Crustacea Decapoda from the Rhodes Island Area (Eastern Mediterranean): New Records and an Updated Checklist
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Work
3. Results
3.1. New Records and Updated Checklist
3.2. Systematics of Species with Specimens Examined
- Family ARISTEIDAE Wood-Mason in Wood-Mason & Alcock, 1891
- Aristaeomorpha foliacea (Risso, 1827) *
- Aristaeomorpha foliacea—Zariquiey Álvarez [22]: 42, Figures 22a,b and 24a.
- Material examined: T3, 600–650 m (BT), 8.xi.2019: 3♂ CL 32.1–38.2 mm, 6♀ CL 34.3–55.9 mm; T4, 650–750 m (BT), 19.xi.2019: 2♂ CL 30.7–31.4 mm, 3♀ CL 27.6–54.8 mm.
- Remarks: A cosmopolitan species known from Indo-Pacific, western and eastern Atlantic, and Mediterranean [58]. This highly prized shrimp is the target of commercial trawlers fishing off Rhodes.
- Aristeus antennatus (Risso, 1816) *
- Aristeus antennatus—Zariquiey Álvarez [22]: 46, Figures 17b, 22c,d and 23a–c.
- Material examined: T3, 600–650 m (BT), 8.xi.2019: 1♂ CL 29.3 mm, 9♀ CL 27–53.6 mm; T4, 650–750 m (BT), 19.xi.2019: 8♂ CL 19.1–32.2 mm, 7♀ CL 18–49.5 mm.
- Remarks: Known from Indian Ocean, eastern Atlantic, and Mediterranean [58]. This highly prized shrimp is the target of commercial trawlers fishing off Rhodes.
- Family PENAEIDAE Rafinesque, 1815
- Penaeus aztecus Ives, 1891—(A)
- Farfantepenaeus aztecus (Ives, 1891)—Deval et al. [59]: 1534, Figure 1.
- Material examined: S7, 1–2 m on sand and pebbles (HN), 20.iii.2015: 1♂ CL 24.1 mm.
- Family SERGESTIDAE Dana, 1852
- Robustosergia robusta (Smith, 1882) *
- Sergestes robustus—Zariquiey Álvarez [22]: 61, Figure 18a.
- Robustosergia robusta—Vereshchaka et al. [63]: 22.
- Material examined: T2, ~570 m in the mouth of Lampanyctus crocodilus (Risso, 1810) (BT), 29.ix.2018: 1♂ CL 15.2 mm.
- Remarks: A mesopelagic species known from northern Atlantic and Mediterranean [64].
- Family PASIPHAEIDAE Dana, 1852
- Pasiphaea multidentata Esmark, 1866
- Pasiphaea multidentata—Zariquiey Álvarez [22]: 73, Figures 8a, 10a and 31.
- Pasiphaea sivado (Risso, 1816) *
- Pasiphaea sivado—Zariquiey Álvarez [22]: 70, Figures 6a and 30a–d.
- Material examined: T1, ~720 m (BT), 28.ix.2018: 2♀ CL 18.6–19.3 mm.
- Remarks: Known from eastern Atlantic and Mediterranean [58]. On meso-bathyal grounds Pasiphaea sivado stay close to the bottom at daytime, when it is caught by bottom-trawlers, whereas it migrates in the upper water column at night.
- Family PALAEMONIDAE Rafinesque, 1815
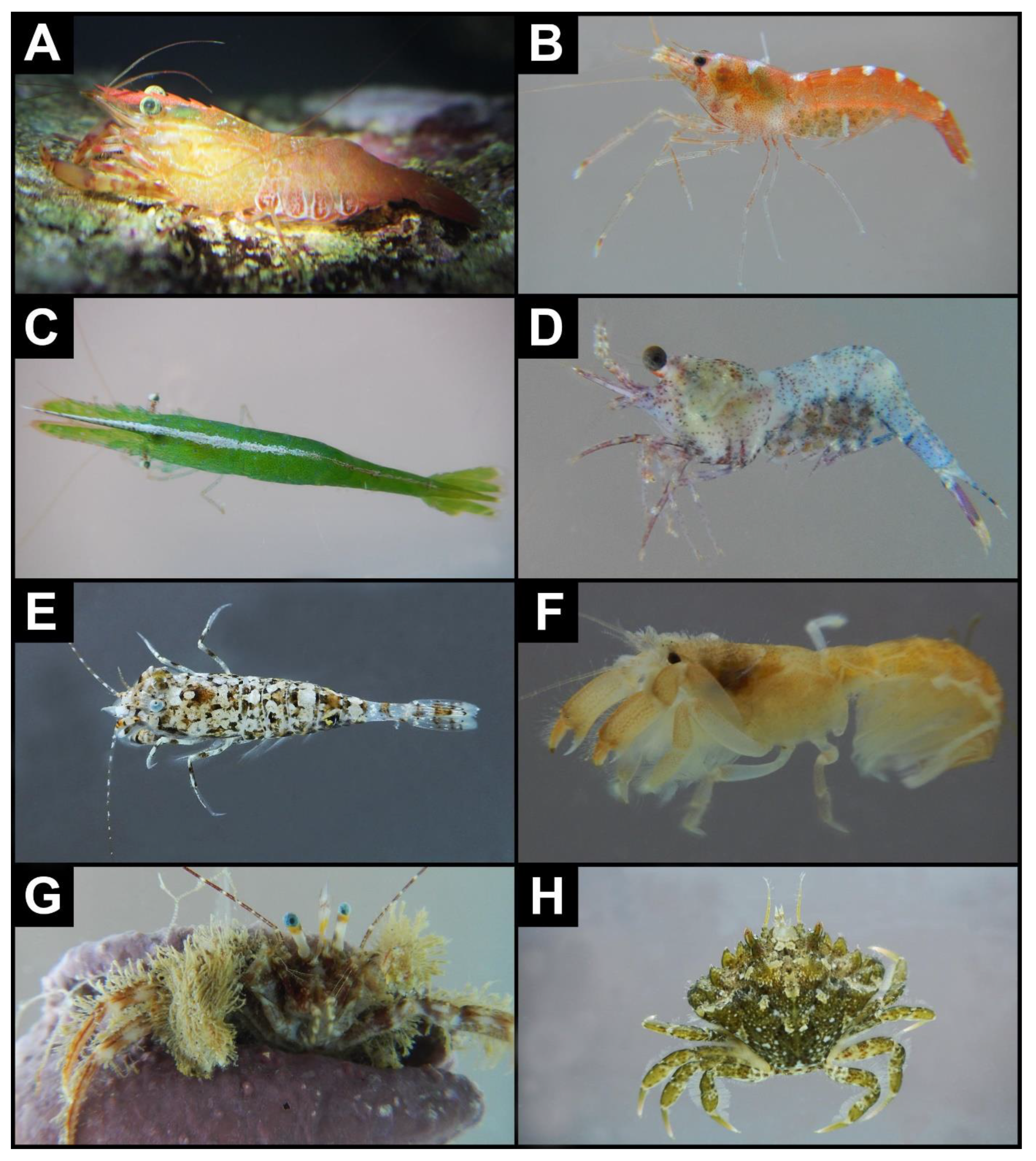
- Brachycarpus biunguiculatus (Lucas, 1846) *—Figure 2A
- Brachycarpus biunguiculatus—Zariquiey Álvarez [22]: 161, figures 64b and 69a.
- Material examined: S3, 1 m on sand and rocks with algal cover, during night, 15.ix.2015: 1 specimen (not measured); S12, 0.5–4 m on hard substrate with photophilic algae, 3.xi.2016: 1 specimen (not measured).
- Remarks: A circumtropical species known from Red Sea, Indian Ocean, eastern Pacific, eastern and western Atlantic, and Mediterranean [58]. This species is considered uncommon in the Mediterranean as it may pass unnoticed due to its behaviour, hiden in crevices or caves during daytime and mostly active at night. According to local divers, the species is present in Rhodes since at least 2010.
- Palaemon serratus (Pennant, 1777) *
- Periclimenes scriptus (Risso, 1822) *
- Family ALPHEIDAE Rafinesque, 1815
- Athanas nitescens (Leach, 1814) *—Figure 2B
- Family HIPPOLYTIDAE Spence Bate, 1888
- Hippolyte inermis Leach, 1816 *—Figure 2C
- Hippolyte inermis—Zariquiey Álvarez [22]: 119, Figures 3b, 4d, 5c,d, 49a, 51b,c and 52f; d’Udekem d’Acoz [67]: 27, Figures 11–15.
- Material examined: S2, 12–14.5 m (ES), 20.vi.2018: 4♀ ov. CL 2.5–2.6 mm; 19.5–21 m (ES), 20.vi.2018: 11♀ CL 2.2–5.3 mm; S5, 15–19.5 m (ES), 21.vi.2018: 2♀ ov. CL 3.1–3.3 mm; 14–17.5 m (ES), 21.vi.2018: 1♂ CL 2.8 mm, 3♀ ov. CL 3–3.5 mm; S19, 4–7.5 m (ES), 18.vi.2018: 20♀ ov. CL 2.6–5.2 mm, 4 unsexed CL 2.2–2.8 mm; 5.5–6 m (ES), 22.vi.2018: 4♀ ov. CL 2.6–4.5 mm.
- Remarks: A common inhabitant of seagrass meadows, known from eastern Atlantic, Mediterranean, and Black Sea [58].
- Family THORIDAE Kingsley, 1879
- Eualus cranchii (Leach, 1817) *—Figure 2D
- Thoralus cranchii—Zariquiey Álvarez [22]: 125, Figures 5a,b, 49d, 51a and 52c–d.
- Material examined: S2, 12–14 m (ES), 20.vi.2018: 1♀ ov. CL 2.1 mm; 19.5–21 m (ES), 20.vi.2018: 3♀ ov. CL 1.8–2.1 mm; S5, 14–19.5 m (ES), 21.vi.2018: 2♀ ov. CL 1.9–4.0 mm; S19, 6–8 m (ES), 18.vi.2018: 2♀ ov. CL 2–2.9 mm; 4.5-8 m (ES), 19.vi.2018: 1 unsexed (broken) CL 2 mm.
