Vocalization Analyses of Nocturnal Arboreal Mammals of the Taita Hills, Kenya
Abstract
:1. Introduction
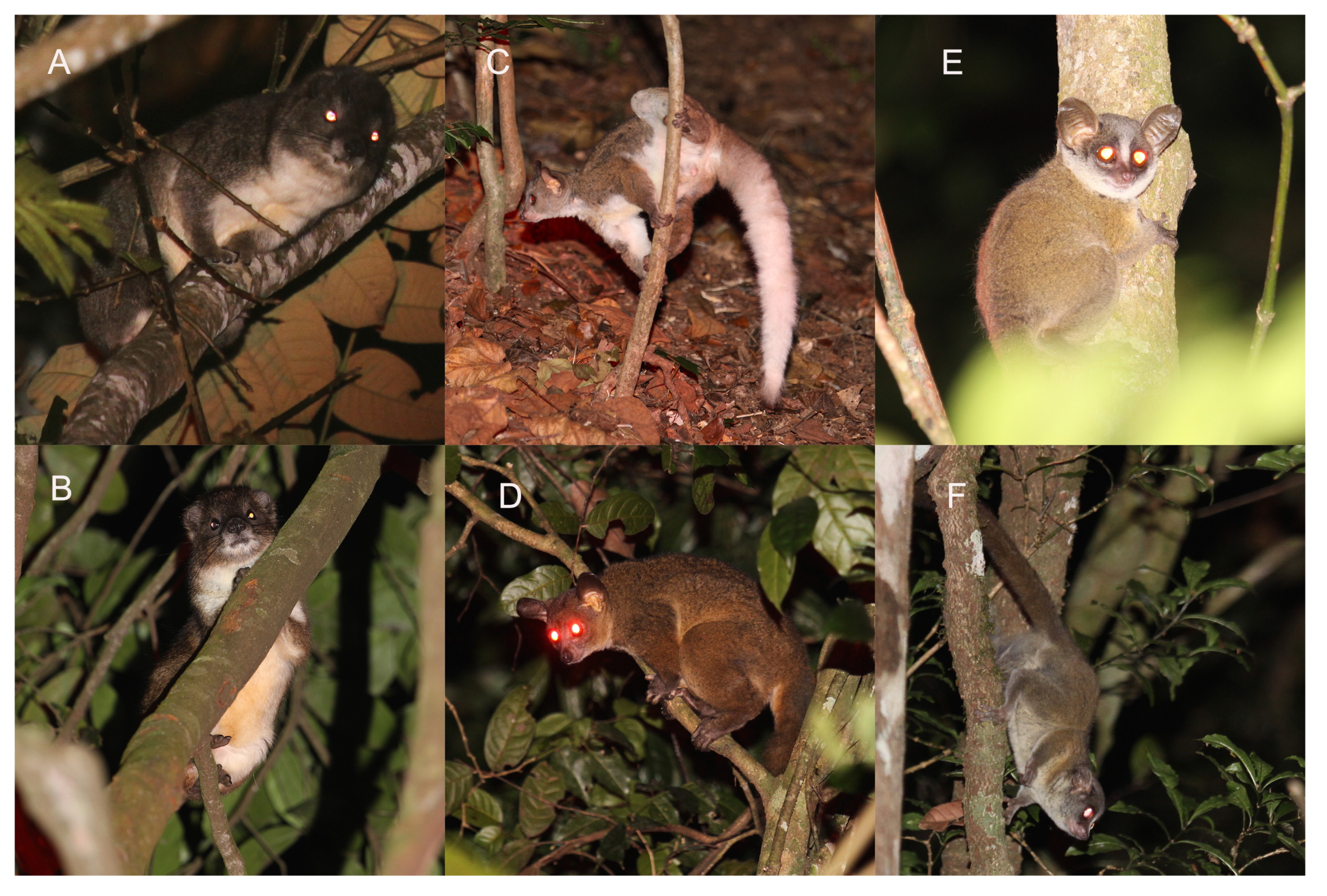
1.1. Tree Hyrax Taxonomy
1.2. Galago Taxonomy
2. Materials and Methods
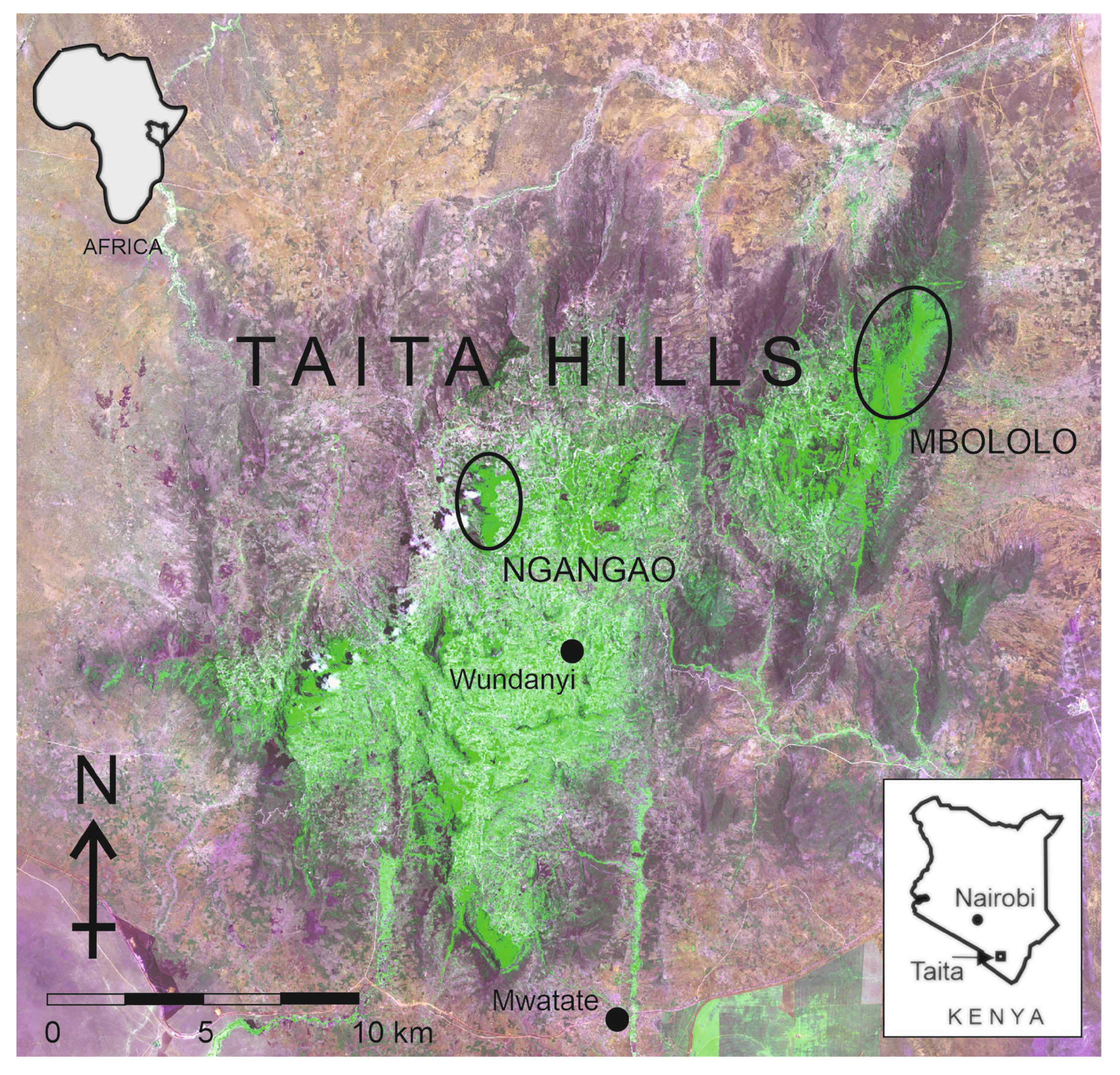
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
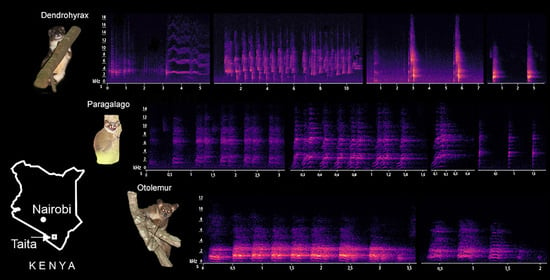
3. Results
3.1. Characterization of Dendrohyrax Vocalizations
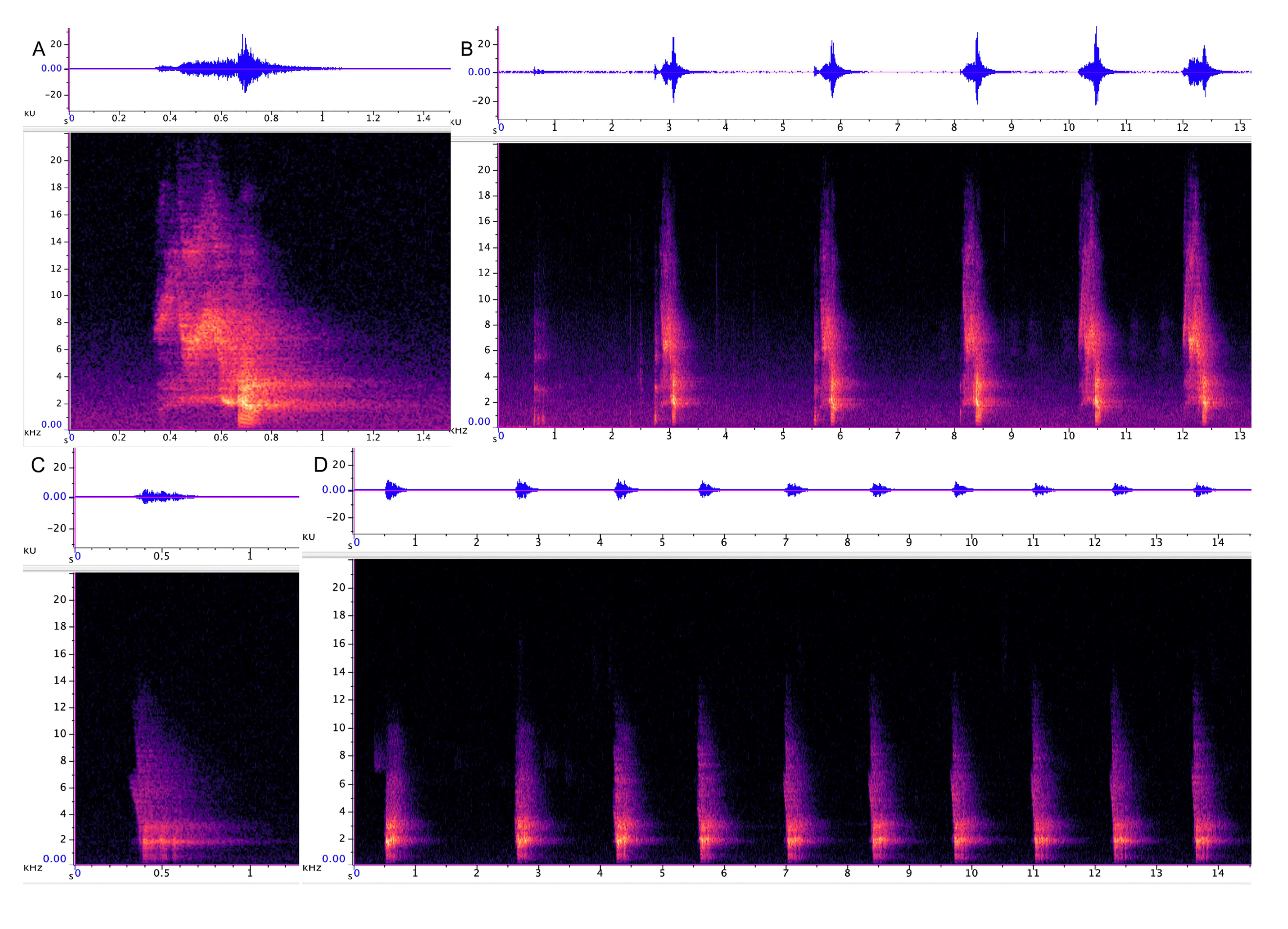
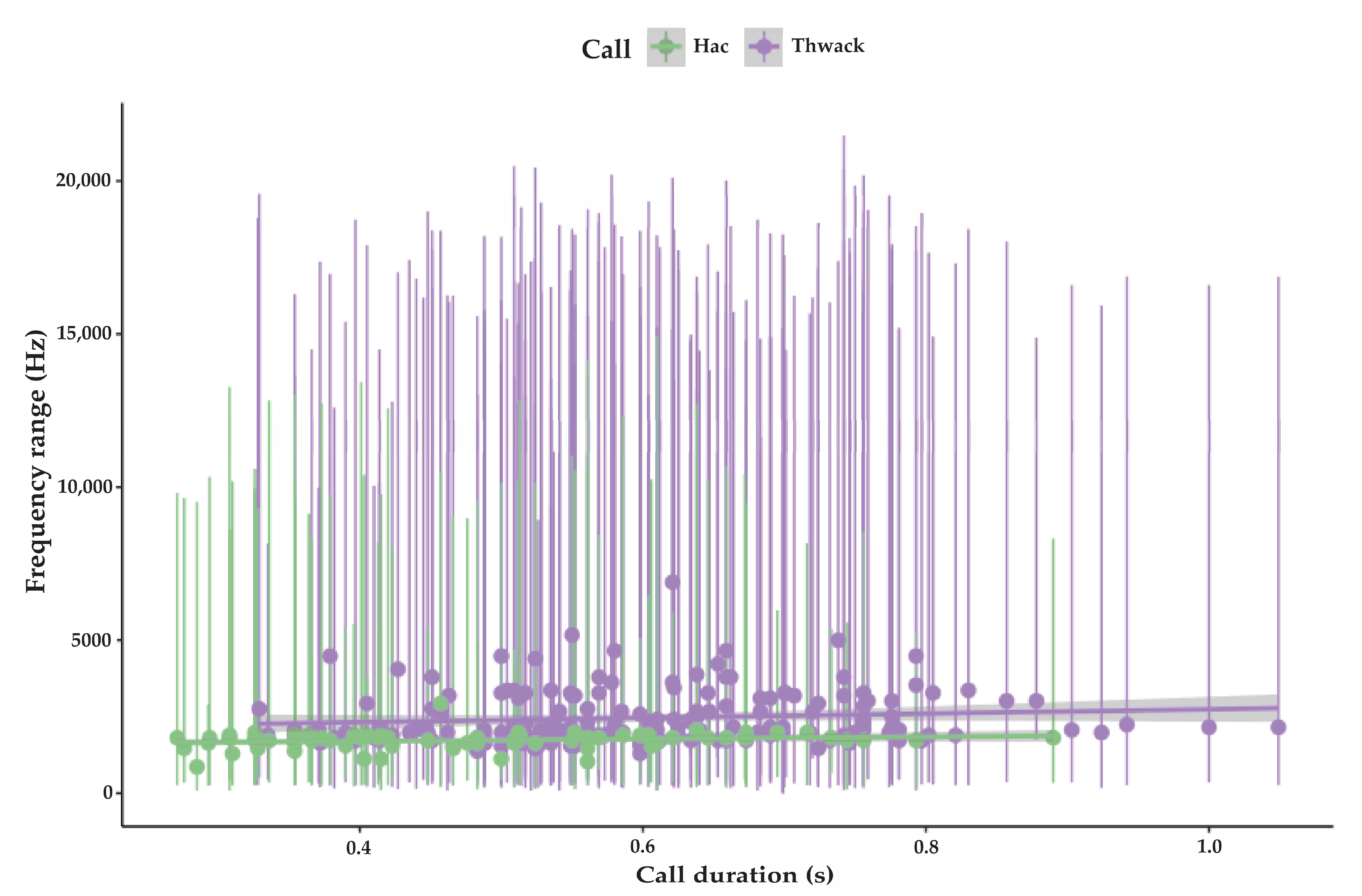
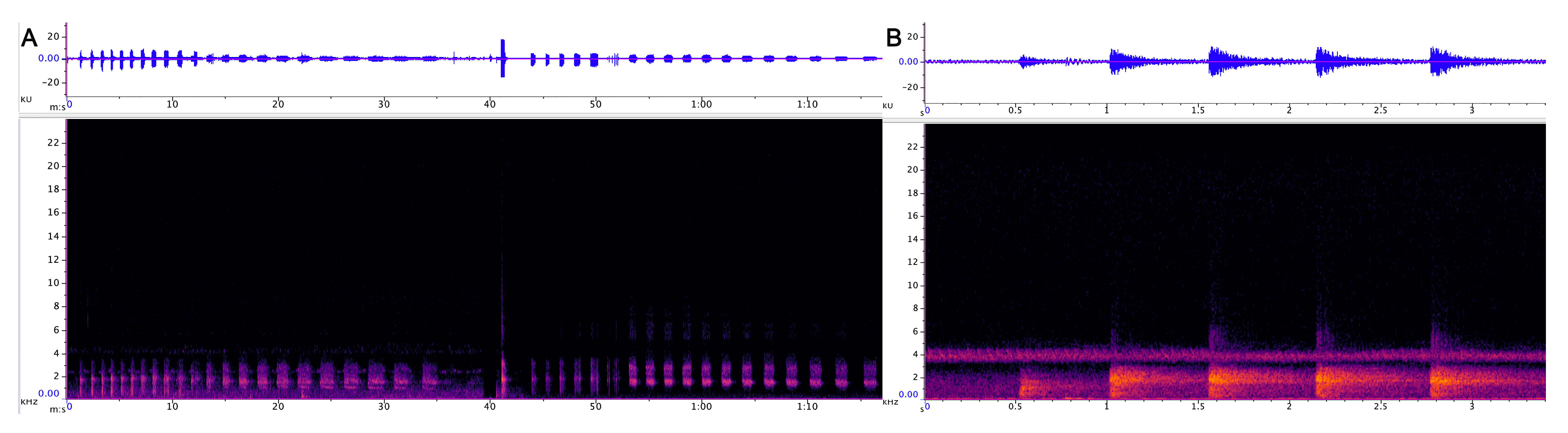
3.1.1. Strangled Thwack
3.1.2. Hac
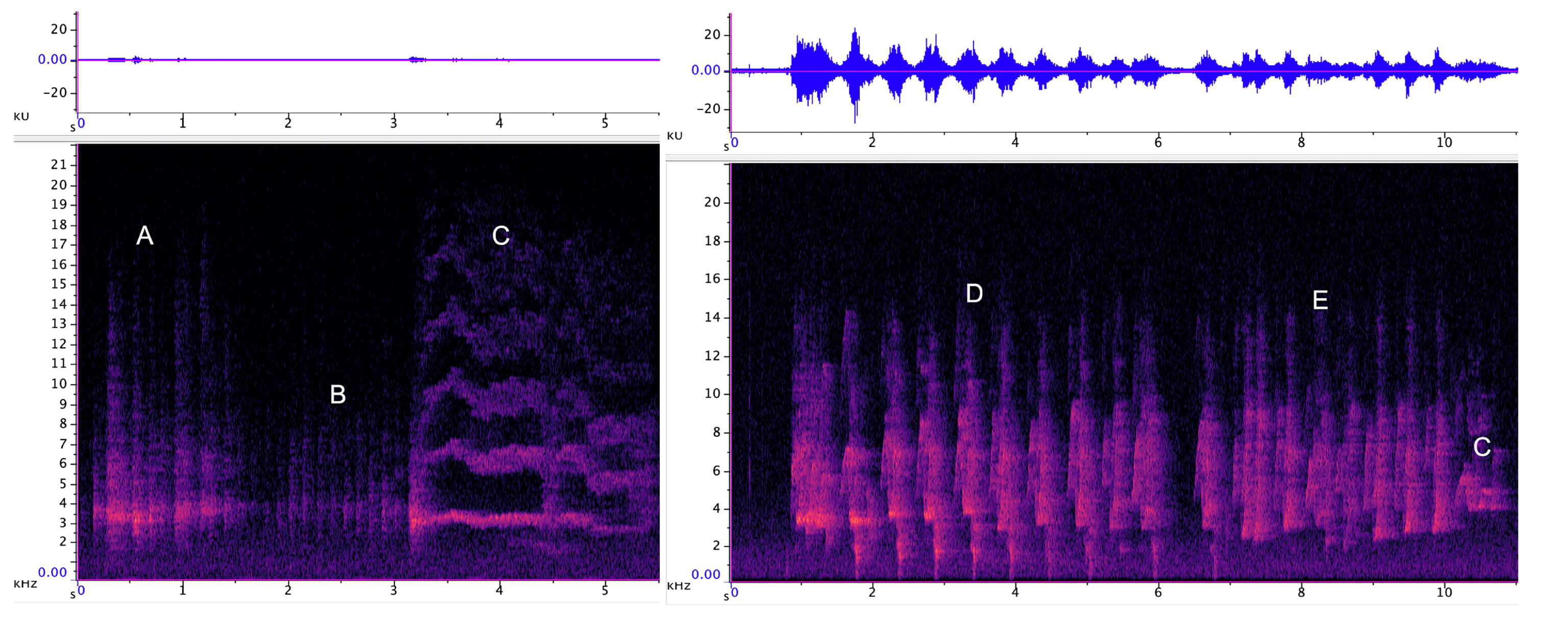
3.1.3. Songs
3.1.4. Behavioural Observations
3.2. Characterization of Otolemur Vocalizations
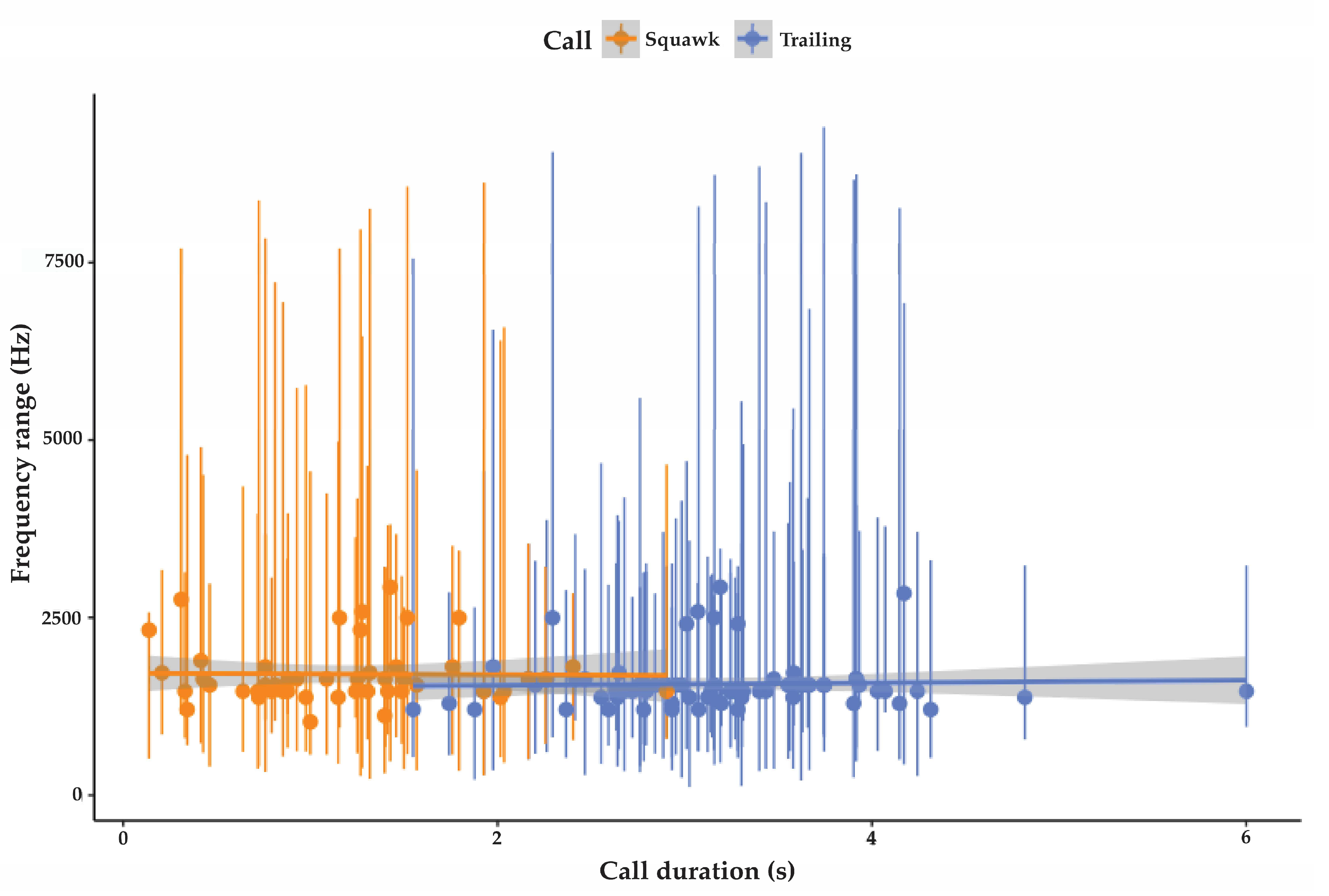
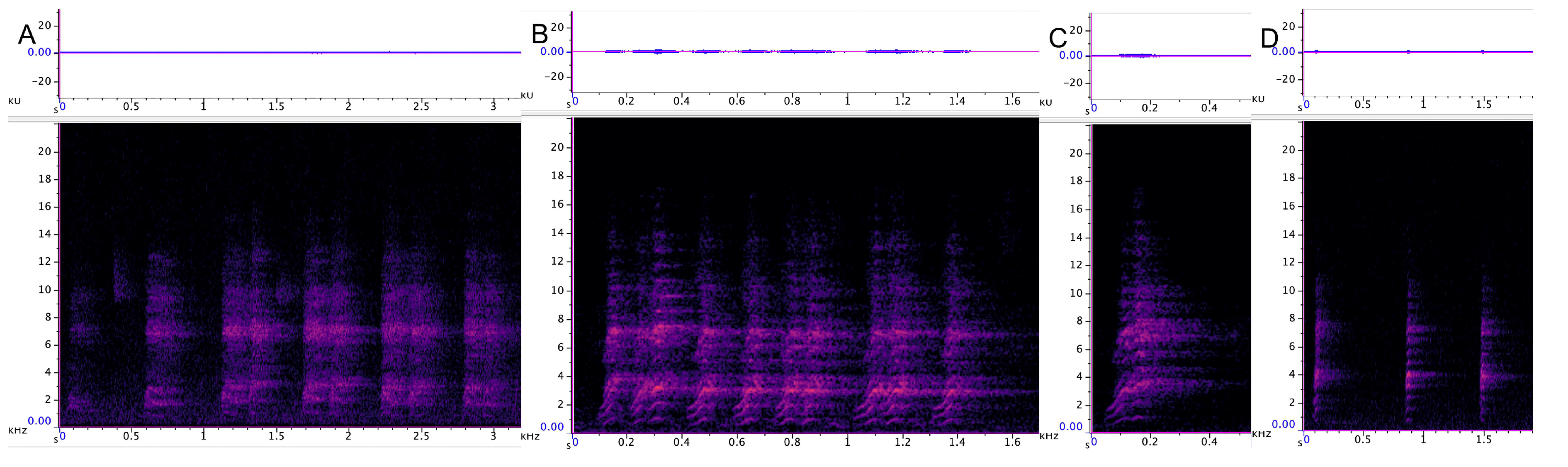
3.2.1. Trailing Call
3.2.2. Cluster Squawk
3.2.3. Behavioural Observations
3.3. Characterization of Paragalago Vocalizations
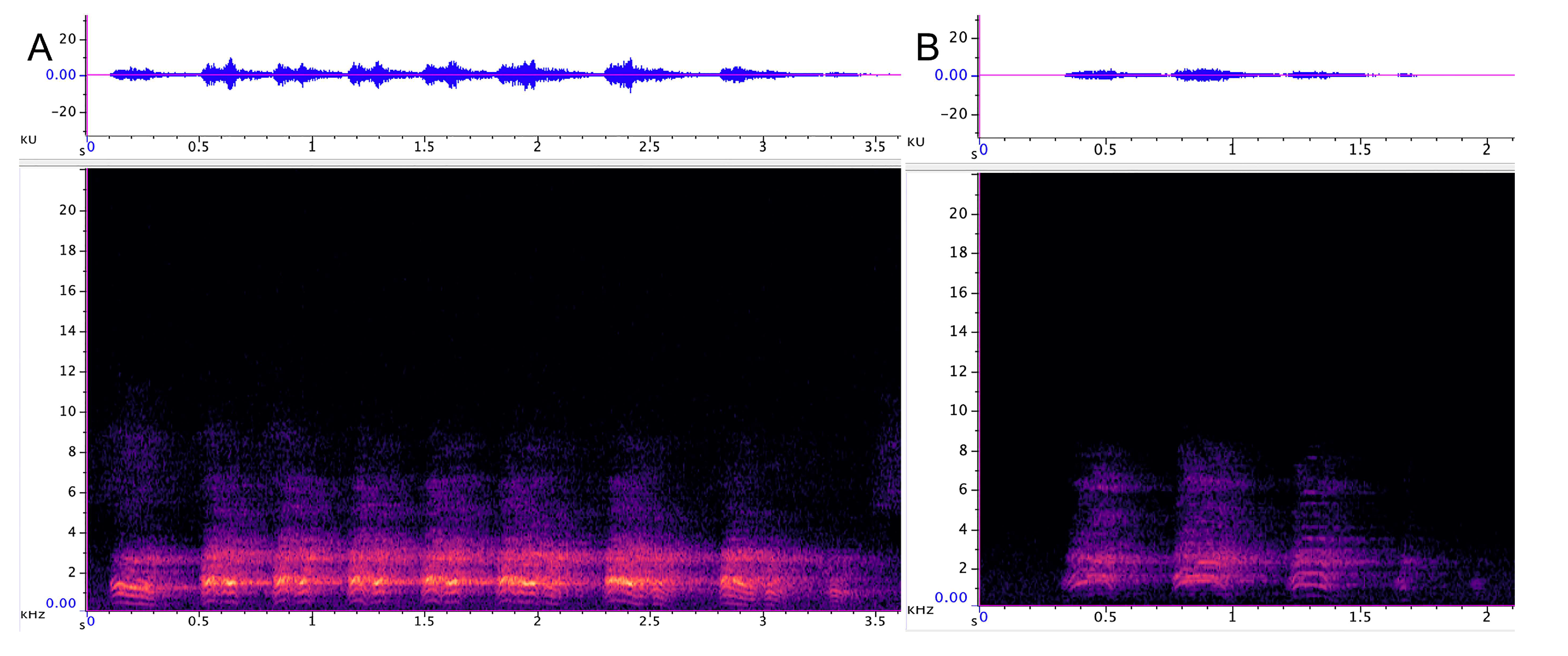
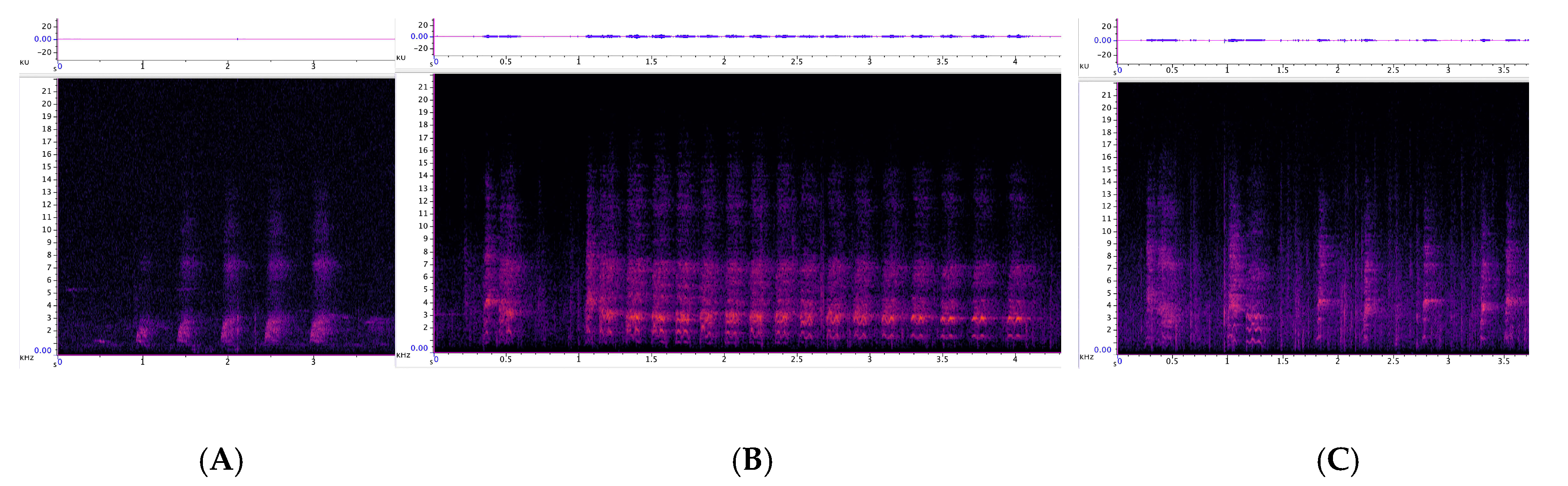
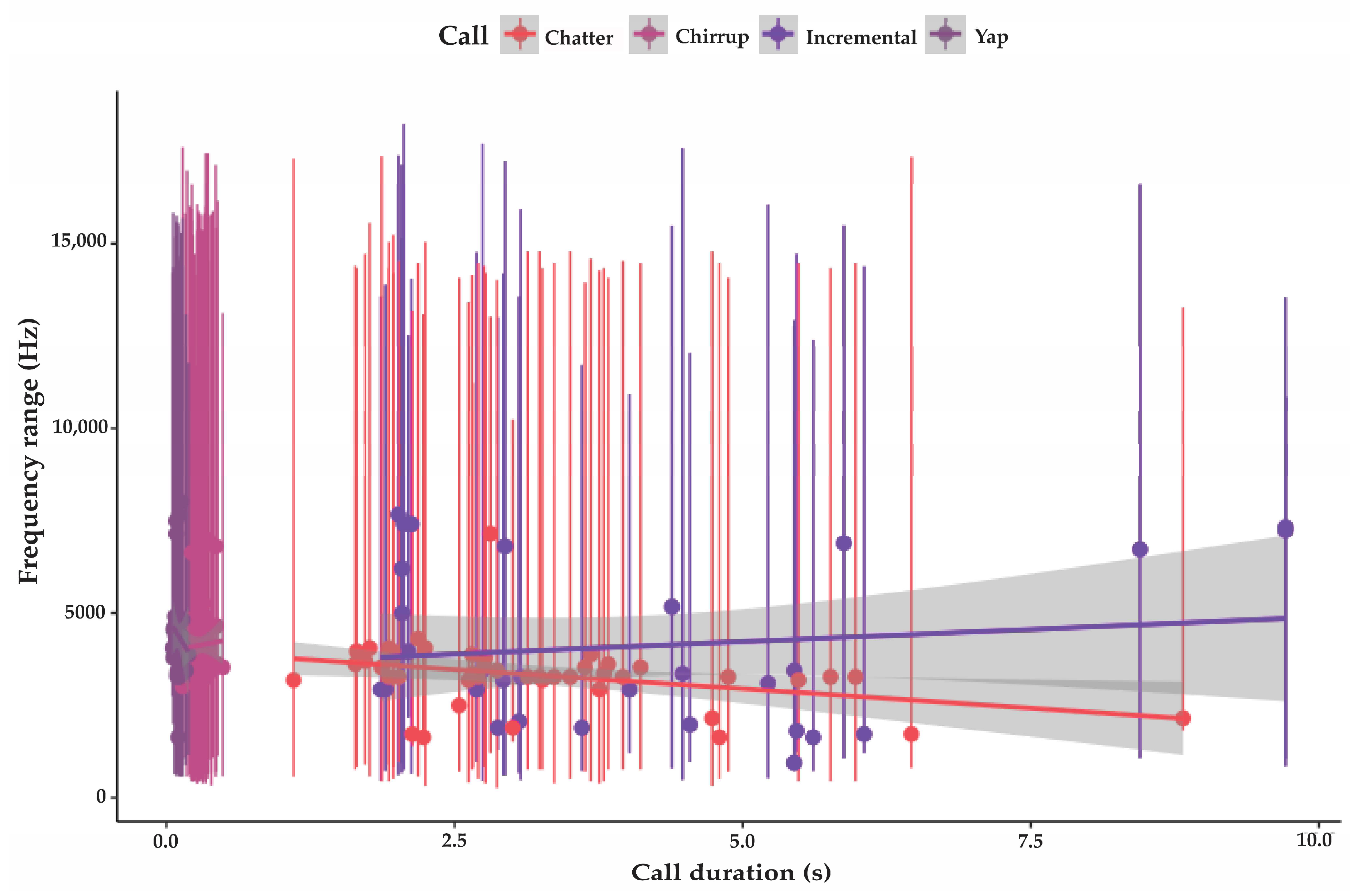
3.3.1. Incremental Call
3.3.2. Chatter
3.3.3. Chirrup
3.3.4. Yap
3.3.5. Behavioural Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snowden, C. Is speech special? Lessons from New World primates. In New World Primates: Ecology, Evolution and Behavior; Kinzey, W.G., Ed.; Aldine De Gruyter: New York, NY, USA, 1997; pp. 75–94. [Google Scholar]
- Braune, P.; Schmidt, S.; Zimmermann, E. Acoustic divergence in the communication of cryptic species of nocturnal primates (Microcebus ssp.). BMC Biol. 2008, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gursky, S.; Nekaris, K.A.I. Primate communication: Ecology, evolution and conservation. Folia Primatol. 2019, 90, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Masters, J. Speciation in the greater galagos (Prosimii: Galaginae): Review and synthesis. Biol. J. Linn. Soc. 1988, 34, 149–174. [Google Scholar] [CrossRef]
- Roberts, D. Geographic Variation in the Loud Calls of Tree Hyrax—Dendrohyrax validus (True 1890) in the Eastern Arc Mountains, East Africa: Taxonomic and Conservation Implications. Master’s Thesis, University of Reading, Reading, UK, 2001. [Google Scholar]
- Paterson, H.E.H. The recognition concept of species. In Species and Speciation; Vrba, E.S., Ed.; Transvaal Museum Monograph No. 4: Pretoria, South Africa, 1985; pp. 21–29. [Google Scholar]
- Masters, J.C. Speciation in the lesser galagos. Folia Primatol. 1998, 69 (Suppl. 1), 357–370. [Google Scholar] [CrossRef]
- Masters, J.C.; Boniotto, M.; Crovella, S.; Roos, C.; Pozzi, L.; Delpero, M. Phylogenetic relationships among the Lorisoidea as indicated by craniodental morphology and mitochondrial sequence data. Am. J. Primatol. 2007, 69, 6–15. [Google Scholar] [CrossRef]
