Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Acoustic Monitoring
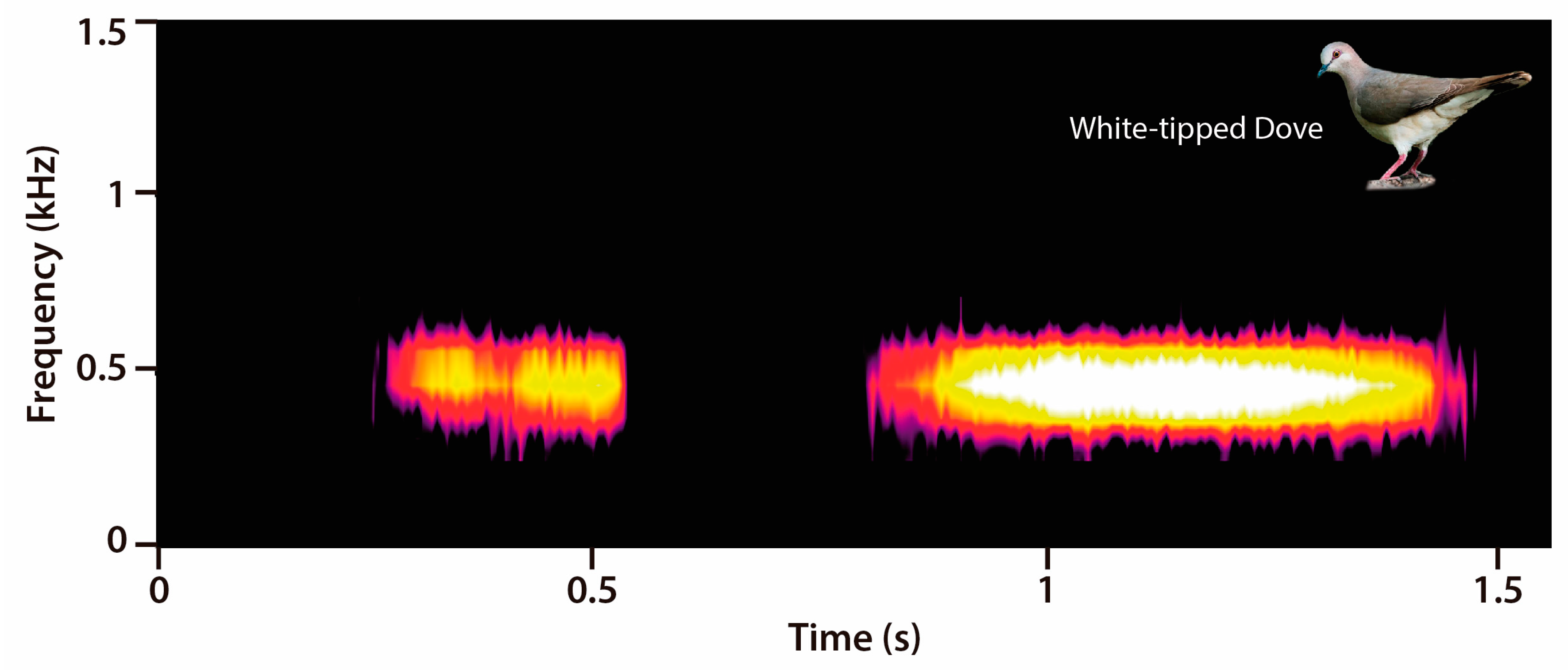
2.3. Acoustic Data Analyses
2.4. Weather Data
2.5. Statistical Analyses
3. Results
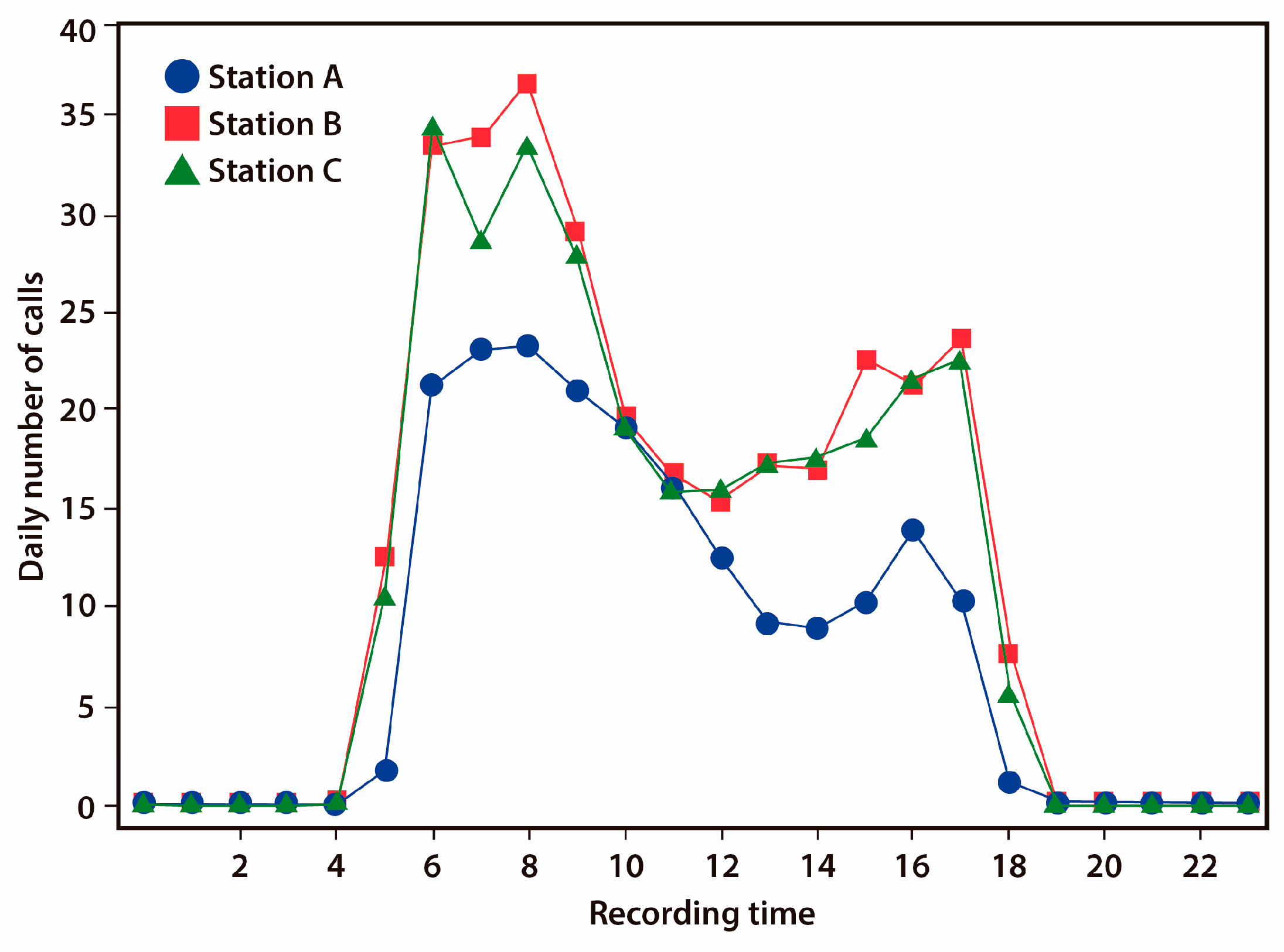
3.1. Diel Activity Pattern
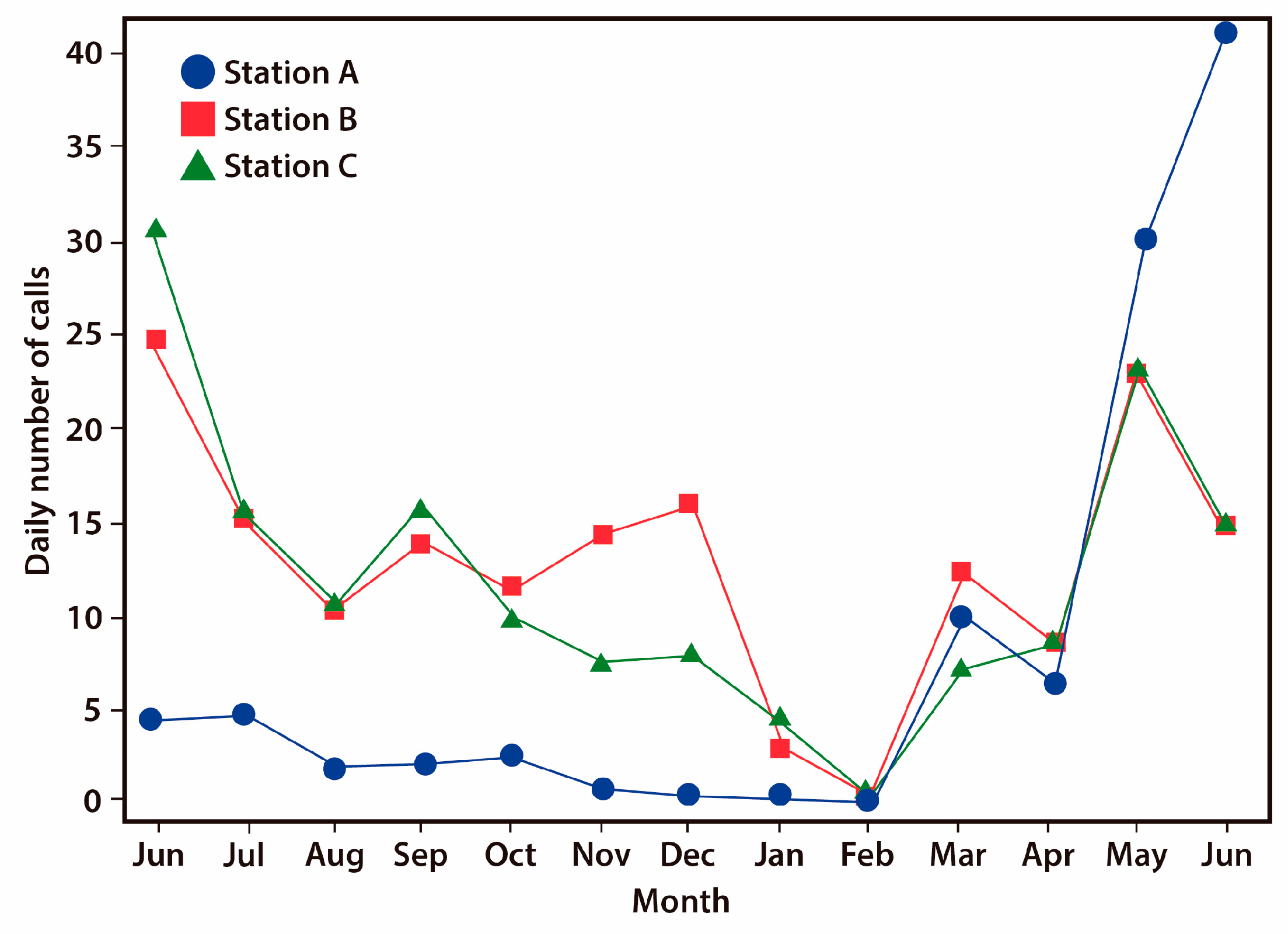
3.2. Seasonal Activity Pattern
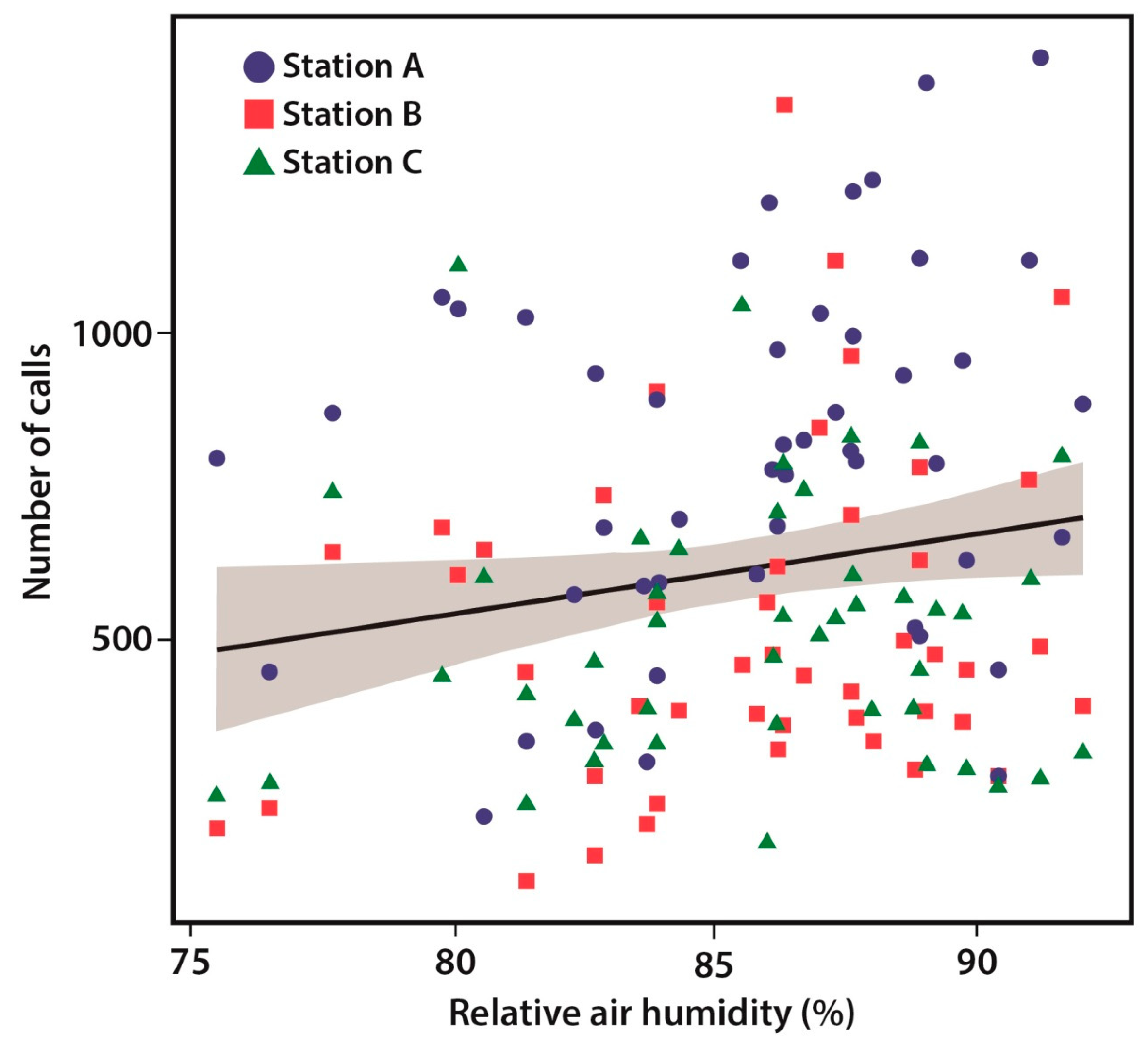
3.3. Environmental Predictors
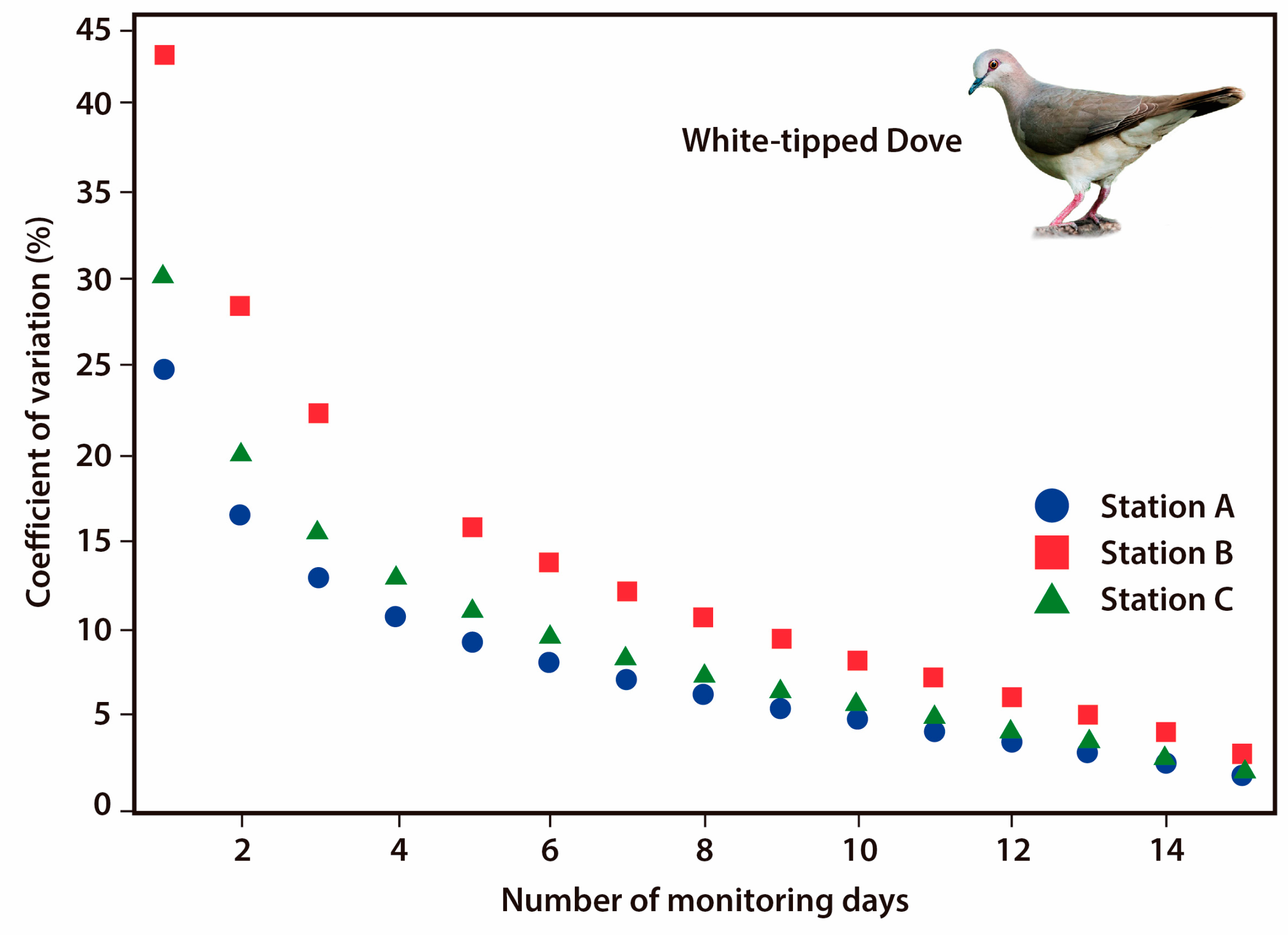
3.4. Monitoring Protocol
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brewster, W. Notes and song-flight of the Woodcock (Philohela minor). Auk 1894, 11, 291–298. [Google Scholar] [CrossRef]
- Hawkins, C.J. Sexual selection and bird song. Auk 1918, 35, 421–437. [Google Scholar]
- Marler, P. Bird calls: Their potential for behavioral neurobiology. Ann. Acad. Sci. 2004, 1016, 31–44. [Google Scholar] [CrossRef]
- Farnsworth, A. Flight calls and their value for future ornithological studies and conservation research. Auk 2005, 122, 733–746. [Google Scholar] [CrossRef]
- Gil, D.; Llusia, D. The bird dawn chorus revisited. In Coding Strategies in Vertebrate Acoustic Communication. Animal Signals and Communication; Aubin, T., Mathevon, N., Eds.; Springer: Cham, Switzerland, 2020; Volume 7, pp. 45–90. [Google Scholar]
- Amrhein, V.; Korner, P.; Naguib, M. Nocturnal and diurnal singing activity in the nightingale: Correlations with mating status and breeding cycle. Anim. Behav. 2002, 64, 939–944. [Google Scholar] [CrossRef]
- Catchpole, C.K.; Slater, P.J. Bird Song: Biological Themes and Variations, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Barker, N.K. Bird song structure and transmission in the Neotropics: Trends, methods and future directions. Ornitol. Neotrop. 2008, 19, 175–199. [Google Scholar]
- Riebel KOdom, K.J.; Langmore, N.E.; Hall, M.L. New insights from female bird song: Towards an integrated approach to studying male and female communication roles. Biol. Lett. 2019, 15, 20190059. [Google Scholar] [CrossRef]