- Remarks: Known from eastern Atlantic, Mediterranean, and Marmara Sea [58].
- Eualus occultus (Lebour, 1936) *
- Eualus occultus—Zariquiey Álvarez [22]: 127, Figures 4b,c, 51d and 52a,b.
- Family PANDALIDAE Haworth, 1825
- Plesionika martia (A. Milne-Edwards, 1883) *
- Plesionika martia—Zariquiey Álvarez [22]: 105, Figures 36a, 38b, 39a,b and 40a–c; Crosnier & Forest [69]: 212, Figures 63d, 64c and 66a.
- Material examined: T4, 650–750 m (BT), 19.xi.2019: 6♂ CL 17.1–20.1 mm, 4♀ CL 17.5–20 mm, 4♀ ov. 17.5–20.2 mm.
- Remarks: A cosmopolitan species, common in the Mediterranean on mesobathyal grounds [58]. It constitutes, together with Plesionika edwardsii (Brandt, 1851), a significant fraction of the commercial bycatch of the bottom trawlers targeting “red shrimps” off Rhodes.
- Family PROCESSIDAE Ortmann, 1896
- Processa edulis (Risso, 1816) *
- Processa edulis edulis—Zariquiey Álvarez [22]: 153, Figures 65 a–g.
- Material examined: S2, 12–14.5 m (ES), 20.vi.2018: 1♀ov. CL 6.1 mm; S5, 14–18 m (ES), 21.vi.2018: 1♂ CL 6.1 mm, 1♀ CL 6.9 mm; S19, 6–8 m (ES), 18.vi.2018: 6♂ CL 3.3–5.4 mm, 2♀ CL 5.1 mm, 4♀ ov. CL 4.9–6.4 mm; 4.6–11 m (ES), 19.vi.2018: 1♀ 6.6 mm, 3♀ ov. CL 5.5–7.6 mm.
- Remarks: A common inhabitant of seagrass meadows, known from eastern Atlantic, Mediterranean, and Black Sea [58]. In our samples it was found in sympatry with Processa macrophthalma Nouvel & Holthuis, 1957 and Processa acutirostris Nouvel & Holthuis, 1957.
- Family CRANGONIDAE Haworth, 1825
- Philocheras fasciatus (Risso, 1816) *
- Philocheras trispinosus (Hailstone in Hailstone & Westwood, 1835) *—Figure 2E
- Family UPOGEBIIDAE Borradaile, 1903
- Upogebia deltaura (Leach, 1816) *—Figure 2F
- Upogebia deltaura—Ngoc-Ho [37]: 508, Figures 26 and 27.
- Material examined: S19, 6–8 m (ES), 18.vi.2018: 1♂ CL 4.1 mm; 5.5–6 m (ES), 22.vi.2018: 1♀ CL 5.0 mm.
- Remarks: Known from eastern Atlantic and Mediterranean [58], although some earlier records may refer to the closely related Upogebia mediterranea Noël, 1992, also living in Rhodes [36]. Identified on the basis of the pleura of the first abdominal segment pointed and the third article of the antennal peduncle with a distal spine on the ventral margin (vs pleura with rounded margin and unarmed antennal peduncle in U. mediterranea) [37].
- Family POLYCHELIDAE Wood-Mason, 1875
- Family PAGURIDAE Latreille, 1802
- Anapagurus breviaculeatus Fenizia, 1937 *
- Material examined: S19, 9.0–11 m (ES), 19.vi.2018: 1♀ov. SL 1 mm.
- Remarks: A Mediterranean species, with a single record from Atlantic northern Spain [71]. Holthuis & Gottlieb [72], regarded Anapagurus breviaculeatus as a junior synonym of Anapagurus laevis (Bell, 1845) and reported the material collected off Israel under the latter name. Lewinsohn [12], followed the previous authors and reported A. laevis as the only Anapagurus species collected from Rhodes, although highlighting that ‘trotzdem es den Merkmalen nach auch zu A. breviaculeatus (Fenizia, 1937) gehoren konnte’ [it has the characteristics of A. breviaculeatus]. Both Ingle [73] and García-Gomez [71] remarked that all the eastern Mediterranean specimens previously identified as A. laevis that they could re-examine did not belong to that species and most of them were A. breviaculeatus. Based on the present material, A. breviaculeatus is added to the fauna of Rhodes and the early record of A. laevis is considered a probable misidentification.
- Pagurus cuanensis Bell, 1845 *—Figure 2G
- Pagurus excavatus (Herbst, 1791) *
- Family DORIPPIDAE MacLeay, 1838
- Medorippe lanata (Linnaeus, 1767) *
- Family INACHIDAE MacLeay, 1838
- Macropodia czernjawskii (Brandt, 1880) *
- Macropodia longirostris (Fabricius, 1775) *
- Family PIRIMELIDAE Alcock, 1899
- Pirimela denticulata (Montagu, 1808) *—Figure 2H
- Family CARCINIDAE MacLeay, 1838
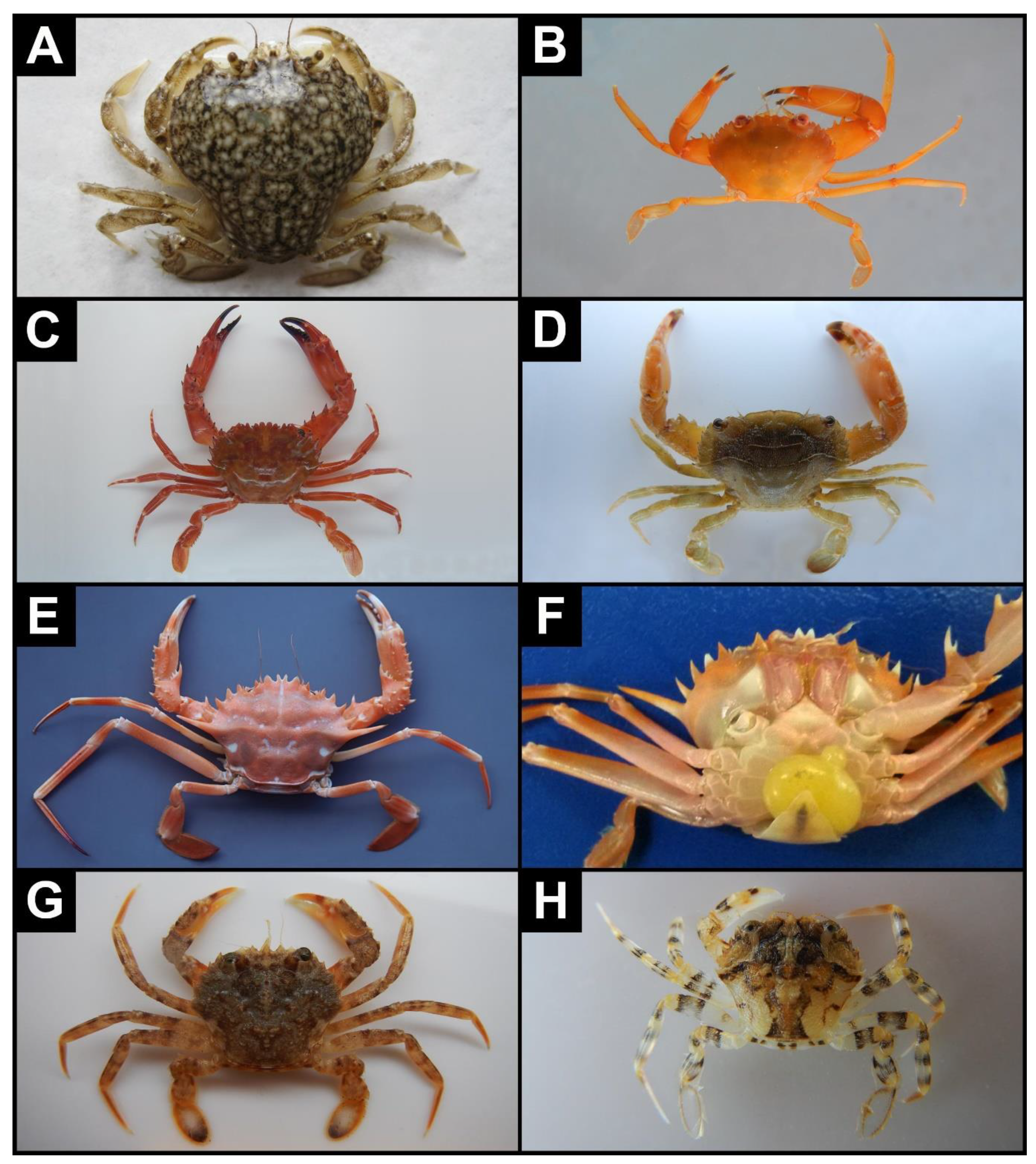
- Portumnus latipes (Pennant, 1777) *—Figure 3A
- Portumnus latipes—Forest [76]: 8, Figures 3, 4 and 6a,b, Pl II Figure 2, Pl. III Figures 2 and 4, Pl IV Figures 4 and 5; Zariquiey Álvarez [22]: 357, Figures 1f, 12h, 14c and 116a,b.
- Material examined: S9, 1 m on sandy bottom with sparse small rocks with algal cover (HN), 14.v.2013: 1♂ CL 27.2 mm.
- Remarks: Known from northeastern Atlantic, Mediterranean, and Black Sea [58]. Noteworthy, in a study on the distribution in Greece of Portumnus latipes and its congeneric species Portunus lysianassa (Herbst, 1796), Chartosia et al. [77] did not find any of the two species in the three sites sampled in eastern Rhodes.
- Family PORTUNIDAE Rafinesque, 1815
- Carupa tenuipes Dana, 1852—(A)—Figure 3B
- Carupa tenuipes—Apel & Spiridonov [78]: 172, Figure 4, Pl. 1.
- Material examined: S7, 8–10 m in the mouth of a Scorpaena species (GN), 3.iv.2012: 1♀ CL 25.2 mm; 25–30 m (TN), 27.iii.2018: 1♂ CL 15.2 mm; S15, 8–10 m (TN), 29.viii.2011: 1♂ CL 12.7 mm; S18, 8–10 m (GN), 11.iv.2011: 1♀ CL 25.1 mm.
