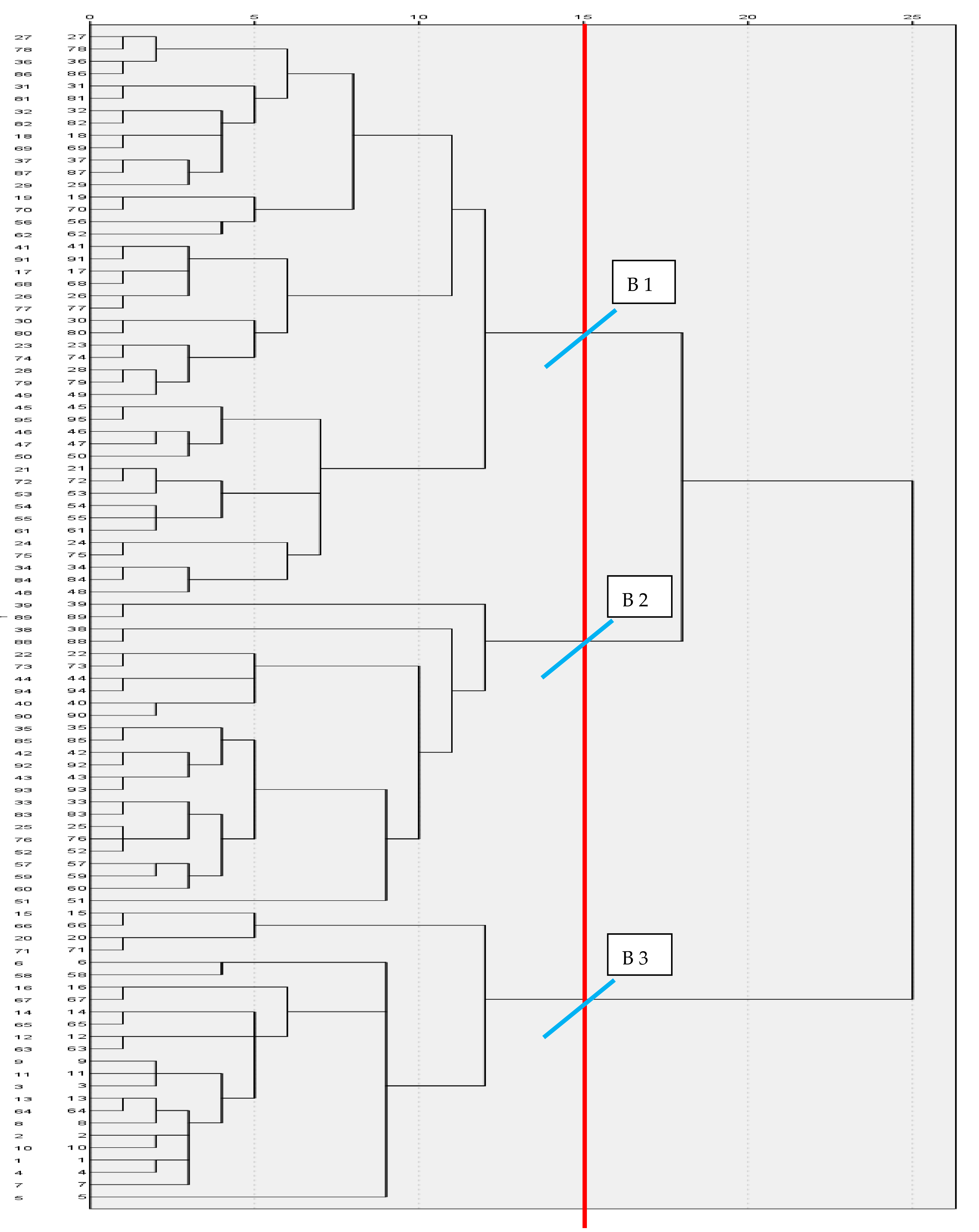
3.1. Byotipe Classification
In this study, three biotypes of Creole cows were differentiated: biotypes I, II, and III grouped 46, 20, and 29 cows, respectively (
Figure 2). The validation of biotypes differences by analysis of variance of hypothesis test, was highly significant (
p < 0.001), which showed that there were differences among biotypes (
Table S5). South American creole bovine has evolved by natural selection and adapted to different conditions such as humid tropical forests, subtropical dry forests, and mountainous and Patagonian steppe. Nowadays, most South and Central American countries have Creole bovine with specific characteristics (milk, meat, or dual purpose) with broad genetic diversity and phenotypic variability. Unfortunately, in recent years, there has been a drastic reduction in their population [
25,
26]. Similarly, four biotypes of Creole bovine were identified in the Patagonian (Northeast of Argentina), differentiating northern animals from the southern ones [
27].
