Regional Pelagic Rotifer Biodiversity in a Tropical Karst Lake District
Abstract
:1. Introduction
2. Study Site
3. Methods
4. Data Analysis
5. Results
5.1. Species Richness and Composition
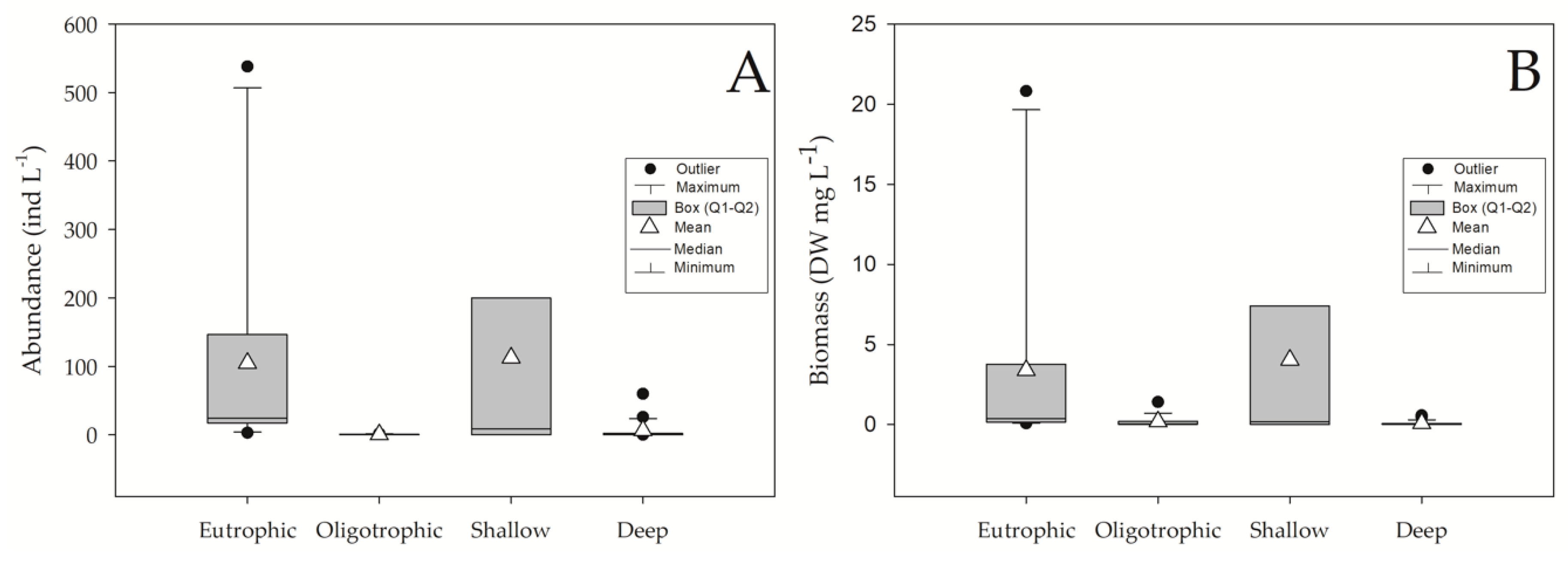
5.2. Abundance and Biomass
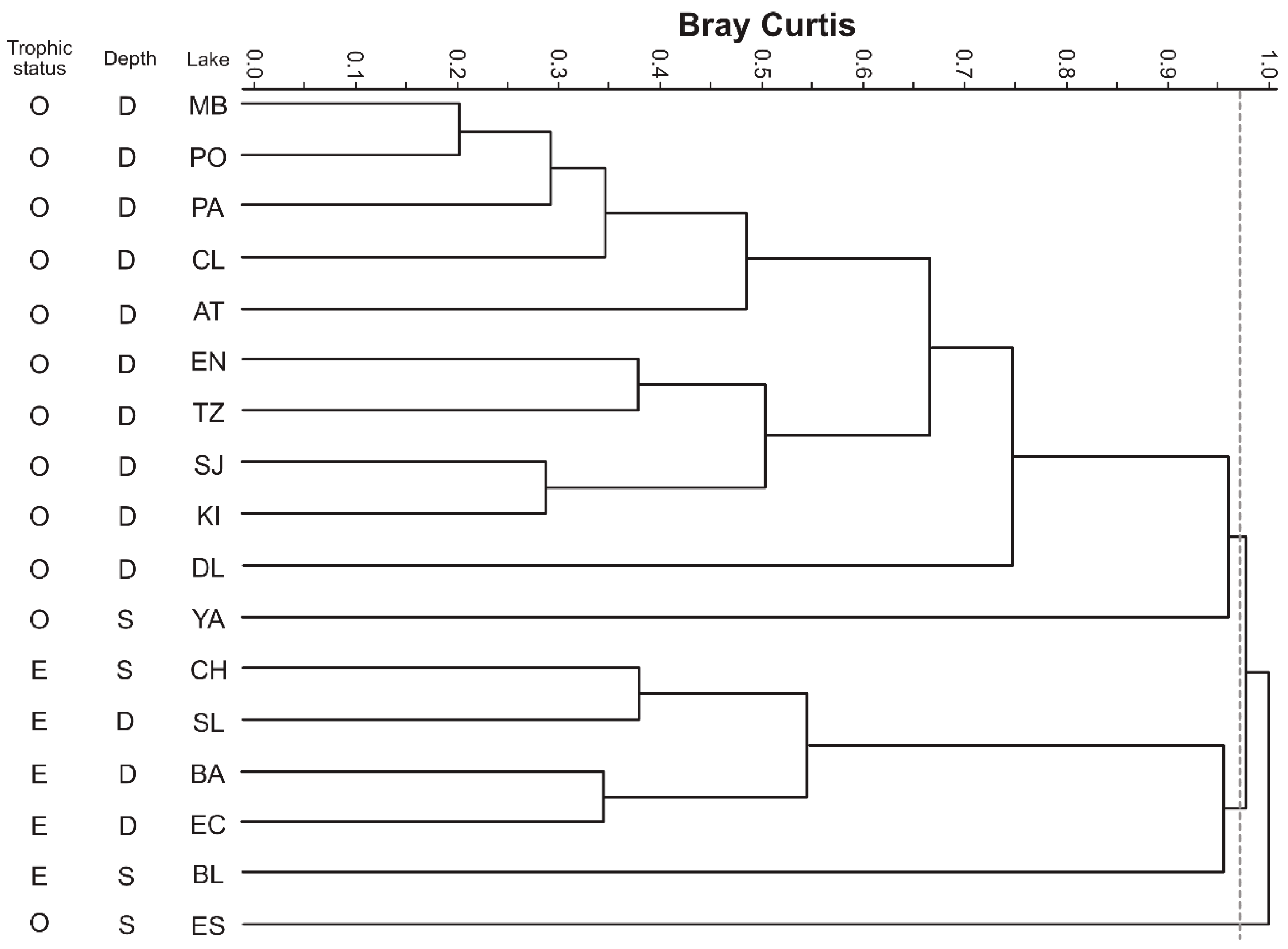
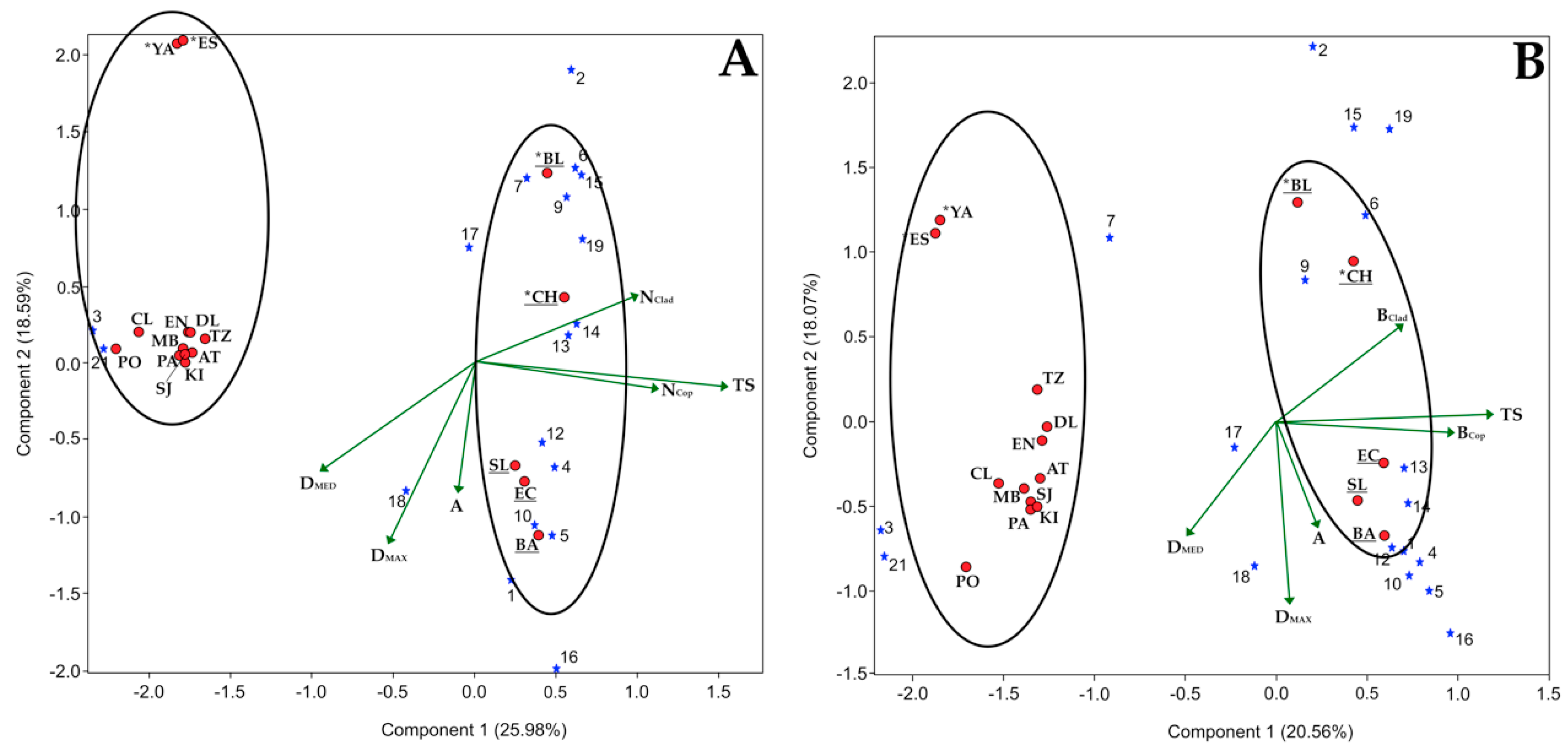
5.3. Multivariate Analysis
6. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanly, P.J.; Mittelbach, G.G.; Schemske, D.W. Speciation and the latitudinal diversity gradient: Insights from the global distribution of endemic fish. Am. Nat. 2017, 189, 604–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleff, P.; Urquiza-Haas, T.; Ruiz-González, S.P.; Hernández-Robles, D.R.; Mastretta-Yanes, A.; Quintero, E.; Sarukhán, J. Biodiversity in Mexico: State of Knowledge. In Global Biodiversity: Volume 4: Selected Countries in the Americas and Australia; Pullaiah, T., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Alcocer, J.; Aguilar-Sierra, V. Biodiversity in inland waters. In Mexican Aquatic Environments. A General View from Hydrobiology to Fisheries; Ibañez, A.L., Ed.; Springer: Berlin, Germany, 2019. [Google Scholar]
- Alcocer, J.; Azpra, E.; Falcón, L.; Gallegos, A.; García, F.; García-Oliva, F.; Jaramillo, V.; Lecuanda, R.; Magaña, V.; Martínez-Yrizar, A.; et al. Diversidad de procesos funcionales en los ecosistemas. In Capital natural de México Vol. 1 Conocimiento actual de la biodiversidad; Escobar, E., Maas, M., Eds.; CONABIO: Ciudad de México, Mexico, 2008. [Google Scholar]
- Martínez-Meyer, E.; Sosa-Escalante, J.E.; Álvarez, F. El estudio de la biodiversidad en México: ¿una ruta con dirección? Rev. Mex. Biodivers. 2014, 85, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sarma, S.S.S.; Nandini, S. Rotíferos Mexicanos (Rotifera); Manual de Enseñanza; UNAM: Toluca, Mexico, 2017. [Google Scholar]
- Nandini, S.; Merino-Ibarra, M.; Sarma, S.S.S. Seasonal changes in the zooplankton abundances of the reservoir Valle de Bravo (State of Mexico, Mexico). Lake Reserv. Manag. 2008, 24, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Sánchez, A.; Reyes-Vanegas, G.; Nandini, S.; Sarma, S.S.S. Diversity and abundance of rotifers during an annual cycle in the reservoir Valerio Trujano (Tepecoacuilco, Guerrero, Mexico). Inland Waters 2014, 4, 293–302. [Google Scholar] [CrossRef]
