First Study on Marine Heterobranchia (Gastropoda, Mollusca) in Bangka Archipelago, North Sulawesi, Indonesia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Animal Collections
3.1.1. Cephalaspidea (Five Species in Five Genera Belonging to Four Families)
3.1.2. Aplysiida (= Anaspidea) (Two Species in Two Genera Belonging to One Family)
3.1.3. Sacoglossa (15 Species in Four Genera Belonging to Three Families)
3.1.4. Pleurobranchida (Two Species in One Genus Belonging to One Family)
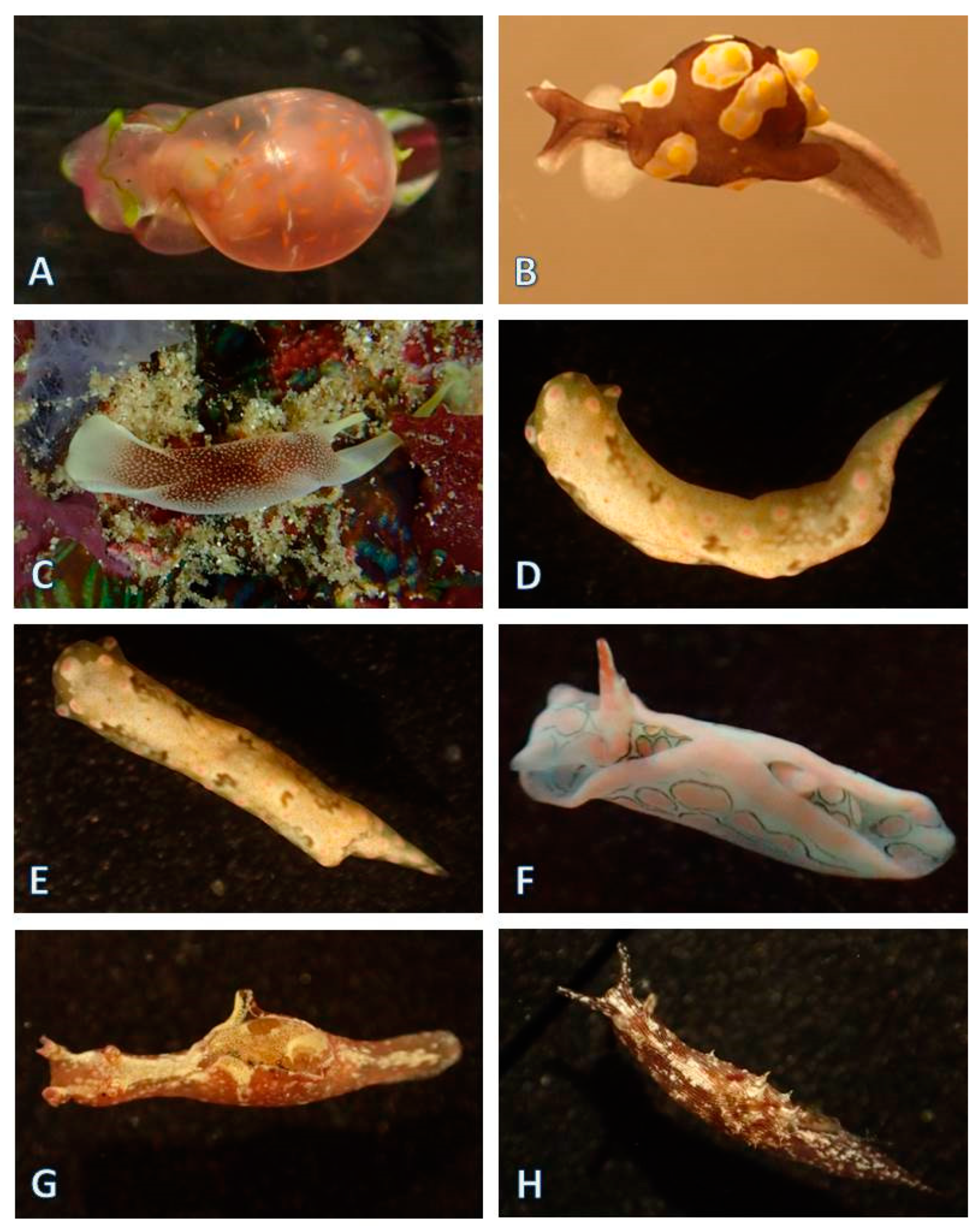
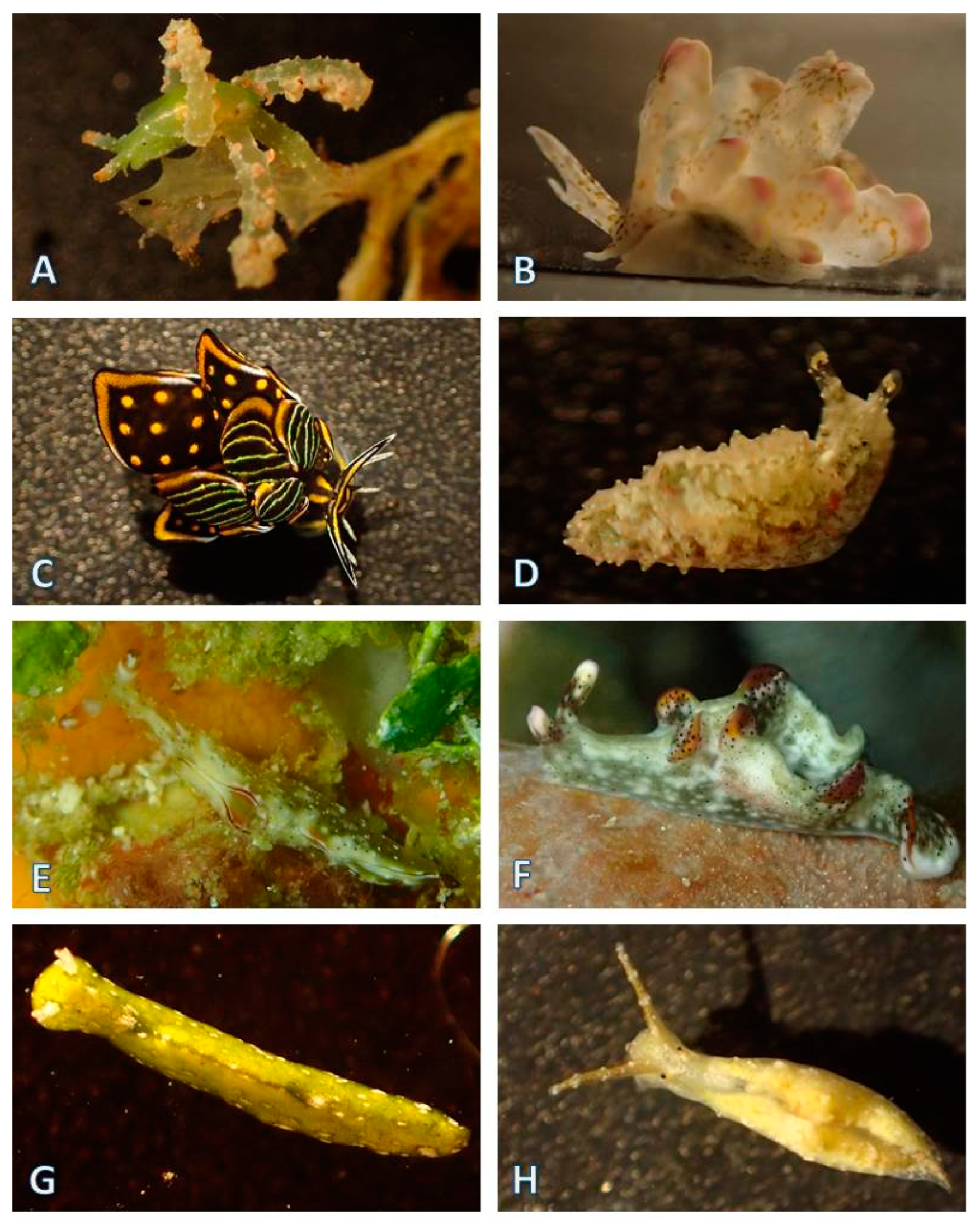
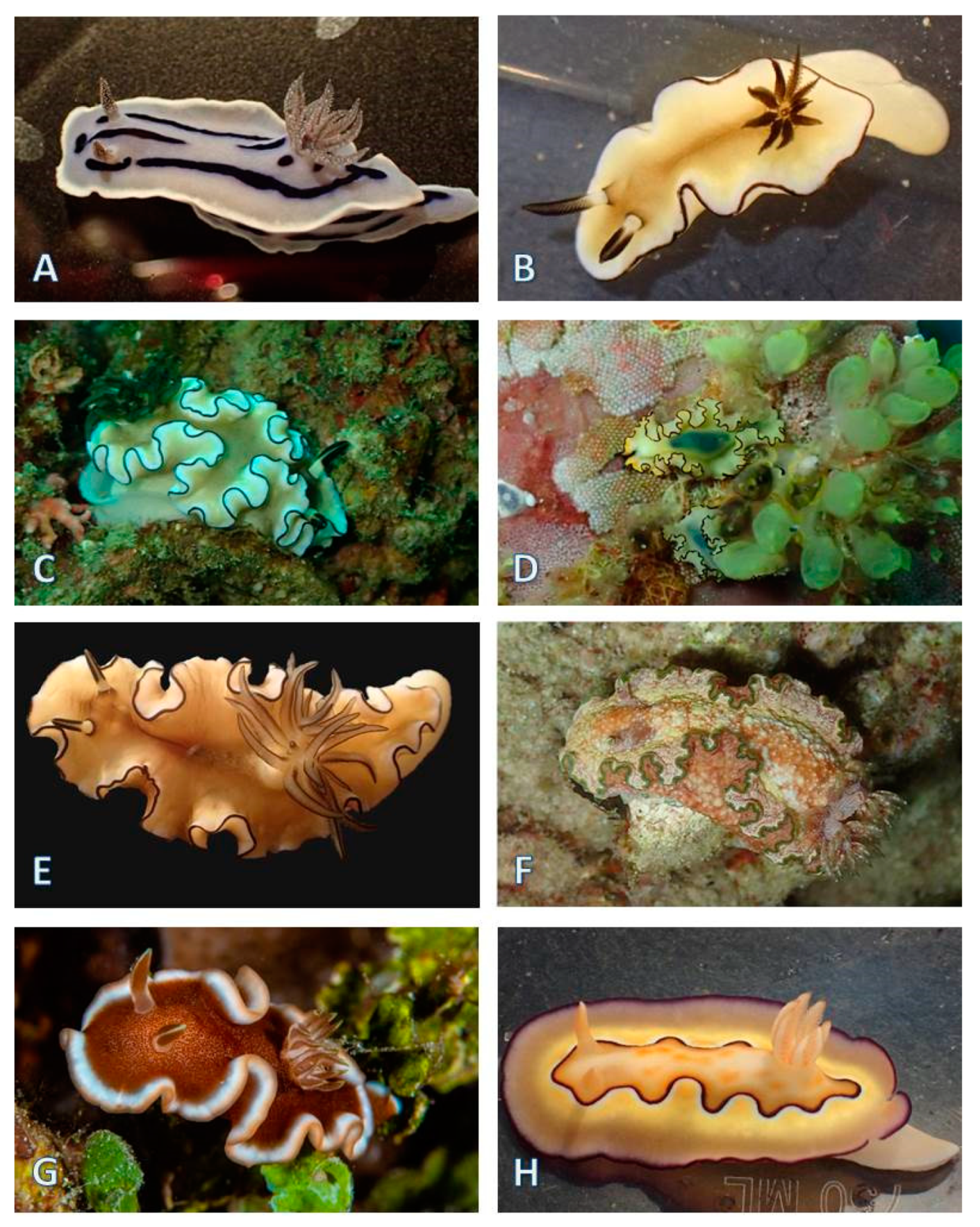
3.1.5. Nudibranchia (124 Species)
3.1.6. Eupulmonata (One Species in One Genus Belonging to One Family)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaligis, F.; Eisenbarth, J.H.; Schillo, D.; Dialao, J.; Schäberle, T.F.; Böhringer, N.; Bara, R.; Reumschüssel, S.; König, G.M.; Wägele, H. Second survey of heterobranch sea slugs (Mollusca, Gastropoda, Heterobranchia) from Bunaken National Park, North Sulawesi, Indonesia—How much do we know after 12 years. Mar. Biodiver. Rec. 2018, 11, 1–20. [Google Scholar] [CrossRef]
- Naturalis Biodiversity Center, Repository, Rumphius Biohistorical. Available online: http://www.repository.naturalis.nl/cgi/b/bib/bib-idx?type=simple;c=naturalis;rgn1=entire%20record;q1=Rumphius%20Biohistorical;Submit.x=14;Submit.y=11;lang=en;sort=year;cc=naturalis;view=reslist;fmt=short;page=reslist;size=10;start=1;sid= (accessed on 9 February 2019).