- Topp, S.M.; Mennill, D.J. Seasonal variation in the duetting behaviour of Rufous-and-white Wrens (Thryothorus rufalbus). Behav. Ecol. Sociobiol. 2008, 62, 1107–1117. [Google Scholar] [CrossRef]
- Jahn, O.; Ganchev, T.D.; Marques, M.I.; Schuchmann, K.L. Automated sound recognition provides insights into the behavioral ecology of a tropical bird. PLoS ONE 2017, 12, e0169041. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.L.; Marques, M.I. Vocal behavior of the Undulated Tinamou (Crypturellus undulatus) over an annual cycle in the Brazilian Pantanal: New ecological information. Biotropica 2020, 52, 165–171. [Google Scholar] [CrossRef]
- Kirschel, A.N.; Blumstein, D.T.; Cohen, R.E.; Buermann, W.; Smith, T.B.; Slabbekoorn, H. Birdsong tuned to the environment: Green Hylia song varies with elevation, tree cover, and noise. Behav. Ecol. 2009, 20, 1089–1095. [Google Scholar] [CrossRef]
- Sosa-López, J.R.; Mennill, D.J. The vocal behavior of the Brown-throated Wren (Troglodytes brunneicollis): Song structure, repertoires, sharing, syntax, and diel variation. J. Ornithol. 2014, 155, 435–446. [Google Scholar] [CrossRef]
- Sandoval, L.; Méndez, C.; Mennill, D.J. Vocal behaviour of White-eared Ground-Sparrows (Melozone leucotis) during the breeding season: Repertoires, diel variation, behavioural contexts, and individual distinctiveness. J. Ornithol. 2016, 157, 1–12. [Google Scholar] [CrossRef]
- Baldo, S.; Mennill, D.J. Vocal behavior of Great Curassows, a vulnerable Neotropical bird. J. Field Ornithol. 2011, 82, 249–258. [Google Scholar] [CrossRef]
- Braga, A.C.R.; Motta-Junior, J.C. Weather conditions and moon phase influence on Tropical Screech Owl and Burrowing Owl detection by playback. Ardea 2009, 97, 395–401. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.L. Monitoring the annual vocal activity of two enigmatic nocturnal Neotropical birds: The Common Potoo (Nyctibius griseus) and the Great Potoo (Nyctibius grandis). J. Ornithol. 2020, 161, 1129–1141. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.L.; Marques, M.I. Vocal activity of the Ferruginous Pygmy-Owl (Glaucidium brasilianum) is strongly correlated with moon phase and nocturnal temperature. Ethol. Ecol. Evol. 2020. [Google Scholar] [CrossRef]
