Competitive Interactions of Flowering Rush (Butomus umbellatus L.) Cytotypes in Submersed and Emergent Experimental Aquatic Plant Communities
Abstract
1. Introduction
2. Materials and Methods
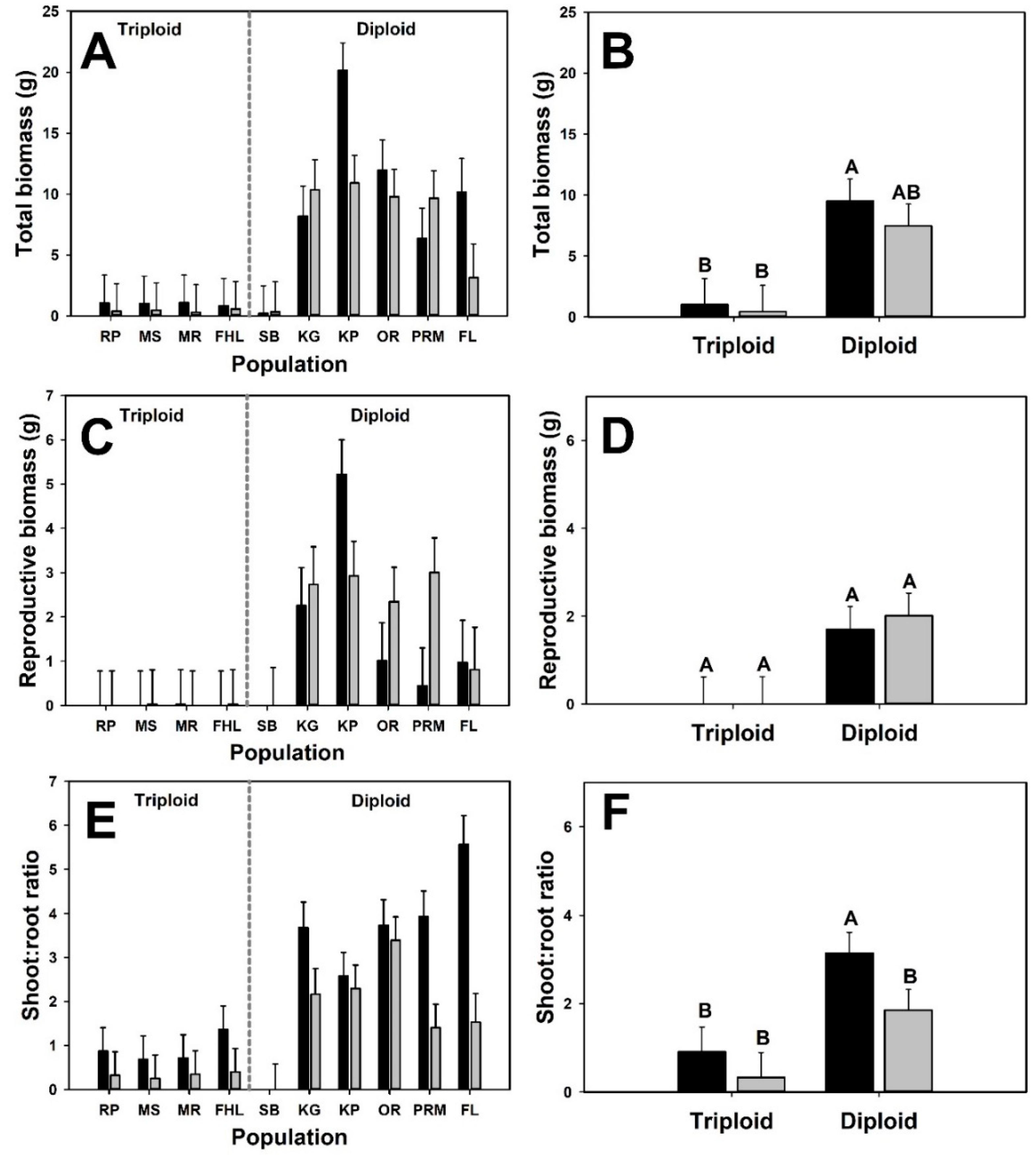
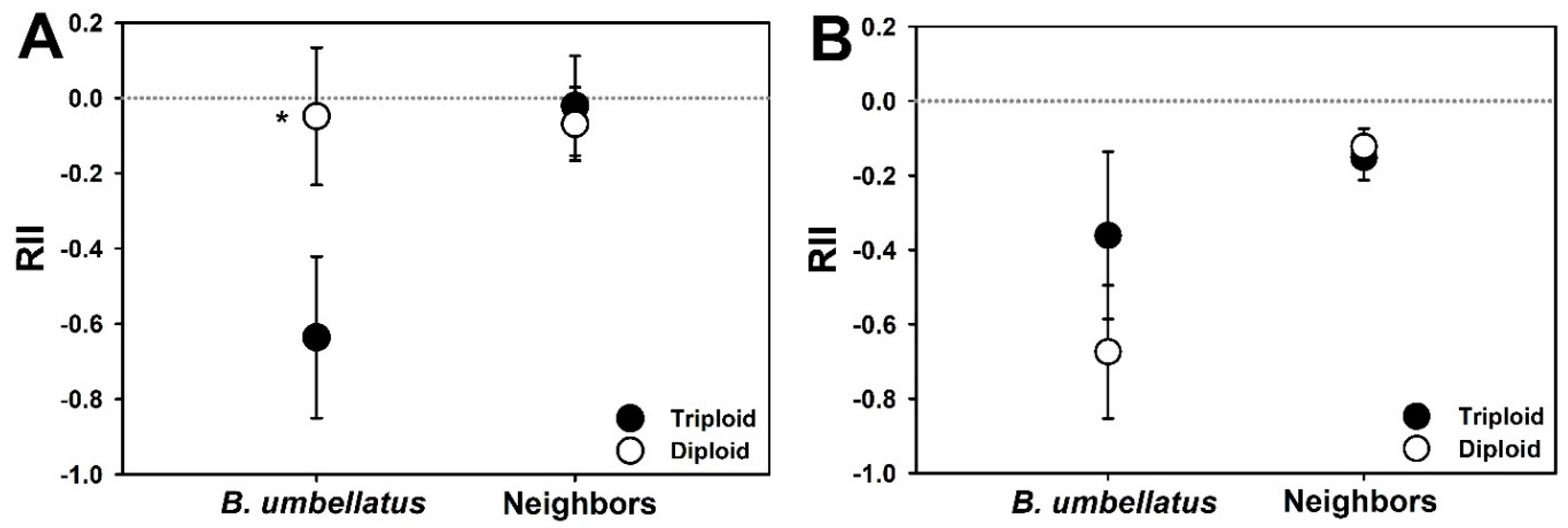
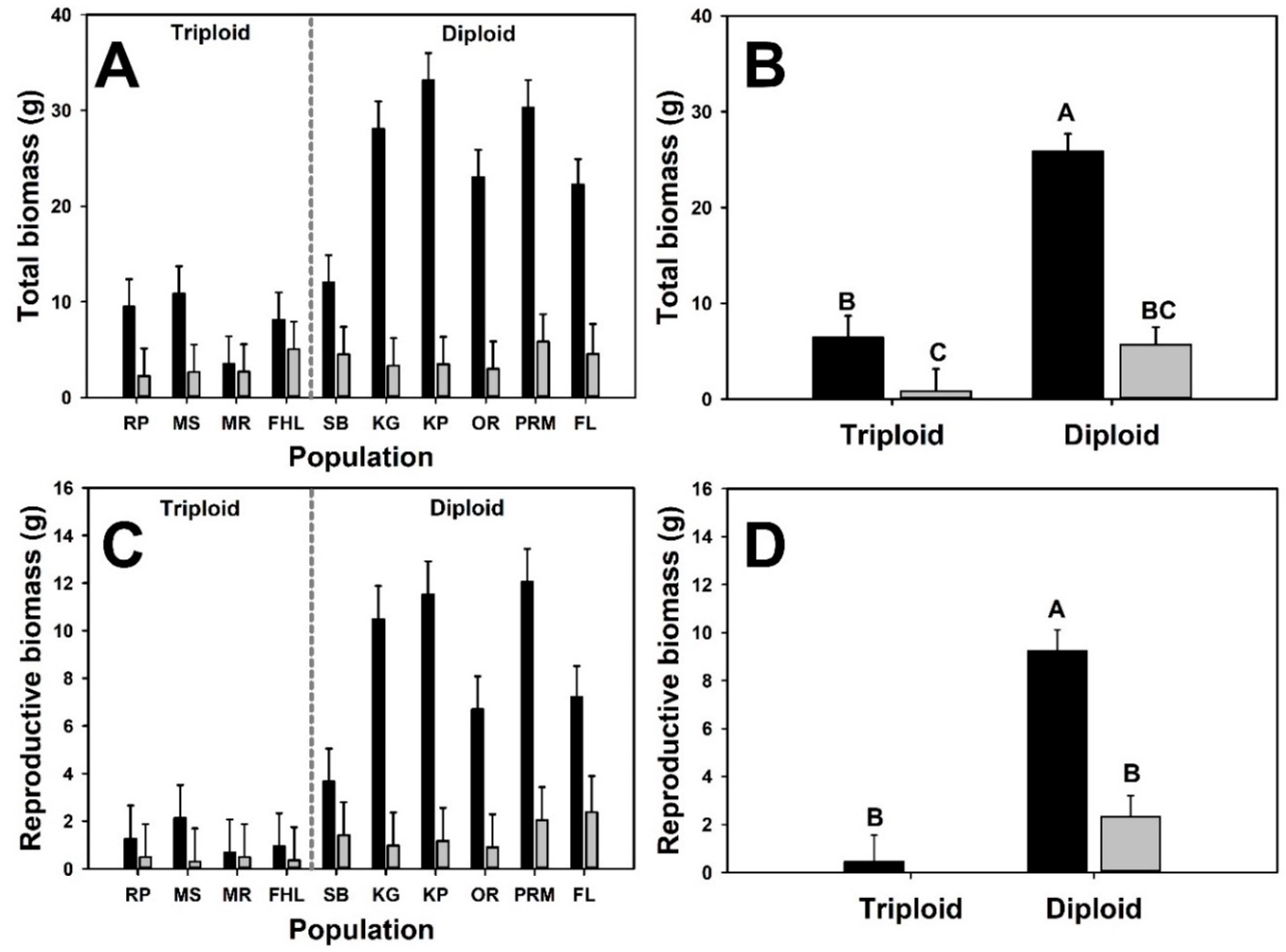
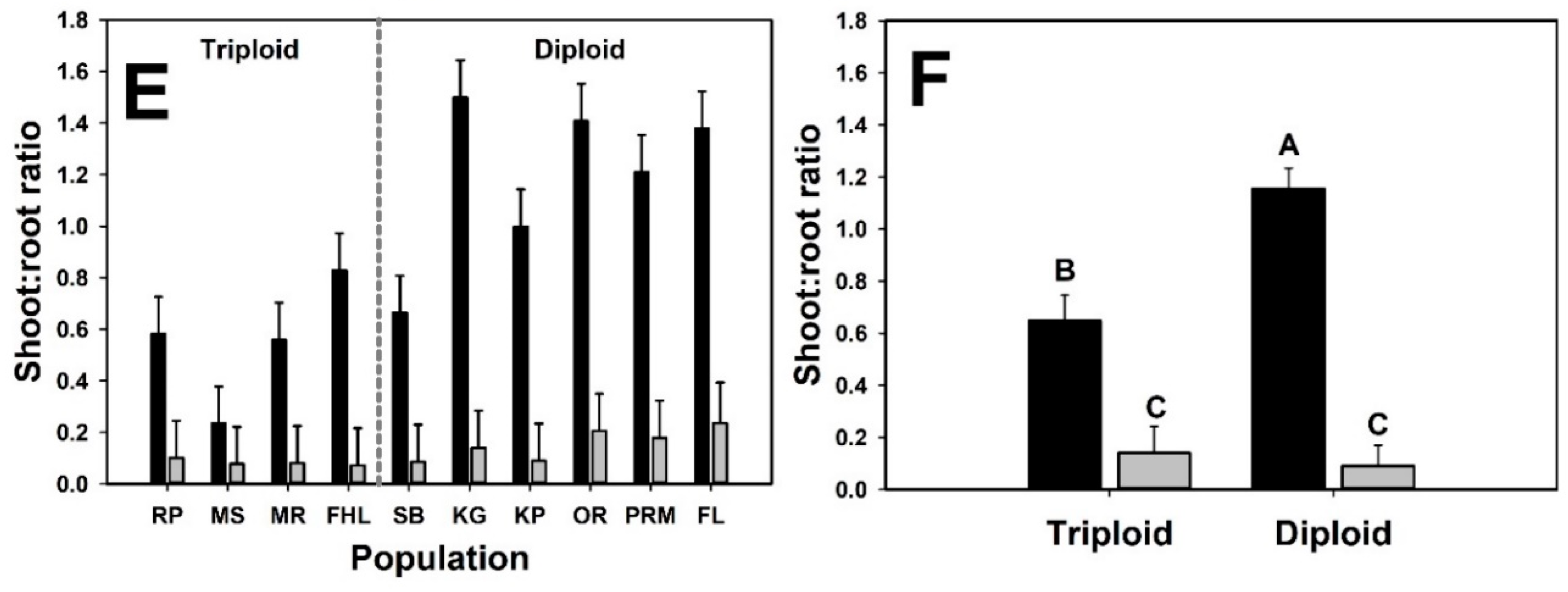
3. Results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Field Observations on Water Depth of B. umbellatus Populations
| Location | Year | Cytotype | Water Depth (cm) |
|---|---|---|---|
| Aberdeen Golf Course Canal, ID, USA | 2014 | Triploid | 100 |
| Columbia River @ Kennewick, WA, USA | 2014 | Triploid | 200 |
| Flathead Lake, MT, USA | 2014 | Triploid | 100 |
| Lake Pend Oreille, ID, USA | 2014 | Triploid | 125 |
| Lake Spokane, WA, USA | 2014 | Triploid | 160 |
| Pend Oreille River, WA, USA | 2014 | Triploid | 100 |
| Rose Pond, ID, USA | 2014 | Triploid | 100 |
| Yakima River, Horn Rapids Park, WA, USA | 2014 | Triploid | 100 |
| Forest Lake, MN, USA | 2015 | Diploid | 115 |
| Kildeer Pond 33, OH, USA | 2015 | Diploid | 100 |
| Olentangy River, OH, USA | 2015 | Diploid | 0 |
| Point Rosa Marsh, MI, USA | 2015 | Diploid | 0 |
| Sterling State Park, MI, USA | 2015 | Diploid | 0 |
| Bertram Lake, WI, USA | 2015 | Triploid | 0 |
| Lake Kawaguesaga, WI, USA | 2015 | Triploid | 100 |
| Lanes Lake, MI, USA | 2015 | Triploid | 0 |
| MS River near Galena, IL, USA | 2015 | Triploid | 0 |
| Oconto Falls, WI, USA | 2015 | Triploid | 100 |
| Village Park, Fremont, WI, USA | 2015 | Triploid | 0 |
| Cayuga Lake, NY, USA | 2016 | Diploid | 10 |
| East Bay Wildlife Management Area, NY, USA | 2016 | Diploid | 0 |
| Forest Lake, MN, USA | 2016 | Diploid | 100 |
| Kildeer Pond 33, OH, USA | 2016 | Diploid | 0 |
| Oswegatchie River, VT, USA | 2016 | Diploid | 10 |
| Point Rosa Marsh, MI, USA | 2016 | Diploid | 10 |
| Shelburne Bay, VT, USA | 2016 | Diploid | 0 |