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 16. Nudibranchia—Dendronotina, Arminina, Aeolidina, and Doridina (Mollusca: Gastropoda: Heterobranchia). Arch. Molluskenkd. 2017, 146, 135–172. [Google Scholar] [CrossRef]
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 15. The suborder Doridina (Mollusca, Gastropoda, Opisthobranchia, Nudibranchia). Zool. Meded. 2011, 85, 905–956. [Google Scholar]
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 11. Doridacea of the families Chromodorididae and Hexabranchidae (Mollusca, Gastropoda, Opisthobranchia, Nudibranchia). Zool. Meded. 2001, 75, 1–50. [Google Scholar]
- Yonow, N.; Jensen, K.R. Results of the Rumphius Biohistorical Expedition to Ambon (1990). Part 17. The Cephalaspidea, Anaspidea, Pleurobranchida, and Sacoglossa (Mollusca: Gastropoda: Heterobranchia). Arch. Molluskenkd. 2018, 147, 1–48. [Google Scholar] [CrossRef]
- Böhringer, N.; Fisch, K.M.; Schillo, D.; Bara, R.; Hertzer, C.; Grein, F.; Eisenbarth, J.-H.; Kaligis, F.; Schneider, T.; Wägele, H.; et al. Antimicrobial potential of bacteria associated with marine sea slugs from North Sulawesi, Indonesia. Front. Microbiol. 2017, 8, 1092. [Google Scholar] [CrossRef]
- Burghardt, I.; Gosliner, T.M. Phyllodesmium rudmani (Mollusca: Nudibranchia: Aeolidoidea), a new solar powered species from the Indo-West Pacific with data on its symbiosis with zooxanthellae. Zootaxa 2008, 1308, 31–47. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New antifouling sesquiterpenes from four nudibranchs of the family Phyllidiidae. Tetrahedron 1996, 52, 9447–9454. [Google Scholar] [CrossRef]
- Fisch, K.M.; Hertzer, C.; Böhringer, N.; Wuisan, Z.G.; Schillo, D.; Bara, R.; Kaligis, F.; Wägele, H.; König, G.M.; Schäberle, T.F. The potential of Indonesian gastropods found around Bunaken Island for production of bioactive compounds. Mar. Drugs 2017, 15, 384. [Google Scholar] [CrossRef]
- Haber, M.; Cerfeda, S.; Carbone, M.; Calado, G.; Gaspar, H.; Neves, R.; Maharajan, V.; Cimino, G.; Gavagnin, M.; Ghiselin, M.T.; et al. Coloration and defense in the nudibranch gastropod Hypselodoris fontandraui. Biol. Bull. 2010, 218, 181–188. [Google Scholar] [CrossRef]
- Wells, F.E.; Bryce, C.W. Sea Slugs and Their Relatives of Western Australia; Western Australian Museum: Perth, Australia, 1993; p. 184. [Google Scholar]
- Nimbs, M.J.; Larkin, M.; Davis, T.R.; Harasti, D.; Willan, R.C.; Smith, S.D.A. Southern range extensions for twelve heterobranch sea slugs (Gastropoda: Heterobranchia) on the eastern coast of Australia. Mar. Biodivers. Rec. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Smith, S.D.A. Beyond Captricornia: Tropical sea slugs (Gastropoda, Heterobranchia) extend their distributions into the Tasman Sea. Diversity 2018, 10, 99. [Google Scholar] [CrossRef]
- Burghardt, I.; Carvalho, R.; Eheberg, D.; Gerung, G.; Kaligis, F.; Mamangkey, G.; Schrödl, M.; Schwabe, E.; Vonnemann, V.; Wägele, H. Molluscan diversity at Bunaken National Park, Sulawesi. J. Zool. Soc. Wallacea 2006, 2, 29–43. [Google Scholar]
- Eisenbarth, J.-H.; Undap, N.; Papu, A.; Schillo, D.; Dialao, J.; Reumschüssel, S.; Kaligis, F.; Bara, R.; Schäberle, T.F.; König, G.M.; et al. Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A follow-up diversity study. Diversity 2018, 10, 127. [Google Scholar] [CrossRef]
- Ompi, M.; Lumoindong, F.; Undap, N.; Papu, A.; Wägele, H. Monitoring marine Heterobranchia in Lembeh Strait, North Sulawesi (Indonesia), in a changing environment. AACL Bioflux 2019, 12, 664–667. [Google Scholar]
- Undap, N.; Papu, A.; Schillo, D.; Ijong, F.G.; Kaligis, F.; Lepar, M.; Hertzer, C.; Böhringer, N.; König, G.M.; Schäberle, T.F.; et al. First survey of heterobranch sea slugs (Mollusca, Gastropoda) from the island Sangihe, North Sulawesi, Indonesia. Diversity 2019, 11, 1–30. [Google Scholar] [CrossRef]
- Ponti, M.; Fratangeli, F.; Dondi, N.; Reinach, M.S.; Serra, C.; Sweet, M.J. Baseline reef health surveys at Bangka Island (North Sulawesi, Indonesia) reveal new threats. PeerJ 2016, 4, e2614. [Google Scholar] [CrossRef]
- Layton, K.K.S.; Gosliner, T.M.; Wilson, N.G. Flexible color check patterns obscure identification and mimicry in Indo–Pacific Chromodoris nudibranchs (Gastropoda: Chromodorididae). Mol. Phyl. Evol. 2018, 124, 27–36. [Google Scholar] [CrossRef]
- Sea Slug Forum Home Page. Available online: http://www.seaslugforum.net/ (accessed on 10 December 2019).
- Martynov, A.V.; Korshunova, T.A. Opisthobranch molluscs of Vietnam (Gastropoda: Opisthobranchia). In Benthic Fauna of the Bay of Nhatrang, Southern Vietnam 2; Britayev, T.Z., Pavlov, D.S., Eds.; KMK Scientific Press: Moscow, Russia, 2012; pp. 142–257. [Google Scholar]
- Burghardt, I.; Wägele, H. The symbiosis between the ‘solar-powered’ nudibranch Melibe engeli Risbec, 1937 (Dendronotoidea) and Symbiodinium sp. (Dinophyceae). J. Moll. Stud. 2014, 80, 508–517. [Google Scholar] [CrossRef]
- Gosliner, T.M.; Valdés, A.; Behrens, D.W. Nudibranch & Sea Slug Identification Indo-Pacific; New World Publications: Jacksonville, FL, USA, 2015; p. 408. [Google Scholar]
- Stoffels, B.E.M.W.; van der Meij, S.E.T.; Hoeksema, B.W.; van Alphen, J.; van Alen, T.; Meyers-Munoz, M.A.; de Voogd, N.J.; Tuti, Y.; van der Velde, G. Phylogenetic relationships within the Phyllidiidae (Opisthobranchia, Nudibanchia). ZooKeys 2016, 605, 1–35. [Google Scholar] [CrossRef]
- World Register of Marine Species. Available online: http://www.marinespecies.org/ (accessed on 17 January 2020).
- Epstein, H.E.; Hallas, J.M.; Johnson, R.F.; Lopez, A.; Gosliner, T.M. Reading between the lines: Revealing cryptic species diversity and color patterns in Hypselodoris nudibranchs (Mollusca: Heterobranchia: Chromodorididae). Zool. J. Linn. Soc. 2019, 186, 116–189. [Google Scholar] [CrossRef]
- Diepenbroek, M.; Glökner, F.O.; Grobe, P.; Güntsch, A.; Huber, R.; König-Ries, B.; Kostadinov, I.; Nieschulze, J.; Seeger, B.; Tolksdorf, R.; et al. Towards an integrated biodiversity and ecological research data management and archiving platform: The German Federation for the Curation of Biological Data (GFBio). In Proceedings of the GI, Informatik 2014—Big Data Komplexität meistern, Stuttgart, Germany, January 2014; Plödereder, E., Grunske, L., Schneider, E., Ull, D., Eds.; Köllen Verlag: Bonn, Germany, 2014; pp. 1711–1724. [Google Scholar]
- Golestani, H.; Crocetta, F.; Padula, V.; Camacho, Y.; Langeneck, J.; Poursanidis, D.; Pola, M.; Yokes, M.B.; Cervera, J.L.; Jung, D.W.; et al. The little Aplysia coming of age: From one species to a complex of species complexes in Aplysia parvula (Mollusca: Gastropoda: Heterobranchia). Zool. J. Linn. Soc. 2019, 187, 279–330. [Google Scholar] [CrossRef]
- Bouchet, P.; Rockroi, J.-P.; Hausdorf, B.; Kaim, A.; Kano, Y.; Nützel, A.; Parkvaev, P.; Schrödl, M.; Strong, E.E. Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 2017, 61, 1–526. [Google Scholar] [CrossRef]
- Martín-Hervás, M.R.; Carmona, L.; Malaquias, M.A.E.; Krug, P.J.; Gosliner, T.; Cervera, J.L. Integrative Systematics of the Genus Thuridilla Bergh, 1872 (Mollusca, Gastropoda, Heterobranchia) reveals a cryptic radiation of polymorphic sea slug species. In Proceedings of the World Congress of Malacology, Pacific Grove, CA, USA, 11–16 August 2019; California Academy of Sciences: San Francisco, CA, USA; p. 119. Available online: https://www.calacademy.org/sites/default/files/final_abstract_volume_2019_1.pdf (accessed on 13 January 2020).
- Matsuda, S.B.; Gosliner, T.M. Glossing over cryptic species: Descriptions of four new species of Glossodoris and three new species of Doriprismatica (Nudibranchia: Chromodorididae). Zootaxa 2018, 4444, 501–529. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.E.; Wilson, N.G.; van den Berg, C.P.; How, M.J.; Endler, J.A.; Marshall, N.J.; White, A.M.; Garson, M.J.; Cheney, K.L. Toxicity and taste: Unequal chemical defences in a mimicry ring. Proc. R. Soc. B Biol. Sci. 2018, 285. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Cobb, G.; Mullins, D. Nudibranchs Encyclopedia, Catalogue of Asia/Indo Pacific Sea Slugs, 2nd ed.; Masalai Press: Oakland, CA, USA, 2015; p. 312. ISBN 978-0-9714127-9-8. [Google Scholar]
- Korshunova, T.; Martynov, A.; Picton, B. Ontogeny as an important part of integrative taxonomy in tergipedid aeolidaceans (Gastropoda: Nudibranchia) with a description of a new genus and species from the Barents Sea. Zootaxa 2017, 4324, 1–22. [Google Scholar] [CrossRef]
- Schillo, D.; Wipfler, B.; Undap, N.; Böhringer, N.; Eisenbarth, J.-H.; Kaligis, F.; Bara, R.; Schäberle, T.F.; König, G.M.; Wägele, H. Description of a new Moridilla species from North Sulawesi, Indonesia (Mollusca: Nudibranchia: Aeolidioidea)—based on MicroCT, histological and molecular analyses. Zootaxa 2019, 4652, 265–295. [Google Scholar] [CrossRef]
- Yasman. Observation on the feeding of nudibranch Phyllidia varicosa Lamarck, 1801 on the sponge Axinyssa cf. aculeata Wilson, 1925 in coral reefs of Pramuka Island, Thousands Islands National Park, Indonesia. Makara Sains 2003, 7, 15–21. [Google Scholar]
- Alqudah, A.A.; Shahbudin, S.; Deny, S.; Hadry, N.F.; Khodzori, M.F.A.; Yusof, M.H.; Rani, M.H. Observations on nudibranch behaviour patterns under laboratory conditions. J. Teknol. 2016, 78, 167–171. [Google Scholar] [CrossRef][Green Version]
- Tonozuka, T. Opisthobranchs of Bali and Indonesia; Hankyu Communications Co. Ltd.: Tokyo, Japan, 2003; p. 164. [Google Scholar]
- Pasternak, G.; Galil, B.S. An established population of the alien sea slug Elysia grandifolia Kelaart, 1858 (Mollusca, Opisthobranchia, Elysiidae) off the Mediterranean coast of Israel. BioInvasions Rec. 2012, 1, 221–223. [Google Scholar] [CrossRef]
- Padula, V.; Bahia, J.; Stöger, I.; Camacho-Garcia, Y.; Malaquias, M.A.E.; Cervera, J.L.; Schrödl, M. A test of color-based taxonomy in nudibranchs: Molecular phylogeny and species delimitation of the Felimida clenchi (Mollusca: Chromodorididae) species complex. Mol. Phyl. Evol. 2016, 103, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.A.; Davis, T.R. Slugging it out for science: Volunteers provide valuable data on the diversity and distribution of heterobranch sea slugs. Moll. Res. 2019, 39, 214–233. [Google Scholar] [CrossRef]
- Pola, M.; Cervera, J.L.; Gosliner, T.M. Revision of the Indo-Pacific genus Nembrotha (Nudibranchia: Dorididae: Polyceridae), with a description of two new species. Sci. Mar. 2008, 72, 145–183. [Google Scholar] [CrossRef]
- Tibiriçá, Y.; Pola, M.; Cervera, J.L. Astonishing diversity revealed: An annotated and illustrated inventory of Nudipleura (Gastropoda: Heterobranchia) from Mozambique. Zootaxa 2017, 4359, 1–133. [Google Scholar] [CrossRef]
- Fetzer, I. Reproduction strategies and distribution of larvae and juveniles of benthic soft-bottom invertebrates in the Kara Sea (Russian Arctic). The influence of river discharge on the structure of benthic communities: A larval approach. Ph.D. Thesis, Bremen University, Bremen, Germany, 2004. Available online: http://hdl.handle.net/10013/epic.21857.d001 (accessed on 28 January 2020).
