Habitat Suitability Modeling to Inform Seascape Connectivity Conservation and Management
Abstract
:1. Introduction
2. Materials and Methods
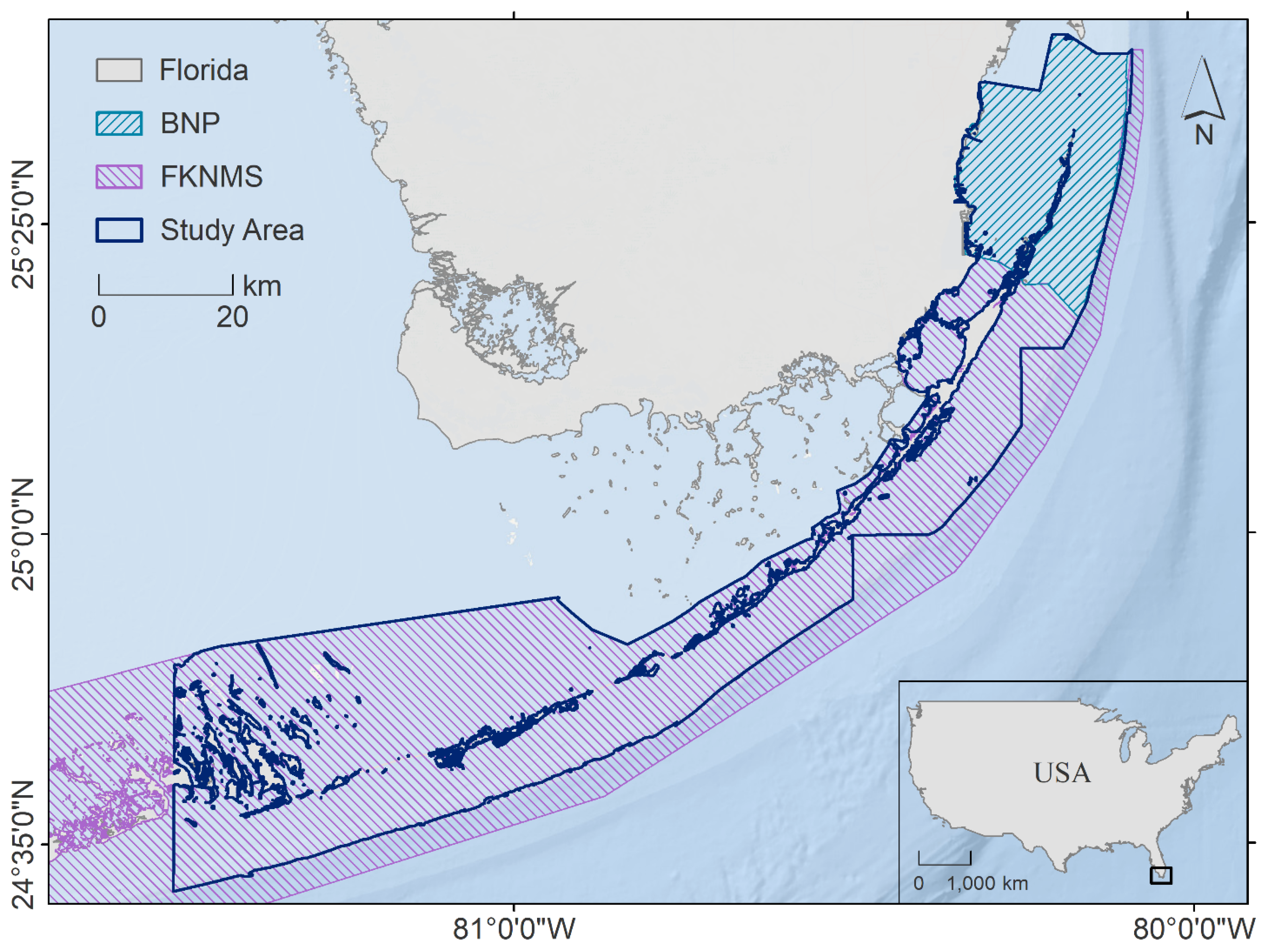
2.1. Study Area
2.2. Focal Species
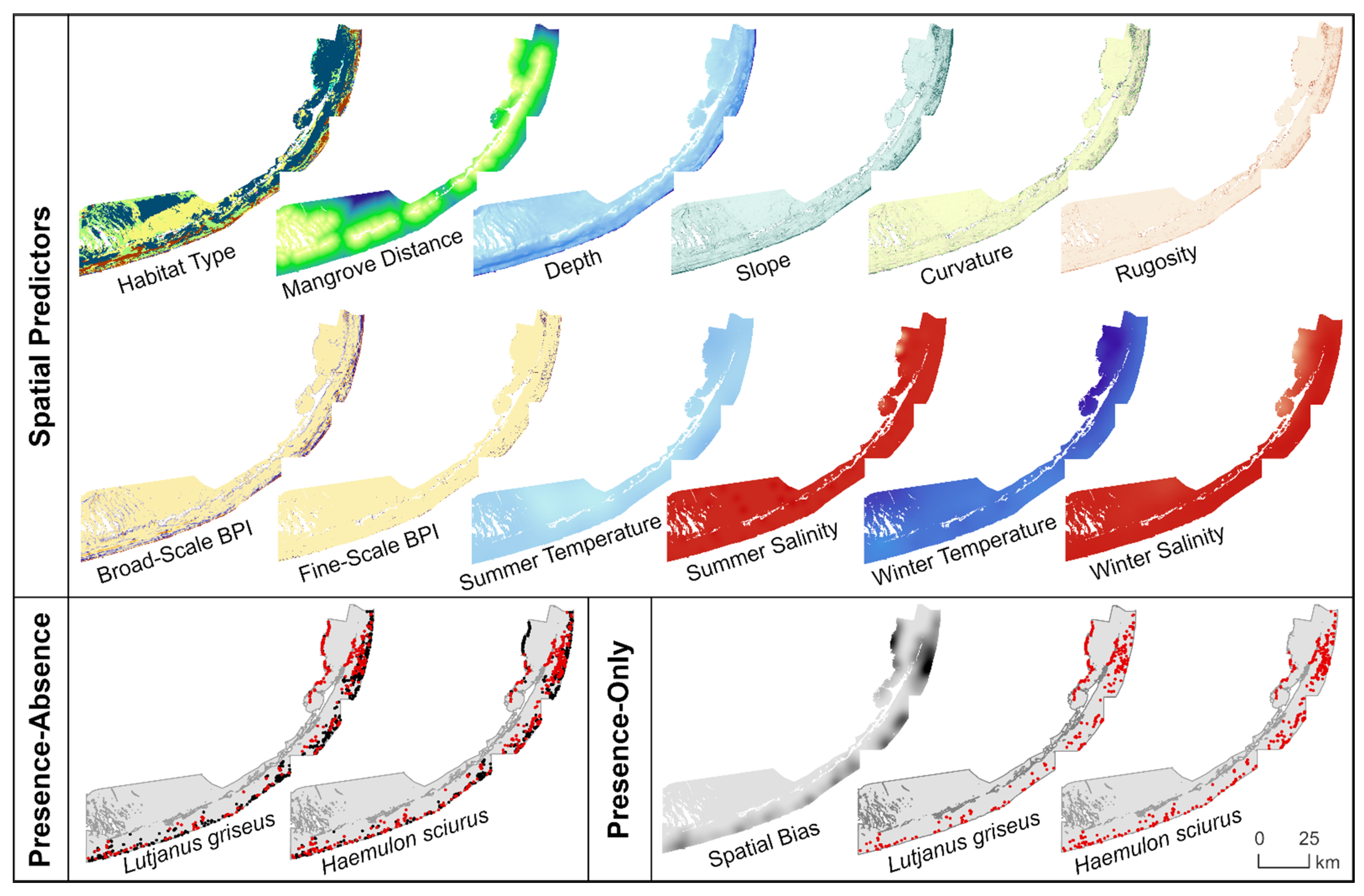
2.3. Spatial Predictors
2.3.1. Benthic Habitat
2.3.2. Bathymetry and Seafloor Morphology
2.3.3. Water Conditions
2.4. Model Development
2.4.1. Filtering and Partitioning of Occurrence Records
2.4.2. Selection of Spatial Predictors
2.4.3. Penalized Logistic Regressions
2.4.4. MaxEnt Models
2.5. Model Assessment
2.5.1. Discriminatory Ability
2.5.2. Binary Predictive Performance
2.5.3. Variable Importance
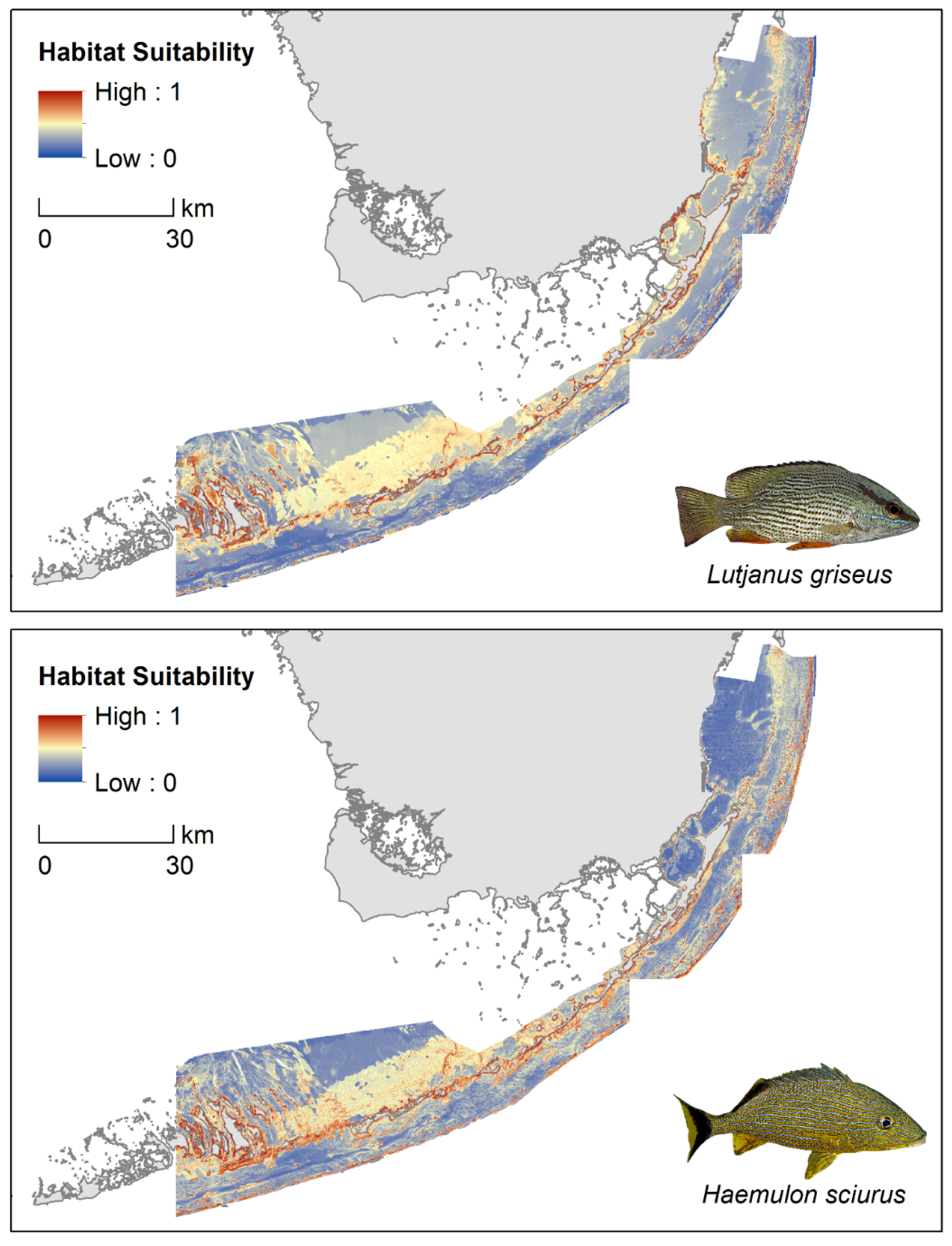
3. Results
3.1. Discriminatory Ability
3.2. Binary Predictive Performance
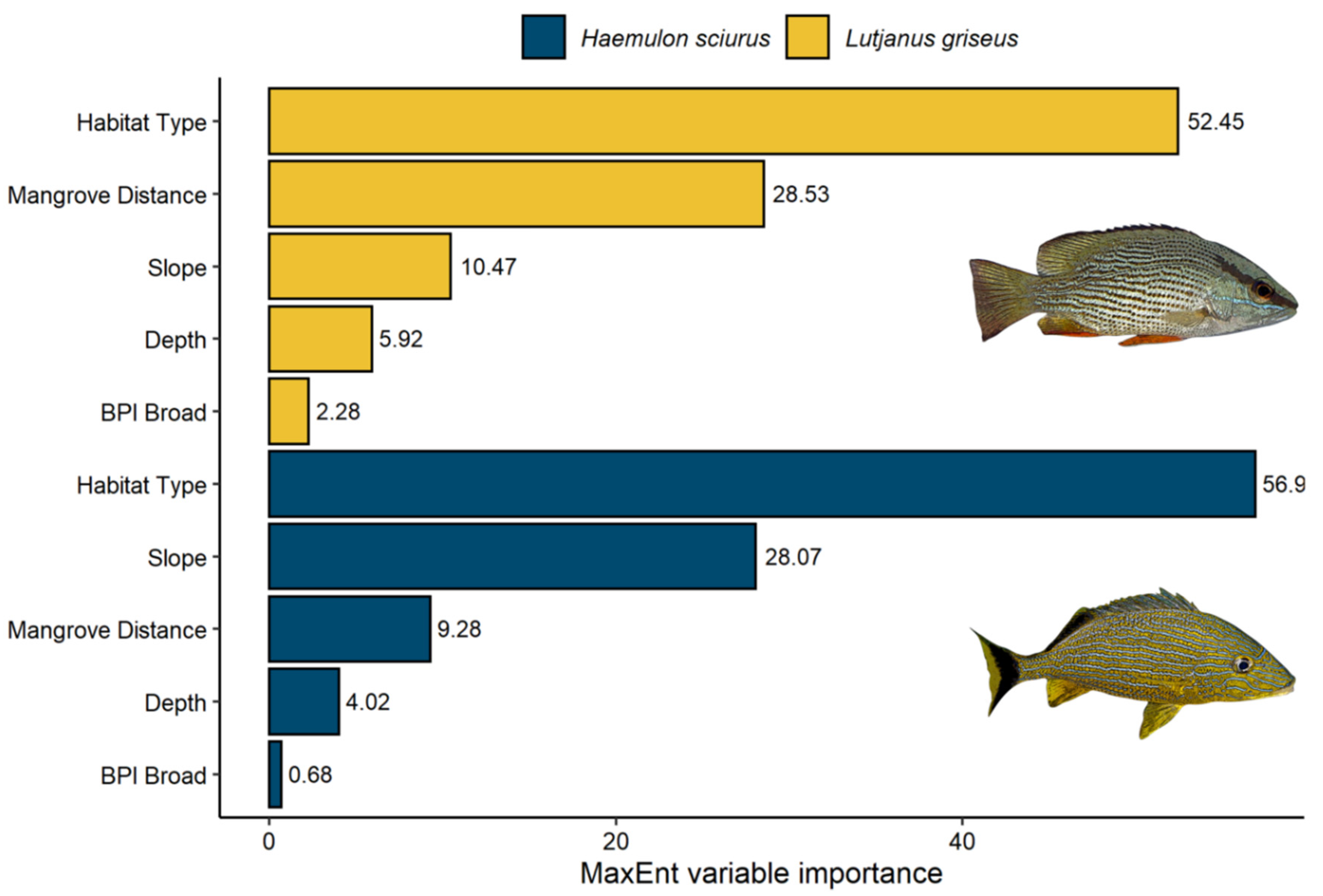
3.3. Variable Importance
4. Discussion
4.1. Model Performance
4.2. Variable Importance
4.3. Implications for Seascape Connectivity Modeling and Conservation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, C.E.; Glegg, G.A.; Howell, K.L. Species Distribution Modelling to Support Marine Conservation Planning: The next Steps. Mar. Policy 2014, 45, 330–332. [Google Scholar] [CrossRef]
- Villero, D.; Pla, M.; Camps, D.; Ruiz-Olmo, J.; Brotons, L. Integrating Species Distribution Modelling into Decision-Making to Inform Conservation Actions. Biodivers. Conserv. 2017, 26, 251–271. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine Heatwaves Threaten Global Biodiversity and the Provision of Ecosystem Services. Nat. Clim. Chang. 2019, 9, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A Review of the Combined Effects of Climate Change and Other Local Human Stressors on the Marine Environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef] [PubMed]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating Loss of Seagrasses across the Globe Threatens Coastal Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-Term Region-Wide Declines in Caribbean Corals. Science 2003, 301, 958–960. [Google Scholar] [CrossRef] [Green Version]
- Deegan, L.A.; Johnson, D.S.; Warren, R.S.; Peterson, B.J.; Fleeger, J.W.; Fagherazzi, S.; Wollheim, W.M. Coastal Eutrophication as a Driver of Salt Marsh Loss. Nature 2012, 490, 388–392. [Google Scholar] [CrossRef]
- Duffy, J.E.; Lefcheck, J.S.; Stuart-Smith, R.D.; Navarrete, S.A.; Edgar, G.J. Biodiversity Enhances Reef Fish Biomass and Resistance to Climate Change. Proc. Natl. Acad. Sci. USA 2016, 113, 6230–6235. [Google Scholar] [CrossRef] [Green Version]