| Springbrook Pond, IL, USA | 2016 | Diploid | 0 |
| Sterling State Park, MI, USA | 2016 | Diploid | 0 |
| Three Mile Bay, NY, USA | 2016 | Diploid | 25 |
| Unity Island, NY, USA | 2016 | Diploid | 30 |
| Aberdeen Golf Course Canal, ID, USA | 2016 | Triploid | 40 |
| Columbia River @ Kennewick, WA, USA | 2016 | Triploid | 125 |
| Flathead Lake, MT, USA | 2016 | Triploid | 70 |
| Lake Pend Oreille, ID, USA | 2016 | Triploid | 80 |
| Missisquoi River, VT, USA | 2016 | Triploid | 0 |
| Pend Oreille River, WA, USA | 2016 | Triploid | 80 |
| Rose Pond, ID, USA | 2016 | Triploid | 70 |
| Sabattus Creek, ME, USA | 2016 | Triploid | 20 |
| Yakima River @ Prosser, WA, USA | 2016 | Triploid | 15 |

References
- Gopal, B. Water Hyacinth; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Wainger, L.A.; Harms, N.E.; Magen, C.; Liang, N.; Nesslage, G.M.; McMurray, A.M.; Cofrancesco, A.F. Evidence-based economic analysis demonstrates that ecosystem service benefits of water hyacinth management greatly exceed research and control costs. PeerJ 2018, 6, e4824. [Google Scholar] [CrossRef] [PubMed]
- Villamagna, A.M.; Murphy, B.R. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshw. Biol. 2010, 55, 282–298. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.R. Effects of invasive, non-indigenous plant species on ecosystem processes: Lessons from Florida. Ecol. Appl. 1998, 8, 975–989. [Google Scholar] [CrossRef]
- Evans, J.M. Ecosystem implications of invasive aquatic plants and aquatic plant control in florida springs. In Summary and Synthesis of Available Literature on the Effects of Nutrients on Springs Organisms and Systems; Florida Department of Environmental Protection: Tallahassee, FL, USA, 2008; pp. 249–270. [Google Scholar]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Zhang, C.; Boyle, K.J. The effect of an aquatic invasive species (Eurasian watermilfoil) on lakefront property values. Ecol. Econ. 2010, 70, 394–404. [Google Scholar] [CrossRef]