- Charybdis hellerii (A. Milne-Edwards, 1867)—(A)
- Charybdis hellerii—Apel & Spiridonov [78]: 194, Figures 13–15 and 17.
- Material examined: S10, 20 m on mixed bottom with sand and rocks (GN), 18.iv.2011: 1♀ CL 40.3 mm; S13, 10–15 m on mixed bottom with sand and rocks (TN), 2.xii.2010: 1♀ CL 19.9 mm; S17, 10 m on mixed bottom with sand and rocks (TN), 23.xii.2010: 2♂ CL 38.1–43.5 mm; 10–15 m on mixed bottom with sand and rocks (TN), 30.v.2013: 1♂ CL 44.9 mm.
- Remarks: Native from Indo-Pacific, including Red Sea [78], this alien species has demonstrated a high invasive capacity, not only in the eastern Mediterranean, where it entered via the Suez Canal, but also in the western Atlantic, where it arrived with ballast waters [80]. First observed in Rhodes in 2004 [48], it is now established all around the island on rocky substrates with algal cover and is displayed in the HSR Aquarium [81].
- Charybdis (Goniohellenus) longicollis Leene, 1938—(A)
- Material examined: S8, 130–187 m (ST), 26.vi.2019: 1♀ CL 19.9 mm; S11, 80 m (ST), iv.2010: 1♀ ov. CL 23.2 mm; S13, 8–10 m (TN), 8.iv.2008: 1♀ CL 21.4 mm.
- Remarks: Native from western Indian Ocean, including Red Sea [78], this alien species is now abundant off Turkey and off Israel on sandy and muddy bottoms in 30–60 m depths, although it was collected down to 250 m [82]. First observed in Rhodes in 1996 [7], the species is only known so far from scattered specimens collected over a wide bathymetric range.
- Gonioinfradens giardi (Nobili, 1905)—(A)—Figure 3C
- Material examined: S7, 8–20 m rocky bottoms with sandy patches (GN), 24.vi.2011: 2♂ CL 25.2–35.3 mm, 1♀ CL 21.2 mm, 1♀ ov. CL 23.8 mm; 16.xi.2012: 2♂ CL 39–49.9 mm; 12.xi.2014: 2♀ CL 19.8–21.3 mm; 4.iv.2017: 4♂ CL 31.3–37.9 mm, 1♀ CL 20 mm; vii.2017: 1♂ CL 40.5 mm; 8.viii.2017: 1♂ CL 35.0 mm; 3.iii.2018: 3♂ CL 28.2–36.9 mm; iv.2018: 2♂ CL 29.9–30.5 mm; S15, 8–20 m rocky bottoms with sandy patches (GN), 29.viii.2011: 1♂ CL 17.4 mm; S4, 10.vi.2011: 1♂ CL 16.2 mm.
- Remarks: Native from western Arabian Sea, including Persian Gulf and Red Sea, this alien species recently spread in the eastern Mediterranean [83]. First recorded in the Mediterranean from Rhodes in 2010 as Gonioinfradens paucidentatus (A. Milne Edwards, 1861) [49], and then from other localities in the eastern Mediterranean [84,85]. More recently, Galil et al. [83], from results of molecular analysis, reinstated the validity of Gonioinfradens giardi, a species previously considered a junior synonym of G. paucidentatus, and reported it from Israel, suggesting that previous Mediterranean records of the latter species should be referred to G. giardi. We keep here present specimens as G. giardi, although this should be confirmed by molecular data as they could not be unequivocably identified on the distinctive morphological characters illustrated in Galil et al. [83].
- Thalamita poissonii (Audouin, 1826)—(A)—Figure 3D
- Thalamita poissonii—Apel & Spiridonov [78]: 253, Figures 73–75.
- Material examined: S1, 2 m (HN), 3.i.2011: 1♀ CL 9.0 mm; S3, 0–1 m (HN), 3.xi.2011: 1♂ CL 20.0 mm; S4, 5 m on rocks (HN), 9.iv.2010: 1♂ CL 13 mm; S7, 3 m (HN), 1.xii.2010: 2♂ CL 20.4-23.8 mm; 3 m (HN), 9.xii.2010: 2♂ CL 24.9–25.2 mm, 1♀ CL 21.0 mm; 1 m (HN), 15.xii.2010: 1♀ ov. CL 20.8 mm; 1 m (HN), 12.xi.2014: 1♂ CL 21.4 mm; 1 m (HN), 10.xi.2017: 1♂ CL 23.5 mm; S11, 100 m (ST), 8.xii.2013: 1♂ CL 11.2 mm, 1♀ CL 7.3 mm; S13, 100 m (ST), 2.xii.2010: 1♂ (not measured); S16, 23 m sandy-rocky bottom with vegetation (HN), 5.x.2009: 1♂ CL 15.1 mm; S18, 5–30 m in the stomach of a Lagocephalus sceleratus (Gmelin, 1789) (BS), 7.iii.2008: 1 carapace CL 6.3 mm; 5–30 m, sandy-muddy bottom with vegetation (BS), 10.viii.2008: 1♂ CL 19.3 mm; 5–30 m (BS), 22.i.2015: 1♀ CL 9.4 mm.
- Family POLYBIIDAE Ortmann, 1893
- Bathynectes maravigna (Prestandrea, 1839) * Figure 3E,F
- Bathynectes superbus—Zariquiey Álvarez [22]: 382, Figure 127g.
- Bathynectes maravigna—Deval & Froglia [87]: 328, Figure 3(left).
- Material examined: T3, 600–650 m, 8.xi.2019: 1♀ CL 26.5 mm; T4, 650–750 m, 19.xi.2019: 5♂ CL 17.5–42.2 mm, 4♀ CL 18.2–28.0 mm, 1♀ ov. 26.7 mm.
- Remarks: Known from eastern Atlantic and Mediterranean Sea [58]. Two females examined here (CL 19 mm, the other smashed) were parasitized by Sacculina (see Figure 3F). Øksnebjerg [88], reported one specimen of B. maravigna from eastern Ionian Sea parasitized by Sacculina carcini Thompson, 1836. However, Polybiidae hosting S. carcini usually occur on shallow grounds in depths of less than 50 m, whereas B. maravigna is restricted to bathyal grounds, from 300 to 1000 m. Moreover, the present externae (4.6 and 4.8 mm in size) have the mantle opening placed at the top of a rather long papilla, less developed in S. carcini [88] (front-cover), and thus we suspect that these specimens do not belong to S. carcini.
- Liocarcinus bolivari (Zariquiey Álvarez, 1948) *—Figure 3G,H
- Macropipus bolivari—Zariquiey Álvarez [22]: 375, Figure 127a,b.
- Material examined: S5, 14–17.5 m (ES), 21.vi.2018: 1♂ CL 8.6 mm; S8, 130–187 m (ST), 26.vi.2019: 2♂ CL 20.6–21.8 mm, 1♀ CL 17.7 mm; S11, 100 m (ST), 15.v.2010: 2♂ CL 15.6–19.6 mm, 1♀ CL 18.7 mm; 150 m (ST), 21.vi.2011: 1♀ CL 17 mm.
- Remarks: Known from western and south-central Mediterranean, with single records from the Gulf of Cadiz (outside Gibraltar) and northern Adriatic [58]. Here, it is recorded for the first time in the eastern Mediterranean. Liocarcinus bolivari is closely related to Liocarcinus depurator (Linnaeus, 1758) and Liocarcinus vernalis (Risso, 1827). In addition to the morphological differences that allow separation of long-time preserved specimens [22], L. bolivari can be recognized at a glance in the field for the presence of dark bands on articles of walking legs (Figure 3H), versus a uniform colour in L. depurator and L. vernalis, and the distal part of dactylus of the fifth pereopod, blue-violet only in L. bolivari and L. depurator.
- Family GERYONIDAE Colosi, 1923
- Family XANTHIDAE MacLeay, 1838
- Atergatis roseus (Rüppell, 1830)—(A)
- Atergatis roseus—Serène [89]: 147, Figure 86, Pl. XXIA.
- Material examined: S12, 8–15 m (TN), 2.v.2010: 1♂ CL 61.5 mm; S18, 8–15 m (TN), 20.xi.2015: 1♀ CL 49.3 mm.
- Remarks: Native from Indo-West Pacific, including Red Sea [89], this alien species recently spread in the eastern Mediterranean (e.g., [90]). First observed in Rhodes in 2009 [51], it is now established all around the island on rocky substrates and it is regularly displayed in the HSR Aquarium since 2009 [81].
- Family GRAPSIDAE MacLeay, 1838
- Pachygrapsus transversus (Gibbes, 1850) *
- Pachygrapsus transversus—Zariquiey Álvarez [22]: 425, Figure 140c; Poupin et al. [91]: 44 (partim), Figures 13a–e, 14l and 15l.
- Material examined: S1, 1 m on hard substrate with algae (HN), 10.iv.2014: 1♀ CL 7.4 mm.
- Remarks: Known from western and eastern Atlantic, including many Islands groups, and Mediterranean [92]. Recorded only once in the Aegean Sea, at Karpathos Island [93]. The specimen herein reported was collected in a local marina, suggesting that the intense maritime traffic in the area may have played a role in this finding.
- Planes minutus (Linnaeus, 1758)
- Material examined: S6, near a stranded male Caretta caretta (Linnaeus, 1758) (carapace curved length 81 cm), 4.iii.2014: 1♂ CL 15.2 mm; S20, tidal rocky substrate (HN), 3.xi.2014: 1♂ CL 9.6 mm.
- Remarks: Known from eastern and western Atlantic and Mediterranean [58]. The species is usually associated with floatsam (natural or artificial) and sea turtles in the pelagic realm [95] and was first collected in the Aegean in 2010 at Rhodes [14]. The present findings represent the second and third records of the species in the Aegean Sea.
- Family OCYPODIDAE Rafinesque, 1815
- Ocypode cursor (Linnaeus, 1758)
- Ocypode cursor—Holthuis & Gottlieb [72]: 99, plate 3, Figure 14; Sakai & Türkay [96]: 702, Figures 2B,13 and 35.
- Material examined: S14, on a sandy beach during night (HN), 14.ix.2016: 9♂ CL 14.4–27.1 mm, 1♀ CL 28.8 mm (specimens released after measurements and photos).