- Benkwitt, C.E.; Wilson, S.K.; Graham, N.A.J. Biodiversity Increases Ecosystem Functions despite Multiple Stressors on Coral Reefs. Nat. Ecol. Evol. 2020, 4, 919–926. [Google Scholar] [CrossRef]
- Kennedy, E.V.; Perry, C.T.; Halloran, P.R.; Iglesias-Prieto, R.; Schönberg, C.H.L.; Wisshak, M.; Form, A.U.; Carricart-Ganivet, J.P.; Fine, M.; Eakin, C.M.; et al. Avoiding Coral Reef Functional Collapse Requires Local and Global Action. Curr. Biol. 2013, 23, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Waltham, N.J.; Elliott, M.; Lee, S.Y.; Lovelock, C.; Duarte, C.M.; Buelow, C.; Simenstad, C.; Nagelkerken, I.; Claassens, L.; Wen, C.K.-C.; et al. UN Decade on Ecosystem Restoration 2021–2030—What Chance for Success in Restoring Coastal Ecosystems? Front. Mar. Sci. 2020, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Olds, A.D.; Connolly, R.M.; Pitt, K.A.; Pittman, S.J.; Maxwell, P.S.; Huijbers, C.M.; Moore, B.R.; Albert, S.; Rissik, D.; Babcock, R.C.; et al. Quantifying the Conservation Value of Seascape Connectivity: A Global Synthesis. Glob. Ecol. Biogeogr. 2016, 25, 3–15. [Google Scholar] [CrossRef]
- Carr, M.H.; Robinson, S.P.; Wahle, C.; Davis, G.; Kroll, S.; Murray, S.; Schumacker, E.J.; Williams, M. The Central Importance of Ecological Spatial Connectivity to Effective Coastal Marine Protected Areas and to Meeting the Challenges of Climate Change in the Marine Environment. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 6–29. [Google Scholar] [CrossRef] [Green Version]
- Weeks, R. Incorporating Seascape Connectivity in Conservation Prioritisation. PLoS ONE 2017, 12, e0182396. [Google Scholar] [CrossRef] [PubMed]
- Balbar, A.C.; Metaxas, A. The Current Application of Ecological Connectivity in the Design of Marine Protected Areas. Glob. Ecol. Conserv. 2019, 17, e00569. [Google Scholar] [CrossRef]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity Is a Vital Element of Landscape Structure. Oikos 1993, 68, 571–573. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.D.; Fahrig, L.; With, K.A. Landscape connectivity: A return to the basics. In Connectivity Conservation; Crooks, K.R., Sanjayan, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 29–43. ISBN 978-0-511-75482-1. [Google Scholar]
- Sambrook, K.; Hoey, A.S.; Andréfouët, S.; Cumming, G.S.; Duce, S.; Bonin, M.C. Beyond the Reef: The Widespread Use of Non-Reef Habitats by Coral Reef Fishes. Fish. Fish. 2019, 20, 903–920. [Google Scholar] [CrossRef]