- García, F.J.; Bertsch, H. Diversity and distribution of the Gastropoda Opisthobranchia from the Atlantic Ocean: A global biogeographic approach. Sci. Mar. 2009, 73, 153–160. [Google Scholar] [CrossRef]
- Brodie, G.D.; Brodie, J.E. Species diversity and habitat selection in opisthobranch gastropods on two adjacent reefs in Fiji. South Pacific, J. Nat. Sci. 1995, 14, 97–113. [Google Scholar]
- Gosliner, T.M. Biodiversity of tropical opisthobranch gastropod faunas. In Proceedings of the Seventh International Coral Reef Symposium, Guam, Micronesia, 22–27 June 1992; pp. 702–709. [Google Scholar]
- Larkin, M.F.; Smith, S.D.A.; Willan, R.C.; Davis, T.R. Diel and seasonal variation in heterobranch sea slug assemblages within an embayment in temperate eastern Australia. Mar. Biodiv. 2018, 48, 1541–1550. [Google Scholar] [CrossRef]
- Smith, S.D.A.; Nimbs, M.J. Quantifying temporal variation in heterobranch (Mollusca: Gastropoda) sea slug assemblages: Tests of alternate models. Moll. Res. 2017, 37, 140–147. [Google Scholar] [CrossRef]
- Gosliner, T.M.; Valdés, A.; Behrens, D.W. Nudibranch & Sea Slug Identification Indo-Pacific; New World Publications: Jacksonville, FL, USA, 2018; p. 451. [Google Scholar]
- Usman, M.S.; Kusen, J.D.; Rimper, J.R.T.S.L. Struktur Komunitas Plankton di Perairan Pulau Bangka Kabupaten Minahasa Utara. J. Pesisir dan Laut Trop. 2013, 2, 51–57. [Google Scholar] [CrossRef]
- Qu, C.F.; Song, J.M.; Li, N. Causes of jellyfish blooms and their influence on marine environment. Ying Yong Sheng Tai Xue Bao. 2014, 25, 3701–3712. [Google Scholar]
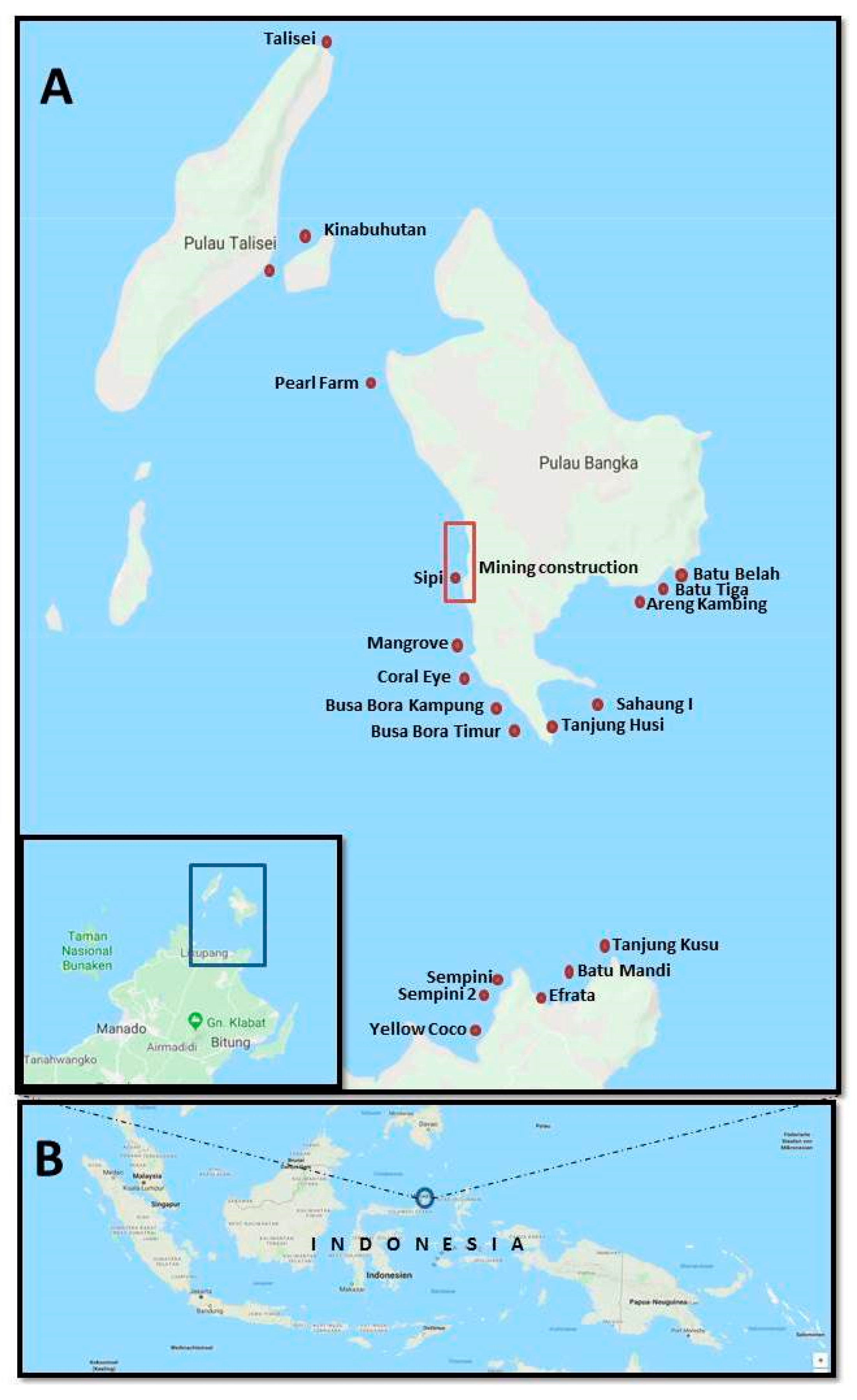









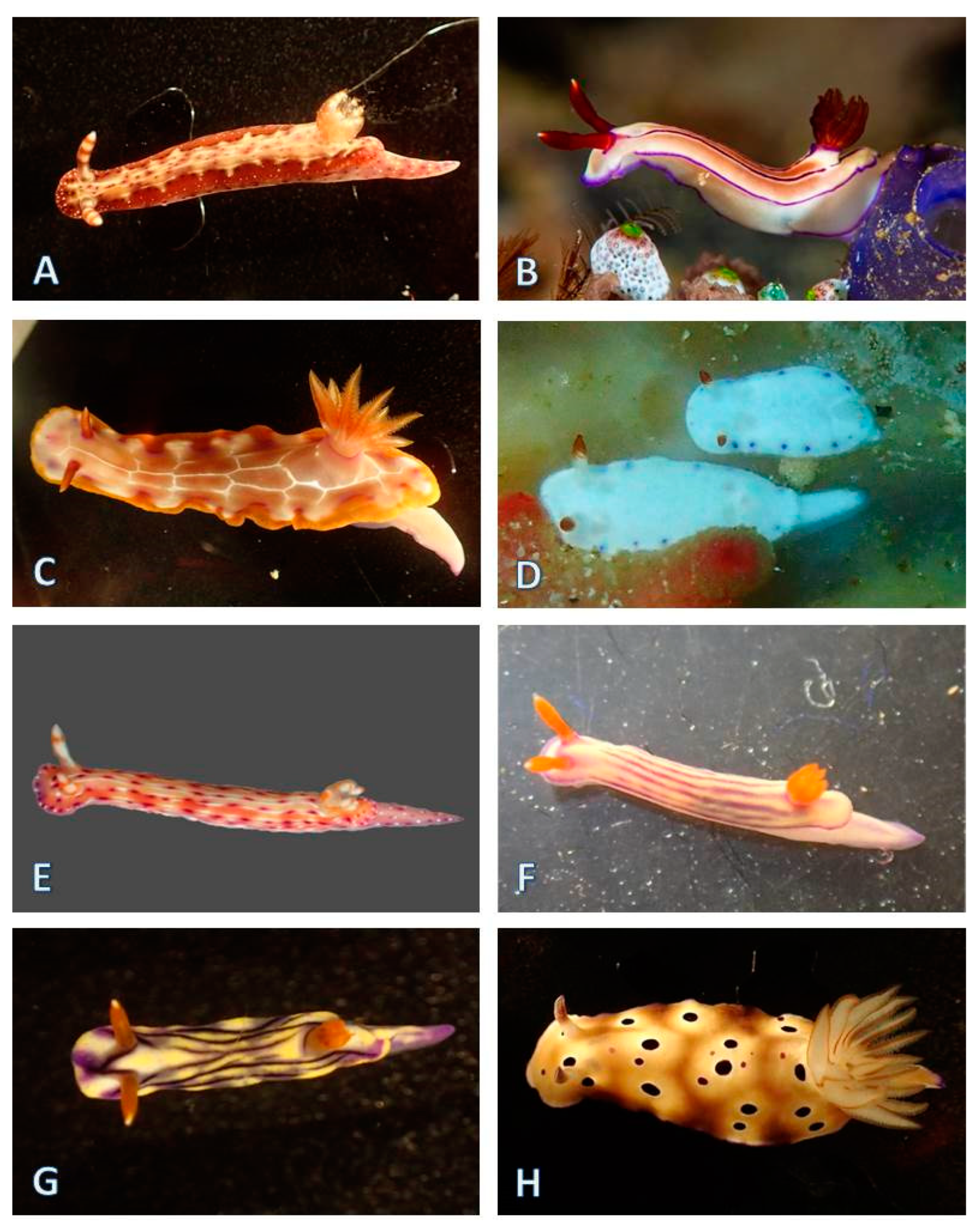



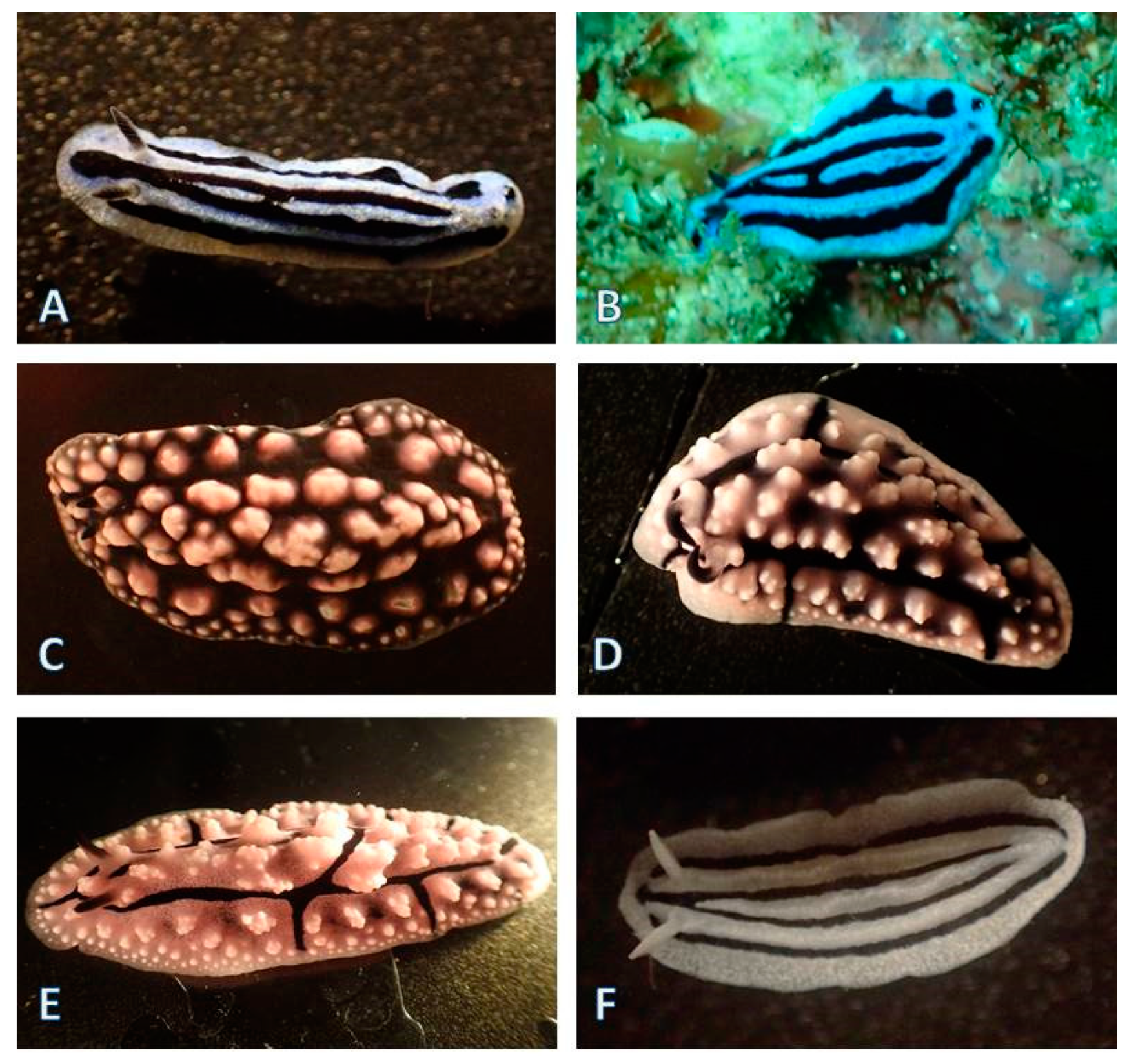

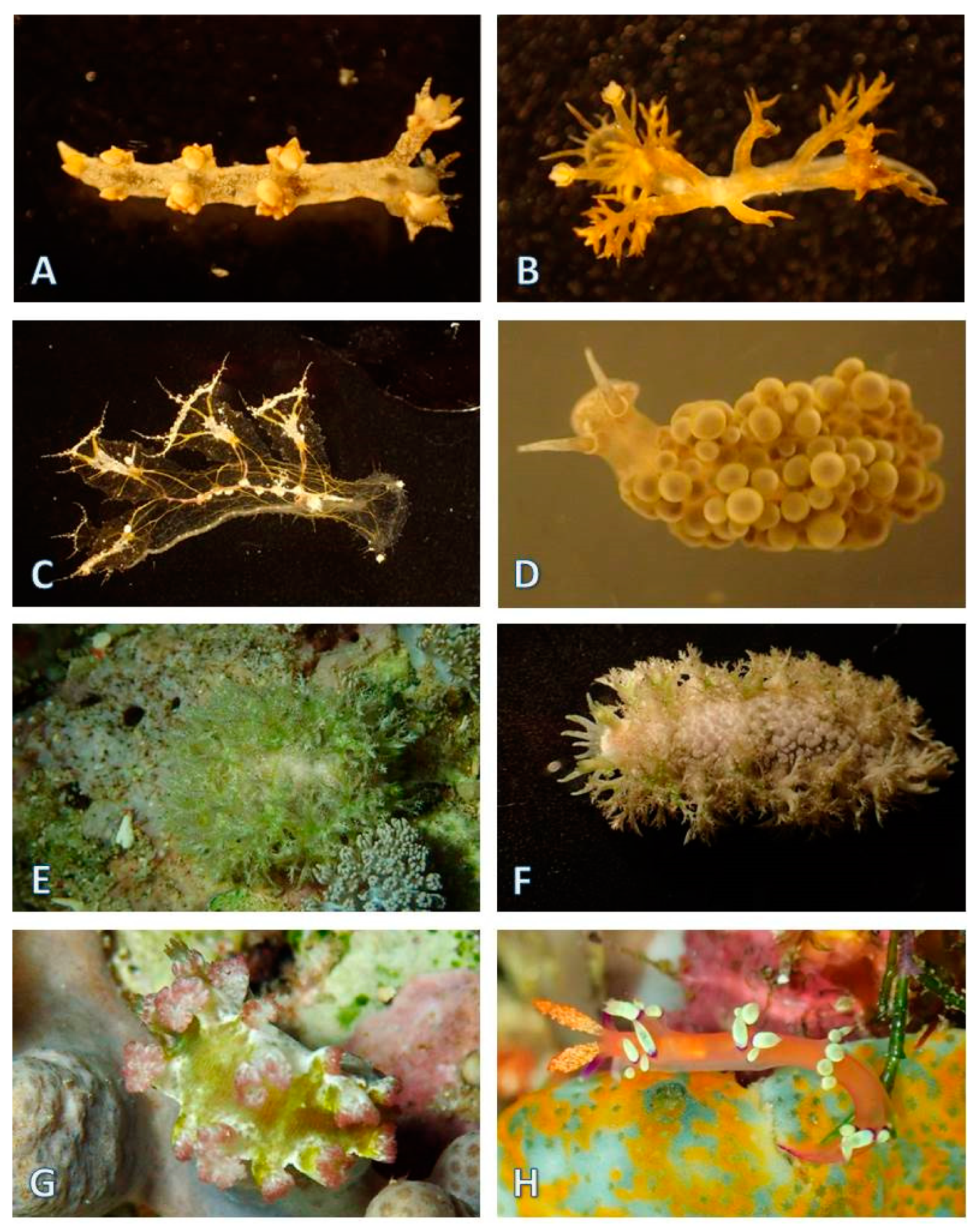





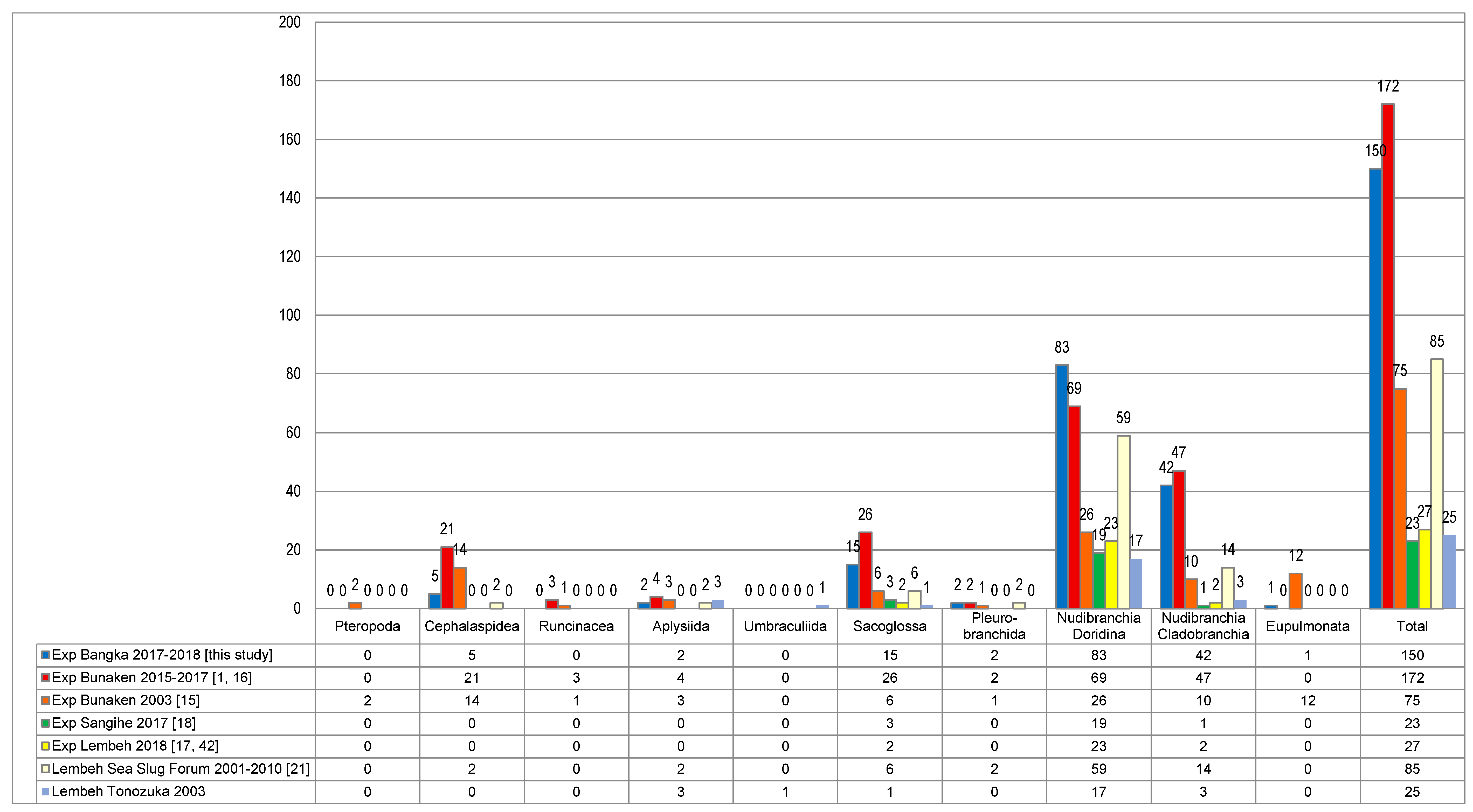
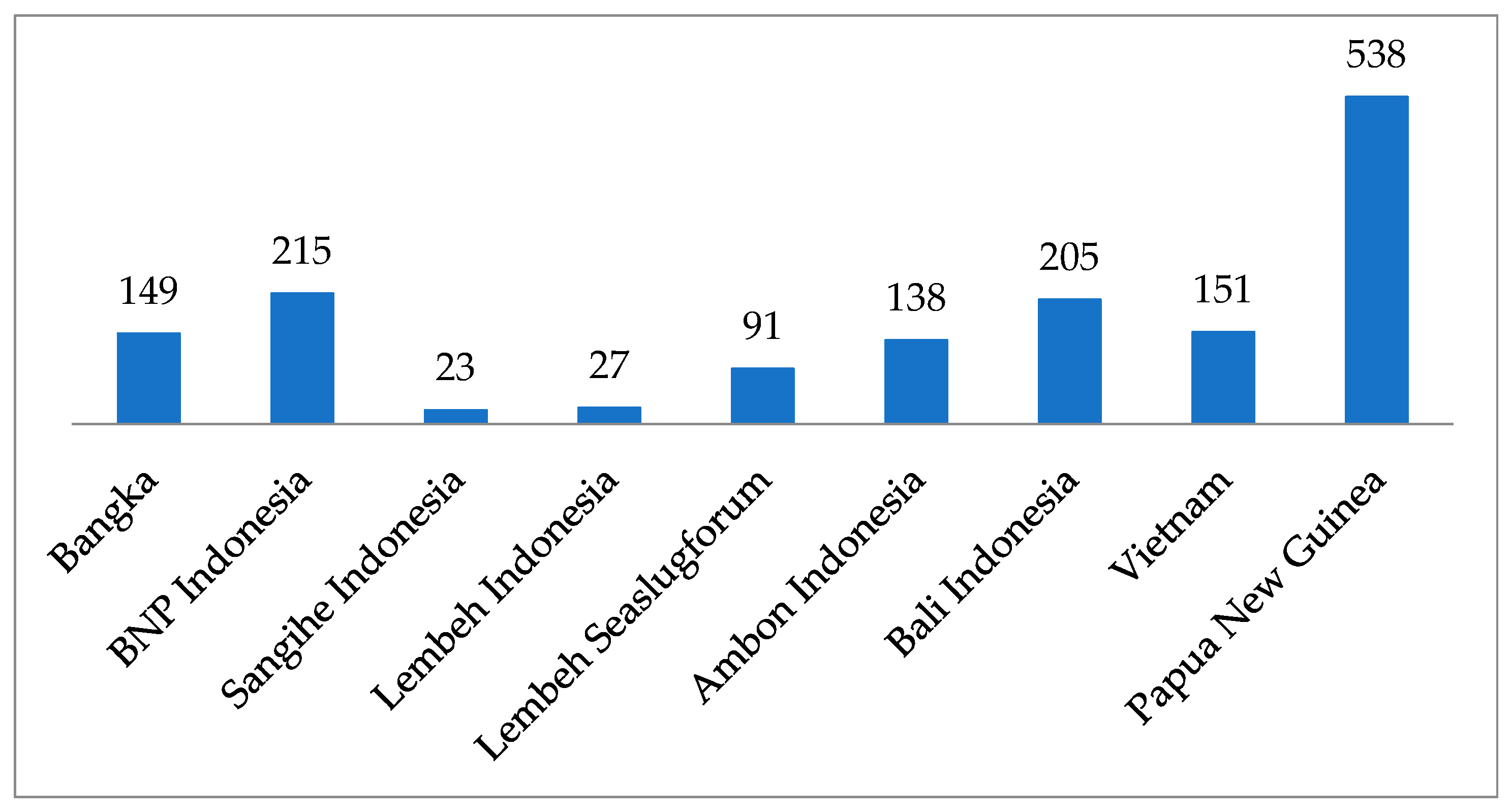
| Area of Collection Site | Location | Characteristics of the Habitat | Year of Collection |
|---|---|---|---|
| Bangka Archipelago | |||
| Batu Belah | 1°46′22.20″ N 125°10′57.38″ E | Sandy bottom with a deposit of large formations of stones richly covered with corals | 2017–2018 |
| Batu Tiga * | 1°46′07.6″ N 125°10′35.7″ E | Coral pinnacles on a deep coral sand bottom | 2018 |
| Areng Kambing | 1°46′07.8″ N 125°10′34.6″ E | Coral sand and coral rabble slope | 2018 |
| Sahaung | 1°44′35.11″ N 125°09′43.5″ E | A shallow area made up of rocks and pinnacles of volcanic origin, richly covered with coral formations | 2017 |
| Tanjung Husi | 1°44′07.74″ N 125°09′05.59″ E | Volcanic rockslide, characterized by the presence of strong tidal currents and ocean swell | 2017–2018 |
| Busa Bora Timur | 1°44′27.4″ N 125°08′35.1″ E | Fringing reef on a coral sand slope | 2018 |
| Busa Bora Kampung | 1°44′39.8″ N 125°08′24.4″ E | Fringing reef on a coral sand slope in front of a little village | 2018 |
| Coral Eye | 1°45′03.61″ N 125°07′58.85″ E | Fringing reef in front of a resort; 2012 was the last storm [1]. Dominant branching coral and little rubble. Coral grown over the tide surface. | 2017–2018 |
| Mangrove | 1°45′46.9″ N 125°07′50.0″ E | Fringing reef on a coral sand slope that starts from the lagoon in front of the mangrove | 2017–2018 |
| Sipi | 1°47′02.8″ N 125°07′42.4″ E | River mouth: mud/fringing reef, site impacted by mining operations | 2018 |
| Pearl Farm | 1°48′41.3″ N 125°06′45.1″ E | Former abandoned pearl farm, fringing reef on a coral sand slope | 2018 |
| Kinabuhutan | 1°50′45.44″ N 125°5′55.26″ E | Fringing reef on a shallow coral sand slope | 2018 |
| Talisei | 1°53′38.32″ N 125°05′53.82″ E | A vertical wall of volcanic rock that ends on a sandy bottom. The spot is subject to strong tidal currents and ocean swell, so the coral formations are small and compact. | 2018 |
| Mainland | |||
| Yellow Coco | 1°40′21.67″ N 125°8′6.60″ E | Fringing reef on a mix of volcanic and coral sand slopes | 2018 |
| Sempini | 1°41′4.83″ N 125°8′32.65″ E | Fringing reef on a coral sand slope | 2018 |
| Sempini 2 | 1°40′51.06″ N 125°8′22.45″ E | Fringing reef on a coral sand slope | 2018 |
| Efrata | 1°40′52.34″ N 125°9′10.55″ E | Volcanic rock beach, followed by volcanic sand | 2018 |
| Batu Mandi | 1°41′16.71″ N 125°09′42.79″ E | Volcanic rock covered by coral reef, sponge, and other invertebrates. Various topographies. | 2017–2018 |
| Tanjung Kusu-Kusu | 1°41′23.01″ N 125°09′55.26″ E | Volcanic rock wall on a backdrop of rock and coral, characterized by the presence of strong tidal currents and ocean swell | 2017 |
| Expedition in Bangka Archipelago and Mainland Nearby | Other Expeditions | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher Taxon Affiliation | Identifier | Species Name | Number of Specimens | Size (mm) | Depth (m) | Bangka Archipelago | Mainland | Exp Bunaken | Exp Sangihe | Exp Lembeh | ||||||||||||||||
| Batu Belah | Batu Tiga | Areng Kambing | Sahaung | Tanjung Husi | Busa Bora Timur | Busa Bora Kampung | Coral Eye | Mangrove | Sipi | Pearl Farm | Kinabuhutan | Talisei | Yellow Coco | Sempini | Efrata | Batu Mandi | Tanjung Kusu-Kusu | |||||||||
| Cephalaspidea (5 species) | ||||||||||||||||||||||||||
| Haminoeidae Pilsbry, 1895 | Hasp18Ba-1 | Haminoea sp. (Haminoea sp. 3 in Gosliner et al. [24]: 30) | 1 | 2 | 7 | 1 | x | - | - | |||||||||||||||||
| Colpodaspididae Oskars, Bouchet and Malaquias, 2015 | Colpodaspis thompsoni Brown, 1979 | 5 | 2–5 | 1.5–9.2 | 2 | 1 | 1 | 1 | x | - | - | |||||||||||||||
| Aglajidae Pilsbry, 1895 (1847) | Chelidonura amoena Bergh, 1905 | 2 | 11–25 | 4–9.1 | 2 | x | - | - | ||||||||||||||||||
| Odsp.a18Ba-1 | Odontoglaja sp. a | 1 | 15 | 1.5 | 1 | - | - | - | ||||||||||||||||||
| Gastropteridae Swainson, 1840 | Sagaminopteron psychedelicum Carlson and Hoff, 1974 | 2 | 3 | 1 | 1 | x | - | - | ||||||||||||||||||
| Aplysiida (2 species) | ||||||||||||||||||||||||||
| Aplysiidae Lamarck, 1809 | Aplysia cf. nigrocincta von Martens, 1880 | 4 | 4–10 | 1 | 4 | x | - | - | ||||||||||||||||||
| Stylocheilus striatus (Quoy and Gaimard, 1832) | 2 | 5–15 | 7–7.5 | 1 | 1 | x | - | - | ||||||||||||||||||
| Sacoglossa (15 species) | ||||||||||||||||||||||||||
| Oxynoidae Stoliczka, 1868 (1847) | Losp1-18Ba1; Losp1-18Ba2 | Lobiger sp. (Lobiger sp. 1 in Gosliner et al. [24]: 70) | 2 | 2–11 | 8.4 | 2 | x | - | - | |||||||||||||||||
| Hermaeidae H. Adams and A. Adams, 1854 | Cyerce bourbonica Yonow, 2012 | 2 | 3–4 | 1–1.5 | 2 | x | - | - | ||||||||||||||||||
| Cyerce nigra Bergh, 1871 | 1 | 4 | 8.6 | 1 | - | - | - | |||||||||||||||||||
| Plakobranchidae Gray, 1840 | Elysia asbecki Wägele, Stemmer, Burghardt and Händeler, 2010 | 1 | 3 | 8.5 | 1 | x | - | - | ||||||||||||||||||
| Elysia marginata (Pease, 1871) | 1 | 3 | 11.6 | 1 | x | - | - | |||||||||||||||||||
| Elysia cf. nigropunctata (Pease, 1871) | 1 | 21 | 2.6 | 1 | - | - | - | |||||||||||||||||||
| Elysia pusilla (Bergh, 1871) | 2 | 7–11 | 1–1.5 | 1 | 1 | x | x | - | ||||||||||||||||||
| Elsp24-18Ba-1 | Elysia sp. 24 (in Gosliner et al. [24]: 89) | 1 | 7 | 7 | 1 | - | - | - | ||||||||||||||||||
| Elsp27-18Ba-1 | Elysia sp. 27 (in Gosliner et al. [24]: 89) | 1 | 5 | 7 | 1 | - | - | - | ||||||||||||||||||
| Elsp.a18Ba-1 | Elysia sp. a | 1 | 8 | 1 | 1 | x | - | - | ||||||||||||||||||
| Elsp.b18Ba-1 | Elysia sp. b | 1 | 9 | 20 | 1 | x | - | - | ||||||||||||||||||
| Thuridilla carlsoni Gosliner, 1995 | 2 | 10–17 | 7.6–8.3 | 1 | 1 | - | - | - | ||||||||||||||||||
| Thuridilla flavomaculata Gosliner, 1995 | 1 | 5 | 5.3 | 1 | x | - | - | |||||||||||||||||||
| Thuridilla vataae (Risbec, 1928) | 2 | 7–9 | 1.5–3 | 2 | x | - | - | |||||||||||||||||||
