Diatoms in Kamchatka’s Hot Spring Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Area
2.2. Samples Collections
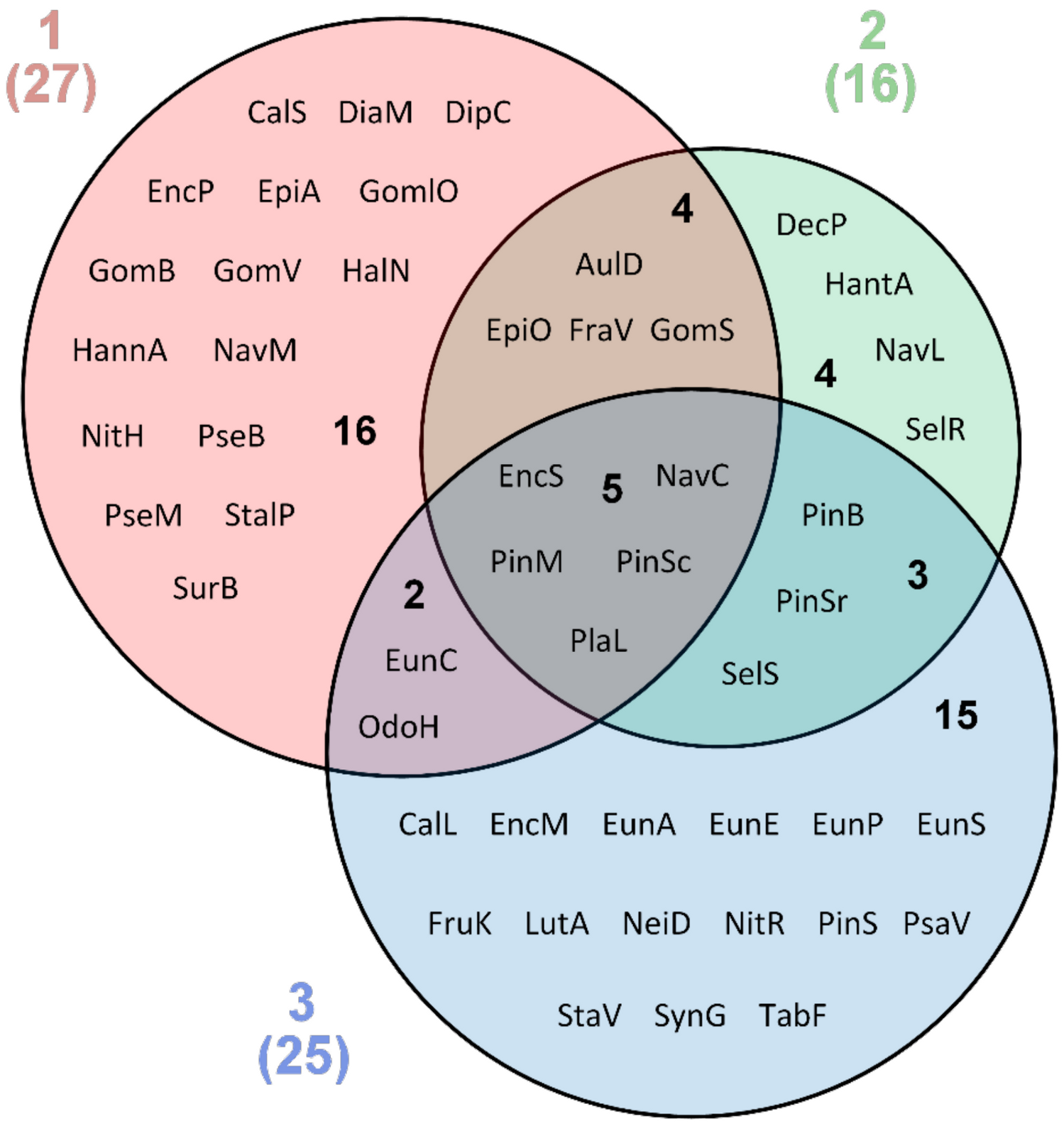
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cowan, D.; Tuffin, M.; Mulako, I.; Cass, J. Terrestrial hydrothermal environments. In Life at Extremes: Environments, Organisms, and Strategies for Survival; Bell, E., Ed.; CABI Publishing: Wallingford, UK; Oxon, UK, 2012; pp. 219–241. [Google Scholar]
- Valtonen, M.; Nurmi, P.; Zheng, J.-Q.; Cucinotta, F.C.; Wilson, J.W.; Horneck, G.; Lindegren, L.; Melosh, J.; Rickman, H.; Mileikowsky, C. Natural transfer of viable microorganisms in space from planets in extrasolar systems to a planet in our solar system and vice versa. Astrophys. J. 2009, 690, 210–215. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef]
- Rampelotto, P.H. Resistance of microorganisms to extreme environmental contribution to astrobiology. Sustainability 2010, 2, 1602–1623. [Google Scholar] [CrossRef]
- Gesheva, V.; Vasileva-Tonkova, E. Production of enzymes and antimicrobial compounds by halophilic Antarctic Nocardioides spp. grown on different carbon sources. World J. Microbiol. Biotechnol. 2012, 28, 2069–2076. [Google Scholar] [CrossRef]
- Lopatina, A.; Medvedeva, S.; Shmakov, S.; Logacheva, M.D.; Krylenkov, V.; Severinov, K. Metagenomic analysis of bacterial communities of Antarctic surface snow. Front. Microbiol. 2016, 7, 398. [Google Scholar] [CrossRef]
- Lavin, P.L.; Yong, S.T.; Wong, C.M.V.L.; De Stefano, M. Isolation and characterization of Antarctic psychrotroph Streptomyces spp. strain INACH3013. Antarct. Sci. 2016, 28, 433–442. [Google Scholar] [CrossRef]
- Solon, A.J.; Vimercati, L.; Darcy, J.L.; Arán, P.; Porazinska, D.; Dorador, C.; Farías, M.E.; Schmidt, S.K. Microbial communities of high-elevation fumaroles, penitentes, and dry tephra “soils” of the Puna de Atacama Volcanic Zone. Microb. Ecol. 2018, 76, 340–351. [Google Scholar] [CrossRef]
- Gaete, A.; Mandakovic, D.; González, M. Isolation and identification of soil bacteria from extreme environments of Chile and their plant beneficial characteristics. Microorganisms 2020, 8, 1213. [Google Scholar] [CrossRef]
- Martínez-Espinosa, R.M. Microorganisms and their metabolic capabilities in the context of the biogeochemical nitrogen cycle at extreme environments. Int. J. Mol. Sci. 2020, 21, 4228. [Google Scholar]
- Reysenback, A.R.; Shock, E. Merging genomes with geochemistry in hydrothermal vent ecosystems. Science 2002, 296, 1077–1082. [Google Scholar] [CrossRef]