- Hackett, S.J.; Kimball, R.; Reddy, S.; Bowie, R.C. A phylogenomic study of birds reveals their evolutionary history. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef]
- Odom, K.; Mennill, D. Vocal duets in a nonpasserine: An examination of territory defence and neighbour–stranger discrimination in a neighbourhood of Barred Owls. Behaviour 2010, 147, 619–639. [Google Scholar]
- Sugai, L.S.M.; Silva, T.S.F.; Ribeiro, J.W., Jr.; Llusia, D. Terrestrial passive acoustic monitoring: Review and perspectives. BioScience 2019, 69, 15–25. [Google Scholar] [CrossRef]
- Marques, T.A.; Thomas, L.; Martin, S.W.; Mellinger, D.K.; Ward, J.A.; Moretti, D.J.; Harris, D.; Tyack, P.L. Estimating animal population density using passive acoustics. Biol. Rev. 2013, 88, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgenburg, S.L.; Sólymos, P.; Kardynal, K.J.; Frey, M.D. Paired sampling standardizes point count data from humans and acoustic recorders. Avian Conserv. Ecol. 2017, 12, 13. [Google Scholar] [CrossRef]
- Bombaci, S.P.L.; Pejchar, L. Using paired acoustic sampling to enhance population monitoring of New Zealand’s forest birds. J. Ecol. 2019, 43, 3356. [Google Scholar] [CrossRef]
- Oppel, S.; Hervias, S.; Oliveira, N.; Pipa, T.; Silva, C.; Geraldes, P.; Goh, M.; Immler, E.; McKown, M. Estimating population size of a nocturnal burrow-nesting seabird using acoustic monitoring and habitat mapping. Nat. Conserv. 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Deichmann, J.L.; Hernandez-Serna, A.; Campos-Cerqueira, M.; Aide, T.M. Soundscape analysis and acoustic monitoring document impacts of natural gas exploration on biodiversity in a tropical forest. Ecol. Indic. 2017, 74, 39–48. [Google Scholar] [CrossRef]
- Schroeder, K.M.; Mcrae, S.B. Automated auditory detection of a rare, secretive marsh bird with infrequent and acoustically indistinct vocalisations. Ibis 2020, 162, 1033–1046. [Google Scholar] [CrossRef]