| Thuridilla gracilis (Risbec, 1928) | 12 | 8–24 | 1–12.8 | 1 | 2 | 3 | 4 | 1 | 1 | - | x | - | ||||||||||||||
| Pleurobranchida (2 species) | ||||||||||||||||||||||||||
| Pleurobranchidae Gray, 1827 | Pleurobranchus forskalii Rüppell and Leuckart, 1828 | 2 | 23–25 | 4–12 | 1 | 1 | x | - | - | |||||||||||||||||
| Pleurobranchus peronii Cuvier, 1804 | 2 | 19–140 | 2–12 | 1 | 1 | - | - | - | ||||||||||||||||||
| Nudibranchia, Doridina (82 species) | ||||||||||||||||||||||||||
| Hexabranchidae Bergh, 1891 | Glsp1_17Ba-1 | Hexabranchus sanguineus (Rüppell and Leuckart, 1830) | 1 | 10 | 6.6 | 1 | x | - | - | |||||||||||||||||
| Polyceridae Alder and Hancock, 1845 | Nembrotha chamberlaini Gosliner and Behrens, 1997 | 5 | 11–52 | 2–11.7 | 1 | 1 | 2 | 1 | - | - | - | |||||||||||||||
| Nembrotha cristata Bergh, 1877 | 5 | 48–65 | 1–8.1 | 3 | 2 | x | - | - | ||||||||||||||||||
| Nembrotha kubaryana Bergh, 1877 | 13 | 23–80 | 4.2–20 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 | x | - | x | |||||||||||
| Nembrotha lineolata Bergh, 1905 | 5 | 30–58 | 4–16.2 | 4 | 1 | - | - | - | ||||||||||||||||||
| Nembrotha milleri Gosliner and Behrens, 1997 | 1 | 80 | 6 | 1 | - | - | - | |||||||||||||||||||
| Nembrotha mullineri Gosliner and Behrens, 1997 | 2 | 17–20 | 7.2–11.7 | 2 | - | - | - | |||||||||||||||||||
| Nesp1_17Ba-1 | Nembrotha sp. 1 (in Gosliner et al. [24]: 122) | 1 | 11 | 8.7 | 1 | - | - | - | ||||||||||||||||||
| Tambja gabrielae Pola, Cervera and Gosliner, 2005 | 1 | 51 | 11.8 | 1 | - | - | - | |||||||||||||||||||
| Tambja morosa (Bergh, 1877) | 4 | 52–90 | 8–16.6 | 2 | 1 | 1 | - | - | - | |||||||||||||||||
| Gymnodoris aurita (Gould, 1852)* | X | X | - | - | - | |||||||||||||||||||||
| Gymnodoris tuberculosa Knutson and Gosliner, 2014 | 1 | 3 | 1 | 1 | x | - | - | |||||||||||||||||||
| Gysp20-18Ba-1 | Gymnodoris sp. 20 (in Gosliner et al. [24]: 156) | 1 | 3 | 5 | 1 | - | - | - | ||||||||||||||||||
| Gysp25-18Ba-1-2 | Gymnodoris sp. 25 (in Gosliner et al. [24]: 157) | 2 | 4–5 | 1 | 2 | - | - | - | ||||||||||||||||||
| Goniodorididae H. Adams and A. Adams, 1854 | Gosp7-18Ba-1 | Goniodoris sp. 7 (in Gosliner et al. [24]: 153) | 1 | 6 | 10 | 1 | - | - | - | |||||||||||||||||
| Trapania armilla Gosliner and Fahey, 2008 | 1 | 7 | 1 | - | - | - | ||||||||||||||||||||
| Trapania safracornia Fahey, 2004 | 1 | 7 | 13.4 | 1 | - | - | - | |||||||||||||||||||
| Aegiridae P. Fischer, 1883 | Aesp7-18Ba-1 | Aegires sp. 7 (in Gosliner et al. [24]: 149) | 1 | 4 | 7 | 1 | - | - | - | |||||||||||||||||
| Notodoris minor Eliot, 1904 * | X | X | - | - | - | |||||||||||||||||||||
| Discodorididae Bergh, 1891 | Asteronotus cespitosus (van Hasselt, 1824) * | X | X | - | - | - | ||||||||||||||||||||
| Asteronotus mimeticus Gosliner and Valdés, 2002 | 9 | 5–25 | 3–11.9 | 8 | 1 | x | - | - | ||||||||||||||||||
| Atagema intecta (Kelaart, 1859) | 2 | 12–55 | 7–8 | 2 | - | - | - | |||||||||||||||||||
| Discodoris cebuensis Bergh, 1877 | 2 | 48–53 | 11.2 | 2 | - | - | - | |||||||||||||||||||
| Halgerda batangas Carlson and Hoff, 2000 | 10 | 32–57 | 6.2–19.2 | 1 | 1 | 2 | 5 | 1 | x | - | x | |||||||||||||||
| Halgerda carlsoni Rudman, 1978 | 2 | 33–40 | 11.8–12 | 1 | 1 | x | - | |||||||||||||||||||
| Jorunna funebris Kelaart, 1859 | 2 | 15–20 | 2 | 2 | - | - | x | |||||||||||||||||||
| Paradoris liturata (Bergh, 1905) | 3 | 22–43 | 8–8.5 | 1 | 1 | 1 | - | - | - | |||||||||||||||||
| Platydoris sanguinea Bergh, 1905 | 1 | 12 | 5.5 | 1 | x | - | - | |||||||||||||||||||
| Chromodorididae Bergh, 1891 | Ceratosoma tenue (Abraham, 1876) * | X | X | - | - | - | ||||||||||||||||||||
| Chromodoris dianae Gosliner and Behrens, 1998 | 2 | 16–20 | 13.6–15 | 1 | 1 | x | x | - | ||||||||||||||||||
| Chromodoris annae Bergh, 1877 | 46 | 18–50 | 1.5–12.1 | 3 | 4 | 10 | 6 | 19 | 1 | 1 | 2 | x | x | x | ||||||||||||
| Chromodoris elisabethina Bergh, 1877 * | X | X | - | x | - | |||||||||||||||||||||
| Chromodoris lochi Rudman, 1982 | 15 | 17–47 | 4–20.4 | 3 | 1 | 1 | 1 | 2 | 2 | 4 | 1 | x | - | x | ||||||||||||
| Chromodoris magnifica (Quoy and Gaimard, 1832) | 2 | 37–60 | 2–5.9 | 1 | 1 | - | - | - | ||||||||||||||||||
| Chromodoris quadricolor (Rüppell and Leuckart, 1830) | 1 | 38 | 15 | 1 | - | - | - | |||||||||||||||||||
| Chromodoris strigata Rudman, 1982 | 2 | 22–28 | 7.4–13.6 | 1 | 1 | x | x | - | ||||||||||||||||||
| Chromodoris willani Rudman, 1982 | 2 | 37–40 | 8 | 2 | x | - | - | |||||||||||||||||||
| Doriprismatica atromarginata (Cuvier, 1804) | 4 | 17–74 | 7.1–10 | 1 | 2 | 1 | - | - | - | |||||||||||||||||
| Doriprismatica sibogae Bergh, 1905 | 1 | 85 | 2 | 1 | - | - | - | |||||||||||||||||||
| Glossodoris cincta (Bergh, 1888) | 2 | 34–60 | 1–6 | 2 | x | x | x | |||||||||||||||||||
| Glossodoris rufomarginata (Bergh, 1890)* | X | X | - | - | - | |||||||||||||||||||||
| Goniobranchus coi (Risbec, 1956) | 2 | 30–40 | 4 | 2 | - | - | - | |||||||||||||||||||
| Goniobranchus fidelis (Kelaart, 1858) | 1 | 13 | 17 | 1 | x | - | x | |||||||||||||||||||
| Goniobranchus geometricus (Risbec, 1928) | 6 | 613–22 | 5–14.8 | 1 | 3 | 1 | 1 | x | x | x | ||||||||||||||||
| Goniobranchus kuniei (Pruvot-Fol, 1930) | 1 | 46 | 12 | 1 | - | - | - | |||||||||||||||||||
| Goniobranchus reticulatus (Quoy and Gaimard, 1832) | 2 | 48–66 | 10.3 | 1 | 1 | x | x | x | ||||||||||||||||||
| Goniobranchus verrieri (Crosse, 1875) | 1 | 3 | 20 | 1 | - | - | - | |||||||||||||||||||
| Hypselodoris apolegma (Yonow, 2001) | 1 | 33 | 16.4 | 1 | x | - | - | |||||||||||||||||||
| Hypselodoris bullockii (Collingwood, 1881) * | X | X | - | - | - | |||||||||||||||||||||
| Hysp1_17Ba-1 | Hypselodoriscerisae Gosliner and Johnson, 2018 | 1 | 16 | 6.6 | 1 | - | - | - | ||||||||||||||||||
| Hypselodorisdecorata Gosliner and Johnson, 2018 | 1 | 20 | 8.1 | 1 | 1 | 1 | 1 | x | - | - | ||||||||||||||||
| Hypselodoris emma Rudman, 1977 * | X | X | - | - | - | |||||||||||||||||||||
| Chromos18Ba-1 | Hypselodoris iacula Gosliner and Johnson, 1999 | 1 | 50 | 15 | 1 | - | - | - | ||||||||||||||||||
| Hysp19_17Ba-1-2 | Hypselodoris lacuna Gosliner and Johnson, 2018 | 2 | 6–8 | 6.8 | 2 | - | - | - | ||||||||||||||||||
| Hypselodoris maculosa (Pease, 1871) | 4 | 18–23 | 3–10.3 | 1 | - | - | - | |||||||||||||||||||
| Hypselodoris maridadilus Rudman, 1977 | 1 | 10 | 6.5 | 1 | - | - | - | |||||||||||||||||||
| Hypselodoris zephyra (Eliot, 1904) | 1 | 9 | 14.2 | 1 | - | - | - | |||||||||||||||||||
| Hypselodoris tryoni (Garret 1873) | 7 | 26–54 | 4–14.1 | 2 | 2 | 2 | 1 | x | x | x | ||||||||||||||||
| Thsp1-18Ba-1 | Hypselodoris sp. a | 1 | 8 | 11.5 | 1 | - | - | - | ||||||||||||||||||
| Mexichromis aurora (Johnson and Gosliner, 1998) | 1 | 11 | 8.6 | 1 | - | - | - | |||||||||||||||||||
| Mexichromis trilineata (A. Adams and Reeve, 1850) * | X | X | - | - | - | |||||||||||||||||||||
| Dorid18Ba-1 | Miamira magnifica | 1 | 2.5 | 20 | 1 | x | - | - | ||||||||||||||||||
| Misp17Ba_1 | Miamira sp. a | 1 | 10 | 4 | 1 | - | - | - | ||||||||||||||||||
| Verconia simplex Pease, 1871 | 1 | 6 | 5.2 | 1 | - | - | - | |||||||||||||||||||
| Thfu18Ba-1-2 | Thorunna furtiva Bergh, 1878 | 2 | 14–16 | 13.8 | 2 | x | - | - | ||||||||||||||||||
| Dendrodorididae O’Donoghue, 1924 (1864) | Dendrodoris nigra (Stimpson, 1855) | 1 | 6 | 1 | 1 | - | - | - | ||||||||||||||||||
| Phyllidiidae Rafinesque, 1814 | Phyllidia cf. babai Brunckhorst, 1993 * | X | X | - | - | - | ||||||||||||||||||||
| Phyllidia coelestis Bergh, 1905 | 8 | 12–43 | 1.5–20 | X | 1 | 1 | 1 | 1 | 3 | 1 | x | x | - | |||||||||||||
| Phyllidia elegans Bergh, 1869 | 3 | 34–38 | 2 | 1 | 2 | x | - | x | ||||||||||||||||||