- Rico-Martínez, R.; Silva-Briano, M. Contribution to the knowledge of the rotifera of Mexico. In Rotifer Symposium VI. Developments in Hydrobiology; Gilbert, J.J., Lubzens, E., Miracle, M.R., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 467–474. [Google Scholar]
- Sarma, S.S.S.; Elías-Gutiérrez, M. Rotifers (Rotifera) from four natural water bodies of Central Mexico. Limnologica 1999, 29, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Nandini, S.; Sarma, S.S.S.; Gulati, R.D. A seasonal study reveals the occurrence of exotic rotifers, the River Antigua, Veracruz, close to the Gulf of Mexico. River Res. Appl. 2017, 33, 970–982. [Google Scholar] [CrossRef]
- Nandini, S.; Ramírez-García, P.; Sarma, S.S.S. Seasonal variations in the species diversity of planktonic rotifers in Lake Xochimilco, Mexico. J. Freshw. Ecol. 2005, 20, 287–294. [Google Scholar] [CrossRef]
- Walsh, E.J.; Schröder, T.; Wallace, R.L.; Ríos-Arana, J.V.; Rico-Martinez, R. Rotifers from selected inland saline waters in the Chihuahuan Desert of Mexico. Saline Syst. 2008, 4. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Arana, J.V.; Agüero-Reyes, L.C.; Wallace, R.L.; Walsh, E.J. Limnological characteristics and rotifer community composition of Northern México Chihuahuan Desert springs. J. Arid Environ. 2019, 160, 32–41. [Google Scholar] [CrossRef]
- Arroyo-Castro, J.L.; Alvarado-Flores, J.; Uh-Moo, J.C.; Koh-Pasos, C.G. Monogonont rotifers species of the Island Cozumel, Quintana Roo, Mexico. Biodivers. Data J. 2019, 7, e34719. [Google Scholar] [CrossRef]
- Quintana, X.D.; Arim, M.; Badosa, A.; Blanco, J.M.; Boix, D.; Brucet, S.; Compte, J.; Egozcue, J.J.; de Eyto, E.; Gaedke, U.; et al. Predation and competition effects on the size diversity of aquatic communities. Aquat. Sci. 2014, 77, 45–57. [Google Scholar] [CrossRef]