- Remarks: Known from the western coasts of Africa and eastern and central Mediterranean [56,97]. As consequence of global warming, the species is currently expanding westwards to the central Mediterranean from the Levant Sea, which may have acted as refugium during the last glacial period [57]. Kinzelbach [56] reported Ocypode cursor from southern Aegean (including Rhodes) based on a specimen displayed at the HSR museum and another one from Karpathos Island.
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, S.C.; Mills Flemming, J.; Watson, R.; Lotze, H.K. Rapid global expansion of invertebrate fisheries: Trends, drivers, and ecosystem effects. PLoS ONE 2011, 6, e14735. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Corsini-Foka, M.; Morri, C.; Zenetos, A. Thirty years after—Dramatic change in the costal marine habitats of Kos Island (Greece), 1981–2013. Mediterr. Mar. Sci. 2014, 15, 482–497. [Google Scholar] [CrossRef]
- Nykjaer, L. Mediterranean Sea surface warming 1985 to 2006. Clim. Res. 2009, 39, 11–17. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, M.A.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Sisma-Ventura, G.; Yam, R.; Shemesh, A. Recent unprecedented warming and oligotrophy of the eastern Mediterranean Sea within the last millennium. Geophys. Res. Lett. 2014, 41, 5158–5166. [Google Scholar] [CrossRef]
- Theocharis, A.; Klein, B.; Nittis, K.; Roether, W. Evolution and status of the Eastern Mediterranean Transient (1997–1999). J. Mar. Syst. 2002, 33, 91–116. [Google Scholar] [CrossRef]
- Galil, B.S.; Kevrekidis, K. Exotic decapods and a stomatopod off Rhodes (Rodos, Greece) and the Eastern Mediterranean Transient. Crustaceana 2002, 75, 925–930. [Google Scholar]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 1–55. [Google Scholar]
- Pancucci-Papadopoulou, M.A.; Raitsos, D.E.; Corsini-Foka, M. Biological invasions and climatic warming: Implications for South Eastern Aegean ecosystem functioning. J. Mar. Biol. Assoc. UK 2012, 92, 777–789. [Google Scholar] [CrossRef]
- Skliris, N. Past, present and future patterns of the thermohaline circulation and characteristic water masses of the Mediterranean Sea. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: London, UK, 2014; pp. 29–48. [Google Scholar] [CrossRef]
- Lewinsohn, C. Crustacea decapoda von der Insel Rhodos, Griechenland. Zool. Meded. 1976, 49, 237–254. [Google Scholar]
- Kevrekidis, K.; Galil, B.S. Decapoda and Stomatopoda (Crustacea) of Rodos island (Greece) and the Erythrean expansion NW of the Levantine Sea. Mediterr. Mar. Sci. 2003, 4, 57–66. [Google Scholar] [CrossRef][Green Version]
- Corsini-Foka, M.; Pancucci-Papadopoulou, M.A. Inventory of Crustacea Decapoda and Stomatopoda from Rhodes Island (Eastern Mediterranean Sea), with emphasis on rare and newly recorded species. J. Biol. Res. (Thessalon.) 2012, 18, 359–371. [Google Scholar]
- Corsini-Foka, M.; Kalogirou, S. First record of Albunea carabus (Linnaeus, 1758) (Decapoda: Anomura: Hippoidea) in the Aegean Sea. Cah. Biol. Mar. 2013, 54, 297–299. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Kondylatos, G.; Pancucci-Papadopoulou, M.A. A new alien crab for the Mediterranean Sea: Xanthias lamarckii (H. Milne Edwards, 1834) (Crustacea: Decapoda: Brachyura: Xanthidae). Mediterr. Mar. Sci. 2013, 14, 295–297. [Google Scholar] [CrossRef]
- Zenetos, A.; Koutsogiannopoulos, D.; Ovalis, P.; Poursanidis, D. The role played by citizen scientists in monitoring marine alien species in Greece. Cah. Biol. Mar. 2013, 54, 419–426. [Google Scholar] [CrossRef]
- Giovos, I.; Kleitou, P.; Poursanidis, D.; Batjakas, I.; Bernardi, G.; Crocetta, F.; Doumpas, N.; Kalogirou, S.; Kampouris, T.E.; Keramidas, I.; et al. Citizen-science for monitoring marine invasions and stimulating public engagement: A case project from the eastern Mediterranean. Biol. Invasions 2019, 21, 3707–3721. [Google Scholar] [CrossRef]
- Hatiris, G.A. INTEREG III GR-CY Project “AKTI”; Technical Report; HCMR: Athens, Greece, 2014. [Google Scholar]
- Corsini-Foka, M.; Zenetos, A.; Crocetta, F.; Çinar, M.E.; Koçak, F.; Golani, D.; Katsanevakis, S.; Tsiamis, K.; Cook, E.; Froglia, C.; et al. Inventory of alien and cryptogenic species of the Dodecanese (Aegean Sea, Greece): Collaboration through COST action training school. Manag. Biol. Invasion 2015, 6, 351–366. [Google Scholar] [CrossRef]
- Crocetta, F.; Gofas, S.; Salas, C.; Tringali, L.P.; Zenetos, A. Local ecological knowledge versus published literature: A review of non-indigenous Mollusca in Greek marine waters. Aquat. Invasions 2017, 12, 415–434. [Google Scholar] [CrossRef]
- Zariquiey Álvarez, R. Crustàceos Decapodos Ibéricos. Inv. Pesq. 1968, 32, 1–510. [Google Scholar]
- World Register of Marine Species (WoRMS). World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org (accessed on 20 March 2020).
- Stephensen, K. Decapoda-Macrura. excl. Sergestidae. (Penaeidae, Pasiphaeidae, Hoplophoridae, Nematocarcinidae, Scyllaridae, Eryonidae, Nephropsidae, Appendix). In Report on the Danish Oceanographical Expeditions 1908–1910 to the Mediterranean and Adjacent Seas; Schmidt, J., Ed.; Carlsbergfondet: Copenhagen, Denmark, 1923; Volume 2, pp. 1–85. [Google Scholar]
- Kevrekidis, K.; Galil, B.S.; Kevrekidis, T. Three lessepsian migrant penaeids (Decapoda) in Rodos Island (Greece). Crustaceana 1998, 71, 474–478. [Google Scholar] [CrossRef]
- Zenetos, A.; Akel, E.; Apostolidis, C.; Bilecenoglu, M.; Bitar, G.; Buchet, V.; Chalari, N.; Corsini-Foka, M.; Crocetta, F.; Dogrammatzi, A.; et al. New Mediterranean Biodiversity Records (April 2015). Mediterr. Mar. Sci. 2015, 16, 266–284. [Google Scholar] [CrossRef]
- Kondylatos, G.; Corsini-Foka, M. Penaeus hathor (Burkenroad, 1959) (Crustacea: Decapoda: Penaeidae) in Rhodian waters (Aegean Sea). Cah. Biol. Mar. 2017, 58, 491–495. [Google Scholar] [CrossRef]
- Maldura, C.M. La pesca nelle Isole Italiane dell’Egeo. Boll. Pesca Piscic. Idrobiol. 1938, 14, 460–481. [Google Scholar]
- Kevrekidis, K.; Kevrekidis, T. The occurrence of Penaeus japonicus Bate (Decapoda, Penaeidae) in the Aegean Sea. Crustaceana 1996, 69, 925–928. [Google Scholar] [CrossRef]
- Adensamer, T. Zoologische Ergebnisse. XI. Decapoden gesammelt auf S.M. Schiff “Pola” in den Jahren 1890-1894. Berichte der Commission fur Erforschung des Ostlichen Mittelmeres, XXII. Denkschr. Kaiserl. Akad. Wiss. Math. Naturwiss. (Kl.) 1898, 65, 597–628. [Google Scholar]
- Kitsos, M.; Doulgeraki, S.; Tselepides, A.; Koukouras, A. Diet composition of the bathyal crabs, Chaceon mediterraneus Manning & Holthuis and Geryon longipes A. Milne-Edwards (Decapoda, Geryonidae) collected at different depths in the eastern Mediterranean. Crustaceana 2005, 78, 171–184. [Google Scholar] [CrossRef]
- Santucci, R. Alcuni crostacei decapodi delle isole Egee. Arch. Zool. Ital. 1928, 12, 345–354. [Google Scholar]
- Thessalou-Legaki, M.; Frantzis, A.; Nassiokas, K.; Hatzinikolaou, S. Observations on Parapandalus narval (Fabricius, 1787) (Crustacea, Decapoda, Pandalidae) from Rhodos Island (Greece). Rapp. Comm. Int. Mer Medit. 1986, 30, 13. [Google Scholar]