Moreover, in another research Chilean Patagonian Creole bovines were grouped according to their meat or milk production characteristics [
28]. In our case, we found Creole cows from the Amazonas region that have a slight inclination for meat and dual purpose (meat and milk). Same as ours, three Creole bovine biotypes from Argentina showed differences in head width, head length, thoracic perimeter, total length, anterior rump width, and rump length [
29].
Highly significant differences were found between biotypes found for quantitative traits. Biotype I has greater teats length, fore udder length, central ligament, and heels. Biotype III is characterized by its superiority in body depth, back length, teats thickness, and pelvic tilt with respect to other biotypes (
Table 1). Udder depth and body condition did not influence the clusters formation, so the values of these traits were not significantly different among groups.
We found four significant and highly significant associations of qualitative traits according to identified biotypes (
Table 2). A greater number of biotype I cows have a simple coloration pattern, while in biotype III there is a greater presence of compound color. Creole bovines are characterized by peculiar morphometry and morphology, such as coat color diversity and large horns [
30]. Although it is true in this study, the horns’ length was not measured, they were observed in 100% of the population, which agrees with the reports for Uruguayan bovine [
30].
Front teat placement was significantly associated with biotypes (
p < 0.01), biotype II is characterized by slightly protruding teats, biotype III by centered teats, and biotype I by slightly inwardly tucked teats. Rear teat orientation is also significantly associated with biotypes, biotype II presents inside teats and biotype I presents slightly tucked teats. Hock cleanliness was significantly associated with biotypes, where biotype II presents slightly undefined hock cleanliness and biotype III presents clear hock (
Table 2).
The description of defects associated with biotypes is detailed in
Table 3. Backline impressed, kidney impressed, rump arched, and rolled hoof are associated with biotype III bovines; and chest narrow is associated with biotype II bovines.
Based on
Table 4 resulting from multivariate analysis, the variables can be reduced to six factors or components and explained total accumulative variance of 92.1%. The first component explained 49%, 18% for the second component, 9% for the third component, 6.5% for the fourth component, 5% for the fifth component, and 4% for the sixth component.
Correlation matrix of principal components is detailed in
Table 5. The first component highly correlated with fore udder length and udder depth. For the second component, traits with the highest correlation are hip width and rump length. The third component correlates highly with raised to sacrum and rump arched, but body depth shows negative and high correlation. Fourth component correlates highly with hock angle and pelvic tilt, back length, and udder depth, and fifth component correlates highly with coat color and coloring pattern. Lastly, sixth component is highly correlated with back length and pasterns.
According to
Table 5, biotype III can be explained up to 67% by traits of components 1 and 3, biotype II is explained up to 66% by traits of components 1, 2, and 6 and the biotype I can be explained up to 67% by traits of components 1, 4. and 5.
3.2. Age and Lactation of Creole Cow
According to
https://www.fleckscore.com/ (accessed on 18 November 2020), the age of Creole cows was significantly associated (
p < 0.01) with muscularity, fore udder attachment, and front teats placement (
Table 6). As expected, more than 60% of six- and eight- teeth cows and more than 40% of full-mouth cows had straight muscularity. More than 65% of six-teeth cows and more than 50% of eight-teeth cows had a fore udder attachment of 30–40°. Furthermore, more than 40% of full-mouth cows had a fore udder attachment of 40–50°. Finally, 38.7% and 48.8% of eight-teeth and full-mouth cows had centered teats, respectively.
Table 4 shows associations of qualitative zoometric traits according to number of lactations. A more significant number of animals with slightly protruding, centered and inward teats were found in second lactation Creole cows (
p < 0.01), and more than 60% of third-lactating cows had front teats placement slightly inward. Among the regions of western hemisphere, the characteristics of milking ease, stature, and body condition of the Creole cows differ significantly. Compared to animals initially imported, these differences are due to time, natural selection, and breeding preferences of local breeders [
31]. Non-significant associations according to age and lactation are detailed in
Table S6).