- Grober-Dunsmore, R.; Pittman, S.J.; Caldow, C.; Kendall, M.S.; Frazer, T.K. A Landscape Ecology Approach for the Study of Ecological Connectivity Across Tropical Marine Seascapes. In Ecological Connectivity among Tropical Coastal Ecosystems; Nagelkerken, I., Ed.; Springer: Dordrecht, The Netherland, 2009; pp. 493–530. ISBN 978-90-481-2405-3. [Google Scholar]
- Tischendorf, L.; Fahrig, L. On the Usage and Measurement of Landscape Connectivity. Oikos 2000, 90, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Urban, D.; Keitt, T. Landscape Connectivity: A Graph Theoretic Perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- McRae, B.H.; Hall, S.A.; Beier, P.; Theobald, D.M. Where to Restore Ecological Connectivity? Detecting Barriers and Quantifying Restoration Benefits. PLoS ONE 2012, 7, e52604. [Google Scholar] [CrossRef] [PubMed]
- Daigle, R.M.; Metaxas, A.; Balbar, A.C.; McGowan, J.; Treml, E.A.; Kuempel, C.D.; Possingham, H.P.; Beger, M. Operationalizing Ecological Connectivity in Spatial Conservation Planning with Marxan Connect. Methods Ecol. Evol. 2020, 11, 570–579. [Google Scholar] [CrossRef] [Green Version]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Duflot, R.; Avon, C.; Roche, P.; Bergès, L. Combining Habitat Suitability Models and Spatial Graphs for More Effective Landscape Conservation Planning: An Applied Methodological Framework and a Species Case Study. J. Nat. Conserv. 2018, 46, 38–47. [Google Scholar] [CrossRef]
- Stevenson-Holt, C.D.; Watts, K.; Bellamy, C.C.; Nevin, O.T.; Ramsey, A.D. Defining Landscape Resistance Values in Least-Cost Connectivity Models for the Invasive Grey Squirrel: A Comparison of Approaches Using Expert-Opinion and Habitat Suitability Modelling. PLoS ONE 2014, 9, e112119. [Google Scholar] [CrossRef] [Green Version]
- Lepczyk, C.A.; Wedding, L.M.; Asner, G.P.; Pittman, S.J.; Goulden, T.; Linderman, M.A.; Gang, J.; Wright, R. Advancing Landscape and Seascape Ecology from a 2D to a 3D Science. BioScience 2021, 71, 596–608. [Google Scholar] [CrossRef]
- Wedding, L.M.; Jorgensen, S.; Lepczyk, C.A.; Friedlander, A.M. Remote Sensing of Three-Dimensional Coral Reef Structure Enhances Predictive Modeling of Fish Assemblages. Remote Sens. Ecol. Conserv. 2019, 5, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Poor, E.E.; Loucks, C.; Jakes, A.; Urban, D.L. Comparing Habitat Suitability and Connectivity Modeling Methods for Conserving Pronghorn Migrations. PLoS ONE 2012, 7, e49390. [Google Scholar] [CrossRef]
- Penjor, U.; Kaszta, Ż.; Macdonald, D.W.; Cushman, S.A. Prioritizing Areas for Conservation Outside the Existing Protected Area Network in Bhutan: The Use of Multi-Species, Multi-Scale Habitat Suitability Models. Landsc. Ecol. 2021, 36, 1281–1309. [Google Scholar] [CrossRef]