- Chan, T.-Y.; Crosnier, A. Crustacea decapoda: Studies of the Plesionika narval (Fabricius, 1787) group (Pandalidae) with descriptions of six new species. Mém. Mus. Natl. Hist. Nat. Ser. A Zool. 1991, 152, 413–461. [Google Scholar]
- Thessalou-Legaki, M. Preliminary data on the occurrence of Thalassinidea (Crustacea, Decapoda) in the Greek seas. Biol. Gallo Hell. 1986, 12, 181–187. [Google Scholar]
- Asgaard, U.; Bromley, R.G.; Hanken, N.M. Recent firmground burrows produced by a upogebiid crustacean: Paleontological implications. Cour. Forsch. Inst. Senckenberg 1997, 201, 23–28. [Google Scholar]
- Ngoc-Ho, N. European and Mediterranean Thalassinidea (Crustacea, Decapoda). Zoosystema 2003, 25, 439–555. [Google Scholar]
- Tortonese, E. Note intorno alla fauna e flora marine dell’Isola di Rodi (Mar Egeo). Boll. Pesca Piscic. Idrobiol. 1947, 23, 13–20. [Google Scholar]
- Pancucci-Papadopoulou, M.A.; Simboura, N.; Zenetos, A.; Thessalou-Legaki, M.; Nicolaidou, A. Benthic invertebrate communities of NW Rodos (Rhodes) island (SE Aegean Sea) as related to hydrological regime and geographical location. Isr. J. Zool. 1999, 45, 371–393. [Google Scholar] [CrossRef]
- Koukouras, A.; Dounas, C. Decapod crustaceans new to the fauna of the Aegean Sea. Crustaceana 2000, 73, 497–502. [Google Scholar] [CrossRef]
- Kondylatos, G.; Corsini-Foka, M.; Perakis, E. First record of the isopod Idotea hectica (Pallas, 1772) (Idoteidae) and of the brachyuran crab Matuta victor (Fabricius, 1781) (Matutidae) in the Hellenic waters. Mediterr. Mar. Sci. 2018, 19, 656–661. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Margies, P.; Santorinios, E. First record of the exotic brachyuran Leucosia signata from Rhodes. In Proceedings of the 8th Pan-Hellenic Symposium of Oceanography and Fisheries, Thessaloniki, Greece, 4–8 June 2006; p. 4. [Google Scholar]
- Corsini, M.; Kondylatos, G. On the occurrence of two brachyurans, Myra subgranulata and Herbstia condyliata, on Rhodes Island (SE Aegean Sea). Crustaceana 2006, 79, 167–174. [Google Scholar] [CrossRef]
- Parisi, B. Escursioni zoologiche del Dott. Enrico Festa nell’Isola di Rodi. Decapodi. Boll. Mus. Zool. Anat. Comp. R. Univ. Torino 1913, 28, 1–2. [Google Scholar]
- Pretzmann, G. Ergebnisse der von Dr. O. Paget und Dr. E. Kritscher auf Rhodos durchgeführten zoologischen Exkursionen. X. Brachyura. Ann. Nat. Hist. Mus. Wien 1964, 67, 661–666. [Google Scholar]
- Kinzelbach, R. Die Blaue Schwimmkrabbe (Callinectes sapidus), ein Neuburger im Mittelmeer. Nat. Mus. 1965, 95, 293–296. [Google Scholar]
- Pancucci-Papadopoulou, M.A.; Corsini-Foka, M.; Tsiamis, K.; Kalogirou, S. The occurrence of Carupa tenuipes Dana, 1851 (Crustacea: Brachyura: Portunidae) from Rhodos Island (SE Aegean Sea, Greece). Aquat. Invasions 2009, 4, 713–714. [Google Scholar] [CrossRef]
- Kirmitzoglou, I.; Kitsos, M.-S.; Thessalou-Legaki, M.; Tselepides, A.; Koukouras, A. Investigation of the progress and possible expansion of the limits of the lessepsian migratory current regarding Decapoda (Crustacea). In Proceedings of the 10th International Congress on the Zoogeography and Ecology of Greece and Adjacent Regions (ICZEGAR), Patras, Greece, 26–30 June 2006; p. 51. [Google Scholar]
- Corsini-Foka, M.; Pancucci-Papadopoulou, M.A.; Kondylatos, G.; Kalogirou, S. Gonioinfradens paucidentatus (A. Milne Edwards, 1861) (Crustacea, Decapoda, Portunidae): A new alien crab in the Mediterranean Sea. Mediterr. Mar. Sci. 2010, 11, 331–340. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Kondylatos, G.; Economidis, P.S. Occurrence of the lessepsian species Portunus pelagicus (Crustacea) and Apogon pharaonis (Pisces) in the marine area of Rhodes Island. Mediterr. Mar. Sci. 2004, 5, 83–89. [Google Scholar] [CrossRef][Green Version]
- Corsini-Foka, M.; Pancucci-Papadopoulou, M.A. The alien brachyuran Atergatis roseus (Rüppell, 1830) (Decapoda, Xanthidae) in Rhodes Island (Greece). Mar. Biodivers. Rec. 2010, 3, e76. [Google Scholar] [CrossRef]
- Koukouras, A.; Kitsos, M.; Tselepides, A. The genera Chaceon Manning and Holthuis and Geryon Krøyer (Decapoda, Geryonidae) in the Eastern Mediterranean. Crustaceana 2000, 73, 107–113. [Google Scholar]
- Corsini-Foka, Μ.; Kondylatos, G. First occurrence of Actaeodes tomentosus (H. Milne Edwards, 1834) (Brachyura: Xanthidae: Actaeinae) in the Mediterranean Sea. Mediterr. Mar. Sci. 2015, 16, 201–205. [Google Scholar] [CrossRef][Green Version]
- Thessalou-Legaki, M.; Zenetos, A.; Kambouroglou, V.; Corsini-Foka, M.; Kouraklis, P.; Dounas, C.; Nicolaidou, A. The establishment of the invasive crab Percnon gibbesi (H. Milne Edwards, 1853) (Crustacea: Decapoda: Grapsidae) in Greek waters. Aquat. Invasions 2006, 1, 133–136. [Google Scholar] [CrossRef]
- Pancucci-Papadopoulou, M.A.; Corsini-Foka, M.; Naletaki, M. Macrophthalmus graeffei A. Milne Edwards, 1873 (Crustacea: Brachyura: Macrophthalmidae): A new Indo-Pacific guest off Rhodes Island (SE Aegean Sea, Greece). Mediterr. Mar. Sci. 2010, 11, 195–200. [Google Scholar] [CrossRef]
- Kinzelbach, R. Neue Nachweise der Reiterkrabbe, Ocypode cursor (Linnaeus, 1758), in der Ägäis (Decapoda, Brachyura, Ocypodidae). Crustaceana 1970, 18, 318–320. [Google Scholar] [CrossRef]
- Vecchioni, L.; Marrone, F.; Deidun, A.; Adepo-Guerene, A.B.; Froglia, C.; Sciberras, A.; Bariche, M.; Ciçek, B.A.; Corsini-Foka, M.; Arculeo, M. DNA Taxonomy confirms the identity of the widely-disjunct Mediterranean and Atlantic populations of the tufted ghost crab Ocypode cursor (Crustacea: Decapoda: Ocypodidae). Zoolog. Sci. 2019, 36, 322–329. [Google Scholar] [CrossRef]
- d’Udekem d’Acoz, C. Inventaire et distribution des crustacés décapodes de l’Atlantique nord-oriental, de la Méditerranée et des eaux continentales adjacentes au nord de 25°N. Patrim. Nat. (Mus. Natl. Hist. Nat. Serv. Patrim. Nat.) 1999, 40, 1–383. [Google Scholar]
- Deval, M.C.; Kaya, Y.; Güven, O.; Gökoglu, M.; Froglia, C. An unexpected find of the western Atlantic shrimp, Farfantepenaeus aztecus (Ives, 1891) (Decapoda, Penaeidae) in Antalya Bay, eastern Mediterranean Sea. Crustaceana 2010, 83, 1531–1537. [Google Scholar] [CrossRef]
- Pérez Farfante, I.; Kensley, B. Penaeoid and sergestoid shrimps and prawns of the world, keys and diagnoses for the families and genera. Mem. Mus. Natl. Hist. Nat. (Paris) 1997, 175, 1–233. [Google Scholar]
- Mytilineou, C.; Akel, E.; Babali, N.; Balistreri, P.; Bariche, M.; Boyaci, Y.; Cilenti, L.; Constantinou, C.; Crocetta, F.; Çelik, M.; et al. New Mediterranean Biodiversity Records (November, 2016). Mediterr. Mar. Sci. 2016, 17, 794–821. [Google Scholar] [CrossRef]
- Scannella, D.; Falsone, F.; Geraci, M.L.; Froglia, C.; Fiorentino, F.; Giusto, G.B.; Colloca, F. First report of Northern brown shrimp Penaeus aztecus Ives, 1891 in Strait of Sicily. BioInvasions Rec. 2017, 6, 67–72. [Google Scholar] [CrossRef]
- Vereshchaka, A.L.; Olesen, J.; Lunina, A.A. Global diversity and phylogeny of pelagic shrimps of the former genera Sergestes and Sergia (Crustacea, Dendrobranchiata, Sergestidae), with definition of eight new genera. PLoS ONE 2014, 9, e112057. [Google Scholar] [CrossRef]
- Judkins, D.C. Geographical distribution of pelagic decapod shrimp in the Atlantic Ocean. Zootaxa 2014, 3895, 301–345. [Google Scholar] [CrossRef]