- Bearder, S.K.; Honess, P.E.; Ambrose, L. Species Diversity Among Galagos with Special Reference to Mate Recognition. In Creatures of the Dark: The Nocturnal Prosimians; Alterman, L., Doyle, G.A., Izard, M.K., Eds.; Springer US: Boston, MA, USA, 1995; pp. 331–352. ISBN 978-1-4419-3250-1. [Google Scholar]
- Bearder, S.K. Physical and social diversity among nocturnal primates: A new view based on long term research. Primates 1999, 40, 267–282. [Google Scholar] [CrossRef]
- Masters, J.C. Loud calls of Galago crassicaudatus and G. garnettii and their relation to habitat structure. Primates 1991, 32, 153–167. [Google Scholar] [CrossRef]
- Butynski, T.M.; de Jong, Y.A.; Perkin, A.W.; Bearder, S.K.; Honess, P.E. Taxonomy, distribution, and conservation status of three species of dwarf galagos (Galagoides) in eastern Africa. Primate Conserv. 2006, 21, 63–79. [Google Scholar] [CrossRef]
- Bettridge, C.M.; Kenworthy, S.P.; Butynski, T.M.; de Jong, Y.A.; de Kort, S.R. Vocal repertoire and intraspecific variation within two loud calls of the small-eared greater galago (Otolemur garnettii) in Tanzania and Kenya. Folia Primatol. 2019, 90, 319–335. [Google Scholar] [CrossRef]
- Pozzi, L.; Disotell, T.R.; Bearder, S.K.; Karlsson, J.; Perkin, A.; Gamba, M. Species boundaries within morphologically cryptic galagos: Evidence from acoustic and genetic data. Folia Primatol. 2019, 90, 279–299. [Google Scholar] [CrossRef]
- Masters, J.C.; Génin, F.; Couette, S.; Groves, C.P.; Nash, S.D.; Delpero, M.; Pozzi, L. A new genus for the eastern dwarf galagos (Primates: Galagidae). Zool. J. Linn. Soc. 2017, 181, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Butynski, T.M.; Chapman, C.A.; Chapman, L.J.; Weary, D.M. Use of male blue monkey “pyow” calls for long-term individual identification. Am. J. Primatol. 1992, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ey, E.; Fischer, J. The “Acoustic Adaptation Hypothesis”—A review of the evidence from birds, anurans and mammals. Bioacoustics 2009, 19, 21–48. [Google Scholar] [CrossRef]
- Bradbury, J.W.; Vehrencamp, S.L. Principles of Animal Communication, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2011; ISBN 978-0-87893-045-6. [Google Scholar]
- Patricelli, G.L.; Hebets, E.A. New dimensions in animal communication: The case for complexity. Curr. Opin. Behav. Sci. 2016, 12, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Gustison, M.L.; le Roux, A.; Bergman, T.J. Derived vocalizations of geladas (Theropithecus gelada) and the evolution of vocal complexity in primates. Phil. Trans. Roy. Soc. B 2012, 367, 1847–1859. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Wadewitz, P.; Hammerschmidt, K. Structural variability and communicative complexity in acoustic communication. Anim. Behav. 2017, 134, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Grubb, P. English common names for subspecies and species of African primates. Primate Conserv. 2006, 20, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Kundaeli, J.N. Distribution of tree hyrax (Dendrohyrax validus validus True) on Mt Kilimanjaro, Tanzania. Afr. J. Ecol. 1976, 14, 253–264. [Google Scholar] [CrossRef]