- Green, E.P.; Mumby, P.J.; Edwards, A.J.; Clark, C.D. A Review of Remote Sensing for the Assessment and Management of Tropical Coastal Resources. Coast. Manag. 1996, 24, 1–40. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. Evaluating the Predictive Performance of Habitat Models Developed Using Logistic Regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is My Species Distribution Model Fit for Purpose? Matching Data and Models to Applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.; Barrett, B.; Burbidge, A. Dealing with Uncertain Absences in Habitat Modelling: A Case Study of a Rare Ground-Dwelling Parrot. Divers. Distrib. 2007, 13, 704–713. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Brotons, L.; Peterson, A.T.; Thuiller, W. Consensual Predictions of Potential Distributional Areas for Invasive Species: A Case Study of Argentine Ants in the Iberian Peninsula. Biol. Invasions 2009, 11, 1017–1031. [Google Scholar] [CrossRef]
- Marini, M.Â.; Barbet-Massin, M.; Lopes, L.E.; Jiguet, F. Predicting the Occurrence of Rare Brazilian Birds with Species Distribution Models. J. Ornithol. 2010, 151, 857–866. [Google Scholar] [CrossRef]
- Ogden, J.C.; Porter, J.W.; Smith, N.P.; Szmant, A.M.; Jaap, W.C.; Forcucci, D. A Long-Term Interdisciplinary Study of the Florida Keys Seascape. Bull. Mar. Sci. 1994, 54, 1059–1071. [Google Scholar]
- NOAA Fisheries Restoring Seven Iconic Reefs: A Mission to Recover the Coral Reefs of the Florida Keys; Iconic Reefs–Summary: Mission. Available online: https://www.fisheries.noaa.gov/southeast/habitat-conservation/restoring-seven-iconic-reefs-mission-recover-coral-reefs-florida-keys (accessed on 18 May 2020).
- Mumby, P.J.; Hastings, A. The Impact of Ecosystem Connectivity on Coral Reef Resilience. J. Appl. Ecol. 2008, 45, 854–862. [Google Scholar] [CrossRef]
- Ladd, M.C.; Miller, M.W.; Hunt, J.H.; Sharp, W.C.; Burkepile, D.E. Harnessing Ecological Processes to Facilitate Coral Restoration. Front. Ecol. Environ. 2018, 16, 239–247. [Google Scholar] [CrossRef]
- Ladd, M.C.; Shantz, A.A. Trophic Interactions in Coral Reef Restoration: A Review. Food Webs 2020, 24, e00149. [Google Scholar] [CrossRef]
- Meyer, J.L.; Schultz, E.T. Tissue Condition and Growth Rate of Corals Associated with Schooling Fish1. Limnol. Oceanogr. 1985, 30, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, S.J.; Brooks, A.J.; Schmitt, R.J.; Stewart, H.L. Effects of Sheltering Fish on Growth of Their Host Corals. Mar. Biol. 2008, 155, 521–530. [Google Scholar] [CrossRef]