- Renaut, R.W.; Jones, B. Hydrothermal environments, terrestrial. In Encyclopedia of Geobiology, Encyclopedia of Earth Sciences Series; Reitner, J., Thiel, V., Eds.; Springer: Berlin, Germany, 2011; pp. 467–479. [Google Scholar]
- Owen, R.; Renaut, R.; Jones, B. Geothermal diatoms: A comparative study of floras in hot spring systems of Iceland, New Zealand and Kenya. Hydrobiologia 2008, 610, 175–192. [Google Scholar] [CrossRef]
- Ehrlich, H. Life in extreme environments: From bacteria to diatoms. In Biological Materials of Marine Origin; Springer: Dordrecht, The Netherlands, 2010; pp. 485–498. [Google Scholar]
- Leira, M.; Meijide-Failde, R.; Torres, E. Diatom communities in thermo-mineral springs of Galicia (NW Spain). Diatom Res. 2017, 32, 1–14. [Google Scholar] [CrossRef]
- Pumas, C.; Pruetiworanan, S.; Peerapornpisal, Y. Diatom diversity in some hot springs of Northern Thailand. Botanica 2018, 24, 69–86. [Google Scholar] [CrossRef]
- Lai, G.G.; Beauger, A.; Wetzel, C.E.; Padedda, B.M.; Voldoire, O.; Lugliè, A.; Allain, E.; Ector, L. Diversity, ecology and distribution of benthic diatoms in thermo-mineral springs in Auvergne (France) and Sardinia (Italy). PeerJ 2019, 7, e7238. [Google Scholar] [CrossRef]
- Kumar, B.; Trivedi, P.; Mishra, A.K.; Pandey, A.; Palni, L.M.S. Microbial diversity of soil from two hot springs in Uttaranchal Himalaya. Microbiol. Res. 2004, 159, 141–146. [Google Scholar] [CrossRef]
- Huang, Z.; Wiegel, J.; Zhou, J.; Hedlund, B.; Zhang, C.L. Molecular phylogeny of uncultivated Crenarchaeota in Great Basin hot springs of moderately elevated temperature. Geomicrobiol. J. 2007, 24, 535–542. [Google Scholar] [CrossRef]
- Nikulina, T.V.; Grishchenko, O.V. Diatom flora of Dachnye Thermal Springs (Kamchatka Peninsula, Russia). In Vladimir Ya. Levanidov’s Biennial Memorial Meetings; 2017; Volume 7, pp. 185–193. Available online: http://www.ibss.febras.ru/files/00015192.pdf (accessed on 18 November 2020). (In Russian)
- Nikulina, T.V.; Kalitina, E.G.; Vakh, E.A.; Kharitonova, N.A. List of diatoms from three hot springs from Kamchatka—Malkinskiye, Nachikinskiye and Verhne-Paratunskiye (Russia). In Freshwater Life; Bogatov, V.V., Ed.; Dalnauka: Vladivostok, Russia, 2016; Volume 2, pp. 108–115. (In Russian) [Google Scholar]
- Koloskov, A.V.; Flerov, G.B.; Perepelov, A.B.; Melekestsev, I.V.; Puzankov, M.Y.; Filosofova, T.M. Evolution stages and petrology of the Kekuknai volcanic massif as reflecting the Magmatismin Backarc Zone of Kuril-Kamchatka Island Arc System. Part 1. Geological position and geochemistry of volcanic rocks. J. Volcanol. Seismol. 2011, 5, 312–334. [Google Scholar] [CrossRef]
