Shell Anomalies in the European Aquatic Stem Turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae)
Abstract
1. Introduction
2. Materials and Methods
3. Identification of Shell Deviations
3.1. Presence of Additional Plates and Scutes
3.1.1. Additional Plates
3.1.2. Additional Scutes
3.2. Absence of Regular Shell Plates
3.3. Unusual Shape of Plates and Scutes
3.3.1. Unusual Shape of Plates
3.3.2. Unusual Shape of Scutes
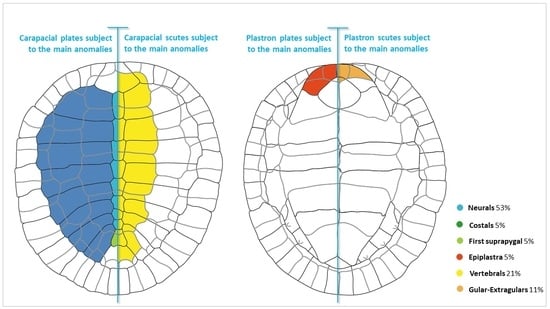
4. Discussion
4.1. Morphogenetic Origin of the Pleurosternon bullockii Shell Anomalies
4.1.1. Additional Plates
4.1.2. Additional Scutes
4.1.3. Absence of Regular Shell Plates
4.1.4. Unusual Shape of Plates
4.1.5. Unusual Shape of Scutes
4.2. General Considerations of the Pleurosternon bullockii Shell Anomalies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAMSM | Sedgwick Museum, Department of Geology, University of Cambridge, UK |
| DORCM | Dorset County Museum, Dorchester, UK |
| NHMUK | Natural History Museum, London, UK |
| OUMNH | Oxford University Museum, Oxford, UK |
| YPM | Peabody Museum of Natural History, Yale University, USA |
References
- Mast, R.B.; Carr, J.L. Carapacial scute variation in Kemp’s ridley sea turtle (Lepidochelys kempi) hatchlings and juveniles. In Proceedings of the First International Symposium on Kemp’s Ridley Sea Turtle Biology, Conservation and Management, Galveston, TX, USA, 1–4 October 1989; pp. 202–219. [Google Scholar]
- Cherepanov, G.O. Ontogenesis and evolution of horny parts of the turtle shell. Russ. J. Herpetol. 2006, 1, 19–33. [Google Scholar]
- Cherepanov, G.O. Patterns of scute development in turtle shell: Symmetry and asymmetry. Paleontol. J. 2014, 48, 1275–1283. [Google Scholar] [CrossRef]
- Cherepanov, G.O. Nature of the turtle shell: Morphogenetic causes of bone variability and its evolutionary implication. Paleontol. J. 2016, 50, 1641–1648. [Google Scholar] [CrossRef]
- Loehr, V.J.T. Wide variation in carapacial scute patterns in a natural population of speckled tortoises, Homopus signatus. Afr. J. Herpetol. 2016, 65, 47–54. [Google Scholar] [CrossRef]
- Moustakas-Verho, J.E.; Cherepanov, G.O. The integumental appendages of the turtle shell: An evo-devo perspective. J. Exp. Zool. 2015, 324, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zimm, R. On the Development of the Turtle Scute Pattern and the Origins of Its Variation. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 20 February 2019. [Google Scholar]
- Parker, G.H. Correlated abnormalities in the scutes and bony plates of the carapace of the sculptured tortoise. Am. Nat. 1901, 35, 17–24. [Google Scholar] [CrossRef]
- Newman, H.H. The significance of scute and plate “abnormalities” in Chelonia. A contribution to the evolutionary history of the chelonian carapace and plastron, Part, I. Biol. Bull. 1906, 10, 99–114. [Google Scholar] [CrossRef][Green Version]
- Coker, R. Diversity in the scutes of Chelonia. J. Morphol. 1910, 21, 1–75. [Google Scholar] [CrossRef]
- Lynn, W.G. Variation in scutes and plates in the box-turtle, Terrapene carolina. Am. Nat. 1937, 71, 421–426. [Google Scholar] [CrossRef]
- Zangerl, R.; Johnson, R.G. The nature of shield abnormalities in the turtle shell. Fieldiana Geol. 1957, 10, 341–362. [Google Scholar]
- McEwan, B. Bone anomalies in the shell of Gopherus polyphemus. Fla. Sci. 1982, 45, 189–195. [Google Scholar]
- Velo-Antón, G.; Becker, C.G.; Cordero-Rivera, A. Turtle carapace anomalies: The roles of genetic diversity and environment. PLoS ONE 2011, 6, e18714. [Google Scholar] [CrossRef] [PubMed]
- Farke, C.M.; Distler, C. Ontogeny and abnormalities of the tortoise carapace: A computer tomography and dissection study. Salamandra 2015, 51, 231–244. [Google Scholar]
- Saçdanaku, E.; Haxhiu, I. Accessory scutes and asymmetries in European pond turtle, Emys orbicularis (Linnaeus, 1758) and Balkan terrapin, Mauremys rivulata (Valenciennes, 1833) from Vlora Bay, western Albania. Int. J. Fauna Biol. Stud. 2016, 3, 127–132. [Google Scholar]
- Hay, O.P. The Fossil Turtles of North America; Carnegie Institution of Washington Publication No. 75: Washington, DC, USA, 1908; p. 568. [Google Scholar]