- Maloiy, G.M.O.; Eley, R.M. The Hyrax; R.M. Eley: Nairobi, Kenya, 1992; ISBN 978-92-9055-702-9. [Google Scholar]
- Gaylard, A.; Kerley, G.I.H. Habitat assessment for a rare, arboreal forest mammal, the tree hyrax Dendrohyrax arboreus. Afr. J. Ecol. 2001, 39, 205–212. [Google Scholar] [CrossRef]
- Fey, V. A note on the behavior of the tree hyrax. East Afr. Geogr. Rev. 1960, 23, 244–246. [Google Scholar]
- Rudnai, J. Activity cycle and space utilization in captive Dendrohyrax arboreus. S. Afr. J. Zool. 1984, 19, 124–128. [Google Scholar] [CrossRef]
- Milner, J. Relationships between the forest dwelling people of South-West Mau and tree hyrax Dendrohyrax arboreus. J. East Afr. Nat. Hist. 1994, 83, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Milner, J.M.; Harris, S. Activity patterns and feeding behaviour of the tree hyrax, Dendrohyrax arboreus, in the Parc National des Volcans, Rwanda. Afr. J. Ecol. 1999, 37, 267–280. [Google Scholar] [CrossRef]
- Richard, P. Notes sur la biologie du daman des arbes (Dendrohyrax dorsalis). Biol. Gabonica 1964, 1, 73–84. [Google Scholar]
- Rahm, U. Notes sur le cri du Dendrohyrax dorsalis (Hyracoidea). Mammalia 1969, 33, 68–79. [Google Scholar] [CrossRef]
- Djossa, B.A.; Zachee, B.A.; Sinsin, B.A. Activity patterns and habitat use of the western tree hyrax (Dendrohyrax dorsalis), within forest patches and implications for conservation. Ecotropica 2012, 2012, 65–72. [Google Scholar]
- Roberts, D.; Topp-Jørgensen, E.; Moyer, D.C. Dendrohyrax validus Eastern tree hyrax. In Mammals of Africa; Kingdon, J., Ed.; Bloomsbury: London, UK, 2013; Volume I, pp. 158–161. [Google Scholar]
- Hahn, H. Die Familie der Procaviidae. Zeitschr. Säugetierk. 1934, 9, 207–358. [Google Scholar]
- Hahn, H. Baumschliefer, Buschschliefer, Klippschliefer; Die Neue Brehm-Bücherei, Heft 246; A. Ziemsen Verlag: Wittenberg Lutherstadt, German Democratic Republic, 1959. [Google Scholar]
- Jones, C. Dendrohyrax dorsalis. Mamm. Species 1978, 113, 1–4. [Google Scholar] [CrossRef]
- Hoeck, H. Some thoughts on the distribution of the tree hyraxes (genus Dendrohyrax) in northern Tanzania. Afrotherian Conserv. 2017, 13, 47–49. [Google Scholar]
- Kingdon, J. Where have the colonists come from? A zoogeographical examination of some mammalian isolates in eastern Africa. Afr. J. Ecol. 1981, 19, 115–124. [Google Scholar] [CrossRef]
- Kingdon, J. Island Africa: The Evolution of Africa’s Rare Animals and Plants; Collins: London, UK, 1990; ISBN 978-0-00-219914-8. [Google Scholar]
- Seibt, U.; Hoeck, H.; Wickler, W. Dendrohyrax validus True, 1890 in Kenya. Zeitschr. Säugetierk. 1977, 42, 115–118. [Google Scholar]
- Bloomer, P. Extant hyrax diversity is vastly underestimated. Afrotherian Conserv. 2009, 7, 11–16. [Google Scholar]