- Prytkov, A.S.; Vasilenko, N.F.; Frolov, D.I. Recent geodynamics of the Kuril subduction zone. Russ. J. Pacif. Geol. 2017, 11, 19–24. [Google Scholar] [CrossRef]
- Merkel, A.Y.; Pimenov, N.V.; Rusanov, I.I.; Slobodkin, A.I.; Slobodkina, G.B.; Tarnovetckii, I.Y.; Frolov, E.N.; Dubin, A.V.; Perevalova, A.A.; Bonch-Osmolovskaya, E.A. Microbial diversity and autotrophic activity in Kamchatka hot springs. Extremophiles 2017, 21, 307–317. [Google Scholar] [CrossRef]
- Nenasheva, E.N. Spiders (Arachnida: Aranei) of thermal habitats in Kamchatka: Preliminary research experience. Actual Issues Mod. Sci. 2016, 4, 28–32. [Google Scholar]
- Kalitina, E.G.; Nikulina, T.V.; Kharitonova, N.A.; Wah, E.A. Materials for the study of the diversity of microorganisms in the thermal springs of Kamchatka (Russia). In Proceedings of the All Russian Conference with International Participation “Modern Problems of Hydrogeology, Engineering Geology and Hydrogeoecology Eurasia” with Elements of a Scientific School; Publishing House of Tomsk Polytechnic University: Tomsk, Russia, 2015; pp. 510–513. (In Russian) [Google Scholar]
- Bakalin, V.A.; Chernyagina, О.A.; Kirichenko, V.E. Features of the flora of Liverworts (Hepaticae) of termal habitats in Kamchatka. Sib. Ekol. Zhurnal 2011, 1, 43–50. [Google Scholar]
- Barragán, C.; Wetzel, C.E.; Ector, L. A standard method for the routine sampling of terrestrial diatom communities for soil quality assessment. J. Appl. Phycol. 2018, 30, 1095–1113. [Google Scholar] [CrossRef]
- Acker, F.; Russell, B.; Morales, E. Preparation of Diatom Slides Using Naphrax™ Mounting Medium. Protocol P-13-49; Academy of Natural Sciences of Philadelphia, Patrick Center for Environmental Research: Philadelphia, PA, USA, 1999; pp. 13–42. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Naviculaceae. Süβwasserflora von Mitteleuropa. Band 2/1; Spectrum Academiche Verlag: Berlin, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 2. Epithemiaceae, Bacillariaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa; Pascher, A., Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Stuttgart, Germany, 1988; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 3. Centrales, Fragilariaceae, Eunotiaceae, Achnanthaceae. In Süβwasserflora von Mitteleuropa; Pascher, A., Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Stuttgart, Germany, 1991; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 4. Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Süsswasserflora von Mitteleuropa; Pascher, A., Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; VEB Gustav Fisher Verlag: Stuttgart, Germany, 1991; p. 436. [Google Scholar]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser—Benthos von Mitteleuropa. In Bestimmungsflora Kieselalgen für die ökologische Praxis. Über 700 der häufigsten Arten und ihre Ökologie; Koeltz Scientific Books: Königstein, Germany, 2013; p. 908. [Google Scholar]
- Paula, C.F.; Lowe, R.L.; Johansen, J.R. Teratology in Eunotia taxa in The Great Smoky Mountains National Park and description of Eunotia macroglossa spp. nov. Diatom Res. 2009, 24, 273–290. [Google Scholar]
- Solak, C.; Wojtal, A. Diatoms in springs and streams of Turkmen Mt (Sakarya River Basin) common in Turkish Inland waters. Pol. Bot. J. 2012, 57, 345–425. [Google Scholar]
- Wetzel, C.E.; Ector, L.; Vijver, B.; Compère, P.; Mann, D.G. Morphology, typification and critical analysis of some ecologically important small naviculoid species (Bacillariophyta). Fottea 2015, 15, 203–234. [Google Scholar] [CrossRef]
- Potapova, M.G. Diatoms of Bering Island, Kamchatka, Russia. Nova Hedwig. 2014, 143, 63–102. [Google Scholar]
- Beauger, A.; Wetzel, C.E.; Voldoire, O.; Garreau, A.; Ector, L. Sellaphora labernardierei (Sellaphoraceae, Bacillariophyta), a new epilithic species from French spring and four new combinations within the genus Sellaphora. Phytotaxa 2016, 260, 235–246. [Google Scholar] [CrossRef]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft-und Flechtenalgen; Gustav Fischer Verlag: Stuttgart, Germany, 1995; p. 721. [Google Scholar]
- Kociolek, J.P.; Spaulding, S.A. Eunotioid and asymmetrical naviculoid diatoms. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 655–668. [Google Scholar]
- Kociolek, J.P.; Spaulding, S.A. Symmetrical naviculoid diatoms. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 637–652. [Google Scholar]
- Lowe, R.L. Keeled and Canalled Raphid Diatoms. In Freshwater Algae of North America. Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: New York, NY, USA, 2003; pp. 669–684. [Google Scholar]
- Stoermer, E.F.; Julius, M.L. Centric diatoms. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 559–652. [Google Scholar]
- Flechtner, V.R.; Johansen, J.R.; Belnap, J. The biological soil crusts of the San Nicolas Island: Enigmatic algae from a geographically isolated ecosystem. West. N. Am. Nat. 2008, 68, 405–436. [Google Scholar] [CrossRef]