- Baptista, L.F.; Trail, P.W.; Horblit, H.M. White-tipped Dove (Leptotila verreauxi). In Handbook of the Birds of the World Alive; Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Bird Life International. Leptotila verreauxi. In IUCN Red List of Threatened Species; Bird Life International: Cambridge, UK, 2016. [Google Scholar]
- Skutch, A.F. Life histories of Central American pigeons. Wilson Bull. 1964, 76, 211–247. [Google Scholar]
- Fraga, R.M. Vocal and reproductive behavior of Leptotila verreauxi in Lobos, Buenos Aires, Argentina. Hornero 1983, 12, 89–95. [Google Scholar]
- Giese, J.C.; Oldenburger, S.L.; Mathewson, H.A.; Schwertner, T.W.; Breeden, J.B. Survival and longevity of a White-tipped Dove (Leptotila verreauxi) population in south Texas. J. Ornithol. 2018, 130, 996–999. [Google Scholar] [CrossRef]
- Hall, J.D.; Giese, J.C.; Mathewson, H.A.; Schwertner, T.W.; Oldenburger, S.L.; Breeden, J.B. Parental behavior and attendance patterns of nesting White-tipped Doves (Leptotila verreauxi) in the lower Rio Grande Valley of Texas. Southwest. Nat. 2019, 63, 142–145. [Google Scholar] [CrossRef]
- Larkin, R.P.; Evans, W.R.; Diehl, R.H. Nocturnal flight calls of Dickcissels and Doppler radar echoes over south Texas in spring. J. Field Ornithol. 2002, 73, 2–9. [Google Scholar] [CrossRef]
- Abrahams, C. Comparison between lek counts and bioacoustic recording for monitoring Western Capercaillie (Tetrao urogallus L.). J. Ornithol. 2019, 19, 685–697. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Bota, G.; Giralt, D.; Barrero, A.; Gómez-Catasús, J.; Bustillo-De La Rosa, D.; Traba, J. Vocal activity rate index: A useful method to infer terrestrial bird abundance with acoustic monitoring. Ibis 2019, 161, 901–907. [Google Scholar] [CrossRef]
- Buxton, R.T.; Jones, I.L. Measuring nocturnal seabird activity and status using acoustic recording devices: Applications for island restoration. J. Field Ornithol. 2012, 83, 47–60. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Gómez-Catasús, J.; Bustillo-de la Rosa, D.; Barrero, A.; Reverter, M.; Traba, J. Effort needed to accurately estimate vocal activity rate index using acoustic monitoring: A case study with a dawn-time singing passerine. Ecol. Indic. 2019, 107, 105608. [Google Scholar] [CrossRef]