- Aránguiz-Acuña, A.; Pérez-Portilla, P.; De la Fuente, A.; Fontaneto, D. Life-history strategies in zooplankton promote coexistence of competitors in extreme environments with high metal content. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kim, S.-K. Responses of Rotifer Community to Microhabitat Changes Caused by Summer-Concentrated Rainfall in a Shallow Reservoir, South Korea. Diversity 2020, 12, 113. [Google Scholar] [CrossRef] [Green Version]
- Karpowicz, M.; Ejsmont-Karabin, J.; Kozłowska, J.; Feniova, I.; Dzialowski, A.R. Zooplankton Community Responses to Oxygen Stress. Water 2020, 12, 706. [Google Scholar] [CrossRef] [Green Version]
- Shurin, J.B.; Winder, M.; Adrian, R.; Keller, W.; Matthews, B.; Paterson, A.M.; Paterson, M.J.; Pinel-Alloul, B.; Rusak, J.A.; Yan, N.D. Environmental stability and lake zooplankton diversity–contrasting effects of chemical and thermal variability. Ecol. Lett. 2010, 13, 453–463. [Google Scholar] [CrossRef]
- Kaya, M.; Fontaneto, D.; Segers, H.; Altindağ, A. Temperature and salinity as interacting drivers of species richness of planktonic rotifers in Turkish continental waters. J. Limnol. 2010, 69, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, L.; Fretwell, S.D.; Arruda, J.; Niemela, P. Exploitation ecosystems in gradients of primary productivity. Am. Nat. 1981, 131, 424–444. [Google Scholar] [CrossRef]
- Hoffmann, M.D.; Dodson, S.I. Land use, primary productivity, and lake area as descriptors of zooplankton diversity. Ecology 2005, 86, 255–261. [Google Scholar] [CrossRef]
- Schuler, M.S.; Chase, J.M.; Knight, T.M. Habitat size modulates the influence of heterogeneity on species richness patterns in a model zooplankton community. Ecology 2017, 98, 1651–1659. [Google Scholar] [CrossRef]
- Scherer, L.; Pfister, S. Global biodiversity loss by freshwater consumption and eutrophication from Swiss food consumption. Environ. Sci. Technol. 2016, 50, 7019–7028. [Google Scholar] [CrossRef]
- Monchamp, M.E.; Spaak, P.; Domaizon, I.; Dubois, N.; Bouffard, D.; Pomati, F. Homogenization of lake cyanobacterial communities over a century of climate change and eutrophication. Nat. Ecol. Evol. 2018, 2, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, A.; Beisner, B.E. Zooplankton biodiversity and lake trophic state: Explanations invoking