- Keller, R.P.; Masoodi, A.; Shackleton, R.T. The impact of invasive aquatic plants on ecosystem services and human well-being in Wular Lake, India. Reg. Environ. Chang. 2018, 18, 847–857. [Google Scholar] [CrossRef]
- Wu, H.; Ding, J.Q. Global Change Sharpens the Double-Edged Sword Effect of Aquatic Alien Plants in China and Beyond. Front. Plant Sci. 2019, 10, 787. [Google Scholar] [CrossRef]
- Dukes, J.S.; Mooney, H.A. Disruption of ecosystem processes in western North America by invasive species. Rev. Chil. Hist. Nat. 2004, 77, 411–437. [Google Scholar] [CrossRef]
- Charles, H.; Dukes, J.S. Impacts of Invasive Species on Ecosystem Services. In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 217–237. [Google Scholar]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 7905–7910. [Google Scholar] [CrossRef]
- Divíšek, J.; Chytrý, M.; Beckage, B.; Gotelli, N.J.; Lososová, Z.; Pyšek, P.; Richardson, D.M.; Molofsky, J. Similarity of introduced plant species to native ones facilitates naturalization, but differences enhance invasion success. Nat. Commun. 2018, 9, 4631. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Mack, R.N. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Keller, R.P.; Lodge, D.M.; Finnoff, D.C. Risk assessment for invasive species produces net bioeconomic benefits. Proc. Natl. Acad. Sci. USA 2007, 104, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.M.; Williams, S.; MacIsaac, H.J.; Hayes, K.R.; Leung, B.; Reichard, S.; Mack, R.N.; Moyle, P.B.; Smith, M.; Andow, D.A.; et al. Biological invasions: Recommendations for U.S. policy and management. Ecol. Appl. 2006, 16, 2035–2054. [Google Scholar] [CrossRef]