- Stenina, A.S. Diatoms (Bacillariophyta) in the Lakes of the East of the Bolshezemelskaya Tundra; Komi NTS UrO RAN Publishing House: Syktyvkar, Russia, 2009; p. 179. [Google Scholar]
- Poradowska, A. Diatoms (Bacillariophyta) from the genus Eunotia and Pinnularia developing on soils in the open landscape of the Low Beskids. J. Ecol. Eng. 2020, 21, 257–270. [Google Scholar] [CrossRef]
- Kostikov, I.Y.; Romanenko, P.O.; Demchenko, E.M.; Darienko, T.M.; Mikhailyuk, T.I.; Rybchinsky, O.V.; Solonenko, A.M. Algae in Soils of Ukraine: History and Methods of Studies, System, and List of the Algal Flora; Fitosotsiotsentr: Kiev, Ukraine, 2001; p. 300. (In Ukrainian) [Google Scholar]
- Venn, J. On the diagrammatic and mechanical representation of propositions and reasonings. Philos. Mag. J. Sci. 1880, 9, 1–18. [Google Scholar] [CrossRef]
- Hulsen, T.; de Vlieg, J.; Alkema, W. BioVenn—A web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Kabirov, R.R.; Safiulina, L.M. Peculiarities of ecology and distribution of unicellular soil alga Eustigmatos magnus (J.B. Petersen) Hibberd in Southern Ural (Russia). Int. J. Algae 2008, 10, 105–116. [Google Scholar]
- Ishikawa, S.; Kashima, K. Diatoms in Bekanbeushi Wetland, Eastern Hokkaido. Diatom 2009, 25, 106–110. [Google Scholar]
- Nikulina, T.V.; Kociolek, J.P. Diatoms from hot springs from Kuril and Sakhalin Islands (Far East, Russia). In Cellular Origin, Life in Extreme Habitats and Astrobiology. The Diatom World, Part 3; Seckbach, J., Kociolek, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 19, pp. 333–363. [Google Scholar]
- Werum, M.; Lange-Bertalot, H. Diatoms in Springs from Central Europe and elsewhere under the influence of hydrogeology and anthropogenic impacts. In Iconographia Diatomologica; Lange-Bertalot, H., Ed.; Koeltz Scientific Books: Koenigstein, Germany, 2004; Volume 13, pp. 1–417. [Google Scholar]
- Cantonati, M.; Angeli, N.; Bertuzzi, E.; Spitale, D.; Lange–Bertalot, H. Diatoms in springs of the Alps: Spring types, environmental determinants, and substratum. Freshw. Sci. 2012, 31, 499–524. [Google Scholar] [CrossRef]
- Wojtal, A.Z. Species composition and distribution of diatom assemblages in spring waters from various geological formations in southern Poland. Bibl. Diatomol. 2013, 59, 1–436. [Google Scholar]
- Cantonati, M.; Angeli, N.; Spitale, D.; Lange–Bertalot, H. A new Navicula (Bacillariophyta) species from low-elevation carbonate springs affected by anthropogenic disturbance. Fottea 2016, 162, 255–265. [Google Scholar] [CrossRef]