| Phyllidia exquisita Brunckhorst, 1993 | 2 | 30–32 | 4.5–12.4 | 1 | 1 | - | - | - | ||||||||||||||||||
| Phyllidia ocellata Cuvier, 1804 | 8 | 14–40 | 2–13.4 | 1 | 1 | 2 | 1 | 2 | 1 | x | x | - | ||||||||||||||
| Phyllidia picta Pruvot-Fol, 1957 | 5 | 18-35 | 4-12 | 2 | 1 | 1 | 1 | - | x | - | ||||||||||||||||
| Phyllidia varicosa Lamarck, 1801 | 18 | 23–85 | 2–20 | 1 | 5 | 1 | 2 | 1 | 5 | 1 | 1 | 1 | x | x | x | |||||||||||
| Phsp18Ba-2-3 | Phyllidia sp. a | 2 | 33–34 | 6–13.5 | 1 | 1 | - | x | - | |||||||||||||||||
| Phyllidiella annulata (Gray, 1853) | 1 | 30 | 10 | 1 | x | - | - | |||||||||||||||||||
| Phyllidiella lizae Brunckhorst, 1993 | 4 | 15.26 | 5-20 | 1 | 1 | 2 | - | - | - | |||||||||||||||||
| Phyllidiella nigra (van Hasselt 1824) | 1 | 56 | 1 | 1 | - | x | - | |||||||||||||||||||
| Phyllidiella pustulosa (Cuvier, 1804) | 39 | 12–75 | 1.5–20 | 5 | 2 | 2 | 1 | 2 | 7 | 1 | 3 | 3 | 2 | 2 | 6 | 1 | 2 | x | x | x | ||||||
| Phyllidiopsis annae Brunckhorst, 1993 | 4 | 3–12 | 9.2–11.9 | 2 | 2 | - | - | - | ||||||||||||||||||
| Phyllidiopsis cf. burni Brunckhorst, 1993 | 1 | 39 | 20 | 1 | - | - | - | |||||||||||||||||||
| Phyllidiopsis krempfi Pruvot-Fol, 1957 | 3 | 17–56 | 9.2–16.2 | 1 | 1 | 1 | - | x | - | |||||||||||||||||
| Phyllidiopsis xishaensis (Lin,1983) | 4 | 9–17 | 3.9–16.8 | 1 | 2 | 1 | x | - | - | |||||||||||||||||
| Nudibranchia, Cladobranchia (42 species) | ||||||||||||||||||||||||||
| Arminidae Iredale and O’Donoghue, 1923 | Dermatobranchus caeruleomaculatus Gosliner and Fahey, 2011 * | X | X | - | - | - | ||||||||||||||||||||
| Dermatobranchus rodmani Gosliner and Fahey, 2011 | 2 | 3–4 | 1 | 2 | - | - | - | |||||||||||||||||||
| Dermatobranchus pustulosus van Hasselt, 1824 | 1 | 70 | 9.1 | 1 | - | - | - | |||||||||||||||||||
| Desp.a18Ba-1-2 | Dermatobranchus sp. a | 2 | 8–11 | 1 | 2 | - | - | - | ||||||||||||||||||
| Desp18Ba-1 | Dermatobranchus sp. b | 1 | 6 | 20.4 | 1 | - | - | - | ||||||||||||||||||
| Desp17Ba-1 | Dermatobranchus sp. c | 1 | 3 | 3 | 1 | - | - | - | ||||||||||||||||||
| Proctonotidae Gray, 1853 | Janolus sp. 1 (in Gosliner et al. [24]: 305) * | X | X | - | - | - | ||||||||||||||||||||
| Bornellidae Bergh, 1874 | Bornella anguilla Johnson, 1984 | 2 | 25–40 | 7.8–8.5 | 2 | - | - | - | ||||||||||||||||||
| Bornella stellifera Adams and Reeve in A. Adams, 1848) | 1 | 8 | 1 | 1 | - | - | - | |||||||||||||||||||
| Tethydidae Rafinesque, 1815 | Mesp.a18Ba-1 | Melibe bucephala Bergh, 1902 | 1 | 9 | 1 | 1 | - | - | - | |||||||||||||||||
| Melibe engeli Riscec, 1937 | 1 | 34 | 7 | 1 | - | - | - | |||||||||||||||||||
| Dotidae Gray, 1853 | Doto ussi Ortea, 1982 | 3 | 4–12 | 4–6.5 | 2 | 1 | x | - | - | |||||||||||||||||
| Tritoniidae Lamarck, 1809 | Masp2.18Ba-1 | Marionia sp. 2 (in Gosliner et al. [24]: 324) | 1 | 40 | 2 | 1 | - | - | - | |||||||||||||||||
| Trsp3_17Ba-1 | Tritonia sp. 3 (in Gosliner et al. [24]: 320) | 1 | 13 | 4 | 1 | - | - | - | ||||||||||||||||||
| Flabellinidae Bergh, 1889 | Coryphellina exoptata (Gosliner and Willan, 1991) | 3 | 16–32 | 2–12.4 | 1 | 1 | 1 | x | - | - | ||||||||||||||||
| Coryphellina rubrolineata O’Donoghue, 1929 | 8 | 20–40 | 6.3–20.4 | 1 | 2 | 2 | 1 | 1 | 1 | x | - | x | ||||||||||||||
| Flsp2-18Ba-1 | Flabellina sp. 2 (in Gosliner et al. [24]: 333) | 1 | 16 | 14.5 | 1 | - | - | - | ||||||||||||||||||
| Flsp3_17Ba-1 | Flabellina sp. 3 (in Gosliner et al. [24]: 333) | 1 | 11 | 13.6 | 1 | - | - | - | ||||||||||||||||||
| Samlidae Korshunova, Martynov, Bakken, Evertsen, Fletcher, Mudianta, Saito, Lundin, Schrödl and Picton, 2017 | Samla riwo (Gosliner and Willan, 1991) | 3 | 9–13 | 4–8.6 | 2 | 1 | x | - | - | |||||||||||||||||
| Eubranchidae Odhner, 1934 | Eubranchus sp. 22 (in Gosliner et al. [24]: 341) | 1 | 6 | 5.1 | 1 | x | - | - | ||||||||||||||||||
| Trinchesiidae F. Nordsieck, 1972 | Trinchesia yamasui (Hamatani, 1993) | X | X | - | - | - | ||||||||||||||||||||
| Cuthonidae Odhner, 1934 | Cuthona sp. 57 (in Gosliner et al. [24]: 353) | 2 | 17–25 | 12.7 | 2 | - | - | - | ||||||||||||||||||
| Facelinidae Bergh, 1889 | Aeol18Ba-1 | Aeolidia sp. a | 1 | 8 | 1.5 | 1 | - | - | - | |||||||||||||||||
| Ansp17Ba-1 | Antonietta sp. a | 1 | 2 | 6.4 | 1 | - | - | - | ||||||||||||||||||
| Caloria indica (Bergh, 1896) | 15 | 2–38 | 5–13.4 | 5 | 2 | 3 | 1 | 1 | 2 | 1 | x | - | - | |||||||||||||
| Crsp17Ba-1 | Cratena sp. a | 1 | 8 | 4 | 1 | - | - | - | ||||||||||||||||||
| Favorinus japonicus Baba, 1949 | 2 | 10–16 | 8.9–20 | 1 | 1 | x | - | - | ||||||||||||||||||
| Fasp1-18Ba-1-2 | Favorinus sp. 1 (in Gosliner et al. [24]: 363) | 2 | 13–15 | 20 | 2 | - | - | - | ||||||||||||||||||
| Favorinus tsuruganus Baba and Abe, 1964 | 1 | 11 | 2 | 1 | - | - | - | |||||||||||||||||||
| Mojo18Ba-1 | Moridilla sp. a | 2 | 12 | 6.8–11.2 | 1 | 1 | - | - | - | |||||||||||||||||
| Noumeaella sp. 2 (in Gosliner et al. [24]: 367) | 1 | 11 | 4 | 1 | - | - | - | |||||||||||||||||||
| Noumeaella sp. 3 (in Gosliner et al. [24]: 367) | 6 | 8–12 | 4–9 | 6 | x | - | - | |||||||||||||||||||
| Noumeaella sp. 13 (in Gosliner et al. [24]: 369) | 7 | 7–16 | 3–9 | 7 | - | - | - | |||||||||||||||||||
| Phyllodesmium briareum (Bergh, 1896) | 17 | 15–60 | 3–20 | 16 | 1 | x | - | - | ||||||||||||||||||
| Phyllodesmium cf. crypticum Rudman, 1981 | 5 | 8–22 | 4 | 5 | - | - | - | |||||||||||||||||||
| Phyllodesmium lizardense Burghardt, Schrödl and Wägele, 2008 | 3 | 10–13 | 1–10 | 1 | 2 | - | - | - | ||||||||||||||||||
| Phyllodesmium longicirrum (Bergh, 1905) | 1 | 125 | 25 | 1 | - | - | - | |||||||||||||||||||
| Phyllodesmium magnum Rudman, 1991 | 2 | 50 | 5.5–11.5 | 1 | 1 | - | - | - | ||||||||||||||||||
| Phyllodesmium parangatum Ortiz and Gosliner, 2003 | 5 | 8–15 | 1–7 | 5 | - | - | - | |||||||||||||||||||
| Phyllodesmium pecten Rudman, 1981 | 1 | 15 | 1 | 1 | - | - | - | |||||||||||||||||||
| Phyllodesmium poindimiei (Risbec, 1928) | 2 | 14–21 | 4–8.9 | 1 | 1 | x | - | - | ||||||||||||||||||
| Pteraeolidia semperi (Bergh, 1870) | 25 | 13–54 | 5–8.9 | 2 | 2 | 1 | 15 | 2 | 3 | x | - | - | ||||||||||||||
| Eupulmonata (1 species) | ||||||||||||||||||||||||||
| Onchidiidae | Peronia sp. a | 1 | 39 | 1 | 1 | - | - | - | ||||||||||||||||||
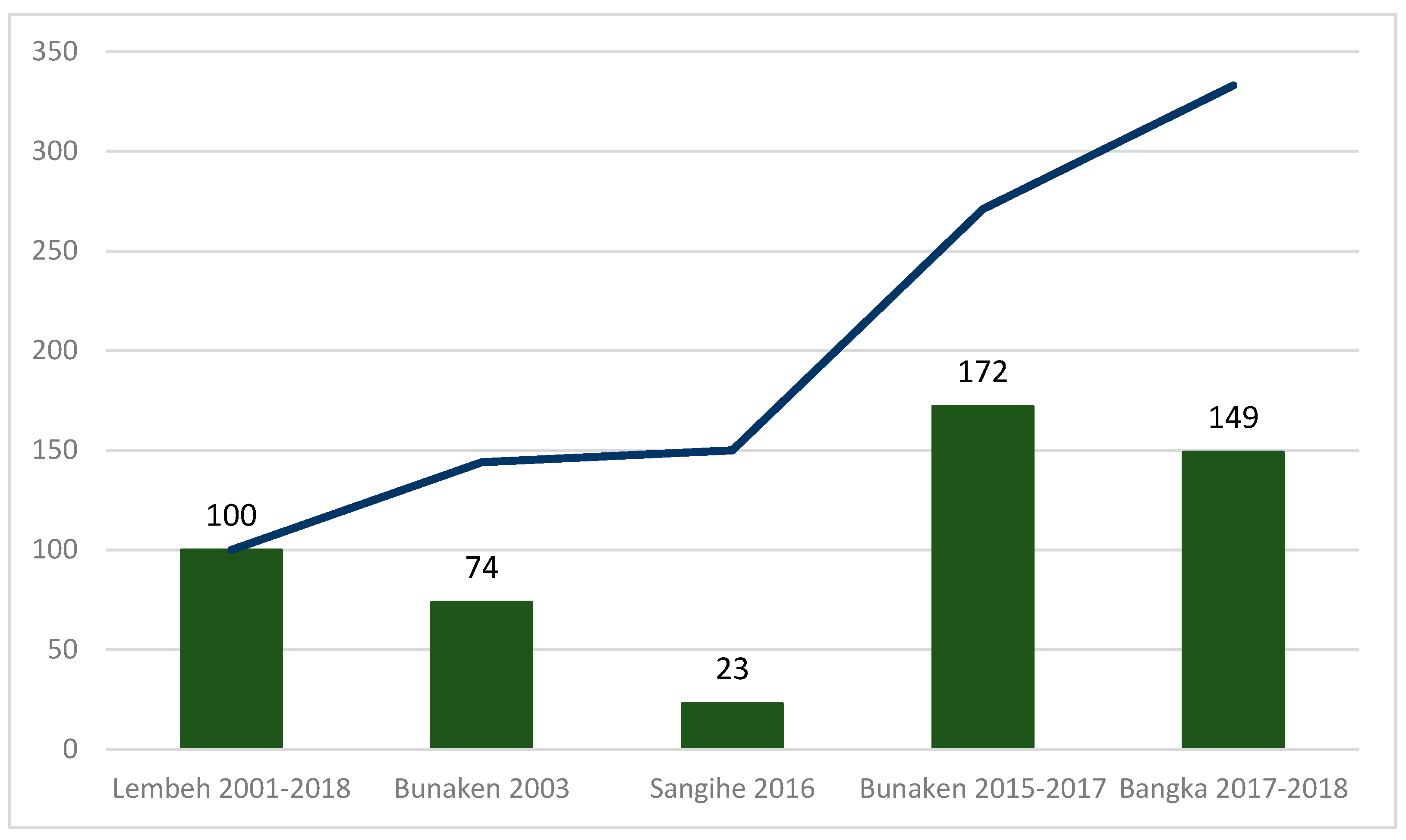
| Total | 149 | 34 | 0 | 11 | 14 | 40 | 14 | 23 | 162 | 8 | 13 | 16 | 7 | 12 | 23 | 41 | 30 | 23 | 14 | - | - | - | ||||
| Expedition in Bangka Archipelago and Adjacent Islands | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Higher Taxon Affiliation | Identifier | Species Name | Exp Bangka 2017–2018 | Exp Bunaken 2015–2017 | Exp Bunaken 2003 | Exp Sangihe 2016 | Exp Lembeh 2018 | Lembeh Sea Slug Forum 2001–2010 | Tonozuka 2003 |
| Pteropoda | |||||||||
| Limacinidae Gray, 1840 | Limacina cf. helicina (Phipps, 1774) | X | |||||||
| Cavoliniidae Gray, 1850 (1815) | Cavolinia globulosa Gray, 1850 | X | |||||||
| Cephalaspidea | |||||||||
| Bullidae Lamarck, 1801 | Bulla cf. ampulla Linnaeus, 1758 | X | |||||||
| Haminoeidae Pilsbry, 1895 | Atys cf. semistriata Pease, 1860 | X | |||||||
| Haminoea curta (Adams, 1850) | X | ||||||||
| Hasp15Bu-1, Hasp16Bu-1 | Haminoea sp. (Haminoea sp. 2 in Gosliner et al. [24]: 30) | X | X | ||||||
| Haminoeid sp. (Haminoeid sp. 2 in Gosliner et al. [24]: 34) | X | ||||||||
| Hasp18Ba-1 | Haminoea sp. (Haminoea sp. 3 in Gosliner et al. [24]: 30) | X | |||||||
| Hasp2_15Bu-1 | Haminoea sp. | X | |||||||
| Hasp2_16Bu-1 | Haminoea sp. | X | |||||||
| Limulatys cf. ooformis Habe, 1952 | X | ||||||||
| Phanerophthalmus cf. albocollaris Heller and Thompson, 1983 | X | X | |||||||
| Phanerophthalmus olivaceus (Ehrenberg, 1828) as P. smaragdinus | X | ||||||||