- Simberloff, D.; Parker, I.M.; Windle, P.N. Introduced species policy, management, and future research needs. Front. Ecol. Environ. 2005, 3, 12–20. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Humphries, S.E. An Integrated Approach to the Ecology and Management of Plant Invasions. Conserv. Biol. 1995, 9, 761–770. [Google Scholar] [CrossRef]
- Zanden, M.J.V.; Olden, J.D. A management framework for preventing the secondary spread of aquatic invasive species. Can. J. Fish. Aquat. Sci. 2008, 65, 1512–1522. [Google Scholar] [CrossRef]
- Zanden, M.J.V.; Hansen, G.J.; Higgins, S.N.; Kornis, M.S. A pound of prevention, plus a pound of cure: Early detection and eradication of invasive species in the Laurentian Great Lakes. J. Great Lakes Res. 2010, 36, 199–205. [Google Scholar] [CrossRef]
- Byers, J.E.; Noonburg, E.G. Scale dependent effects of biotic resistance to biological invasion. Ecology 2003, 84, 1428–1433. [Google Scholar] [CrossRef]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Parker, J.D.; Hay, M.E. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol. Lett. 2005, 8, 959–967. [Google Scholar] [CrossRef]
- Byun, C.; Lee, E.J. Ecological application of biotic resistance to control the invasion of an invasive plant, Ageratina altissima. Ecol. Evol. 2017, 7, 2181–2192. [Google Scholar] [CrossRef]
- Byun, C.; de Blois, S.; Brisson, J. Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. J. Ecol. 2013, 101, 128–139. [Google Scholar] [CrossRef]
- Maron, J.L.; Vilà, M. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 2001, 95, 361–373. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Naturalization of introduced plants: Ecological drivers of biogeographical patterns. New Phytol. 2012, 196, 383–396. [Google Scholar] [CrossRef]
- Barbosa, P.; Hines, J.; Kaplan, I.; Martinson, H.; Szczepaniec, A.; Szendrei, Z. Associational Resistance and Associational Susceptibility: Having Right or Wrong Neighbors. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 1–20. [Google Scholar] [CrossRef]