- Bearder, S.K.; Oates, J.F.; Dowsett-Lemaire, F.; Dowsett, R. Evidence of an undescribed form of tree hyrax in the forests of western Nigeria and the Dahomey Gap. Afrotherian Conserv. 2015, 11, 2–5. [Google Scholar]
- True, F.W. Description of Two New Species of Mammals from Mt. Kilima-Njaro, East Africa; US Government Printing Office: Washington, DC, USA, 1890; Volume 13, pp. 227–229. [Google Scholar] [CrossRef]
- True, F.W. An Annotated Catalogue of the Mammals Ccollected by Dr. W. L. Abbott in the Kilimanjaro Region, East Africa; US Government Printing Office: Washington, DC, USA, 1892; Volume 15, pp. 445–480. [Google Scholar]
- Matschie, P. Über anscheinend neue afrikanische Säugethiere (Leimacomys n. g.). Sitz. Ber. Ges. Nat. Freunde Berlin 1893, 1893, 107–114. [Google Scholar]
- Brauer, A. Neue Procaviiden. Sitz. Ber. Ges. Nat. Freunde Berlin 1917, 1917, 294–303. [Google Scholar]
- Mollison, T. Dendrohyrax nova species, aff. D. neumanni. Zool. Anz. 1905, 24, 417–424. [Google Scholar]
- Beentje, H. An ecological and floristical study of the forests of the Taita Hills, Kenya. Utafiti 1988, 1, 23–66. [Google Scholar]
- Bytebyer, B. Taita Hills Biodiversity Project Report; National Museums of Kenya: Nairobi, Kenya, 2001. [Google Scholar]
- Nash, L.T.; Harcourt, C.S. Social organization of galagos in Kenyan coastal forests: II. Galago garnettii. Am. J. Primatol. 1986, 10, 357–369. [Google Scholar] [CrossRef]
- Harcourt, C.S.; Perkin, A.W. Otolemur garnettii Small-eared greater galago. In Mammals of Africa; Kingdon, J., Ed.; Bloomsbury: London, UK, 2013; Volume II, pp. 413–416. [Google Scholar]
- Harcourt, C.S.; Perkin, A.W. Galagoides cocos Kenya coast dwarf galago (Diani dwarf galago). In Mammals of Africa; Kingdon, J., Ed.; Bloomsbury: London, UK, 2013; Volume II, pp. 457–459. [Google Scholar]
- Masters, J. Geographic distributions of karyotypes and morphotypes within the greater galagines. Folia Primatol. 1986, 46, 127–141. [Google Scholar] [CrossRef]
- Nash, L.T.; Bearder, S.K.; Olson, T.R. Synopsis of Galago species characteristics. Int. J. Primatol. 1989, 10, 57–80. [Google Scholar] [CrossRef]
- Anderson, M.J. The use of hand morphology in the taxonomy of galagos. Primates 1999, 40, 469–478. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ambrose, L.; Bearder, S.K.; Dixson, A.F.; Pullen, S. Intraspecific variation in the vocalizations and hand pad morphology of southern lesser bush babies (Galago moholi): A comparison with G. senegalensis. Int. J. Primatol. 2000, 21, 537–555. [Google Scholar] [CrossRef]
- DelPero, M.; Masters, J.C.; Zuccon, D.; Cervella, P.; Crovella, S.; Ardito, G. Mitochondrial sequences as indicators of generic classification in bush babies. Int. J. Primatol. 2000, 21, 889–904. [Google Scholar] [CrossRef]