- Bird Life International. Columbina cyanopis. In Red List of Threatened Species; Bird Life International: Cambridge, UK, 2019. [Google Scholar]
- Junk, W.J.; Da Cunha, C.N.; Wantzen, K.M.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- De Deus, F.F.; Schuchmann, K.L.; Arieira, J.; de Oliveira Tissiani, A.S.; Marques, M.I. Avian eta diversity in a Neotropical wetland: The effects of flooding and vegetation structure. Wetlands 2020, 1–15. [Google Scholar] [CrossRef]
- Yip, D.; Leston, L.; Bayne, E.; Sólymos, P.; Grover, A. Experimentally derived detection distances from audio recordings and human observers enable integrated analysis of point count data. Avian Conserv. Ecol. 2017, 12, 11. [Google Scholar] [CrossRef]
- Darras, K.; Batáry, P.; Furnas, B.; Celis-Murillo, A.; Van Wilgenburg, S.L.; Mulyani, Y.A.; Tscharntke, T. Comparing the sampling performance of sound recorders versus point counts in bird surveys: A meta-analysis. J. Appl. Ecol. 2018, 55, 2575–2586. [Google Scholar] [CrossRef]
- Rempel, R.S.; Francis, C.M.; Robinson, J.N.; Campbell, M. Comparison of audio recording system performance for detecting and monitoring songbirds. J. Field Ornithol. 2013, 84, 86–97. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Bota, G.; Giralt, D.; Albarracín, J.; Traba, J. Cost-effectiveness assessment of five audio recording systems for wildlife monitoring: Differences between recording distances and singing direction. Ardeola 2019, 66, 311–325. [Google Scholar] [CrossRef]
- Bioacoustics Research Program Raven Pro. Interactive Sound Analysis Software. Version 1.5. Computer Software. Available online: http://www.birds.cornell.edu/raven (accessed on 3 April 2020).
- Knight, E.; Hannah, K.; Foley, G.; Scott, C.; Brigham, R.; Bayne, E. Recommendations for acoustic recognizer performance assessment with application to five common automated signal recognition programs. Avian Conserv. Ecol. 2017, 12, 14. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, G.R.J.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.L. Illuminating the nightlife of two Neotropical nightjars: Vocal behavior over a year and monitoring recommendations. Ethol. Ecol. Evol. 2020, 32, 466–480. [Google Scholar] [CrossRef]