| Phanerophthalmus sp. (Phanerophthalmus sp. 3 in Gosliner et al. [24]: 33) | X | ||||||||
| Philinidae Gray, 1850 (1815) | Ilsp17Bu-1 | Philine sp. | X | X | |||||
| Philinoglossidae Hertling, 1932 | Philinoglossa marcusi Challis, 1969 | X | |||||||
| Philinoglossa sp. (in Gosliner et al. [24]: 103) | X | ||||||||
| Colpodaspididae Oskars, Bouchet and Malaquias, 2015 | Colpodaspis thompsoni Brown, 1979 | X | X | X | |||||
| Aglajidae Pilsbry, 1895 (1847) | Agsp15Bu-1 | Aglajidae sp. | X | ||||||
| Chelidonura amoena Bergh, 1905 | X | X | X | X | |||||
| Chelidonura hirundinina (Quoy and Gaimard, 1833) | X | X | |||||||
| Odsp.a18Ba-1 | Odontoglaja cf. guamensis Rudman, 1978 | X | X | ||||||
| Philinopsis speciosa Pease, 1860 | X | ||||||||
| Phisp16Bu-1 | Tubulophilinopsis sp. | X | |||||||
| Gastropteridae Swainson, 1840 | Gasp5_16Bu-1 | Gastropteron sp. (Gastropteron sp. 5 in Gosliner et al. [24]: 56) | X | ||||||
| Sagaminopteron psychedelicum Carlson and Hoff, 1974 | X | X | |||||||
| Sasp17Bu-1 | Sagaminopteron sp. | X | |||||||
| Siphopteron brunneomarginatum (Carlson and Hoff, 1974) | X | ||||||||
| Siphopteron ladrones (Carlson and Hoff, 1974) | X | ||||||||
| Siphopteron nigromarginatu mGosliner, 1989 | X | ||||||||
| Siphopteron tigrinum Gosliner, 1989 | X | X | |||||||
| Sini15Bu-19+20 | Siphopteron sp. | X | |||||||
| Runcinacea | |||||||||
| Runcinidae H. Adams and A. Adams, 1854 | Rusp15Bu-1 | Runcina sp. | X | X | |||||
| Rusp16Bu-1 | Runcina sp. | X | |||||||
| Rusp2_16Bu-1 Rusp3_16Bu-1 | Runcina sp. | X | |||||||
| Aplysiida | |||||||||
| Aplysia oculifera A. Adams and Reeve, 1850 | X | ||||||||
| Aplysiidae Lamarck, 1809 | Bursatella leachii Blainville, 1817 | X | |||||||
| Dolabella auricularia (Lightfoot, 1786) | X | ||||||||
| Petalifera ramosa Baba, 1959 | X | ||||||||
| Aplysia cf. nigrocincta von Martens, 1880 | X | X | |||||||
| Stylocheilus striatus (Quoy and Gaimard, 1832) | X | X | X | ||||||
| Dolabella auricularia (Lightfoot, 1786) | X | X | |||||||
| Dolabrifera dolabrifera (Rang, 1828) | X | ||||||||
| Phyllaplysia sp. | X | ||||||||
| Syphonota geographica (A. Adams and Reeve, 1850) | X | ||||||||
| Umbraculida | |||||||||
| Umbraculiidae Dall, 1889 (1827) | Umbraculum umbraculum (Lightfoot, 1786) | X | |||||||
| Sacoglossa | |||||||||
| Cylindrobullidae Thiele, 1931 | Assp1_17Bu-1-4 | Cylindrobulla sp. | X | ||||||
| Oxynoidae Stoliczka, 1868 (1847) | Lobiger nevilli Pilsbry, 1896 | X | |||||||
| Losp1-18Ba1; Losp1-18Ba2 | Lobiger sp. (Lobiger sp. 1 in Gosliner et al. [24]: 70) | X | X | ||||||
| Hermaeidae H. Adams and A. Adams, 1854 | Cyerce bourbonica Yonow, 2012 | X | X | X as C. sp. 1 | |||||
| Cyerce elegans Bergh, 1870 | X | ||||||||
| Cyerce nigra Bergh, 1871 | X | ||||||||
| Cysp2_15Bu-5 | Cyerce sp. | X | |||||||
| Hermaea sp. (Hermaea sp. 2 in Gosliner et al. [24]: 81); Aplysiopsis sp. 1 in Sea Slug Forum) | X | ||||||||
| Sohgenia palauensis Hamatani, 1991 | X | ||||||||
| Costasiellidae Clarke, 1984 | Cosp17Bu-1 | Costasiella kuroshimae Ichikawa, 1993 | X | X as Chromodoris sp. 3 | |||||
| Cosp1_17Bu-1-2 | Costasiella sp. (Costasiella sp. 1 in Gosliner et al. [24]: 79) | X | |||||||
| Cosp8_17Bu-1 | Costasiella sp. (Costasiella sp. 8 in Gosliner et al. [24]: 81) | X | |||||||
| Cosp3_16Bu-1-5 | Costasiella sp. | X | |||||||
| Plakobranchidae Gray, 1840 | Elysia asbecki Wägele, Stemmer, Burghardt and Händeler, 2010 | X | X | ||||||
| Elysia grandifolia Kelaart, 1858 | X | ||||||||
| Elysia marginata (Pease, 1871) | X | X | |||||||
| Elysia mercieri (Pruvot-Fol, 1930) | X | ||||||||
| Elysia cf nigropunctata (Pease, 1871) | X | ||||||||
| Elysia ornata (Swainson, 1840) | X | ||||||||
| Elysia pusilla (Bergh, 1871) | X | X | X | X | |||||
| Elsp.a18Ba-1 | Elysia sp. (Elysia sp. a) | X | X | ||||||
| Elsp.b18Ba-1 | Elysia sp. (Elysia sp. b) | X | X | ||||||
| Elsp1_16Bu-1 | Elysia sp. | X | |||||||
| Elsp16Bu-1 | Elysia sp. | X | |||||||
| Elsp4_16Bu-1 | Elysia sp. | X | |||||||
| Elsp24-18Ba-1 | Elysia sp. 24 (in Gosliner et al. [24]: 89) | X | |||||||
| Elsp27-18Ba-1 | Elysia sp. 27 (in Gosliner et al. [24]: 89) | X | |||||||
| Plakobranchus ocellatus van Hasselt, 1824 | X | ||||||||
| Plakobranchus cf. papua Meyers-Muños and van der Velde, 2016 | X | ||||||||
| Thuridilla albopustulosa Gosliner, 1995 | X | X | |||||||
| Thuridilla carlsoni Gosliner, 1995 | X | ||||||||
| Thuridilla flavomaculata Gosliner, 1995 | X | X | |||||||
| Thuridilla vataae (Risbec, 1928) | X | X | |||||||
| Thuridilla gracilis (Risbec, 1928) | X | X | X as T.bayeri | X | X | ||||
| Thuridilla cf. hoffae Gosliner, 1995 | X | ||||||||
| Thuridilla lineolate (Bergh, 1905) | X | X | X | ||||||
| Thuridilla livida (Baba, 1955) | X | ||||||||
| Thuridilla undula Gosliner, 1995 | X | ||||||||
| Pleurobranchida | |||||||||
| Pleurobranchidae Gray, 1827 | Berthellina citrina (Pease, 1861) | X | |||||||
| Berthella martensi (Pilsbry, 1896) | X | ||||||||
| Pleurobranchus forskalii Rüppell and Leuckart, 1828 | X | X | |||||||
| Pleurobranchus peronii Cuvier, 1804 | X | X | X | ||||||
| Nudibranchia, Doridina | |||||||||
| Hexabranchidae Bergh, 1891 | Glsp1_17Ba-1 | Hexabranchus sanguineus (Rüppell and Leuckart, 1830) | X | X | X | ||||
| Polyceridae Alder and Hancock, 1845 | Kaloplocamus dokte Vallès and Gosliner, 2006 | X | |||||||
| Kalsp8_16Bu-1 | Kaloplocamus sp. (Kaloplocamus sp. 8 in Gosliner et al. [24]: 116) | X | |||||||
| Kalsp9_16Bu-1 | Kaloplocamus sp. (Kaloplocamus sp. 9 in Gosliner et al. [24]: 116) | X | |||||||
| Nembrotha chamberlaini Gosliner and Behrens, 1997 | X | ||||||||
| Nembrotha cristata Bergh, 1877 | X | X | |||||||
| Nembrotha kubaryana Bergh, 1877 | X | X | X | X | |||||
| Nembrotha lineolata Bergh, 1905 | X | ||||||||
| Nembrotha megalocera Yonow, 1990 | X misidentified? | ||||||||
| Nembrotha milleri Gosliner and Behrens, 1997 | X | ||||||||
| Nembrotha mullineri Gosliner and Behrens, 1997 | X | ||||||||
| Nembrotha purpureolineata O’Donoghue, 1924 | X as N. rutilans | ||||||||
| Nesp1_17Ba-1 | Nembrotha sp. 1 (in Gosliner et al. [24]: 122) | X | |||||||
| Polycera japonica Baba, 1949 | X | ||||||||
| Polycera risbeci Odhner, 1941 | X | ||||||||
| Posp516Bu-1 | Polycera sp. (Polycera sp. 5 in Gosliner et al. [24]: 113) | X | |||||||
| Roboastra gracilis (Bergh, 1877) | X | X | |||||||
| Tambja gabrielae Pola, Cervera and Gosliner, 2005 | X | X | |||||||
| Tambja morosa (Bergh, 1877) | X | X | |||||||
| Thecacera picta Baba, 1972 | X | ||||||||
| Gymnodoris aurita (Gould, 1852) * | X | ||||||||
| Gymnodoris citrina (Bergh, 1877) | X | ||||||||
| Gysp2_16Bu-1 | Gymnodoris sp. (Gymnodoris sp. 2 in Gosliner et al. [24]: 152) | X | |||||||
| Gysp16Bu-1 | Gymnodoris sp. (Gymnodoris cf. sp. 35 in Gosliner et al. [24]: 159) | X | |||||||
| Gysp1_17Bu-1 Gysp22_17Bu-1 | Gymnodoris sp. (Gymnodoris cf. sp. 46 in Gosliner et al. [24]: 161) | X | |||||||
| Gysp1_15Bu-2 | Gymnodoris sp. | X | |||||||
| Gymnodoris tuberculosa Knutson and Gosliner, 2014 | X | X | |||||||
| Thecacera sp. | X | ||||||||
| Gysp20-18Ba-1 | Gymnodoris sp. 20 (in Gosliner et al. [24]: 156) | X | |||||||
| Gysp25-18Ba-1-2 | Gymnodoris sp. 25 (in Gosliner et al. [24]: 157) | X | |||||||
| Goniodorididae H. Adams and A. Adams, 1854 | Gosp7-18Ba-1 | Goniodoris sp. 7 (in Gosliner et al. [24]: 153) | X | ||||||
| Okenia kendi Gosliner, 2004 | X | ||||||||
| Okenia pellucida Burn, 1967 | X | ||||||||
| Trapania armilla Gosliner and Fahey, 2008 | X | X | |||||||
| Trapania euryeia Gosliner and Fahey, 2008 | X | ||||||||
| Trapania safracornia Fahey, 2004 | X | ||||||||