The Creole cows’ age is significantly associated with absence of defects such as chest narrow, loosely shoulders and front legs distorted (
p < 0.05) (
Table 7 and
Table S7). More than 50% of six-teeth cows did not have chest narrow defect. In the same way, more than 70% and 85% of eight-teeth animals and full-mouth animals did not have chest narrow defect. Moreover, more than 60% of six-teeth animals and full-mouth and more than 85% of eight-teeth cows did not present loosely shoulder defect. Finally, 100% of eight-teeth and a full-mouth and more than 90% of Creole cows do not have front legs distorted defect (
p < 0.05). On the other hand, all defects were seen in all animals in different percentages.
The presence of defects in Creole cows could be due to absence of selection programs, and also because these animals are displaced to harsh areas with predomination of woody plants, cacti, and native pastures with low levels of nutrients [
32,
33]. Such is the case of creole cattle within Copper Canyon in Chihuahua, Mexico, isolated by the unlimited road infrastructure. Isolation caused groups with similar characteristics within groups, but different among groups, originating an adaptation process. It was reported that Creole cows show ease of adaptation and survival to harsh conditions, and they can survive with high stress and low nutrients levels [
34]. Moreover, all these environmental characteristics cause defects such as short stature or irregular conformation, among others [
35]. Lactation number was not associated (
p > 0.05) with any of structural defect traits (
Table S7).
From fourteen quantitative traits evaluated, only rear udder length was significantly different among ages (
p < 0.05), being higher in six-teeth cows than eight-teeth and full-mouth cows (
Table 8 and
Table S8). The height at cross (125 cm), body depth (70 cm), hip width (45.6), back length (89 cm), and rump length (45 cm) were similar among ages. Research about zoometric evaluation of Creole bovine from Áncash region reported 115 cm in height at withers [
36], similar to Creole bovine from Puno region [
37] with height 118 cm. Creole cows from Amazonas region has height at cross 125.2 cm, similar to six-teeth and full-mouth Creole cows (122 cm and 124 cm; respectively) from Puno region [
37]. Hip width (45.6 cm) and back length (89.1 cm) of Creole cows are higher than hip width and height at withers of Creole bulls of Mixtec region of Mexico (32.8 cm and 66.1 cm, respectively) [
7]. Rump length was similar to Chinampo Creole bovine from Mexico (45 cm) [
8], and six-teeth and full-mouth (43 cm) in Creole bovine from the Puno region [
37]. Quantitative traits are necessary for biotype classification, such as hip width and length, which facilitate easy calving and potential winning of muscle mass [
38].
Differences between Creole cows of Amazonas region and Creole bovines from other origins could be due to agroclimatic and nutritional factors. Moreover, phenotypic differences between bovines of different latitudes and altitudes could be due to environmental conditions, breeding purpose, functionality, distances among groups, and adaptation to the agroclimatic factors [
5,
39].
Regarding the mammary system, Creole cows from Amazonas region have averages of 5.7, 2.7, 14.1, 18.4, and 2.2 cm for teat length, teat thickness, fore udder length, udder depth, and central ligament, respectively. Hoof height varied from 2.6 to 2.8 cm, rump angle was 5 cm, and body condition of 2.7 points (BC2). This poor body condition was due to limited technical management and food quality intake, leading to low daily weight gains [
12]. A body condition score from 1 to 2.5 is associated with parasites accumulators; meanwhile, animals with body condition score higher than 3 points are considered resilient to diseases [
40].
According to lactations number, differences between hip width and rump length were significant (
p < 0.05). Cows with more than two lactations have 2 cm wider hip than first-lactating cows (
Table 8). Similar phenomenon happens when evaluating rump length. Cows with more than two lactations have greater rump length than first-lactating cows (
p < 0.05). Regarding the hip width, Mixtec bovine has average hip width of 32.82 cm [
7], lower than Creole cows from Amazonas region; this is possible because of different phenotypes and genetic variations [
41]. Differences could also be due to natural selection that bovines have undergone; natural selection of bovine with more environmental adaptation skills to wild areas and rough conditions is essential for survival [
42,
43].
After evaluating the effect of age and lactations number of Creole cows on qualitative and quantitative traits, we determined their association with the classified biotypes through cluster analysis. We found no significant association between age and biotype or lactations number with biotype (
Table 9), which supports the hypothesis that our classification of Creole cow biotypes from the Amazon region does not necessarily correspond to these two factors.