- Shantz, A.A.; Ladd, M.C.; Schrack, E.; Burkepile, D.E. Fish-Derived Nutrient Hotspots Shape Coral Reef Benthic Communities. Ecol. Appl. 2015, 25, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerken, I.; Dorenbosch, M.; Verberk, W.; Cocheret de la Morinière, E.; van der Velde, G. Importance of Shallow-Water Biotopes of a Caribbean Bay for Juvenile Coral Reef Fishes: Patterns in Biotope Association, Community Structure and Spatial Distribution. Mar. Ecol. Prog. Ser. 2000, 202, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Nagelkerken, I.; Sheaves, M.; Baker, R.; Connolly, R.M. The Seascape Nursery: A Novel Spatial Approach to Identify and Manage Nurseries for Coastal Marine Fauna. Fish. Fish. 2015, 16, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Faunce, C.H.; Serafy, J.E. Nearshore Habitat Use by Gray Snapper (Lutjanus griseus) and Bluestriped Grunt (Haemulon sciurus): Environmental Gradients and Ontogenetic Shifts. Bull. Mar. Sci. 2007, 80, 473–495. [Google Scholar]
- Faunce, C.H.; Serafy, J.E. Selective Use of Mangrove Shorelines by Snappers, Grunts, and Great Barracuda. Mar. Ecol. Prog. Ser. 2008, 356, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Serafy, J.E.; Lindeman, K.C.; Hopkins, T.E.; Ault, J.S. Effects of Freshwater Canal Discharge on Fish Assemblages in a Subtropical Bay: Field and Laboratory Observations. Mar. Ecol. Prog. Ser. 1997, 160, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Cocheret de la Morinière, E.; Pollux, B.J.A.; Nagelkerken, I.; van der Velde, G. Diet Shifts of Caribbean Grunts (Haemulidae) and Snappers (Lutjanidae) and the Relation with Nursery-to-Coral Reef Migrations. Estuar. Coast. Shelf Sci. 2003, 57, 1079–1089. [Google Scholar] [CrossRef]
- Nemeth, R.S. Dynamics of Reef Fish and Decapod Crustacean Spawning Aggregations: Underlying Mechanisms, Habitat Linkages, and Trophic Interactions. In Ecological Connectivity among Tropical Coastal Ecosystems; Nagelkerken, I., Ed.; Springer: Dordrecht, The Netherland, 2009; pp. 73–134. ISBN 978-90-481-2406-0. [Google Scholar]
- Ault, J.S.; Bohnsack, J.A.; Smith, S.G.; Luo, J. Towards Sustainable Multispecies Fisheries in the Florida, USA, Coral Reef Ecosystem. Bull. Mar. Sci. 2005, 76, 595–622. [Google Scholar]
- Harper, D.E.; Bohnsack, J.A.; Lockwood, B.R. Recreational Fisheries in Biscayne National Park, Florida, 1976–19. Mar. Fish. Rev. 2000, 62, 8–26. [Google Scholar]
- Bohnsack, J.A.; Bannerot, S.P. A Stationary Visual Census Technique for Quantitatively Assessing Community Structure of Coral Reef Fishes. Available online: http://spo.nwr.noaa.gov/tr41.pdf (accessed on 18 February 2021).