resource abundance and distribution. Ecology 2007, 88, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.C.; Housley, L.; Back, J.A.; King, R.S. Freshwater eutrophication drives sharp reductions in temporal beta diversity. Ecology 2018, 99, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, S.; Lin, J.; Li, H.; Lin, Q.; Han, B.P. Urbanization increases biotic homogenization of zooplankton communities in tropical reservoirs. Ecol. Indic. 2020, 110, 105899. [Google Scholar] [CrossRef]
- Alcocer, J.; Oseguera, L.A.; Sanchez, G.; Gonzalez, C.G.; Martinez, J.R.; Gonzalez, R. Bathymetric and morphometric surveys of the Montebello Lakes, Chiapas. J. Limnol. 2016, 75, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Alcocer, J.; Merino-Ibarra, M.; Oseguera, L.A.; Escolero, O. Anthropogenic impacts on tropical karst lakes: “Lagunas de Montebello” Chiapas. Ecohydrology 2018, 11, e2029. [Google Scholar] [CrossRef]
- CONANP. Estudio para el Monitoreo de Calidad del agua de las Lagunas en el Parque Nacional Lagunas de Montebello, Chiapas; Comisión Nacional de Áreas Naturales Protegidas: Ciudad de México, Mexico, 2009; pp. 15–35. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Instituto de Geografía, UNAM: Ciudad de México, Mexico, 2004; p. 97. [Google Scholar]
- Durán Calderón, I.; Escolero-Fuentes, O.A.; Muñoz Salinas, E.; Castillo-Rodríguez, M.; Silva-Romo, G. Cartografía geomorfológica a escala 1:50,000 del Parque Nacional Lagunas de Montebello, Chiapas (México). Boletín de la Sociedad Geológica Mexicana 2014, 66, 263–277. [Google Scholar] [CrossRef]
- Vera-Franco, M.; Hernández-Victoria, P.; Alcocer, J.; Ardiles-Gloria, V.; Oseguera, L.A. Concentración y distribución vertical de la clorofila-a fitoplanctónica en los lagos de Montebello, Chiapas. In Tendencias de Investigación en Limnología Tropical: Perspectivas Universitarias en Latinoamérica; Instituto de Ciencias del Mar y Limnología, UNAM; Alcocer, J., Merino-Ibarra, M., Escobar-Briones, E., Eds.; y Consejo Nacional de Ciencia y Tecnología: Ciudad de México, Mexico, 2015; pp. 107–114. Available online: http://cc-catalogo.org/site/pdf/epifania.pdf (accessed on 1 October 2020).