- Stiner, E.; Turmelle, A. Galagid taxonomy and the placement of the needle-clawed galago (Euoticus): Based on cytochrome b, 12S and 16S partial sequences. Afr. Primates 2002, 6, 3–10. [Google Scholar]
- Kingdon, J. The Kingdon Field Guide to African Mammals; Natural World Academic Press: London, UK, 1997; ISBN 978-0-69111-692-1. [Google Scholar]
- Groves, C.P. Primate Taxonomy; Smithsonian Institution Press: Washington, DC, USA, 2001; ISBN 978-1-56098-872-4. [Google Scholar]
- Olson, T. Systematics and zoogeography of the greater galagos. Am. J. Phys. Anthropol. 1981, 54, 259. [Google Scholar] [CrossRef]
- Grubb, P.; Butynski, T.M.; Oates, J.F.; Bearder, S.K.; Disotell, T.R.; Groves, C.P.; Struhsaker, T.T. Assessment of the diversity of African primates. Int. J. Primatol. 2003, 24, 1301–1357. [Google Scholar] [CrossRef]
- Pozzi, L.; Disotell, T.R.; Masters, J.C. A multilocus phylogeny reveals deep lineages within African galagids (Primates: Galagidae). BMC Evol. Biol. 2014, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, L.; Nekaris, K.A.-I.; Perkin, A.; Bearder, S.K.; Pimley, E.R.; Schulze, H.; Streicher, U.; Nadler, T.; Kitchener, A.; Zischler, H.; et al. Remarkable ancient divergences amongst neglected lorisiform primates. Zool. J. Linn. Soc. 2015, 175, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Perkin, A.; Bearder, S.; Butynski, T.M.; Agwanda, B.; Bytebier, B. The Taita Mountain dwarf galago Galagoides sp: A new primate for Kenya. J. East Afr. Nat. Hist. 2002, 91, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rosti, H.; Rikkinen, J.; Pellikka, P.; Bearder, S.; Mwang’ombe, J. Taita Mountain dwarf galago is extant in the Taita Hills of Kenya. Oryx 2020, 54, 152–153. [Google Scholar] [CrossRef] [Green Version]
- Wagura, L. A Guide to Taita Hills: Unique Natural History; ABC: Nairobi, Kenya, 2014; ISBN 978-9966-074-61-4. [Google Scholar]
- Pellikka, P.K.E.; Lötjönen, M.; Siljander, M.; Lens, L. Airborne remote sensing of spatiotemporal change (1955–2004) in indigenous and exotic forest cover in the Taita Hills, Kenya. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 221–232. [Google Scholar] [CrossRef]
- Wilder, C.; Brooks, T.; Lens, L. Vegetation structure and composition of the Taita Hills forests. J. East Afr. Nat. Hist. 1998, 87, 181–187. [Google Scholar] [CrossRef]
- Aerts, R.; Thijs, K.W.; Lehouck, V.; Beentje, H.; Bytebier, B.; Matthysen, E.; Gulinck, H.; Lens, L.; Muys, B. Woody plant communities of isolated Afromontane cloud forests in Taita Hills, Kenya. Plant Ecol. 2011, 212, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Malombe, I.; Matheka, K.W.; Pócs, T.; Patiño, J. Edge effect on epiphyllous bryophytes in Taita Hills fragmented afromontane forests. J. Bryol. 2016, 38, 33–46. [Google Scholar] [CrossRef]