- Veselá, J. Spatial heterogeneity and ecology of algal communities in an ephemeral sandstone stream in the Bohemian Switzerland National Park, Czech Republic. Nova Hedwig. 2009, 88, 531–547. [Google Scholar] [CrossRef]
- Morais, K.S.; Oliveira, S.A.; Lehmkuhl, E.A.; Silva-Lehmkuhl, A.M.; Bicudo, C.E.M. Criptógamos do parque estadual das fontes do Ipiranga. In Algae: Bacillariophyceae (Surirellales: Epithemia); Hoehnea: São Paulo, Portugal, 2019; p. e262018. [Google Scholar]
- Wyatt, K.H.; Hauer, R.; Pessoney, G.F. Benthic algal response to hyporheic-surface water exchange in an alluvial river. Hydrobiology 2008, 607, 151–161. [Google Scholar] [CrossRef]
- Cantonati, M.; Spitale, D.; Scalfi, A.; Guella, G. Exploring the contrasting seasonal strategies of two crenic macroalgae. Fottea 2016, 16, 133–143. [Google Scholar] [CrossRef]
- Hobbs, W.O.; Wolfe, A.P.; Inskeep, W.P.; Amskold, L.; Konhauser, K.O. Epipelic diatoms from an extreme acid environment: Beowulf Spring, Yellowstone National Park, USA. Nova Hedwig. Beih. 2009, 135, 71–83. [Google Scholar]
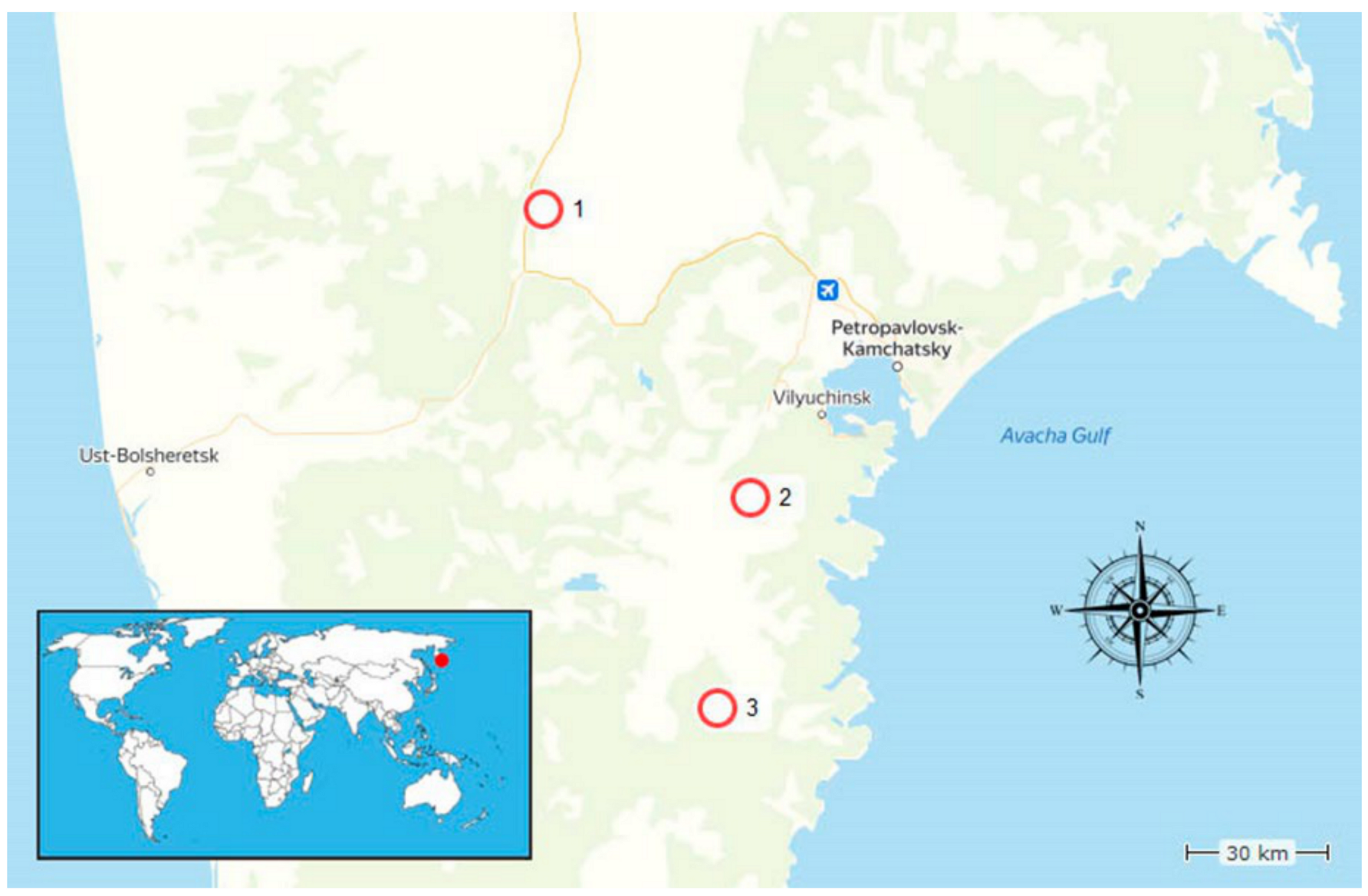


| Studied Area | Temperature, °C | рН | Total Mineralization, g/L |
|---|---|---|---|
| 1 | 65.9 | 3.5 | 0.70 |
| 2 | 39.5 | 8.3 | 1.00 |
| 3 | 30.0–50.0 | 3.2–9.4 | 0.12–0.70 |
| Number | Description | Name | GPS | Area | Sample Description |
|---|---|---|---|---|---|
| 1 | River Kluchevka bank (village Malki) | K1 | 53°19′15.0′′ N 157°31′55.7′′ E | Malki | Soil sample near the spring vent |
| 2 | Village Malki | K2 | 53°19′21.6′′ N 157°32′12.7′′ E | Malki | Soil sample near hot spring vent |
| 3 | Hot springs in Upper Raratunka valley, 19 km from Thermalni village | K3 | 52°45′46.7′′ N 158°10′52.9′′ E | Upper Paratunka | Soil sample 0.2 m from the thermal bath |
| 4 | River Paratunka valley, Paratunka village | K4 | 52°45′33.7′′ N 158°11′02.8′′ E | Upper Paratunka | Soil sample near a small swamp close to the vent |
| 5 | Hot springs in Upper Raratunka valley | K5 | 52°45′39.1′′ N 158°11′00.4′′ E | Upper Paratunka | Soil sample 0.2 m from the spring vent |
| 6 | Small geysers on the plateau on Mutnovski volcano valley | K6 | 52°22′22.4′′ N 158°04′31.5′′ E | Dachnie | Mud sample from the thermal bath |
| 7 | Small geysers on the plateau on Mutnovski volcano valley, fumaroles | K7 | 52°22′10.8′′ N 158°04′33.0′′ E | Dachnie | Soil sample near small boiling mud pool |
| 8 | Small geysers on the plateau near Mutnovski volcano | K8 | 52°22′31.5′′ N 158°04′28.5′′ E | Dachnie | Soil sample near the geyser |
| Taxon | 1 | 2 | 3 | Habitat * | Salinity Tolerance * | рН * | Distribution * | Ecological Group * |