- Alcocer, J.; Prado, B.; Mora, L.; Oseguera, L.A.; Caballero, M. Sedimentary characteristics of tropical, karstic lakes and their relationship with topography, lake morphometry and anthropogenic activities. J. Paleolimnol. 2020. submitted. [Google Scholar]
- Koste, W. Rotatoria. Die Rädertiere Mitteleuropas 1978, 2, 234–673. [Google Scholar]
- Jersabek, C.D.; Leitner, M.F. Rotifer World Catalog. 2013. Available online: http://rotifera.hausdernatur.at/ (accessed on 1 October 2020).
- Bonecker, C.C.; Nagae, M.Y.; Bletller, M.C.M.; Velho, L.F.M.; Lansac-Toˆha, F.A. Zooplankton biomass in tropical reservoirs in southern Brazil. Hydrobiologia 2007, 579, 115–123. [Google Scholar] [CrossRef]
- Ruttner-Kolisko, A. Suggestions for biomass calculation of plankton rotifers. Archiv für Hydrobiologie 1977, 8, 71–76. [Google Scholar]
- Bottrell, H.H.; Duncan, A.; Gliwicz, Z.M.V.; Grygierek, E.; Herzig, A.; Hillbricht-Ilkowska, A.; Kurasawa, H.; Larsson, P.; Weglenska, T. A review of some problems in zooplankton production studies. Norweg. J. Zool. 1976, 24, 419–456. [Google Scholar]
- Pace, M.L.; Orcutt, J.D., Jr. The relative importance of protozoans, rotifers and crustaceans in a freshwater zooplankton community. Limnol. Oceanogr. 1981, 26, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Ugland, K.I.; Gray, J.S.; Ellingsen, K.E. The species–accumulation curve and estimation of species richness. J. Anim. Ecol. 2003, 72, 888–897. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 1 October 2020).
- Sarma, S.S.S.; Elias-Gutiérrez, M.; Serrania-Soto, C. Rotifers from high altitude crater-lales at Nevado de Toluca Volcano, México. Hidrobiologica 1997, 6, 33–38. [Google Scholar]
- Cortés-Guzmán, D.; Alcocer, J.; Oseguera, L.A. Benthic macroinvertebrates community diversity of Montebello Lakes, Chiapas. Rev. Mex. Biodivers 2019, 90, e902769. [Google Scholar] [CrossRef]
- García-Morales, A.E.; Elías-Gutiérrez, M. DNA barcoding of freshwater Rotifera in Mexico: Evidence of cryptic speciation in common rotifers. Mol. Ecol. Resour. 2013, 13, 1097–1107. [Google Scholar] [CrossRef]
- García-Morales, A.E.; Elías-Gutiérrez, M. Rotifera from southeastern México, new records and comments on zoogeography. An. Inst. Biol. 2004, 75, 99–120. [Google Scholar]
- Sarma, S.S.S.; Osnaya-Espinosa, L.R.; Aguilar-Acosta, C.R.; Nandini, S. Seasonal variations in zooplankton abundances in the Iturbide reservoir (Isidro Fabela, State of Mexico, Mexico). J. Environ. Biol. 2011, 32, 473–480. [Google Scholar]
- Muñoz-Colmenares, M.E.; Sarma, S.S.S.; Nandini, S. Seasonal variations of rotifers from the high altitude Llano reservoir (State of Mexico, Mexico). J. Environ. Biol. 2017, 38, 1171–1181. [Google Scholar] [CrossRef]
- Cervantes-Martínez, A.; Gutierrez-Aguirre, M.A. Physicochemistry and zooplankton of two karstic sinkholes in the Yucatan Peninsula, Mexico. J. Limnol. 2015, 74, 382–393. [Google Scholar]
- Matsumara-Tundisi, T.; Rietzler, A.C.; Tundise, J.G. Biomass (dry weight and carbon content) of plankton Crustacea from Broa reservoir (São Carlos, SP, Brazil) and its fluctuation across one year. Hydrobiologia 1989, 149, 229–236. [Google Scholar] [CrossRef]