- Burgess, N.D.; Butynski, T.M.; Cordeiro, N.J.; Doggart, N.H.; Fjeldså, J.; Howell, K.M.; Kilahama, F.B.; Loader, S.P.; Lovett, J.C.; Mbilinyi, B.; et al. The biological importance of the Eastern Arc Mountains of Tanzania and Kenya. Biol. Conserv. 2007, 134, 209–231. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Dimitrov, D.; Nogués-Bravo, D.; Scharff, N. Why do tropical mountains support exceptionally high biodiversity? The Eastern Arc Mountains and the drivers of Saintpaulia diversity. PLoS ONE 2012, 7, e48908. [Google Scholar] [CrossRef] [Green Version]
- Gereau, R.E.; Cumberlidge, N.; Hemp, C.; Hochkirch, A.; Jones, T.; Kariuki, M.; Lange, C.N.; Loader, S.P.; Malonza, P.K.; Menegon, M.; et al. Globally threatened biodiversity of the Eastern Arc Mountains and coastal forests of Kenya and Tanzania. J. East Afr. Nat. Hist. 2016, 105, 115–201. [Google Scholar] [CrossRef] [Green Version]
- Heiskanen, J.; Korhonen, L.; Hietanen, J.; Pellikka, P.K.E. Use of airborne lidar for estimating canopy gap fraction and leaf area index of tropical montane forests. Int. J. Remote Sens. 2015, 36, 2569–2583. [Google Scholar] [CrossRef]
- Adhikari, H.; Heiskanen, J.; Siljander, M.; Maeda, E.; Heikinheimo, V.; KE Pellikka, P. Determinants of aboveground biomass across an Afromontane landscape mosaic in Kenya. Remote Sens. 2017, 9, 827. [Google Scholar] [CrossRef] [Green Version]
- Himberg, N. Traditionally Protected Forests´ Role within Transforming Natural Resource Management Regimes in Taita Hills, Kenya. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2011. Department of Geosciences and Geography A 14. 1798-7911. [Google Scholar]
- Teucher, M.; Schmitt, C.B.; Wiese, A.; Apfelbeck, B.; Maghenda, M.; Pellikka, P.; Lens, L.; Habel, J.C. Behind the fog: Forest degradation despite logging bans in an East African cloud forest. Global Ecol. Conserv. 2020, 22, e01024. [Google Scholar] [CrossRef]
- Thijs, K.W.; Aerts, R.; Musila, W.; Siljander, M.; Matthysen, E.; Lens, L.; Pellikka, P.; Gulinck, H.; Muys, B. Potential tree species extinction, colonization and recruitment in Afromontane forest relicts. Basic Appl. Ecol. 2014, 15, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Thijs, K.W. Tree Community Dynamics and Ecosystem Function in a Tropical Landscape under Deforestation Pressure. Ph.D. Thesis, KU Leuven, Science, Engineering & Technology, Leuven, Belgium, 2015. [Google Scholar]
- Stam, Å.; Enroth, J.; Malombe, I.; Pellikka, P.; Rikkinen, J. Experimental transplants reveal strong environmental effects on the growth of non-vascular epiphytes in Afromontane forests. Biotropica 2017, 49, 862–870. [Google Scholar] [CrossRef]
- Stam, Å.; Anttila, J.; Pellikka, P.; Rikkinen, J. Sensitivity of tropical pendant Bryophytes: Results from a translocation experiment along an elevation gradient. Ann. Bot. Fenn. 2020, 57, 71. [Google Scholar] [CrossRef]