- Serafy, J.E.; Faunce, C.H.; Lorenz, J.J. Mangrove Shoreline Fishes of Biscayne Bay, Florida. Bull. Mar. Sci. 2003, 72, 161–180. [Google Scholar]
- Serafy, J.E.; Teare, B.P. Mangrove Study Examining Seasonal and Spatial Variation in Fish Taxonomic Composition and Diversity in North Atlantic Ocean from 1998-08-17 to 2019-08-12 (NCEI Accession 0159580). [Southern Biscayne Bay, Card Sound, Barnes Sound]. Available online: https://www.ncei.noaa.gov/archive/accession/0159580 (accessed on 30 September 2020).
- FWRI Unified Florida Coral Reef Tract Map v2.0. Available online: https://myfwc.com/research/gis/regional-projects/unified-reef-map/ (accessed on 18 May 2020).
- FWRI Mangrove Habitat in Florida. Available online: https://geodata.myfwc.com/datasets/a78a27e02f9d4a71a3c3357aefc35baf_4?geometry=-95.440%2C23.806%2C-67.689%2C30.638 (accessed on 18 May 2020).
- Hijmans, R.J. raster: Geographic Data Analysis and Modeling; R package version 3.3-13. Available online: https://CRAN.R-project.org/package=raster (accessed on 18 May 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- CIRES; NOAA-NCEI. Continuously Updated Digital Elevation Model (CUDEM)-1/9 Arc-Second Resolution Bathymetric-Topographic Tiles [Florida and East Gulf of Mexico]. Available online: https://www.ncei.noaa.gov/metadata/geoportal/rest/metadata/item/gov.noaa.ngdc.mgg.dem:999919/html (accessed on 18 May 2020).
- Wright, D.J.; Pendleton, M.; Boulware, J.; Walbridge, S.; Gerlt, B.; Eslinger, D.; Sampson, D.; Huntley, E. ArcGIS Benthic Terrain Modeler (BTM), v. 3.0, Environmental Systems Research Institute, NOAA Coastal Services Center, Massachusetts Office of Coastal Zone Management. Available online: http://esriurl.com/5754 (accessed on 18 May 2020).
- Walbridge, S.; Slocum, N.; Pobuda, M.; Wright, D.J. Unified Geomorphological Analysis Workflows with Benthic Terrain Modeler. Geosciences 2018, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Pittman, S.J.; Brown, K.A. Multi-Scale Approach for Predicting Fish Species Distributions across Coral Reef Seascapes. PLoS ONE 2011, 6, e20583. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Taylor, J.C.; Kracker, L.; Battista, T.; Pittman, S. Mapping Reef Fish and the Seascape: Using Acoustics and Spatial Modeling to Guide Coastal Management. PLoS ONE 2014, 9, e85555. [Google Scholar]
- Wedding, L.M.; Friedlander, A.M. Determining the Influence of Seascape Structure on Coral Reef Fishes in Hawaii Using a Geospatial Approach. Mar. Geod. 2008, 31, 246–266. [Google Scholar] [CrossRef]
- Stamoulis, K.A.; Delevaux, J.M.S.; Williams, I.D.; Poti, M.; Lecky, J.; Costa, B.; Kendall, M.S.; Pittman, S.J.; Donovan, M.K.; Wedding, L.M.; et al. Seascape Models Reveal Places to Focus Coastal Fisheries Management. Ecol. Appl. 2018, 28, 910–925. [Google Scholar] [CrossRef]
- Borland, H.P.; Gilby, B.L.; Henderson, C.J.; Leon, J.X.; Schlacher, T.A.; Connolly, R.M.; Pittman, S.J.; Sheaves, M.; Olds, A.D. The Influence of Seafloor Terrain on Fish and Fisheries: A Global Synthesis. Fish. Fish. 2021, 22, 707–734. [Google Scholar] [CrossRef]
- Pebesma, E.J. Multivariable Geostatistics in S: The Gstat Package. Comput. Geosci. 2004, 30, 683–691. [Google Scholar] [CrossRef]
- Munro, J.L.; Gaut, V.C.; Thompson, R.; Reeson, P.H. The Spawning Seasons of Caribbean Reef Fishes. J. Fish. Biol. 1973, 5, 69–84. [Google Scholar] [CrossRef]
- Domeier, M.L.; Koenig, C.; Coleman, F. Reproductive biology of the gray snapper (Lutjanus griseus), with notes on spawning for other western Atlantic snappers (Lutjanidae). In Biology, Fisheries and Culture of Tropical Snappers and Groupers; ICLARM Conference Proceedings; Arreguin-Sanchez, F., Munro, J.L., Balgos, M.C., Pauly, D., Eds.; ICLARM: Manila, Philippines, 1996; Volume 48, pp. 189–201. [Google Scholar]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G.; Vining, G.G. Introduction to Linear Regression Analysis; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2012; ISBN 978-1-118-62736-5. [Google Scholar]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 22. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M. Caret: Classification and Regression Training; R Package Version 6.0-86. Available online: https://CRAN.R-project.org/package=caret (accessed on 18 May 2020).