|---|---|---|---|---|---|---|---|---|
| Aulacoseira distans (Ehrenberg) Simonsen | 12 | 10 | P B | i | ac | b | marine, freshwater | |
| Caloneis lancettula (Schulz) Lange-Bertalot & Witkowski | 8 | B | i | al | c | freshwater | ||
| C. silicula (Ehrenberg) Cleve | 5 | B Ep | i | ac | c | freshwater | ||
| Diatoma moniliformis (Kützing) D.M.Williams | 9 | P B | hl | al | c | marine, freshwater | ||
| Diploneis calcilacustris Lange-Bertalot & Fuhrmann | 3 | B | i | al | c | freshwater | ||
| Decussiphycus placenta (Ehrenberg) Guiry & Gandhi | 3 | B | i | i | c | freshwater, terrestrial | ||
| Encyonema minutum (Hilse) D.G.Mann | 4 | B Ep | i | al | c | marine, freshwater | ||
| E. perpusillum (Cleve-Euler) D.G.Mann | 6 | B Ep | hb | ac | c | freshwater | ||
| E. silesiacum (Bleisch) D.G.Mann | 3 | 1 | 7 | B Ep | i | al | b | freshwater, terrestrial |
| Epithemia adnata (Kützing) Brébisson | 2 | Ep | i | al | c | freshwater, terrestrial | ||
| E. operculata (C.Agardh) Ruck & Nakov | 14 | 15 | Ep | i | al | c | freshwater | |
| Eunotia arcus var. fallax Hustedt | 4 | Ep | hb | i | c | freshwater | ||
| E. curtagrunowii Nörpel-Schempp & Lange-Bertalot | 6 | 10 | L Ep | hb | ac | aa | freshwater | |
| E. exigua (Brébisson ex Kützing) Rabenhorst | 15 | L Ep | hb | ac | c | freshwater | ||
| E. paratridentula Lange-Bertalot & Kulikovskiy | 2 | L Ep | hb | ac | c | freshwater | ||
| Eunotia sp. | 6 | L Ep | hb | ac | c | freshwater | ||
| Fragilariforma virescens (Ralfs) D.M.Williams & Round | 15 | 15 | L Ep | i | i | aa | freshwater | |
| Frustulia krammeri Lange-Bertalot & Metzeltin | 9 | B | hb | ac | aa | freshwater, terrestrial | ||
| Gomphonella olivacea (Hornemann) Rabenhorst | 5 | Ep | i | i | c | freshwater | ||
| Gomphonema brebissonii Kützing | 4 | Ep | i | al | c | freshwater | ||
| G. ventricosum W.Gregory | 4 | Ep | i | i | aa | freshwater | ||
| Gomphonema sp. | 13 | 10 | Ep | i | i | c | marine, freshwater | |
| Halamphora normanii (Rabenhorst) Levkov | 14 | B | i | al | c | freshwater, terrestrial | ||
| Hannaea arcus (Ehrenberg) R.M.Patrick | 2 | L | hb | al | aa | freshwater | ||
| Hantzschia cf .abundans Lange-Bertalot | 5 | B | i | al | c | freshwater | ||
| Luticola acidoclinata Lange-Bertalot | 4 | B | hl | al | c | freshwater | ||
| Navicula cincta (Ehrenberg) Ralfs | 2 | 5 | 1 | B Ep | hl | al | c | marine, brackish, freshwater, terrestrial |
| N. leptostriata Jørgensen | 8 | B | hb | ac | c | freshwater | ||
| N. minima Grunow | 6 | B Ep | i | al | c | freshwater | ||
| Neidium dubium (Ehenberg) Cleve | 8 | B | i | al | c | marine | ||