- Siljander, M.; Kuronen, T.; Johansson, T.; Munyao, M.N.; Pellikka, P.K.E. Primates on the farm—Spatial patterns of human—wildlife conflict in forest-agricultural landscape mosaic in Taita Hills, Kenya. Appl. Geogr. 2020, 117, 102185. [Google Scholar] [CrossRef]
- Hemp, C.; Heller, K.-G.; Warchałowska-Śliwa, E.; Grzywacz, B.; Hemp, A. Biogeography, ecology, acoustics and chromosomes of East African Eurycorypha Stål species (Orthoptera, Phaneropterinae) with the description of new species. Org. Divers. Evol. 2013, 13, 373–395. [Google Scholar] [CrossRef]
- Heller, K.-G.; Hemp, C. Extremely divergent song types in the genus Aerotegmina Hemp (Orthoptera: Tettigoniidae: Hexacentrinae) and the description of a new species from the Eastern Arc Mountains of Tanzania (East Africa). Bioacoustics 2019, 28, 269–285. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems: Data exploration. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Roberts, D. A Preliminary Investigation of the Taxonomic Implications of the Loud Calls of Dendrohyrax (Hyracoidea, Procaviidae) in Africa. Master’s Thesis, Oxford Brookes University, Oxford, UK, 1999. [Google Scholar]
- Koren, L.; Geffen, E. Complex call in male rock hyrax (Procavia capensis): A multi-information distributing channel. Behav. Ecol. Sociobiol. 2009, 63, 581–590. [Google Scholar] [CrossRef]
- Fitch, W.T.; Neubauer, J.; Herzel, H. Calls out of chaos: The adaptive significance of nonlinear phenomena in mammalian vocal production. Anim. Behav. 2002, 63, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Koren, L. Hyrax Socialization: First Evidence for a Matriarchal Society. Master’s Thesis, Department of Zoology, Tel-Aviv University, Tel Aviv, Israel, 2000. [Google Scholar]
- Koren, L. Vocalization as an Indicator of Individual Quality in the Rock Hyrax. Ph.D. Thesis, Department of Zoology, Tel-Aviv University, Tel Aviv, Israel, 2006. [Google Scholar]
- Kershenbaum, A.; Ilany, A.; Blaustein, L.; Geffen, E. Syntactic structure and geographical dialects in the songs of male rock hyraxes. Proc. R. Soc. B 2012, 279, 2974–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demartsev, V.; Kershenbaum, A.; Ilany, A.; Barocas, A.; Bar Ziv, E.; Koren, L.; Geffen, E. Male hyraxes increase song complexity and duration in the presence of alert individuals. Behav. Ecol. 2014, 25, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.L.; Buder, E.H.; Ward, J.P. Spectrographic description of vocalizations in captive Otolemur garnettii. Int. J. Primatol. 2003, 24, 415–446. [Google Scholar] [CrossRef]
- De Yong, Y.; Butynski, T.M. Primates of East Africa: Pocket Identification Guide; Global Wildlife Conservation: Austin, TX, USA, 2018; ISBN 978-0-692-13025-4. [Google Scholar]
- Butynski, T.M.; de Jong, Y.A. Paragalago cocos, The IUCN Red List of Threatened Species 2019: e.T136212A17963050. 2016. Available online: https://www.iucnredlist.org/species/136212/17963050 (accessed on 15 November 2020).