- Sendacz, S.; Caleffi, S.; Santos-Soares, J. Zooplankton biomass of reservoirs in different trophic conations in the State of São Paulo, Brazil. Braz. J. Biol. 2006, 66, 337–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, R.; Alcocer, J.; Oseguera, L.A. Microcystins presence threatens the ecosystem health of a tropical National Park: Lagunas de Montebello, Chiapas. Braz. J. Bot. 2020. under review. [Google Scholar]
- Dodson, S.I.; Arnott, S.E.; Cottingham, K.L. The relationship in lake communities between primary productivity and species richness. Ecology 2000, 81, 2662–2679. [Google Scholar] [CrossRef]
- Chick, J.H.; Levchuk, A.P.; Medley, K.A.; Havel, J.H. Underestiamtion of rotifer abundance a much greater problem than previously appreciated. Limnol. Oceanogr. Methods 2010, 8, 79–87. [Google Scholar] [CrossRef]
- Thomas, S.M.; Chick, J.H.; Czesny, S.J. Underestimation of microzooplankton is a macro problem: One size fits all zooplankton sampling needs alterations. J. Great Lakes Res. 2017, 43, 91–101. [Google Scholar] [CrossRef]
- Brown, P.D.; Schröder, T.; Ríos-Arana, J.V.; Rico-Martinez, R.; Silva-Briano, M.; Wallace, R.L.; Walsh, E.J. Patterns of Rotifer Diversity in the Chihuahuan Desert. Diversity 2020, 12, 393. [Google Scholar] [CrossRef]
- Da Silva Brito, M.T.; Heino, J.; Pozzobom, U.M.; Landeiro, V.L. Ecological uniqueness and species richness of zooplankton in subtropical floodplain lakes. Aquat. Sci. 2020, 82, 1–13. [Google Scholar]
- Wilson, A.E.; Sarnelle, O.; Tilmanns, A.R. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: Meta-analyses of laboratory experiments. Limnol. Oceanogr. 2006, 51, 1915–1929. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Shi, W.; Chen, Q.; Lauridsen, T.L. Spacial and temporal variations reveal the response of zooplankton to cyanobacteria. Harmful Algae 2017, 64, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Barrios, C.A.; Nandini, S.; Sarma, S.S.S. Bioaccumulation of microcystins in seston, zooplankton and fish: A case study in Lake Zumpango, Mexico. Environ. Pollut. 2019, 249, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Elert, E.V.; Martin-Creuzburg, D.; Le Coz, J.R. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc. R. Soc. Lond. Ser. B 2003, 270, 1209–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krztoń, W.; Kosiba, J.; Pociecha, A.; Wilk-Woźniak, E. The effect of cyanobacterial blooms on bio-and functional diversity of zooplankton communities. Biodivers. Conserv. 2019, 28, 1815–1835. [Google Scholar] [CrossRef] [Green Version]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Ortega-Mayagoitia, E.; Ciros-Perez, J.; Sánchez-Martínez, M.A. A story of famine in pelagic realm: Temporal and special patterns of food limitation in rotifers from an oligotrophic tropical lake. J. Plankton Res. 2011, 33, 1574–1585. [Google Scholar] [CrossRef] [Green Version]
- Fernández, R.; Alcocer, J.; Oseguera, L.A. Zooplankton biodiversity in tropical karst lakes of southeast Mexico, Chiapas. Rev. Mex. Biodivers. 2020, 91, e913184. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Miracle, M.R.; Nandini, S.; Vicente, E. Predation by Acanthocyclops americanus (Copepoda: Cyclopoida) in the hypertrophic shallow waterbody, Lake Albufera (Spain): Field and laboratory observations. Hydrobiologia 2019, 829, 5–17. [Google Scholar] [CrossRef]