- R Development Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Lumley, T.; Warnes, M.G.R. Package ‘Gtools’. R Package Version 3.8.1; R Foundation for Statistical Computing: Vienna, Austria, 2018; pp. 1–47. [Google Scholar]
- Laverde, R.O.; Caycedo-Rosales, P.; Pulgarin, R.P.C.; Cadena, C.D. Bird songs on the shelf: Assessing vocal activity and output using data hidden in sound archives. BioRxiv 2017, 202978. [Google Scholar] [CrossRef]
- Boydstun, C.P.; DeYoung, C.A. Distribution and relative abundance of white-tipped doves in South Texas. Southwest. Nat. 1985, 30, 567–571. [Google Scholar] [CrossRef]
- De Pinho, J.B.; Aragona, M.; Hakamada, K.Y.P.; Marini, M.A. Migration patterns and seasonal forest use by birds in the Brazilian Pantanal. Bird Conserv. Int. 2017, 27, 371–387. [Google Scholar] [CrossRef]
- Ragusa-Netto, J. Flowers, fruits, and the abundance of the Yellow-chevroned Parakeet (Brotogeris chiriri) at a gallery forest in the south Pantanal (Brazil). Braz. J. Biol. 2004, 64, 867–877. [Google Scholar] [CrossRef]
- Koloff, J.; Mennill, D.J. Vocal behaviour of Barred Antshrikes, a Neotropical duetting suboscine bird. J. Ornithol. 2013, 154, 51–61. [Google Scholar] [CrossRef]
- Demko, A.D.; Mennill, D.J. Rufous-capped Warblers Basileuterus rufifrons show seasonal, temporal and annual variation in song use. Ibis 2019, 161, 481–494. [Google Scholar] [CrossRef]
- Hüppop, O.; Hilgerloh, G. Flight call rates of migrating thrushes: Effects of wind conditions, humidity and time of day at an illuminated offshore platform. J. Avian Biol. 2012, 43, 85–90. [Google Scholar] [CrossRef]
- Digby, A.; Towsey, M.; Bell, B.D.; Teal, P.D. Temporal and environmental influences on the vocal behaviour of a nocturnal bird. J. Avian Biol. 2014, 45, 591–599. [Google Scholar] [CrossRef]