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions, (Version 3.4.1). Available online: https://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 18 May 2020).
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists: Statistical Explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Anderson, R.P.; Gonzalez, I. Species-Specific Tuning Increases Robustness to Sampling Bias in Models of Species Distributions: An Implementation with Maxent. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM Eval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample Selection Bias and Presence-Only Distribution Models: Implications for Background and Pseudo-Absence Data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping Species Distributions with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398, ISBN 0-470-58247-2. [Google Scholar]
- Liu, C.; White, M.; Newell, G. Selecting Thresholds for the Prediction of Species Occurrence with Presence-Only Data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Gastón, A.; García-Viñas, J.I. Modelling Species Distributions with Penalised Logistic Regressions: A Comparison with Maximum Entropy Models. Ecol. Model. 2011, 222, 2037–2041. [Google Scholar] [CrossRef]
- Monk, J.; Ierodiaconou, D.; Versace, V.; Bellgrove, A.; Harvey, E.; Rattray, A.; Laurenson, L.; Quinn, G. Habitat Suitability for Marine Fishes Using Presence-Only Modelling and Multibeam Sonar. Mar. Ecol. Prog. Ser. 2010, 420, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Nagelkerken, I.; Dorenbosch, M.; Verberk, W.; Cocheret de la Morinière, E.; van der Velde, G. Day-Night Shifts of Fishes between Shallow-Water Biotopes of a Caribbean Bay, with Emphasis on the Nocturnal Feeding of Haemulidae and Lutjanidae. Mar. Ecol. Prog. Ser. 2000, 194, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Serafy, J.E.; Sponaugle, S.; Teare, P.B.; Kieckbusch, D. Movement of Gray Snapper Lutjanus griseus among Subtropical Seagrass, Mangrove, and Coral Reef Habitats. Mar. Ecol. Prog. Ser. 2009, 380, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Huijbers, C.M.; Nagelkerken, I.; Debrot, A.O.; Jongejans, E. Geographic Coupling of Juvenile and Adult Habitat Shapes Spatial Population Dynamics of a Coral Reef Fish. Ecology 2013, 94, 1859–1870. [Google Scholar] [CrossRef] [Green Version]
- Nagelkerken, I.; Huebert, K.B.; Serafy, J.E.; Grol, M.G.G.; Dorenbosch, M.; Bradshaw, C.J.A. Highly Localized Replenishment of Coral Reef Fish Populations near Nursery Habitats. Mar. Ecol. Prog. Ser. 2017, 568, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Pittman, S.; McAlpine, C.; Pittman, K. Linking Fish and Prawns to Their Environment: A Hierarchical Landscape Approach. Mar. Ecol. Prog. Ser. 2004, 283, 233–254. [Google Scholar] [CrossRef]
- Grober-Dunsmore, R.; Frazer, T.K.; Beets, J.P.; Lindberg, W.J.; Zwick, P.; Funicelli, N.A. Influence of Landscape Structure on Reef Fish Assemblages. Landsc. Ecol. 2008, 23, 37–53. [Google Scholar] [CrossRef]
- Green, A.L.; Maypa, A.P.; Almany, G.R.; Rhodes, K.L.; Weeks, R.; Abesamis, R.A.; Gleason, M.G.; Mumby, P.J.; White, A.T. Larval Dispersal and Movement Patterns of Coral Reef Fishes, and Implications for Marine Reserve Network Design. Biol. Rev. 2015, 90, 1215–1247. [Google Scholar] [CrossRef]
- Shideler, G.S.; Araújo, R.J.; Walker, B.K.; Blondeau, J.; Serafy, J.E. Non-Linear Thresholds Characterize the Relationship between Reef Fishes and Mangrove Habitat. Ecosphere 2017, 8, e01943. [Google Scholar] [CrossRef] [Green Version]
- Serrano, X.; Grosell, M.; Serafy, J.E. Salinity Selection and Preference of the Grey Snapper Lutjanus griseus: Field and Laboratory Observations. J. Fish. Biol. 2010, 76, 1592–1608. [Google Scholar] [CrossRef]
- Wuenschel, M.J.; Jugovich, A.R.; Hare, J.A. Effect of Temperature and Salinity on the Energetics of Juvenile Gray Snapper (Lutjanus griseus): Implications for Nursery Habitat Value. J. Exp. Mar. Biol. Ecol. 2004, 312, 333–347. [Google Scholar] [CrossRef]
- Hare, J.A.; Able, K.W. Mechanistic Links between Climate and Fisheries along the East Coast of the United States: Explaining Population Outbursts of Atlantic Croaker (Micropogonias undulatus). Fish. Oceanogr. 2007, 16, 31–45. [Google Scholar] [CrossRef]
- Tolan, J.M.; Fisher, M. Biological Response to Changes in Climate Patterns: Population Increases of Gray Snapper (Lutjanus griseus) in Texas Bays and Estuaries. Fish. Bull. 2009, 107, 36–44. [Google Scholar]
- Almany, G.R.; Connolly, S.R.; Heath, D.D.; Hogan, J.D.; Jones, G.P.; McCook, L.J.; Mills, M.; Pressey, R.L.; Williamson, D.H. Connectivity, Biodiversity Conservation and the Design of Marine Reserve Networks for Coral Reefs. Coral Reefs 2009, 28, 339–351. [Google Scholar] [CrossRef]
- Gilby, B.L.; Olds, A.D.; Connolly, R.M.; Henderson, C.J.; Schlacher, T.A. Spatial Restoration Ecology: Placing Restoration in a Landscape Context. Bioscience 2018, 68, 1007–1019. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Maxent Is Not a Presence–Absence Method: A Comment on Thibaud et Al. Methods Ecol. Evol. 2014, 5, 1192–1197. [Google Scholar] [CrossRef]