- Burgess, N.D.; Fjeldså, J.; Botterweg, R. Faunal importance of the Eastern Arc Mountains of Kenya and Tanzania. J. East Afr. Nat. Hist. 1998, 87, 37–58. [Google Scholar] [CrossRef]
- Stanley, W.T.; Kihaule, P.M.; Howell, K.M.; Hutterer, R. Small mammals of the Eastern Arc Mountains, Tanzania. J. East Afr. Nat. Hist. 1998, 87, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Demos, T.C.; Kerbis Peterhans, J.C.; Agwanda, B.; Hickerson, M.J. Uncovering cryptic diversity and refugial persistence among small mammal lineages across the Eastern Afromontane biodiversity hotspot. Mol. Phylogenet. Evol. 2014, 71, 41–54. [Google Scholar] [CrossRef]
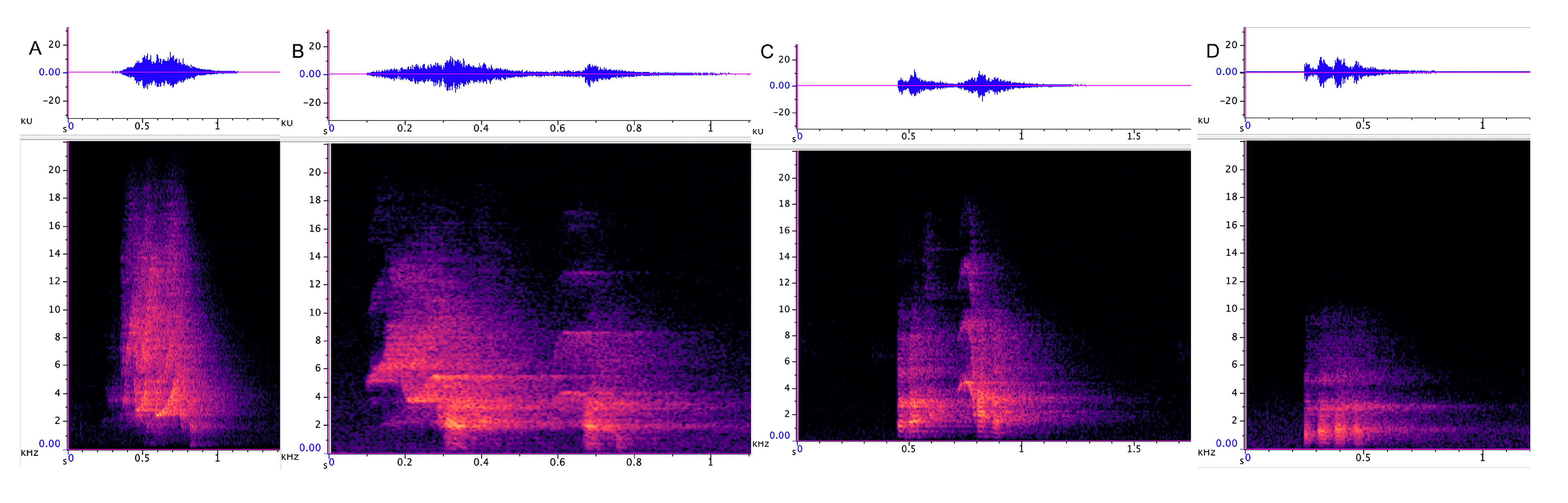
| Dendrohyrax Strangled Thwack n 174 | Dendrohyrax Hac n 80 | Otolemur Trailing n 78 | Otolemur Squawk n 48 | Paragalago Incremental n 31 | Paragalago Chatter n 48 | Paragalago Chirrup n 55 | Paragalago Yap n 45 | |
|---|---|---|---|---|---|---|---|---|
| Percentage | 89.1 | 58.2 | 93.6 | 76.6 | 41.9 | 90.7 | 92.6 | 97.8 |
| Call Type | n | Fundamental Frequency Hz | Highest Frequency | Maximum Frequency Hz | Call Duration s | Redundancy | Unit Distance s (n) | Answer % |
|---|---|---|---|---|---|---|---|---|
| Strangled thwack | 174 | 323 SD 53.5 | 16,206 SD 3469 | 2431 SD 818 | 0.6 SD 0.14 | 1–15 avg. 5.2 | 11.5 (n 248) SD 8.6 | 75 |
| Hac | 79 | 304 SD 124 | 9538 SD 3469 | 1725 SD 269 | 0.49 SD 0.16 | 1–<100 avg. 7.2 | 8.7 (n 115) SD 3.8 | 74 |
| Call Type | n | Fundamental Frequency Hz | Highest Frequency | Maximum Frequency Hz | Length s | Energy dB | Answer % |
|---|---|---|---|---|---|---|---|
| Trailing call | 78 | 227 SD 25.7 | 6195 SD 4026 | 1568 SD 357 | 3.2 SD 0.7 | 98.2 SD 5.9 | 42 |
| Cluster squawk | 47 | 315 SD 52 | 6602 SD 2861 | 1710 SD 428 | 1.5 SD 0.6 | 91.5 SD 6 | NA |
| Call Type. | n | Fundamental Frequency Hz | Highest Frequency Hz | Maximum Frequency Hz | Length s. | Energy dB | Inter-Unit Interval s. |
|---|---|---|---|---|---|---|---|
| Incremental | 31 | 974 SD 360 | 13,734 SD 2085 | 4064 SD 2146 | 4.1 SD 2.2 | 94 SD 4.6 | variable |
| Chatter | 43 | 533 SD 390 | 15,680 SD 2356 | 3230 SD 1218 | 3.3 SD 1.5 | 92.8 SD 5.6 | 1.2 (n 77) |
| Chirrup | 54 | 694 SD 273 | 15,406 SD 3124 | 4144 SD 1133 | 0.29 SD 0.07 | 90.4 SD 2.6 | 0.6 (n 94) |
| Yap | 45 | 1364 SD 316 | 14,020 SD 692 | 4365 SD 954 | 0.1 SD 0.02 | 83 SD 3.9 | 0.47 (n 112) |
| n | Mean Call Length s | Call Range s | Mean Number of Units | Range Number of Units | Low Frequency Hz | Maximum Frequency Hz | |
|---|---|---|---|---|---|---|---|
| O. g. panganiensis (Bettridge et al. 2019) | 226 | 3.08 | 0.9–6.43 | 7.7 | 3–15 | 533 | 819 |
| O. lasiotis (Bettridge et al. 2019) | 10 | 2.42 | 0.87 | 5.6 | 4–8 | 200 | 1320 |
| Otolemur Taita Hills (this study) | 78 | 3.2 | 0.35–3.97 | 7.3 | 5–12 | 227 | 1593 |
| Paragalago, Taita Hills | Paragalago cocos, Diani | |
|---|---|---|
| Fundamental frequency | 800–1200 Hz | 800–1200 Hz |
| Frequency range | 680–14,500 Hz | 650–11,150 |
| Harmonics visible | 9000 Hz | 9300 (10th of 11th harmonic) |
| Duration | 3.55 s range 1.9–9.7 s | 4.3 s range 1.7–8.6 s |
| Mean number of phases | 6.6 (range 1–6) | 6 (range 1–11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosti, H.; Pihlström, H.; Bearder, S.; Pellikka, P.; Rikkinen, J. Vocalization Analyses of Nocturnal Arboreal Mammals of the Taita Hills, Kenya. Diversity 2020, 12, 473. https://doi.org/10.3390/d12120473
Rosti H, Pihlström H, Bearder S, Pellikka P, Rikkinen J. Vocalization Analyses of Nocturnal Arboreal Mammals of the Taita Hills, Kenya. Diversity. 2020; 12(12):473. https://doi.org/10.3390/d12120473
Chicago/Turabian StyleRosti, Hanna, Henry Pihlström, Simon Bearder, Petri Pellikka, and Jouko Rikkinen. 2020. "Vocalization Analyses of Nocturnal Arboreal Mammals of the Taita Hills, Kenya" Diversity 12, no. 12: 473. https://doi.org/10.3390/d12120473