- Zangerl, R.; Turnbull, W.D. Procolpochelys grandaeva (Leidy), an early carettine sea turtle. Fieldiana Geol. 1955, 37, 345–382. [Google Scholar]
- Weems, R.E. Middle miocene sea turtles (Syllomus, Procolpochelys, Psephophorus) from the Calvert formation. J. Paleontol. 1974, 48, 278–303. [Google Scholar]
- Brinkman, D.B.; Nicholls, E.L. Anatomy and relationships of the turtle Boremys pulchra (Testudines: Baenidae). J. Vertebr. Paleontol. 1991, 11, 302–315. [Google Scholar] [CrossRef]
- Sullivan, P.M.; Joyce, W.G. The shell and pelvic anatomy of the Late Jurassic turtle Platychelys oberndorferi based on material from Solothurn, Switzerland. Swiss J. Palaeontol. 2017, 136, 323–343. [Google Scholar] [CrossRef]
- Szczygielski, T.; Słowiak, J.; Dróżdż, D. Shell variability in the stem turtles Proterochersis spp. PeerJ 2018, 6, e6134. [Google Scholar] [CrossRef] [PubMed]
- Garbin, R.C.; Böhme, M.; Joyce, W.G. A new testudinoid turtle from the middle to late Eocene of Vietnam. PeerJ 2019, 7, e6280. [Google Scholar] [CrossRef]
- Joyce, W.G.; Rollot, Y.; Cifelli, R.L. A new species of baenid turtle from the Early Cretaceous Lakota Formation of South Dakota. Foss. Rec. 2020, 23, 1–13. [Google Scholar] [CrossRef]
- Owen, R. Report on British fossil reptiles. Part II. Rep. Br. Ass. Advmt. Sci. 1842, 11, 60–204. [Google Scholar]
- Guerrero, A.; Pérez-García, A. On the validity of the British Upper Jurassic turtle “Pleurosternon portlandicum” (Paracryptodira, Pleurosternidae). J. Iber. Geol. 2020, 46, 419–429. [Google Scholar] [CrossRef]
- Guerrero, A.; Pérez-García, A. Morphological variability and shell characterization of the European uppermost Jurassic to lowermost Cretaceous stem turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae). Cretac. Res. 2021, 125, 104872. [Google Scholar] [CrossRef]
- Cherepanov, G.O. Anomalies of the bony shell of turtles. Zool. Zhurnal 1994, 73, 68–78. [Google Scholar]
- Cherepanov, G.O. New morphogenetic data on the turtle shell: Discussion on the origin of the horny and bony parts. Stud. Palaeocheloniol. 1989, 3, 9–24. [Google Scholar]
- Cherepanov, G.O. Ontogenetic development of the shell in Trionyx sinensis (Trionychidae, Testudinata) and some questions on the nomenclature of bony plates. Russ. J. Herpetol. 1995, 2, 129–133. [Google Scholar]
- Cherepanov, G.O. The origin of the bony shell of turtles as a unique evolutionary model in reptiles. Russ. J. Herpetol. 1997, 4, 155–162. [Google Scholar] [CrossRef]
- Cherepanov, G.O. Scute’s polymorphism as a source of evolutionary development of the turtle shell. Paleontol. J. 2015, 49, 1635–1644. [Google Scholar] [CrossRef]
- Cherepanov, G.; Malashichev, Y.; Danilov, I. Supernumerary scutes verify a segment-dependent model of the horny shell development in turtles. J. Anat. 2019, 235, 836–846. [Google Scholar] [CrossRef]
- Kordikova, E.G. Paedomorphosis in the shell of fossil and living turtles. Neues Jahrb. Geol. Palaontol. Abh. 2000, 218, 399–446. [Google Scholar] [CrossRef]
- Scheyer, T.M.; Brüllmann, B.; Sánchez-Villagra, M.R. The ontogeny of the shell in side-necked turtles, with emphasis on the homologies of costal and neural bones. J. Morphol. 2008, 269, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Moustakas-Verho, J.E.; Zimm, R.; Cebra-Thomas, J.; Lempiainen, N.K.; Kallonen, A.; Mitchell, K.L.; Hamalainen, K.; Salazar-Ciudad, I.; Jernvall, J.; Gilbert, S.F. The origin and loss of periodic patterning in the turtle shell. Development 2014, 141, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Moustakas-Verho, J.E.; Cebra-Thomas, J.; Gilbert, S.F. Patterning of the turtle shell. Curr. Opin. Genet. Dev. 2017, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Pérez-García. Ontogenetic development of the European basal aquatic turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae). Foss. Rec. 2021. accepted. [Google Scholar]
- Webb, R.G. North American Recent Soft-Shelled Turtles (Family Trionychidae); University of Kansas Publications, Museum of Natural History: Lawrence, KS, USA, 1962; Volume 13, pp. 429–611. [Google Scholar]