| Nitzschia hantzschiana Rabenhorst | 15 | B | i | al | b | freshwater, terrestrial | ||
| N. recta Hantzsch ex Rabenhorst | 4 | B | i | al | c | freshwater | ||
| Odontidium hyemale (Roth) Kützing | 6 | 6 | B Ep | hb | i | aa | freshwater | |
| Pinnularia substreptoraphe Krammer | 2 | B | i | i | c | freshwater | ||
| P. borealis Ehrenberg | 6 | 10 | B | i | ac | c | freshwater, terrestrial | |
| P. microstauron (Ehrenberg) Cleve | 9 | 7 | 6 | B | i | i | c | freshwater, terrestrial |
| P.cf. subcapitata W.Gregory | 14 | 15 | 15 | B | hb | ac | c | freshwater, terrestrial |
| P. cf. subrupestris Krammer | 2 | 3 | B | i | i | c | freshwater, terrestrial | |
| Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot | 15 | 14 | 15 | Ep | i | al | c | freshwater |
| Psammothidium ventrale (Krasske) Bukhtiyarova & Round | 1 | Ep | hb | ac | aa | freshwater | ||
| Pseudostaurosira brevistriata (Grunow) D.M.Williams & Round | 1 | L Ep | i | al | c | freshwater | ||
| P. medliniae D.M.Williams & E.A Morales | 2 | L Ep | i | al | c | freshwater | ||
| Sellaphora rectangularis (W.Gregory) Lange-Bertalot & Metzeltin | 10 | B | hl | i | c | freshwater | ||
| S. seminulum (Grunow) D.G.Mann | 3 | 4 | B Ep | i | ac | c | freshwater | |
| Staurosira venter (Ehrenberg) Cleve & J.D.Möller | 4 | 9 | L Ep | i | al | c | freshwater | |
| Staurosirella pinnata (Ehrenberg) D.M.Williams & Round | 5 | L | hl | al | c | marine, freshwater | ||
| Surirella brebissonii Krammer & Lange-Bertalot | 9 | B | i | al | c | freshwater | ||
| Synedra goulardii Brébisson ex Cleve & Grunow | 3 | L Ep | i | al | c | marine, freshwater | ||
| Tabellaria flocculosa (Roth) Kützing | 6 | P Ep | hb | ac | aa | freshwater, terrestrial | ||
| Total number 49 | 27 | 16 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazlutdinova, A.; Gabidullin, Y.; Allaguvatova, R.; Gaysina, L. Diatoms in Kamchatka’s Hot Spring Soils. Diversity 2020, 12, 435. https://doi.org/10.3390/d12110435
Fazlutdinova A, Gabidullin Y, Allaguvatova R, Gaysina L. Diatoms in Kamchatka’s Hot Spring Soils. Diversity. 2020; 12(11):435. https://doi.org/10.3390/d12110435
Chicago/Turabian StyleFazlutdinova, Alfiya, Yunir Gabidullin, Rezeda Allaguvatova, and Lira Gaysina. 2020. "Diatoms in Kamchatka’s Hot Spring Soils" Diversity 12, no. 11: 435. https://doi.org/10.3390/d12110435
APA StyleFazlutdinova, A., Gabidullin, Y., Allaguvatova, R., & Gaysina, L. (2020). Diatoms in Kamchatka’s Hot Spring Soils. Diversity, 12(11), 435. https://doi.org/10.3390/d12110435







