epipelic diatom assemblages in a nutrient-rich lowland stream, Pampa Region, Argentina. Hydrobiologia 2016, 766, 135–150. [Google Scholar] [CrossRef]
- Passy, S.I. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 2007, 86, 171–178. [Google Scholar] [CrossRef]
- Gottschalk, S.; Kahlert, M. Shifts in taxonomical and guild composition of littoral diatom assemblages along environmental gradients. Hydrobiologia 2012, 694, 41–56. [Google Scholar] [CrossRef]
- Béres, V.; Török, P.; Kókai, Z.; Krasznai, E.T.; Tóthmérész, B.; Bácsi, I. Ecological diatom guilds are useful but not sensitive enough as indicators of extremely changing water regimes. Hydrobiologia 2014, 738, 191–204. [Google Scholar] [CrossRef]
- Zelnik, I.; Balanč, T.; Toman, M.J. Diversity and Structure of the Tychoplankton Diatom Community in the Limnocrene Spring Zelenci (Slovenia) in Relation to Environmental Factors. Water 2018, 10, 361. [Google Scholar] [CrossRef]
- Peszek, Ł.; Zgrundo, A.; Noga, T.; Kochman-Kędziora, N.; Poradowska, A.; Rybak, M.; Puchalski, C.; Lee, J. The influence of drought on diatom assemblages in aemperate climate zone: A case study from the Carpathian Mountains, Poland. Ecol. Indic. 2021, 125, 107579. [Google Scholar] [CrossRef]
- Lavoie, I.; Lento, J.; Morin, A. Inadequacy of size distributions of stream benthic diatoms for environmental monitoring. J. N. Am. Benthol. Soc. 2010, 29, 586–601. [Google Scholar] [CrossRef]
- Soininen, J.; Jamoneau, A.; Rosebery, J.; Passy, S.I. Global patterns and drivers of species and trait composition in diatoms: Global compositional patterns in stream diatom. Glob. Ecol. Biogeogr. 2016, 25, 940–950. [Google Scholar] [CrossRef]
- Scinto, L.J.; Reddy, K.R. Biotic and abiotic uptake of phosphorus by periphyton in a subtropical freshwater wetland. Aquat. Bot. 2003, 77, 203–222. [Google Scholar] [CrossRef]
- Haubois, A.G.; Sylvestre, F.; Guarini, J.M.; Richard, P.; Blanchard, G.F. Spatio-temporal structure of the epipelic diatom assemblage from an intertidal mudflat in Marennes-Oléron Bay, France. Estuar. Coast. Shelf Sci. 2005, 64, 385–394. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends. Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- van der Grinten, E.; Janssen, A.P.H.; de Mutsert, K.; Barranguet, C.; Admiraal, W. Temperature- and Light-Dependent Performance of the Cyanobacterium Leptolyngbya Foveolarum and the Diatom Nitzschia Perminuta in Mixed Biofilms. Hydrobiologia 2005, 548, 267–278. [Google Scholar] [CrossRef]
- Jurczak, T.; Wojtal-Frankiewicz, A.; Kaczkowski, Z.; Oleksińska, Z.; Bednarek, A.; Zalewski, M. Restoration of a shady urban pond—The pros and cons. J. Environ. Manag. 2018, 217, 919–928. [Google Scholar] [CrossRef] [PubMed]
- DeNicola, D.M. A review of diatoms found in highly acidic environments. Hydrobiologia 2000, 433, 111–122. [Google Scholar] [CrossRef]
- Krivograd-Klemenčič, A.; Smolar-Žvanut, N.; Istenič, D.; Griessler-Bulc, T. Algal community patterns in Slovenian bogs along environmental gradients. Biologia 2010, 65, 422–437. [Google Scholar] [CrossRef]
- Schmutzer, A.C.; Gray, M.J.; Burton, E.C.; Miller, D.L. Impacts of cattle on amphibian larvae and the aquatic environment. Freshw. Biol. 2008, 53, 2613–2625. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rainforests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Gascón, S.; Boix, D.; Sala, J. Are different biodiversity metrics related to the same factors? A case study from Mediter-ranean wetlands. Biol. Conserv. 2009, 11, 2602–2612. [Google Scholar] [CrossRef]
- Feio, M.; Almeida, S.; Craveiro, S.; Calado, A. Diatoms and macroinvertebrates provide consistent and complementary information on environmental quality. Fundam. Appl. Limnol. 2007, 169, 247–258. [Google Scholar] [CrossRef]
- Lange, K.; Liess, A.; Piggott, J.J.; Townsend, C.R.; Matthaei, C.D. Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure: Diatom responses to light, nutrients and grazing. Freshw. Biol. 2011, 56, 264–278. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).