- Valencia-Vargas, M.A.; Nandini, S.; Sarma, S.S.S. Demographic characteristics of two freshwater cyclopoid copepods in Mexico, fed a plankton diet: The native Mesocyclops longisetus Thiébaud and the invasive Mesocyclops pehpeinsis Hu. Inland Waters 2020, 10, 128–136. [Google Scholar] [CrossRef]
- Balkić, A.G. The importance of environmental differences in the structuring of rotifer functional diversity. J. Limnol. 2019, 78. [Google Scholar] [CrossRef] [Green Version]
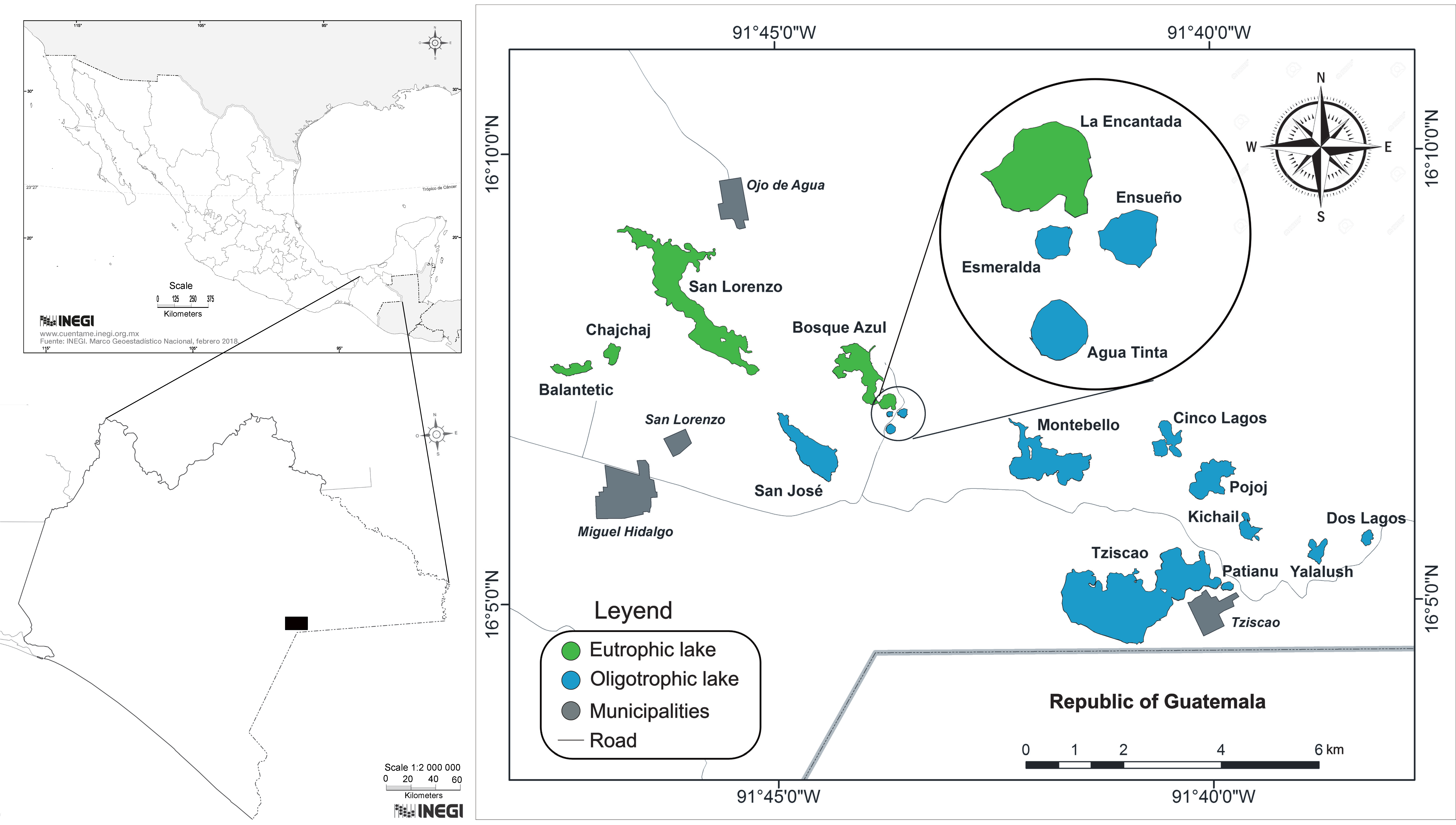
| Lake | Abr. | A (ha) | DMAX (m) | DMEAN (m) | DMEAN (m) | Trophic Status |
|---|---|---|---|---|---|---|
| Balantetic | BL | 13.6 | 3 | 1.7 | Warm polymictic | Eutrophic |
| Chajchaj | CH | 9.2 | 12 | 5.3 | Warm polymictic | Eutrophic |
| San Lorenzo | SL | 181.3 | 67 | 11.8 | Warm monomictic | Eutrophic |
| Bosque Azul | BA | 52.5 | 58 | 7.1 | Warm monomictic | Eutrophic |
| La Encantada | EC | 8.2 | 89 | 27.5 | Warm monomictic | Eutrophic |
| Esmeralda | ES | 1.1 | 7 | 3.6 | Warm polymictic | Oligotrophic |
| Ensueño | EN | 2.7 | 35 | 21.6 | Warm monomictic | Oligotrophic |
| Agua Tinta | AT | 3.0 | 24 | 14.7 | Warm monomictic | Oligotrophic |
| San José | SJ | 60.6 | 30 | 10.3 | Warm monomictic | Oligotrophic |
| Montebello | MB | 96.2 | 45 | 12.3 | Warm monomictic | Oligotrophic |
| Cinco Lagos | CL | 23.7 | 162 | 42.5 | Warm monomictic | Oligotrophic |
| Pojoj | PO | 43.7 | 198 | 35.2 | Warm monomictic | Oligotrophic |
| Kichail | KI | 12.5 | 22 | 9.5 | Warm monomictic | Oligotrophic |
| Tziscao | TZ | 306.6 | 86 | 28.9 | Warm monomictic | Oligotrophic |
| Patianú | PA | 3.4 | 26 | 10.8 | Warm monomictic | Oligotrophic |
| Yalalush | YA | 11.5 | 23 | 9.9 | Warm polymictic | Oligotrophic |
| Dos Lagos | DL | 5.2 | 42 | 25.2 | Warm monomictic | Oligotrophic |
| Phylum Rotifera Cuvier 1798 | Eutrophic | Oligotrophic | Nr. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | CH | SL | BA | EC | ES | EN | AT | SJ | MB | CL | PO | KI | TZ | PA | YA | DL | ||
| Subclass Monogononta Plate, 1889 | ||||||||||||||||||
| Order Flosculariaceae Harring, 1913 | ||||||||||||||||||
| Family Hexarthridae Bartos, 1959 | ||||||||||||||||||
| 1. Hexarthra intermedia (Wisniewski, 1929) | C/W | C/W | W | C | C | C | 6 | |||||||||||
| Family Testudinellidae Harring, 1913 | ||||||||||||||||||
| 2. Testudinella patina (Hermann, 1783) | C | C | C | 3 | ||||||||||||||
| 3. Ptygura sp. | C/W | C/W | C | C | C | C | C | C | 8 | |||||||||
| Family Trochosphaeridae Harring, 1913 | ||||||||||||||||||
| 4. Filinia longiseta (Ehrenberg, 1834) | C/W | C/W | C/W | C | C/W | W | C | 7 | ||||||||||
| 5. F. terminalis (Plate, 1886) | W | C/W | C | 3 | ||||||||||||||
| Order Ploima (Hudson and Gosse, 1886) | ||||||||||||||||||
| Family Asplanchnidae Eckstein, 1883 | ||||||||||||||||||
| 6. Asplanchna girodi de Guerne, 1888 | W | W | C | 3 | ||||||||||||||
| Family Brachionidae Ehrenberg, 1838 | ||||||||||||||||||