- González-Ortegón, E.; Cuesta, J.A. An illustrated key to species of Palaemon and Palaemonetes (Crustacea: Decapoda: Caridea) from European waters, including the alien species Palaemon macrodactylus. J. Mar. Biol. Ass. UK 2006, 86, 93–102. [Google Scholar] [CrossRef]
- Grippa, G.B.; d’Udekem d’Acoz, C. The genus Periclimenes Costa, 1844 in the Mediterranean Sea and the Northeastern Atlantic Ocean: Review of species and description of Periclimenes sagittifer aegylios subsp. nov. (Crustacea, Decapoda, Caridea, Pontoniinae). Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. (Milano) 1996, 135, 401–412. [Google Scholar]
- d’Udekem d’Acoz, C. The genus Hippolyte Leach, 1814 (Crustacea: Decapoda: Caridea: Hippolytidae) in the East Atlantic Ocean and the Mediterranean Sea, with a checklist of all species in the genus. Zool. Verh. 1996, 303, 1–133. [Google Scholar]
- d’Udekem d’Acoz, C.; Wirtz, P. Observations on some interesting coastal Crustacea Decapoda from the Azores, with a key to the genus Eualus Thallwitz, 1892 in the Northeastern Atlantic and the Mediterranean. Arquipelago 2002, 19, 67–84. [Google Scholar]
- Crosnier, A.; Forest, J. Les crevettes profondes de l’Atlantique orientale tropicale. Faune Trop. 1973, 19, 1–409. [Google Scholar]
- Galil, B.S. Crustacea Decapoda: Review of the genera and species of the family Polychelidae Wood-Mason, 1874. Mém. Mus. Natl. Hist. Nat. 2000, 184, 285–387. [Google Scholar]
- García-Gómez, J. The systematics of the genus Anapagurus Henderson, 1886, and a new genus for Anapagurus drachi Forest (Crustacea, Decapoda, Paguridae). Zool. Verh. 1994, 295, 1–131. [Google Scholar]
- Holthuis, L.B.; Gottlieb, E. An annotated list of the Decapod crustacea of the Mediterranean coast of Israel, with an appendix listing the Decapoda of the Eastern Mediterranean. Bull. Res. Counc. Isr. 1958, 7B, 1–126. [Google Scholar]
- Ingle, R.W. Hermit Crabs of the Northeastern Atlantic Ocean and the Mediterranean Sea. An Illustrated Key; Chapman & Hall: London, UK, 1993; pp. 1–495. [Google Scholar]
- Ingle, R.W. Northeastern Atlantic and Mediterranean hermit crabs (Crustacea: Anomura: Paguroidea: Paguridae). I. The genus Pagurus Fabricius, 1775. J. Nat. Hist. 1985, 19, 745–769. [Google Scholar] [CrossRef]
- Manning, R.B.; Holthuis, L.B. West African Brachyuran Crabs (Crustacea: Decapoda). Smithson. Contr. Zool. 1981, 306, 1–379. [Google Scholar] [CrossRef]
- Forest, J. Sur une collection de Crustacés Décapodes de la région de Porto Cesareo. Description de Portumnus pestai sp. nov. Thalass. Salent. 1967, 2, 3–29. [Google Scholar]
- Chartosia, N.; Koukouras, A.; Mavidis, M.; Kitsos, M. Preliminary estimation of the factors influencing the distribution of the midlittoral crab Portumnus lysianassa (Herbst, 1796). Hydrobiologia 2006, 557, 97–106. [Google Scholar] [CrossRef]
- Apel, M.; Spiridonov, V.A. Taxonomy and zoogeography of the portunid crabs (Crustacea: Decapoda: Brachyura: Portunidae) of the Arabian Gulf and adjacent waters. Fauna Arab. 1998, 17, 159–331. [Google Scholar]
- Galil, B.S. Carupa tenuipes Dana, 1851: An Indo-Pacific swimming crab new to the Mediterranean (Decapoda, Brachyura, Portunidae). Crustaceana 2004, 77, 249–251. [Google Scholar]
- Tavares, M. Alien Decapod Crustaceans in the Southwestern Atlantic Ocean. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: London, UK, 2011; pp. 251–268. [Google Scholar]
- Corsini-Foka, M.; Kondylatos, G.; Santorinios, E. The role of the Aquarium of Rhodes (Eastern Mediterranean Sea) on raising public awareness to marine invasions, with a note on the husbandry and trade of marine aliens. Cah. Biol. Mar. 2014, 55, 173–182. [Google Scholar] [CrossRef]
- Innocenti, G.; Stasolla, G.; Goren, M.; Stern, N.; Levitt-Barmats, Y.; Diamant, A.; Galil, B.S. Going down together: Invasive host, Charybdis longicollis (Decapoda: Brachyura: Portunidae) and invasive parasite, Heterosaccus dollfusi (Cirripedia: Rhizocephala: Sacculinidae) on the upper slope off the Mediterranean coast of Israel. Mar. Biol. Res. 2017, 13, 229–236. [Google Scholar] [CrossRef]
- Galil, B.S.; Douek, J.; Gevili, R.; Goren, M.; Yudkovsky, Y.; Paz, G.; Rinekvich, B. The resurrection of Charybdis (Gonioinfradens) giardi (Nobili, 1905), newly recorded from the SE Mediterranean Sea. Zootaxa 2018, 4370, 580–590. [Google Scholar] [CrossRef]
- Karhan, S.U.; Yokes, M.B. An earlier record of the Indo-Pacific swimming crab Gonioinfradens paucidentatus (A. Milne-Edwards, 1861) (Decapoda, Brachyura, Portunidae) off the Mediterranean coast of Turkey. Crustaceana 2012, 85, 117–121. [Google Scholar] [CrossRef]
- Kondylatos, G.; Kampouris, T.; Kouloumperis, V.; Corsini-Foka, M. The Indo-Pacific brachyuran Charybdis (Gonioinfradens) paucidentatus (A. Milne-Edwards, 1861) (Brachyura, Portunidae) in the Cyclades, Aegean Sea. Turk. J. Zool. 2017, 41, 1118–1120. [Google Scholar] [CrossRef]
- d’Udekem d’Acoz, C. Contribution à la connaissance des Crustacés Décapodes Helléniques I: Brachyura. BIOS (Maced. Greece) Sci. Ann. School Biol. 1994, 1, 9–47. [Google Scholar]
- Deval, M.C.; Froglia, C. New records of deep-sea decapod crustaceans in the Turkish Mediterranean Sea (North Levant Sea). Zool. Middle East 2016, 62, 323–330. [Google Scholar] [CrossRef]
- Øksnebjerg, B. The Rhizocephala (Crustacea: Cirripedia) of the Mediterranean and Black Sea: Taxonomy, biogeography and ecology. Isr. J. Zool. 2000, 46, 1–102. [Google Scholar] [CrossRef]
- Serène, R. Crustacés Décapodes Brachyoures de l’Océan Indien occidental et de la Mer Rouge. Xanthoidea: Xanthidae Trapeziidae. Faune Trop. 1984, 24, 1–349. [Google Scholar]
- Crocetta, F.; Agius, D.; Balistreri, P.; Bariche, M.; Bayhan, Y.; Çakir, M.; Ciriaco, S.; Corsini-Foka, M.; Deidun, A.; El Zrelli, R.; et al. New Mediterranean Biodiversity Records (October 2015). Mediterr. Mar. Sci. 2015, 16, 682–702. [Google Scholar] [CrossRef]
- Poupin, J.; Davie, P.J.F.; Cexus, J.C. A revision of the genus Pachygrapsus Randall, 1840 (Crustacea: Decapoda: Brachyura, Grapsidae), with special reference to the Southwest Pacific species. Zootaxa 2005, 1015, 1–66. [Google Scholar] [CrossRef]
- Crocetta, F.; Mifsud, S.; Paolini, P.; Piscopo, J.; Schembri, P.J. New records of the genus Pachygrapsus (Crustacea: Decapoda) from the central Mediterranean Sea with a review of its Mediterranean zoogeography. Mediterr. Mar. Sci. 2011, 12, 75–93. [Google Scholar] [CrossRef]
- Kinzelbach, R. Pachygrapsus transversus (Gibbes, 1850) in der Aegäis (Crustacea Decapoda). Bonn. Zool. Beitr. 1964, 15, 266–267. [Google Scholar]
- Chace, F.A. The oceanic crabs of the genera Planes and Pachygrapsus. Proc. U.S. Natl. Mus. 1951, 101, 65–103. [Google Scholar] [CrossRef][Green Version]
- Dellinger, T.; Davenport, J.; Wirtz, P. Comparison of social structure of Columbus crabs living on loggerhead turtles and inanimate flotsam. J. Mar. Biol. Assoc. UK 1997, 77, 185–194. [Google Scholar] [CrossRef]
- Sakai, K.; Türkay, M. Revision of the genus Ocypode with description of a new genus, Hoplocypode (Crustacea: Decapoda: Brachyura). Mem. Queensl. Mus. Nat. 2013, 56, 665–793. [Google Scholar]