- Andersen, C.P.; Louda, S.M. Abundance of and Floral Herbivory on Exotic Bull Thistle Versus Native Tall Thistle in Western Tallgrass Prairie; University of Nebraska at Kearney: Kearney, NE, USA, 2006. [Google Scholar]
- Yannelli, F.; Koch, C.; Jeschke, J.; Kollmann, J. Limiting similarity and Darwin’s naturalization hypothesis: Understanding the drivers of biotic resistance against invasive plant species. Oecologia 2017, 183, 775–784. [Google Scholar] [CrossRef]
- Kennedy, T.A.; Naeem, S.; Howe, K.M.; Knops, J.M.H.; Tilman, D.; Reich, P. Biodiversity as a barrier to ecological invasion. Nature 2002, 417, 636–638. [Google Scholar] [CrossRef]
- Thiele, J.; Isermann, M.; Otte, A.; Kollmann, J. Competitive displacement or biotic resistance? Disentangling relationships between community diversity and invasion success of tall herbs and shrubs. J. Veg. Sci. 2010, 21, 213–220. [Google Scholar] [CrossRef]
- Britton-Simmons, K.H. Functional group diversity, resource preemption and the genesis of invasion resistance in a community of marine algae. Oikos 2006, 113, 395–401. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through reassembly: Plant traits and invasion resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.L.; Bever, J.D.; Schultz, P.A. The Effect of Restoration Methods on the Quality of the Restoration and Resistance to Invasion by Exotics. Restor. Ecol. 2010, 18, 181–187. [Google Scholar] [CrossRef]
- Iii, B.V.I.; Potter, K.M.; Guo, Q.; Jo, I.; Oswalt, C.M.; Fei, S. Environmental harshness drives spatial heterogeneity in biotic resistance. NeoBiota 2018, 40, 87–105. [Google Scholar]
- Iannone, B.V.; Oswalt, C.M.; Liebhold, A.M.; Guo, Q.; Potter, K.M.; Nunez-Mir, G.C.; Oswalt, S.N.; Pijanowski, B.C.; Fei, S. Region-specific patterns and drivers of macroscale forest plant invasions. Divers. Distrib. 2015, 21, 1181–1192. [Google Scholar] [CrossRef]