- Griffin, D.R. The importance of atmospheric attenuation for the echolocation of bats (Chiroptera). Anim. Behav. 1971, 19, 55–61. [Google Scholar] [CrossRef]
- Colbourne, R.; Baird, K.; Jolly, J. Relationship between invertebrates eaten by little spotted kiwi, Apteryx owenii, and their availability on Kapiti Island, New Zealand. N. Z. J. Zool. 1990, 17, 533–542. [Google Scholar] [CrossRef]
- Brumm, H.; Slabbekoorn, H. Acoustic communication in noise. Adv. Study Behav. 2005, 35, 151–209. [Google Scholar]
- Mennill, D.J. Variation in the vocal behavior of Common Loons (Gavia immer): Insights from landscape-level recordings. Waterbirds 2014, 37, 26–36. [Google Scholar] [CrossRef][Green Version]
- Robbins, C.S. Effect of time and day on bird activity. Stud. Avian Biol. 1981, 6, 275–282. [Google Scholar]
- Lengagne, T.; Slater, P.J. The effects of rain on acoustic communication: Tawny Owls have good reason for calling less in wet weather. Proc. Soc. B Biol. Sci. 2002, 269, 2121–2125. [Google Scholar] [CrossRef]
- Zuberogoitia, I.; Burgos, G.; González-Oreja, J.A.; Morant, J.; Martínez, J.E.; Zabala Albizua, J. Factors affecting spontaneous vocal activity of Tawny Owls Strix aluco and implications for surveying large areas. Ibis 2019, 161, 495–503. [Google Scholar] [CrossRef]
- Larom, D.; Garstang, M.; Payne, K.; Raspet, R.; Lindeque, M. The influence of surface atmospheric conditions on the range and area reached by animal vocalizations. J. Exp. Biol. 1997, 200, 421–431. [Google Scholar] [PubMed]
- Clark, K.A.; Anderson, S.H. Temporal, climatic and lunar factors affecting owl vocalizations of western Wyoming. J. Raptor Res. 1997, 31, 358–363. [Google Scholar]
- Swiston, K.A.; Mennill, D.J. Comparison of manual and automated methods for identifying target sounds in audio recordings of Pileated, Palebilled, and putative Ivory-billed Woodpeckers. J. Field Ornithol. 2009, 80, 42–50. [Google Scholar] [CrossRef]
- Bobay, L.R.; Taillie, P.J.; Moorman, C.E. Use of autonomous recording units increased detection of a secretive marsh bird. J. Field Ornithol. 2018, 89, 384–392. [Google Scholar] [CrossRef]