- Deidun, A.; Crocetta, F.; Sciberras, A.; Sciberras, J.; Insacco, G.; Zava, B. The protected taxon Ocypode cursor (Linnaeus, 1758) (Crustacea: Decapoda: Ocypodidae)—Documenting its well-established presence in the central Mediterranean. Eur. Zool. J. 2017, 84, 96–103. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Galil, B.S.; Froglia, C.; Noël, P. Looking back, looking ahead: The CIESM Atlas, Crustaceans. Manag. Biol. Invasion 2015, 6, 171–175. [Google Scholar] [CrossRef]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, P.K.; Simboura, N.; Tsiamis, K.; Pancucci-Papadopoulou, M.A. Deep cleaning of alien species records in the Greek Seas (2018 update). Manag. Biol. Invasion 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Kondylatos, G.; Santorinios, E. Increase of sea turtles stranding records in Rhodes Island (Eastern Mediterranean Sea): Update of a long-term survey. J. Mar. Biol. Assoc. UK 2013, 93, 1991–2002. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Mastis, S.; Kondylatos, G.; Batjakas, I.E. Alien and native fish in gill nets at Rhodes, eastern Mediterranean (2014–2015). J. Mar. Biol. Assoc. UK 2017, 97, 635–642. [Google Scholar] [CrossRef]
- Crise, A.; Kaberi, H.; Ruiz, J.; Zatsepin, A.; Arashkevich, E.; Giani, M.; Karageorgis, A.P.; Prieto, L.; Pantazi, M.; Gonzalez-Fernandez, D.; et al. A MSFD complementary approach for the assessment of pressures, knowledge and data gaps in Southern European Seas: The PERSEUS experience. Mar. Pollut. Bull. 2015, 95, 28–39. [Google Scholar] [CrossRef]
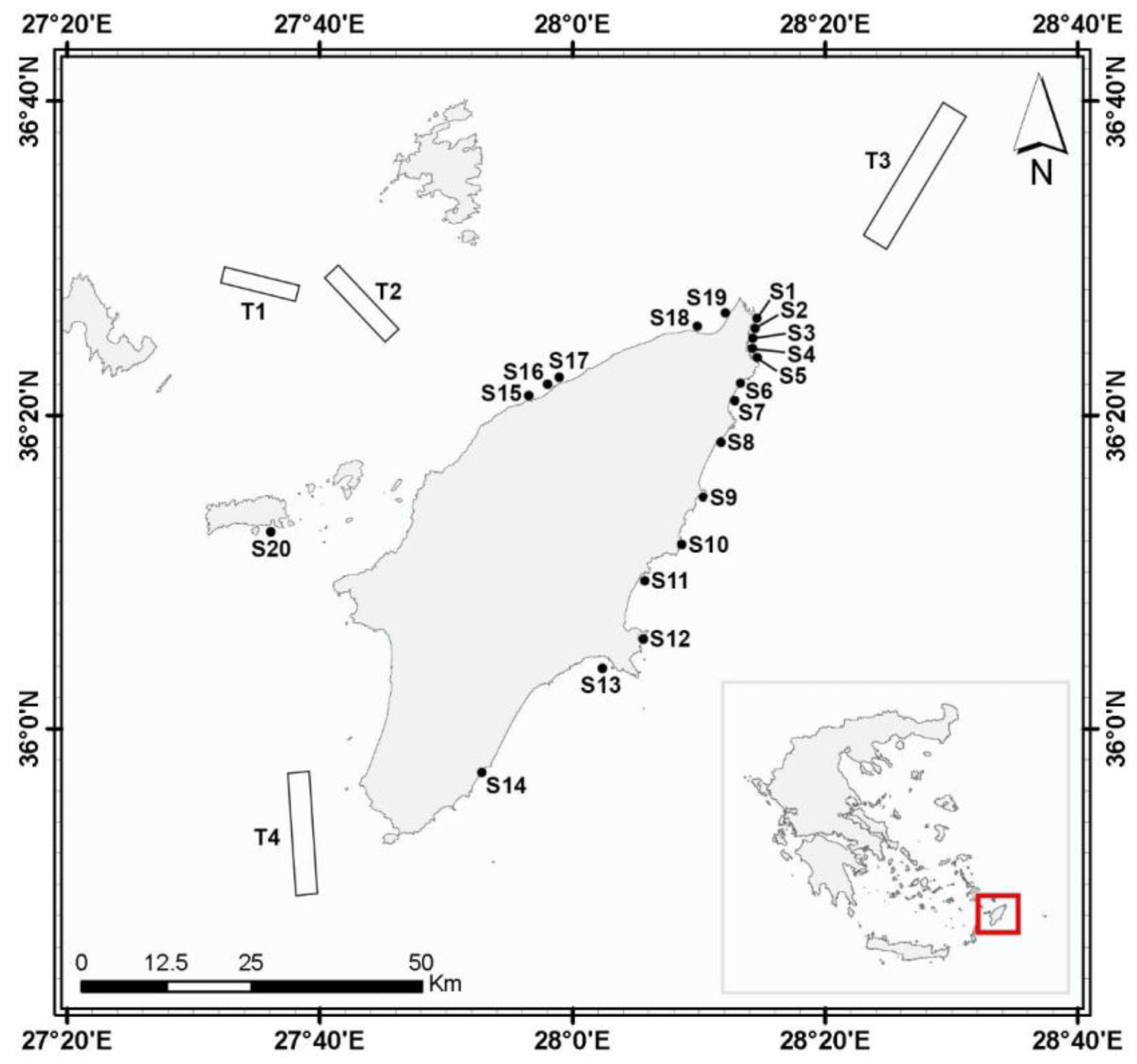
| Sites | Coordinates | Depth Range | |
|---|---|---|---|
| S1 | Mandraki Marina | 36°26′ N–28°14′ E | 1–2 |
| S2 | Zefiros | 36°25′ N–28°14′ E | 0.5–21 |
| S3 | Karakonero | 36°25′ N–28°14′ E | 0–1 |
| S4 | Aghia Marina | 36°24′ N–28°13′ E | 5–20 |
| S5 | Kalithea | 36°24′ N–28°14′ E | 14–20 |
| S6 | Faliraki beach | 36°22′ N–28°13′ E | Tidal |
| S7 | Faliraki | 36°20′ N–28°13′ E | 0.5–30 |
| S8 | Afandou Bay | 36°18′ N–28°12′ E | 130–187 |
| S9 | Kolimbia | 36°15′ N–28°10′ E | 1 |
| S10 | Stegna | 36°11′ N–28°08′ E | 20 |
| S11 | Haraki | 36°09′ N–28°06′ E | 80–150 |
| S12 | Lindos Bay | 36°05′ N–28°05′ E | 0.5–15 |
| S13 | Pefki | 36°04′ N–28°02′ E | 8–20 |
| S14 | Aghios Georgios | 35°57′ N–27°53′ E | 0 |
| S15 | Kalavarda | 36°21′ N–27°56′ E | 8–20 |
| S16 | Fanes | 36°22′ N–27°58′ E | 23 |
| S17 | Soroni | 36°22′ N–27°59′ E | 10–15 |
| S18 | Ixia | 36°26′ N–28°10′ E | 5–30 |
| S19 | Kritika | 36°26′ N–28°12′ E | 4–11 |
| S20 | Chalki Island | 36°12′ N–27°33′ E | Tidal |
| T1 | NW of Rhodes | 36°27′ N–27°38′ E | ~720 |
| T2 | NW of Rhodes | 36°25′ N–27°45′ E | ~570 |
| T3 | NE of Rhodes | 36°30′ N–28°26′ E | 600–650 |
| T4 | SW of Rhodes | 35°55′ N–27°35′ E | 650–750 |
| Taxa | References |
|---|---|
| Suborder DENDROBRANCHIATA Spence Bate, 1888 | |
| Family ARISTEIDAE Wood-Mason in Wood-Mason & Alcock, 1891 | |
| Aristaeomorpha foliacea (Risso, 1827) | present study |
| Aristeus antennatus (Risso, 1816) | present study |
| Family BENTHESICYMIDAE Wood-Mason in Wood-Mason & Alcock, 1891 | |
| Gennadas elegans (Smith, 1882) | [24] |
| Family PENAEIDAE Rafinesque, 1815 | |
| Metapenaeopsis aegyptia Galil & Golani, 1990 (A) | [25] |
| Metapenaeopsis mogiensis consobrina (Nobili, 1904) (A) | [25] |
| Parapenaeus longirostris (Lucas, 1846) | [13] |
| Penaeus aztecus Ives, 1891 (A) | [26] |
| Penaeus hathor (Burkenroad, 1959) (A) | [27] |
| Penaeus kerathurus (Forskål, 1775) | [28] as Penaeus caramote |
| Penaeus pulchricaudatus Stebbing, 1914 (A) | [29] as Penaeus japonicus |
| Trachysalambria palaestinensis (Steinitz, 1932) (A) | [25] as Trachypenaeus curvirostris |
| Family SICYONIIDAE Ortmann, 1898 | |
| Sicyonia carinata (Brünnich, 1768) | [13] |
| Family SOLENOCERIDAE Wood-Mason in Wood-Mason & Alcock, 1891 | |
| Solenocera membranacea (Risso, 1816) | [13] |
| Family SERGESTIDAE Dana, 1852 | |
| Robustosergia robusta (Smith, 1882) | present study |
| Family LUCIFERIDAE De Haan, 1849 | |
| Lucifer typus H. Milne Edwards, 1837 | [30] as Lucifer reynaudii |
| Suborder PLEOCYEMATA Burkenroad, 1963 | |
| Family STENOPODIDAE Claus, 1872 | |
| Stenopus spinosus Risso, 1827 | [14] |
| Family PASIPHAEIDAE Dana, 1852 | |
| Pasiphaea multidentata Esmark, 1866 | [24] |
| Pasiphaea sivado (Risso, 1816) | present study |
| Family ACANTHEPHYRIDAE Spence Bate, 1888 | |
| Acanthephyra pelagica (Risso, 1816) | [24] as Acanthephyra multispina |
| Acanthephyra eximia Smith, 1884 | [31] |
| Family PALAEMONIDAE Rafinesque, 1815 | |
| Gnathophyllum elegans (Risso, 1816) | [14] |
| Brachycarpus biunguiculatus (Lucas, 1846) | present study |
| Palaemon elegans Rathke, 1837 | [32] as Leander squilla var. elegans |
| Palaemon serratus (Pennant, 1777) | present study |
| Palaemon xiphias Risso, 1816 | [12] |
| Periclimenes scriptus (Risso, 1822) | present study |
| Pontonia pinnophylax (Otto, 1821) | [32] as Pontonia custos |
| Family ALPHEIDAE Rafinesque, 1815 | |
| Alpheus dentipes Guérin, 1832 | [12] |
| Athanas nitescens (Leach, 1814) | present study |
| Synalpheus gambarelloides (Nardo, 1847) | [32] as Synalpheus laevimanus |
| Family HIPPOLYTIDAE Spence Bate, 1888 | |
| Hippolyte inermis Leach, 1816 | present study |
| Family THORIDAE Kingsley, 1879 | |
| Eualus cranchii (Leach, 1817) | present study |
| Eualus occultus (Lebour, 1936) | present study |
| Family LYSMATIDAE Dana, 1852 | |
| Lysmata seticaudata (Risso, 1816) | [14] |
| Family PROCESSIDAE Ortmann, 1896 | |
| Processa acutirostris Nouvel & Holthuis, 1957 | [13] |
| Processa edulis (Risso, 1816) | present study |
| Processa macrophthalma Nouvel & Holthuis, 1957 | [13] |
| Family PANDALIDAE Haworth, 1825 | |
| Plesionika edwardsii (Brandt, 1851) | [33] |
| Plesionika martia (A. Milne-Edwards, 1883) | present study |
| Plesionika narval (Fabricius, 1787) | [33,34] |
| Family CRANGONIDAE Haworth, 1825 | |
| Aegaeon cataphractus (Olivi, 1792) | [13] |
| Philocheras fasciatus (Risso, 1816) | present study |
| Philocheras trispinosus (Hailstone, 1853) | present study |
| Family CALLIANASSIDAE Dana, 1852 | |
| Callianassa subterranea (Montagu, 1808) | [35] |
| Gourretia denticulata (Lutze, 1937) | [35] as Gourretia serrata |
| Pestarella candida (Olivi, 1792) | [12] as Callianassa pestai |
| Pestarella tyrrhena (Petagna, 1792) | [13] as Callianassa tyrrhena |
| Family UPOGEBIIDAE Borradaile, 1903 | |
| Gebiacantha talismani (Bouvier, 1915) | [35] as Upogebia talismani |
| Upogebia deltaura (Leach, 1816) | present study |
| Upogebia mediterranea Noël, 1992 | [36,37] |
| Upogebia pusilla (Petagna, 1792) | [13] |
| Upogebia stellata (Montagu, 1808) | [35] |
| Upogebia tipica (Nardo, 1869) | [12] |
| Family POLYCHELIDAE Wood-Mason, 1875 | |
| Polycheles typhlops Heller, 1862 | present study |
| Family PALINURIDAE Latreille, 1802 | |
| Palinurus elephas (Fabricius, 1787) | [28] as Palinurus vulgaris |
| Family SCYLLARIDAE Latreille, 1825 | |
| Scyllarides latus (Latreille, 1803) | [28] |
| Scyllarus arctus (Linnaeus, 1758) | [38] as Scyllarus arctos |
| Scyllarus pygmaeus (Bate, 1888) | [12] |
| Family DIOGENIDAE Ortmann, 1892 | |