- Araújo, M.B.; Rozenfeld, A. The geographic scaling of biotic interactions. Ecography 2014, 37, 406–415. [Google Scholar] [CrossRef]
- Souza, L.; Bunn, W.A.; Simberloff, D.; Lawton, R.M.; Sanders, N.J. Biotic and abiotic influences on native and exotic richness relationship across spatial scales: Favourable environments for native species are highly invasible. Funct. Ecol. 2011, 25, 1106–1112. [Google Scholar] [CrossRef]
- Richards, C.L.; Pennings, S.C.; Donovan, L.A. Habitat range and phenotypic variation in salt marsh plants. Plant Ecol. 2005, 176, 263–273. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.D.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Turner, K.G.; Fréville, H.; Rieseberg, L.H. Adaptive plasticity and niche expansion in an invasive thistle. Ecol. Evol. 2015, 5, 3183–3197. [Google Scholar] [CrossRef] [PubMed]
- Vretare, V.; Weisner, S.E.; Strand, J.A.; Granéli, W. Phenotypic plasticity in Phragmites australis as a functional response to water depth. Aquat. Bot. 2001, 69, 127–145. [Google Scholar] [CrossRef]
- Pendergrass, A.G.; Knutti, R.; Lehner, F.; Deser, C.; Sanderson, B.M. Precipitation variability increases in a warmer climate. Sci. Rep. 2017, 7, 17966. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Q.; Wei, F.; Zeng, L.Y.; Li, X.K.; Tang, S.C.; Zhong, Y.; Geng, Y.P. Multiple introductions are responsible for the disjunct distributions of invasive Parthenium hysterophorus in China: Evidence from nuclear and chloroplast DNA. Weed Res. 2009, 49, 373–380. [Google Scholar] [CrossRef]
- Kelager, A.; Pedersen, J.S.; Bruun, H.H. Multiple introductions and no loss of genetic diversity: Invasion history of Japanese Rose, Rosa rugosa, in Europe. Biol. Invasions 2013, 15, 1125–1141. [Google Scholar] [CrossRef]
- Zhu, X.C.; Gopurenko, D.; Serrano, M.; Spencer, M.A.; Pieterse, P.J.; Skoneczny, D.; Lepschi, B.J.; Reigosa, M.J.; Gurr, G.M.; Callaway, R.M.; et al. Genetic evidence for plural introduction pathways of the invasive weed Paterson’s curse (Echium plantagineum L.) to southern Australia. PLoS ONE 2019, 14, e0222696. [Google Scholar] [CrossRef]