- Pritchard, P.C.H. Evolution and structure of the turtle shell. In Biology of Turtles; Wyneken, J., Godfrey, M.H., Bels, V., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2008; pp. 46–83. [Google Scholar]
- Gaffney, E.S.; Hutchison, J.H.; Jenkins, F.A.; Meeker, L.J. Modern turtle origins: The oldest known cryptodire. Science 1987, 237, 289–291. [Google Scholar] [CrossRef]
- Brinkman, D.B. Anatomy and systematics of Plesiobaena antiqua (Testudines; Baenidae) from the mid-Campanian Judith River Group of Alberta, Canada. J. Vertebr. Paleontol. 2003, 23, 146–155. [Google Scholar] [CrossRef]
- Szczygielski, T. Homeotic shift at the dawn of the turtle evolution. R. Soc. Open Sci. 2017, 4, 160933. [Google Scholar] [CrossRef] [PubMed]
- Gadow, H. On the reproduction of the carapax in tortoises. J. Anat. 1886, 20, 220–224. [Google Scholar]
- Smith, H.M. Total regeneration of the carapace in a box turtle. Turtox News 1958, 36, 234–237. [Google Scholar]
- Rose, F.L. Carapace regeneration in Terrapene (Chelonia: Testudinidae). Southwest Nat. 1986, 31, 131–134. [Google Scholar] [CrossRef]
- Kuchling, G. Restoration of epidermal scute patterns during regeneration of the chelonian carapace. Chelonian Conserv. Biol. 1997, 2, 500–506. [Google Scholar]
- Ayres Fernández, C.; Cordero Rivera, A. Asymmetries and accessory scutes in Emys orbicularis from Northwest Spain. Biologia 2004, 59, 85–88. [Google Scholar]
- Cordero Rivera, A.; Ayres, C.; Velo-Antón, G. High prevalence of accessory scutes and anomalies in Iberian populations of Emys Orbicularis. Rev. Esp. Herp. 2008, 22, 5–14. [Google Scholar]
- Mlynarski, M. Studies on the Morphology of the Shell of Recent and Fossil Tortoises; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1956; Volume 1, pp. 1–16. [Google Scholar]
- Cherepanov, G.O. Morphogenetic and constructional differences of the carapace of aquatic and terrestrial turtles and their evolutionary significance. J. Morphol. 2019, 280, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Gadow, H. Orthogenetic variation in the shells of Chelonia. In Zoological Results Based on Material from New Britain, New Guinea, Loyalty Islands and Elsewhere, Collected during the Years 1895, 1896, and 1897; Willey, A., Ed.; University Press: Cambridge, UK, 1899; Part 3; pp. 207–222. [Google Scholar]
- Frye, F.L. Biomedical and Surgical Aspects of Captive Reptile Husbandry; Krieger Publications: Malabar, FL, USA, 1991; p. 637. [Google Scholar]
- Lynn, W.G.; Ullrich, M.G. Experimental production of shell abnormalities in turtles. Copeia 1950, 1950, 253–262. [Google Scholar] [CrossRef]
- Özdemir, B.; Türkozan, O. Carapacial scute variation in green turtle, Chelonia mydas hatchlings in Northern Cyprus. Turk. Zool. Derg. 2006, 30, 141–146. [Google Scholar]
- Bujes, C.S.; Verrastro, L. Supernumerary epidermal shields and carapace variation in Orbigny’s slider turtles, Trachemys dorbigni (Testudines, Emydidae). Rev. Bras. Zool. 2007, 24, 666–672. [Google Scholar] [CrossRef]
- Caracappa, S.; Pisciotta, A.; Persichetti, M.F.; Caracappa, G.; Alduina, R.; Arculeo, M. Nonmodal scutes patterns in the Loggerhead Sea Turtle (Caretta caretta): A possible epigenetic effect? Can. J. Zool. 2016, 94, 379–383. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, A.; Pérez-García, A. Shell Anomalies in the European Aquatic Stem Turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae). Diversity 2021, 13, 518. https://doi.org/10.3390/d13110518
Guerrero A, Pérez-García A. Shell Anomalies in the European Aquatic Stem Turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae). Diversity. 2021; 13(11):518. https://doi.org/10.3390/d13110518
Chicago/Turabian StyleGuerrero, Andrea, and Adán Pérez-García. 2021. "Shell Anomalies in the European Aquatic Stem Turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae)" Diversity 13, no. 11: 518. https://doi.org/10.3390/d13110518
APA StyleGuerrero, A., & Pérez-García, A. (2021). Shell Anomalies in the European Aquatic Stem Turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae). Diversity, 13(11), 518. https://doi.org/10.3390/d13110518