| Calcinus tubularis (Linnaeus, 1767) | [12] as Calcinus ornatus |
| Clibanarius erythropus (Latreille, 1818) | [32] as Clibanarius misanthropus |
| Dardanus arrosor (Herbst, 1796) | [38] as Pagurus striatus |
| Dardanus calidus (Risso, 1827) | [12] as Dardanus callidus |
| Diogenes pugilator (Roux, 1829) | [12] |
| Paguristes eremita (Linnaeus, 1767) | [32] as Paguristes oculatus |
| Family PAGURIDAE Latreille, 1802 | |
| Anapagurus bicorniger A. Milne-Edwards & Bouvier,1892 | [14] |
| Anapagurus breviaculeatus Fenizia, 1937 | [12] as Anapagurus laevis |
| Anapagurus petiti Dechancé & Forest, 1962 | [14] |
| Cestopagurus timidus (Roux, 1830) | [12] as Catapaguroides timidus |
| Pagurus anachoretus Risso, 1827 | [12] |
| Pagurus cuanensis Bell, 1845 | present study |
| Pagurus excavatus (Herbst, 1791) | present study |
| Pagurus prideaux Leach, 1815 | [12] as Pagurus prideauxi |
| Family GALATHEIDAE Samouelle, 1819 | |
| Galathea intermedia Lilljeborg, 1851 | [39] |
| Galathea machadoi Barrois, 1888 | [40] |
| Galathea squamifera Leach, 1814 | [32] |
| Galathea strigosa (Linnaeus, 1761) | [38] |
| Family MUNIDIDAE Ahyong, Baba, Macpherson & Poore, 2010 | |
| Munida curvimana A. Milne-Edwards & Bouvier, 1894 | [14] |
| Family PORCELLANIDAE Haworth, 1825 | |
| Pisidia bluteli (Risso, 1816) | [12] |
| Porcellana platycheles (Pennant, 1777) | [12] |
| Family ALBUNEIDAE Stimpson, 1858 | |
| Albunea carabus (Linnaeus, 1758) | [15] |
| Family DROMIIDAE De Haan, 1833 | |
| Dromia personata (Linnaeus, 1758) | [38] as Dromia vulgaris |
| Family HOMOLIDAE De Haan, 1839 | |
| Homola barbata (Fabricius, 1793) | [13] |
| Family LATREILLIIDAE Stimpson, 1858 | |
| Latreillia elegans Roux, 1830 | [13] |
| Family ETHUSIDAE Guinot, 1977 | |
| Ethusa mascarone (Herbst, 1785) | [13] |
| Family DORIPPIDAE MacLeay, 1838 | |
| Medorippe lanata (Linnaeus, 1767) | present study |
| Family CALAPPIDAE De Haan, 1833 | |
| Calappa granulata (Linnaeus, 1758) | [38] |
| Family MATUTIDAE De Haan, 1835 | |
| Matuta victor (Fabricius, 1781) (A) | [41] |
| Family LEUCOSIIDAE Samouelle, 1819 | |
| Coleusia signata (Paulson, 1875) (A) | [42] as Leucosia signata |
| Ilia nucleus (Linnaeus, 1758) | [13] |
| Ixa monodi Holthuis & Gottlieb, 1956 (A) | [7] |
| Myra subgranulata Kossmann, 1877 (A) | [43] |
| Family INACHIDAE MacLeay, 1838 | |
| Inachus communissimus Rizza, 1839 | [13] |
| Inachus dorsettensis (Pennant, 1777) | [13] |
| Inachus leptochirus Leach, 1817 | [13] |
| Inachus thoracicus Roux, 1830 | [13] |
| Macropodia czernjawskii (Brandt, 1880) | present study |
| Macropodia longirostris (Fabricius, 1775) | present study |
| Macropodia rostrata (Linnaeus, 1761) | [39] |
| Family MAJIDAE Samouelle, 1819 | |
| Eurynome aspera (Pennant, 1777) | [39] |
| Maja crispata Risso, 1827 | [44] as Maja verrucosa |
| Maja squinado (Herbst, 1788) | [38] |
| Neomaja goltziana (d’Oliveira, 1888) | [14] as Maja goltziana |
| Family EPIALTIDAE MacLeay, 1838 | |
| Acanthonyx lunulatus (Risso, 1816) | [32] |
| Herbstia condyliata (Fabricius, 1787) | [43] |
| Lissa chiragra (Fabricius, 1775) | [14] |
| Pisa armata (Latreille, 1803) | [13] |
| Pisa hirticornis (Herbst, 1804) | [12] as Pisa corallina |
| Pisa muscosa (Linnaeus, 1758) | [13] |
| Pisa tetraodon (Pennant, 1777) | [45] |
| Family PARTHENOPIDAE MacLeay, 1838 | |
| Derilambrus angulifrons (Latreille, 1825) | [14] |
| Spinolambrus macrochelos (Herbst, 1790) | [14] |
| Family PIRIMELIDAE Alcock, 1899 | |
| Pirimela denticulata (Montagu, 1808) | present study |
| Family CARCINIDAE MacLeay, 1838 | |
| Portumnus latipes (Pennant, 1777) | present study |
| Family PORTUNIDAE Rafinesque, 1815 | |
| Callinectes sapidus Rathbun, 1896 (A) | [43,46] |
| Carupa tenuipes Dana, 1852 (A) | [47] |
| Charybdis hellerii (A. Milne-Edwards, 1867) (A) | [48] |
| Charybdis (Goniohellenus) longicollis Leene, 1938 (A) | [7] |
| Gonioinfradens giardi (Nobili, 1905) (A) | [49] as Gonioinfradens paucidentatus |
| Portunus hastatus (Linnaeus, 1767) | [38] as Neptunus hastatus |
| Portunus segnis (Forskål, 1775) (A) | [50] as Portunus pelagicus |
| Thalamita poissonii (Audouin, 1826) (A) | [51] |
| Family POLYBIIDAE Ortmann, 1893 | |
| Bathynectes maravigna (Prestandrea, 1839) | present study |
| Liocarcinus bolivari (Zariquiey Álvarez, 1948) | present study |
| Liocarcinus corrugatus (Pennant, 1777) | [38] as Portunus corrugatus |
| Liocarcinus depurator (Linnaeus, 1758) | [38] as Portunus depurator |
| Liocarcinus navigator (Herbst, 1794) | [32] as Portunus arcuatus |
| Liocarcinus zariquieyi (Gordon, 1968) | [12] as Macropipus zariquieyi |
| Family GERYONIDAE Colosi, 1923 | |
| Chaceon mediterraneus Manning & Holthuis, 1989 | [52] |
| Geryon longipes A. Milne-Edwards, 1882 | present study |
| Family PROGERYONIDAE Števčić, 2005 | |
| Paragalene longicrura (Nardo, 1869) | [14] |
| Family GONEPLACIDAE MacLeay, 1838 | |
| Goneplax rhomboides (Linnaeus, 1758) | [39] |
| Family ERIPHIIDAE MacLeay, 1838 | |
| Eriphia verrucosa (Forskål, 1775) | [44] as Eriphia spinifrons |
| Family XANTHIDAE MacLeay, 1838 | |
| Actaeodes tomentosus (H. Milne Edwards, 1834) (A) | [53] |
| Atergatis roseus (Rüppell, 1830) (A) | [51] |
| Monodaeus guinotae Forest, 1976 | [13] |
| Paractaea monodi Guinot, 1969 | [14] |
| Xanthias lamarckii (H. Milne Edwards, 1834) (A) | [16] |
| Xantho granulicarpus Forest in Drach & Forest,1953 | [12] |
| Xantho poressa (Olivi, 1792) | [32] |
| Family PILUMNIDAE Samouelle, 1819 | |
| Pilumnus hirtellus (Linnaeus, 1761) | [32] |
| Pilumnus villosissimus (Rafinesque, 1814) | [14] |
| Family GRAPSIDAE MacLeay, 1838 | |
| Pachygrapsus marmoratus (Fabricius, 1787) | [32] |
| Pachygrapsus transversus (Gibbes, 1850) | present study |
| Planes minutus (Linnaeus, 1758) | [14] |
| Family PERCNIDAE Števčić, 2005 | |
| Percnon gibbesi (H. Milne Edwards, 1853) (A) | [54] |
| Family MACROPHTHALMIDAE Dana, 1851 | |
| Macrophthalmus indicus Davie, 2012 (A) | [55] as Macrophthalmus graeffei |
| Family OCYPODIDAE Rafinesque, 1815 | |
| Ocypode cursor (Linnaeus, 1758) | [56,57] |
| Family PALICIDAE Bouvier, 1898 | |
| Palicus caronii (Roux, 1828) | [13] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondylatos, G.; Crocetta, F.; Corsini-Foka, M.; Froglia, C. Crustacea Decapoda from the Rhodes Island Area (Eastern Mediterranean): New Records and an Updated Checklist. Diversity 2020, 12, 246. https://doi.org/10.3390/d12060246
Kondylatos G, Crocetta F, Corsini-Foka M, Froglia C. Crustacea Decapoda from the Rhodes Island Area (Eastern Mediterranean): New Records and an Updated Checklist. Diversity. 2020; 12(6):246. https://doi.org/10.3390/d12060246
Chicago/Turabian StyleKondylatos, Gerasimos, Fabio Crocetta, Maria Corsini-Foka, and Carlo Froglia. 2020. "Crustacea Decapoda from the Rhodes Island Area (Eastern Mediterranean): New Records and an Updated Checklist" Diversity 12, no. 6: 246. https://doi.org/10.3390/d12060246
APA StyleKondylatos, G., Crocetta, F., Corsini-Foka, M., & Froglia, C. (2020). Crustacea Decapoda from the Rhodes Island Area (Eastern Mediterranean): New Records and an Updated Checklist. Diversity, 12(6), 246. https://doi.org/10.3390/d12060246