| Aegiridae P. Fischer, 1883 | Aegires citrinus Pruvot-Fol, 1930 | X | |||||||
| Aesp7-18Ba-1 | Aegires sp. 7 (in Gosliner et al. [24]: 149) | X | |||||||
| Notodoris minor Eliot, 1904 * | X | X | |||||||
| Notodoris serenae Gosliner and Behrens, 1997 | X | X | |||||||
| Doridoidea Rafinesque, 1815 | Dosp17Bu-3 | Doridoidea sp. | X | ||||||
| Scsp1_17Bu-1 | Doridoidea sp. | X | |||||||
| Cadlinidae Bergh, 1891 | Aldisa albatrossae Elwood, Valdés and Gosliner, 2000 | X | |||||||
| Discodorididae Bergh, 1891 | Asteronotus cespitosus (van Hasselt, 1824) * | X | X | X | |||||
| Asteronotus mimeticus Gosliner and Valdés, 2002 | X | X | |||||||
| Atagema intecta (Kelaart, 1859) | X | X | |||||||
| Disp1_Bu-1 | Diaulula sp. (Diaulula sp. 1 in Gosliner et al. [24]: 197) | X | |||||||
| Carminodoris estrelyado (Gosliner and Behrens, 1998) | X | X | |||||||
| Discodoris cebuensis Bergh, 1877 | X | ||||||||
| Halgerda batangas Carlson and Hoff, 2000 | X | X | X | X | X | ||||
| Halgerda carlsoni Rudman, 1978 | X | X | |||||||
| Halgerda okinawa Carlson and Hoff, 2000 | X | ||||||||
| Halgerda tessellate (Bergh, 1880) | X | ||||||||
| Jorunna funebris Kelaart, 1859 | X | X | X | ||||||
| Paradoris liturata (Bergh, 1905) | X | X | |||||||
| Platydoris ellioti (Alder and Hancock, 1864) | X | ||||||||
| Platydoris formosa (Alder and Hancock, 1864) | X | ||||||||
| Platydoris inframaculata (Abraham, 1877) | X | ||||||||
| Platydoris sanguinea Bergh, 1905 | X | X | |||||||
| Sclerodoris tuberculate Eliot, 1904 | X | ||||||||
| Scsp2_16Bu-1 | Sclerodoris sp. (Sclerodoris sp. 2 in Gosliner et al. [24]: 195) | X | |||||||
| Taringa halgerda Gosliner and Behrens, 1998 | X | ||||||||
| Dosp17Bu-1 | Discodorididae sp. | X | |||||||
| Dosp17Bu-2 | Discodorididae sp. | X | |||||||
| Chromodorididae Bergh, 1891 | Ardeadoris averni (Rudman, 1985) | X | |||||||
| Ardeadoris cruenta (Rudman, 1986) | X | ||||||||
| Ceratosoma bicolor Baba, 1949 | X | ||||||||
| Ceratosoma tenue Abraham, 1876 * | X | X | X | ||||||
| Ceratosoma trilobatum (J.E. Gray, 1827) | X | X | |||||||
| Cesp2_15Bu-3 Cesp1_17Bu-1 | Ceratosoma sp. (Ceratosoma sp. 1 in Gosliner et al. [24]: 266) | X | |||||||
| Chromodoris annae Bergh, 1877 | X | X | X | X | X | X | |||
| Chromodoris cf. boucheti Rudman, 1982 | X | ||||||||
| Chromodoris colemani Rudman, 1982 | X | ||||||||
| Chromodoris dianae Gosliner and Behrens, 1998 | X | X | X | X | |||||
| Chromodoris elisabethina Bergh, 1877 * | X | ||||||||
| Chromodoris lochi Rudman, 1982 | X | X | X | X | X | X | |||
| Chromodoris magnifica (Quoy and Gaimard, 1832) | X | X | |||||||
| Chromodoris cf. michaeli Gosliner and Behrens, 1998 | X | ||||||||
| Chromodoris quadricolor (Rüppell and Leuckart, 1830) | X | ||||||||
| Chromodoris strigata Rudman,1982 | X | X | X | X | X | ||||
| Chromodoris willani Rudman, 1982 | X | X | X | X | |||||
| Doriprismatica atromarginata (Cuvier, 1804) | X | ||||||||
| Doriprismatica sibogae Berg, 1905 | X | ||||||||
| Doriprismatica stellata (Rudman, 1986) | X | ||||||||
| Glossodoris cincta (Bergh, 1888) | X | X | X | X | X | ||||
| Glossodoris hikuerensis (Pruvot-Fol, 1954) | X | ||||||||
| Glossodoris rufomarginata (Bergh, 1890) * | X | X | |||||||
| Goniobranchus aureopurpureus (Collingwood, 1881) | X | ||||||||
| Goniobranchus coi (Risbec, 1956) | X | ||||||||
| Goniobranchus decorus (Pease, 1860) | X | ||||||||
| Goniobranchus fidelis (Kelaart, 1858) | X | X | X | X | |||||
| Goniobranchus geometricus (Risbec, 1928) | X | X | X | X | X | X | |||
| Goniobranchus hintuanensis (Gosliner and Behrens, 1998) | X | ||||||||
| Goniobranchus kuniei (Pruvot-Fol, 1930) | X | X | |||||||
| Goniobranchus preciosus (Kelaart, 1858) | X | ||||||||
| Goniobranchus reticulatus (Quoy and Gaimard, 1832) | X | X | X | X | X | ||||
| Goniobranchus roboi (Gosliner and Behrens, 1998) | X | ||||||||
| Goniobranchus tinctorius (Rüppell and Leuckart, 1830) | X | ||||||||
| Goniobranchus verrieri (Crosse, 1875) | X | X | |||||||
| Gosp40_16Bu-1 | Goniobranchus sp. (Goniobranchus sp. 40 in Gosliner et al. [24]: 230) | X | |||||||
| Hypselodoris apolegma (Yonow, 2001) | X | X | X | X | |||||
| Hypselodoris bullockii (Collingwood, 1881) * | X | ||||||||
| Hysp1_17Ba-1 | Hypselodoriscerisae Gosliner and Johnson, 2018 | X | X | ||||||
| Hypselodorisdecorata Gosliner and Johnson, 2018 | X | ||||||||
| Hypselodoris emma Rudman, 1977 * | X | ||||||||
| Chromos18Ba-1 | Hypselodoris iacula Gosliner and Johnson, 1999 | X | |||||||
| Hypselodoris iba Gosliner and Johnson, 2018 | X | ||||||||
| Hypselodoris infucata (Rüppell and Leuckart, 1830) | X | ||||||||
| Hypselodoris kanga Rudman, 1977 | X | ||||||||
| Hypselodoris cf. krakatoa Gosliner and Johnson, 1999 | X | ||||||||
| Hysp19_17Ba-1-2 | Hypselodoris lacuna Gosliner and Johnson, 2018 | X | |||||||
| Hypselodoris maculosa (Pease, 1871) | X | X | X | X | |||||
| Hypselodoris zephyra (Eliot, 1904) | X | ||||||||
| Hypselodoris nigrostriata (Eliot, 1904) | X | X | |||||||
| Hypselodoris purpureomaculosa Hamatani, 1995 | X | X | |||||||
| Hypselodoris tryoni (Garret, 1873) | X | X | X | X | X | X | |||
| Thsp1-18Ba-1 | Hypselodoris sp. a | X | X | X | |||||
| Hysp16Bu-1 | Hypselodoris sp. | X | |||||||
| Hysp2_16Bu-1 | Hypselodoris sp. | X | |||||||
| Mexichromis aurora (Johnson and Gosliner, 1998) | X | ||||||||
| Mexichromis multituberculata (Baba, 1953) | X | ||||||||
| Mexichromis trilineata v(A. Adams and Reeve, 1850) | X | X | |||||||
| Miamira sinuata (van Hasselt, 1824) | X | X | |||||||
| Dorid18Ba-1 | Miamira magnifica | X | |||||||
| Misp17Ba_1 | Miamira sp. a | X | |||||||
| Verconia simplex Pease, 1871 | X | ||||||||
| Thorunna florens (Baba, 1949) | X | ||||||||
| Thorunna furtiva Bergh, 1878 | X | X | |||||||
| Verconia varians (Pease, 1871) | X | X | |||||||
| Dosp17Bu-4 | Verconia sp. | X | |||||||
| Dendrodorididae O’Donoghue, 1924 (1864) | Dendrodoris albobrunnea Allan, 1933 | X | |||||||
| Dendrodoris carbunculosa (Kelaart, 1858) | X | ||||||||
| Dendrodoris elongata Baba, 1936 | X | ||||||||
| Dendrodoris guttata (Odhner, 1917) | X | ||||||||
| Dendrodoris nigra (Stimpson, 1855) | X | X | |||||||
| Rosp17Bu-1 | Dendrodoris sp. | X | |||||||
| Phyllidiidae Rafinesque, 1814 | Phyllidia cf. babai Brunckhorst, 1993 * | X | X | X | |||||
| Phyllidia coelestis Bergh, 1905 | X | X | X | X | |||||
| Phyllidia elegans Bergh, 1869 | X | X | X | X | X | ||||
| Phyllidia exquisita Brunckhorst, 1993 | X | X | |||||||
| Phyllidia madangensis Brunckhorst, 1993 | X | ||||||||
| Phyllidia ocellata Cuvier, 1804 | X | X | X | X | X | ||||
| Phyllidia picta Pruvot-Fol, 1957 | X | X | |||||||
| Phyllidia polkadotsa Brunckhorst, 1993 | X | ||||||||
| Phsp18Ba-2-3 | Phyllidia sp. a | X | X | X | |||||
| Phyllidia varicosa Lamarck, 1801 | X | X | X | X | X | X | |||
| Phyllidiella annulata (Gray, 1853) | X | X | |||||||
| Phyllidiella lizae Brunckhorst, 1993 | X | X | X | ||||||
| Phyllidiella nigra (van Hasselt 1824) | X | X | X | ||||||
| Phyllidiella pustulosa (Cuvier, 1804) | X | X | X | X | X | X | |||
| Phyllidiopsis annae Brunckhorst, 1993 | X | X | |||||||
| Phssp.aBa-1 | Phyllidiopsis cf. burni Brunckhorst, 1983 | X | |||||||
| Phyllidiopsis krempfi Pruvot-Fol, 1957 | X | X | |||||||
| Phyllidiopsis pipeki Brunckhorst, 1993 | X | ||||||||
| Phyllidiopsis shireenae Brunckhorst, 1990 | X | X | |||||||
| Phyllidiopsis sphingis Brunckhorst, 1993 | X | ||||||||
| Phyllidiopsis xishaensis (Lin, 1983) | X | X | X | ||||||
| Nudibranchia, Cladobranchia | |||||||||