- Franco, J.N.; Tuya, F.; Bertocci, I.; Rodríguez, L.; Martínez, B.; Sousa-Pinto, I.; Arenas, F. The ‘Golden Kelp’ Laminaria ochroleuca under Global Change: Integrating Multiple Eco-Physiological Responses with Species Distribution Models. J. Ecol. 2018, 106, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Couce, E.; Ridgwell, A.; Hendy, E.J. Future Habitat Suitability for Coral Reef Ecosystems under Global Warming and Ocean Acidification. Glob. Chang. Biol. 2013, 19, 3592–3606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, P.J.; Wagner, D.; Fowle, H.A.; Poti, M.; Kinlan, B.; Georgian, S.E.; Cordes, E.E. Models of Habitat Suitability, Size, and Age-Class Structure for the Deep-Sea Black Coral Leiopathes glaberrima in the Gulf of Mexico. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 150, 218–228. [Google Scholar] [CrossRef]
| Predictor | Units | Description | Tool Used | |
|---|---|---|---|---|
| Habitat | Benthic Habitat | Categorical (12) | Bottom habitat type | Not Applicable |
| Distance to Mangrove | Meters | Euclidean distance to the nearest mangrove habitat | gridDistance function in the raster R package | |
| Bathymetry & Seafloor Morphology | Depth | Meters | Water depth in each cell | Not Applicable |
| Depth (Standard Deviation) | Meters | Local dispersion | Calculate Metrics tool in BTM | |
| Slope | Degrees | Rate of maximum change in depth | Slope tool in BTM | |
| Curvature | th of a meter, convex (+) or concave (−) | Second derivative of the bathymetric surface | Curvature tool in ArcGIS Spatial Analyst | |
| Plan Curvature | th of a meter, convex (+) or concave (−) | Curvature perpendicular to the direction of maximum slope | Curvature tool in ArcGIS Spatial Analyst | |
| Rugosity | Ratio | Local surface roughness calculated as the ratio of surface area to planar area | Surface Area to Planar Area (slope-corrected) tool in BTM | |
| Broad-Scale Bathymetric Position Index (BPI) | Ridge (+), Flat (0), or Valley (−) | Depth of a cell relative to its surroundings, evaluated using concentric rings of 125 m and 1250 m | Broad-Scale BPI tool in BTM (standardized) | |
| Fine-Scale BPI | Ridge (+), Flat (0), or Valley (−) | Depth of a cell relative to its surroundings, evaluated using concentric rings of 5 m and 125 m | Fine-Scale BPI tool in BTM (standardized) | |
| Water Conditions | Winter Temperature | Degrees Celsius | Mean winter (January–March) temperature | Krige function in the gstat R package |
| Winter Salinity | Practical Salinity Units | Mean winter (January–March) salinity | Krige function in the gstat R package | |
| Winter Dissolved Oxygen | Milligrams per Liter | Mean winter (January–March) dissolved oxygen | Krige function in the gstat R package | |
| Summer Temperature | Degrees Celsius | Mean summer (July–September) temperature | Krige function in the gstat R package | |
| Summer Salinity | Practical Salinity Units | Mean summer (July–September) salinity | Krige function in the gstat R package | |
| Summer Dissolved Oxygen | Milligrams per Liter | Mean summer (July–September) dissolved oxygen | Krige function in the gstat R package |
| Size (cm TL) | Presence Sites | Absence Sites | Total | |
|---|---|---|---|---|
| Calibration Data | ||||
| Lutjanus griseus | 9.51–24.71 | 378 | 1129 | 1507 |
| Haemulon sciurus | 11.90–25.33 | 447 | 1060 | 1507 |
| Evaluation Data | ||||
| Lutjanus griseus | 9.51–24.71 | 167 | 479 | 646 |
| Haemulon sciurus | 11.90–25.33 | 216 | 430 | 646 |
| Discrimination | Binary Performance (Standard) | Binary Performance (Max SSS) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | Cut-Off | Accuracy | Sensitivity | Specificity | Cut-Off | Accuracy | Sensitivity | Specificity | |
| Lutjanus griseus | |||||||||
| Lasso Regression | 0.74 | 0.5 | 77.9 | 32.7 | 93.3 | 0.28 | 69.4 | 73.9 | 67.8 |
| Ridge Regression | 0.74 | 0.5 | 77.7 | 32.7 | 93.1 | 0.24 | 65.5 | 80.0 | 60.5 |
| MaxEnt | 0.88 | 0.5 | 50.5 | 84.9 | 38.7 | 0.65 | 59.0 | 80.0 | 51.8 |
| Haemulon sciurus | |||||||||
| Lasso Regression | 0.76 | 0.5 | 73.2 | 37.5 | 91.2 | 0.32 | 72.8 | 71.3 | 73.5 |
| Ridge Regression | 0.75 | 0.5 | 74.2 | 35.7 | 93.5 | 0.32 | 72.8 | 70.8 | 73.7 |
| MaxEnt | 0.86 | 0.5 | 41.3 | 79.1 | 24.3 | 0.65 | 51.7 | 72.6 | 42.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuart, C.E.; Wedding, L.M.; Pittman, S.J.; Green, S.J. Habitat Suitability Modeling to Inform Seascape Connectivity Conservation and Management. Diversity 2021, 13, 465. https://doi.org/10.3390/d13100465
Stuart CE, Wedding LM, Pittman SJ, Green SJ. Habitat Suitability Modeling to Inform Seascape Connectivity Conservation and Management. Diversity. 2021; 13(10):465. https://doi.org/10.3390/d13100465
Chicago/Turabian StyleStuart, Courtney E., Lisa M. Wedding, Simon J. Pittman, and Stephanie J. Green. 2021. "Habitat Suitability Modeling to Inform Seascape Connectivity Conservation and Management" Diversity 13, no. 10: 465. https://doi.org/10.3390/d13100465
APA StyleStuart, C. E., Wedding, L. M., Pittman, S. J., & Green, S. J. (2021). Habitat Suitability Modeling to Inform Seascape Connectivity Conservation and Management. Diversity, 13(10), 465. https://doi.org/10.3390/d13100465