| 7. Brachionus angularis Gosse, 1851 | C/W | C/W | C | C | C | W | 6 | |||||||||||
| 8. B. bidentatus Anderson, 1889 | C | 1 | ||||||||||||||||
| 9. B. calyciflorus species complex Pallas, 1766 | C/W | C/W | W | C | W | W | 6 | |||||||||||
| 10. B. havanaensis Rousselet, 1911 | C/W | C/W | C | C | C | C | 6 | |||||||||||
| 11. B. rubens Ehrenberg, 1838 | C | C | 2 | |||||||||||||||
| 12. Keratella americana Carlin 1943 | C/W | C/W | C/W | C/W | C/W | C/W | C | C/W | C/W | C/W | C/W | C | C/W | 13 | ||||
| 13. K. cochlearis (Gosse, 1851) | C/W | C/W | C/W | C | 4 | |||||||||||||
| 14. K. tropica (Apstein, 1907) | C/W | C/W | C/W | C | C/W | 5 | ||||||||||||
| 15. Plationus patulus (Müller, 1786) | C/W | W | W | 3 | ||||||||||||||
| 16. Platyias quadricornis (Ehrenberg, 1832) | C | 1 | ||||||||||||||||
| Family Lecanidae Remane, 1933 | ||||||||||||||||||
| 17. Lecane bulla (Gosse, 1851) | W | C/W | C | W | C | C | 6 | |||||||||||
| 18. L. papuana (Murray, 1913) | W | W | 2 | |||||||||||||||
| Family Synchaetidae Hudson and Gosse, 1886 | ||||||||||||||||||
| 19. Polyarthra vulgaris Carlin, 1943 | C/W | 1 | ||||||||||||||||
| 20. Synchaeta oblonga Ehrenberg, 1831 | C | 1 | ||||||||||||||||
| Family Trichotriidae Harring, 1913 | ||||||||||||||||||
| 21. Macrochaetus sp. | W | 1 | ||||||||||||||||
| S Subtotal C | 6 | 9 | 8 | 8 | 7 | 1 | 4 | 2 | 1 | 2 | 3 | 2 | 6 | 5 | 5 | 0 | 3 | |
| S Subtotal W | 6 | 11 | 10 | 4 | 4 | 0 | 2 | 1 | 3 | 1 | 1 | 1 | 2 | 1 | 0 | 2 | 3 | |
| S Grand Total | 8 | 12 | 10 | 9 | 8 | 1 | 4 | 2 | 3 | 3 | 3 | 2 | 7 | 5 | 5 | 2 | 4 | |
| Lake | Cold/Dry | Warm/Rainy | |||||
|---|---|---|---|---|---|---|---|
| S | N (ind L−1) | B (µg L−1) | S | N (ind L−1) | B (µg L−1) | ||
| Eutrophic | Balantetic | 6 | 120 | 20.82 | 6 | 17 | 0.31 |
| Chajchaj | 9 | 538 | 9.23 | 11 | 227 | 1.95 | |
| San Lorenzo | 8 | 17 | 0.23 | 10 | 60 | 0.57 | |
| Bosque Azul | 8 | 20 | 0.12 | 4 | 26 | 0.43 | |
| La Encantada | 7 | 23 | 0.16 | 4 | 3 | 0.07 | |
| Oligotrophic | Esmeralda | 1 | <0.1 | <0.01 | 0 | 0.0 | 0.00 |
| Ensueño | 4 | 0.2 | <0.01 | 2 | 1.9 | 0.02 | |
| Agua Tinta | 2 | 0.2 | <0.01 | 1 | 0.3 | 0.01 | |
| San Jose | 1 | 1.4 | 0.04 | 3 | 1.3 | 0.01 | |
| Montebello | 2 | 0.1 | <0.01 | 1 | 0.6 | 0.01 | |
| Cinco Lagos | 3 | 0.1 | <0.01 | 1 | 0.5 | 0.01 | |
| Pojoj | 2 | 0.7 | 0.01 | 1 | 0.2 | <0.01 | |
| Kitchail | 6 | 0.7 | 0.04 | 2 | 1.8 | 0.03 | |
| Tziscao | 5 | 1.1 | 0.02 | 1 | 0.1 | <0.01 | |
| Pataniu | 5 | 1.1 | 0.02 | 0 | 0.0 | 0.00 | |
| Yalalush | 0 | 0.0 | 0.00 | 2 | 0.3 | <0.01 | |
| Dos Lagos | 3 | 0.3 | <0.01 | 3 | 0.3 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, R.; Alcocer, J.; Oseguera, L.A. Regional Pelagic Rotifer Biodiversity in a Tropical Karst Lake District. Diversity 2020, 12, 454. https://doi.org/10.3390/d12120454
Fernández R, Alcocer J, Oseguera LA. Regional Pelagic Rotifer Biodiversity in a Tropical Karst Lake District. Diversity. 2020; 12(12):454. https://doi.org/10.3390/d12120454
Chicago/Turabian StyleFernández, Rocío, Javier Alcocer, and Luis A. Oseguera. 2020. "Regional Pelagic Rotifer Biodiversity in a Tropical Karst Lake District" Diversity 12, no. 12: 454. https://doi.org/10.3390/d12120454