- Shonfield, J.; Heemskerk, S.; Bayne, E.M. Utility of automated species recognition for acoustic monitoring of owls. J. Raptor Res. 2018, 52, 42–55. [Google Scholar] [CrossRef]
- Tomas, W.M.; de Oliveira Roque, F.; Morato, R.G.; Medici, P.E.; Chiaravalloti, R.M.; Tortato, F.R.; Penha, J.M.F.; Izzo, T.J.; Garcia, L.C.; Lourival, R.F.F.; et al. Sustainability agenda for the Pantanal wetland: Perspectives on a collaborative interface for science, policy, and decision-making. Trop. Conserv. Sci. 2019, 12. [Google Scholar] [CrossRef]
- Por, F.D. The Pantanal of Mato Grosso (Brazil). In The World’s Largest Wetlands; Kluwer Academic: London, UK, 1992. [Google Scholar]
- Bird Life International. Claravis geoffroyi. (amended version of 2016 assessment). In The IUCN Red List of Threatened Species 2018; Bird Life International: Cambridge, UK, 2018. [Google Scholar]
| Variable | Sum Sq | Mean Sq | df | Den df | F | p |
|---|---|---|---|---|---|---|
| Hour | 261.60 | 11.38 | 12 | 898 | 104.63 | <0.0001 |
| Month | 31.42 | 2.62 | 23 | 898 | 24.09 | <0.0001 |
| Variable | Estimate | Std. Error | t-Value | p |
|---|---|---|---|---|
| Intercept | −587.80 | 513.41 | −1.145 | 0.254 |
| Mean air temperature (°C) | −11.71 | 8.99 | −1.302 | 0.195 |
| Air humidity (%) | 16.74 | 6.79 | 2.466 | 0.015 |
| Daily rainfall | 2.12 | 4.23 | 0.500 | 0.618 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Granados, C.; Schuchmann, K.-L. Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi). Diversity 2020, 12, 402. https://doi.org/10.3390/d12100402
Pérez-Granados C, Schuchmann K-L. Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi). Diversity. 2020; 12(10):402. https://doi.org/10.3390/d12100402
Chicago/Turabian StylePérez-Granados, Cristian, and Karl-L. Schuchmann. 2020. "Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi)" Diversity 12, no. 10: 402. https://doi.org/10.3390/d12100402
APA StylePérez-Granados, C., & Schuchmann, K.-L. (2020). Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi). Diversity, 12(10), 402. https://doi.org/10.3390/d12100402