| Arminidae Iredale and O’Donoghue, 1923 | Dermatobranchus caeruleomaculatus Gosliner and Fahey, 2011 * | X | |||||||
| Dermatobranchus diagonalis Gosliner and Fahey, 2011 | X | ||||||||
| Dermatobranchus fasciatus Gosliner and Fahey, 2011 | X | ||||||||
| Dermatobranchus cf. kokonas Gosliner and Fahey, 2011 | X | ||||||||
| Dermatobranchus cf. piperoides Gosliner and Fahey, 2011 | X | ||||||||
| Dermatobranchus rodmani Gosliner and Fahey, 2011 | X | ||||||||
| Dermatobranchus pustulosus van Hasselt, 1824 | X | ||||||||
| Desp8_17Bu-1 | Dermatobranchus sp. (Dermatobranchus sp. 8 in Gosliner et al. [24]: 302) | X | |||||||
| Desp17Ba-1 | Dermatobranchus sp. | X | |||||||
| Desp.a18Ba-1-2 | Dermatobranchus sp. a | X | |||||||
| Desp1_16Bu-1Desp18Ba-1 | Dermatobranchus sp. | X | X | ||||||
| Proctonotidae Gray, 1853 | Janolus cf. mirabilis Baba and Abe, 1970 | X | X | ||||||
| Janolus savinkini Martynov and Korshunova, 2012 | X as Janolus sp. 4 | ||||||||
| Janolus sp. 1 (in Gosliner et al. [24]: 305)* | X | X | |||||||
| Scyllaeidae Alder and Hancock, 1855 | Cross16Bu-1-8 | Crosslandia daedali Poorman and Mulliner, 1981 | X | ||||||
| Bornellidae Bergh, 1874 | Bornella anguilla Johnson, 1984 | X | |||||||
| Bornella stellifera (A. Adams and Reeve [in A. Adams], 1848) | X | X | |||||||
| Pseudovermis cf. mortoni Challis, 1969 | X | ||||||||
| Pseudovermidae Thiele, 1931 | Mesp.a18Ba-1 | Melibe bucephala Bergh, 1902 | X | ||||||
| Tethydidae Rafinesque, 1815 | Melibe engeli Risbec, 1937 | X | |||||||
| Melibe viridis (Kelaart, 1858) | X | ||||||||
| Doto ussi Ortea, 1982 | X | X | |||||||
| Dotidae Gray, 1853 | Kabeiro rubroreticulata Shipman and Gosliner, 2015 | X | |||||||
| Dotosp15Bu-1 | Kabeiro sp. | X | |||||||
| Kasp16Bu-1-15+17 Kasp17Bu-1 | Kabeiro sp. | X | |||||||
| Kasp16Bu-16 | Kabeiro sp. | X | |||||||
| Marianina rosea (Pruvot-Fol, 1930) | X | ||||||||
| Tritoniidae Lamarck, 1809 | Masp2.18Ba-1 | Marionia sp. 2 (in Gosliner et al. [24]: 324) | X | ||||||
| Trsp3_17Ba-1 | Tritonia sp. 3 (in Gosliner et al. [24]: 320) | X | |||||||
| Trsp8_16Bu-1 | Tritonia sp. (Tritonia sp. 9 in Gosliner et al. [24]: 321) | X | |||||||
| Trisp10_16Bu-1 | Tritonia sp. (Tritonia sp. 10 in Gosliner et al. [24]: 321) | X | |||||||
| Trsp16Bu-1 | Tritonia sp. | X | |||||||
| Embletonia gracilis Risbec, 1928 | X | ||||||||
| Embletoniidae | Coryphellina delicate (Gosliner and Willan, 1991) | X | |||||||
| Flabellinidae Bergh, 1889 | Coryphellina exoptata (Gosliner and Willan, 1991) | X | X | X | X | ||||
| Coryphellina rubrolineata O’Donoghue, 1929 | X | X | X | X | |||||
| Flsp2-18Ba-1 | Flabellina sp. 2 (in Gosliner et al. [24]: 333) | X | |||||||
| Flsp3_17Ba-1 | Flabellina sp. 3 (in Gosliner et al. [24]: 333) | X | |||||||
| Flabellina sp. 8 (Sea Slug Forum) | X | ||||||||
| Samla bicolor (Kelaart, 1858) | X | ||||||||
| Samlidae Korshunova, Martynov, Bakken, Evertsen, Fletcher, Mudianta, Saito, Lundin, Schrödl and Picton, 2017 | Samla bicolor (Kelaart, 1858) | X | |||||||
| Samla riwo (Gosliner and Willan, 1991) | X | X | X | ||||||
| Phestilla lugubris (Bergh, 1870) | X | ||||||||
| Trinchesiidae F. Nordsieck, 1972 | Phestilla minor Rudman, 1981 | X | |||||||
| Trinchesia yamasui (Hamatani, 1993) | X | ||||||||
| Eubranchus sp. (Eubranchus sp. 22 in Gosliner et al. [24]: 341) | X | X | |||||||
| Eubranchidae Odhner, 1934 | Cusp4_16Bu-1 | Cuthona sp. (Cuthona sp. 4 in Gosliner et al. [24]: 343) | X | ||||||
| Cuthonidae Odhner, 1934 | Cusp54_16Bu-1 | Cuthona sp. (Cuthona sp. 54 in Gosliner et al. [24]: 353) | X | ||||||
| Cuthona sp. 57 (in Gosliner et al. [24]: 353) | X | ||||||||
| Cusp65_16Bu-1 | Cuthona sp. (Cuthona cf. sp. 65 in Gosliner et al. [24]: 343) | X | |||||||
| Aeol18Ba-1 | Aeolidia sp. | X | X | X | |||||
| Aeolidiidae Gray, 1827 | Bulbaeolidia alba (Risbec, 1928) | X | X | ||||||
| Baeolidia australis (Rudman, 1982) | X | ||||||||
| Cerberilla ambonensis Bergh, 1905 | X | ||||||||
| Ansp17Ba-1 | Antonietta sp. | X | |||||||
| Facelinidae Bergh, 1889 | Caloria indica (Bergh, 1896) | X | X | ||||||
| Caloria sp. (Caloria sp. 1 in Gosliner et al. [24]: 362) | X | ||||||||
| Cratena sp. (Cratena sp. 5 in Gosliner et al. [24]: 383) | X | ||||||||
| Crsp17Ba-1 | Cratena sp. | X | |||||||
| Facelina rhodopos Yonow, 2000 | X | ||||||||
| Fasp3_16Bu-1+3-5 | Facelina sp. (Facelina sp. 3 in Gosliner et al. [24]: 359) | X | |||||||
| Fasp3_16Bu-2 | Facelina sp. (Facelina sp. 4 in Gosliner et al. [24]: 359) | X | |||||||
| Fasp8_16Bu-1 | Facelina sp. (Facelina sp. 8 in Gosliner et al. [24]: 360) | X | |||||||
| Favorinus japonicus Baba, 1949 | X | X | |||||||
| Favorinus mirabilis Baba, 1955 | X | ||||||||
| Favorinus tsuruganus Baba and Abe, 1964 | X | ||||||||
| Fasp1-18Ba-1-2 | Favorinus sp. 1 (in Gosliner et al. [24]: 363) | X | |||||||
| Mojo18Ba-1 | Moridilla sp. a | X | |||||||
| Noumeaella sp. 2 (in Gosliner et al. [24]: 367) | X | ||||||||
| Noumeaella sp. 3 (in Gosliner et al. [24]: 367) | X | X | |||||||
| Nosp6_17Bu-1-2 | Noumeaella sp. (Noumeaella cf. sp. 6 in Gosliner et al. [24]: 368) | X | |||||||
| Nosp12_16Bu-1 | Noumeaella sp. (Noumeaella sp. 12 in Gosliner et al. [24]: 367) | X | |||||||
| Noumeaella sp. 13 (in Gosliner et al. [24]: 369) | X | ||||||||
| Nosp2_15Bu 1 Nosp2_16Bu-1-6 | Noumeaella sp. | X | |||||||
| Phyllodesmium briareum (Bergh, 1896) | X | X | X | X | |||||
| Phyllodesmium cf. crypticum Rudman, 1981 | X | ||||||||
| Phyllodesmium jakobsenae Burghardt and Wägele, 2004 | X | ||||||||
| Phyllodesmium lizardense Burghardt, Schrödl and Wägele, 2008 | X | ||||||||
| Phyllodesmium longicirrum (Bergh, 1905) | X | ||||||||
| Phyllodesmium magnum Rudman, 1991 | X | X | |||||||
| Phyllodesmium parangatum Ortiz and Gosliner, 2003 | X | ||||||||
| Phyllodesmium pecten Rudman, 1981 | X | ||||||||
| Phyllodesmium poindimiei (Risbec, 1928) | X | X | |||||||
| Phyllodesmium rudmani Burghardt and Gosliner, 2006 | X as sp 1 | X as sp. 11 | |||||||
| Phyllodesmium undulatum Moore and Gosliner, 2014 | X | ||||||||
| Phyllodesmium sp. 9 (Sea Slug Forum) | X | ||||||||
| Pteraeolidia semperi (Bergh, 1870) as P. ianthina (Burghardt and Sea Slug Forum) | X | X | X | X | X | ||||
| Fasp17Bu-1 | Facelinidae sp. | X | |||||||
| Fasp17Bu-1 | Facelinidae sp. | X | |||||||
| Eupulmonata | |||||||||
| Onchidiidae | Peronia sp. | X | |||||||
| Total number | 149 | 172 | 75 | 23 | 27 | 85 | 25 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papu, A.; Undap, N.; Martinez, N.A.; Segre, M.R.; Datang, I.G.; Kuada, R.R.; Perin, M.; Yonow, N.; Wägele, H. First Study on Marine Heterobranchia (Gastropoda, Mollusca) in Bangka Archipelago, North Sulawesi, Indonesia. Diversity 2020, 12, 52. https://doi.org/10.3390/d12020052
Papu A, Undap N, Martinez NA, Segre MR, Datang IG, Kuada RR, Perin M, Yonow N, Wägele H. First Study on Marine Heterobranchia (Gastropoda, Mollusca) in Bangka Archipelago, North Sulawesi, Indonesia. Diversity. 2020; 12(2):52. https://doi.org/10.3390/d12020052
Chicago/Turabian StylePapu, Adelfia, Nani Undap, Nancy Armas Martinez, Marco R. Segre, Ivan Galton Datang, Rendy Robert Kuada, Marco Perin, Nathalie Yonow, and Heike Wägele. 2020. "First Study on Marine Heterobranchia (Gastropoda, Mollusca) in Bangka Archipelago, North Sulawesi, Indonesia" Diversity 12, no. 2: 52. https://doi.org/10.3390/d12020052
APA StylePapu, A., Undap, N., Martinez, N. A., Segre, M. R., Datang, I. G., Kuada, R. R., Perin, M., Yonow, N., & Wägele, H. (2020). First Study on Marine Heterobranchia (Gastropoda, Mollusca) in Bangka Archipelago, North Sulawesi, Indonesia. Diversity, 12(2), 52. https://doi.org/10.3390/d12020052