- Ward, S.M.; Gaskin, J.F.; Wilson, L.M. Ecological Genetics of Plant Invasion: What Do We Know? Invasive Plant Sci. Manag. 2008, 1, 98–109. [Google Scholar] [CrossRef]
- Williams, D.A.; Overholt, W.A.; Cuda, J.P.; Hughes, C.R. Chloroplast and microsatellite DNA diversities reveal the introduction history of Brazilian peppertree (Schinus terebinthifolius) in Florida. Mol. Ecol. 2005, 14, 3643–3656. [Google Scholar] [CrossRef]
- Larue, E.A.; Zuellig, M.P.; Netherland, M.D.; Heilman, M.A.; Thum, R.A. Hybrid watermilfoil lineages are more invasive and less sensitive to a commonly used herbicide than their exotic parent (Eurasian watermilfoil). Evol. Appl. 2013, 6, 462–471. [Google Scholar] [CrossRef]
- Thum, R.A. Genetic variation and aquatic plant management: Key concepts and practical implications. J. Aquat. Plant Manag. 2018, 56, 101–106. [Google Scholar]
- Gaskin, J.F.; Bon, M.C.; Cock, M.J.; Cristofaro, M.; De Biase, A.; De Clerck-Floate, R.; Ellison, C.A.; Hinz, H.L.; Hufbauer, R.A.; Julien, M.H.; et al. Applying molecular-based approaches to classical biological control of weeds. Biol. Control 2011, 58, 1–21. [Google Scholar] [CrossRef]
- Williams, W.I.; Friedman, J.M.; Gaskin, J.F.; Norton, A.P. Hybridization of an invasive shrub affects tolerance and resistance to defoliation by a biological control agent. Evol. Appl. 2014, 7, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Hroudová, Z.; Zákravský, P. Ecology of two cytotypes of Butomus umbellatus II. Reproduction, growth and biomass production. Folia Geobot. Phytotaxon. 1993, 28, 413–424. [Google Scholar] [CrossRef]
- Anderson, L.C.; Zeis, C.D.; Alam, S.F. Phytogeography and Possible Origins of Butomus in North America. Bull. Torrey Bot. Club 1974, 101, 292–296. [Google Scholar] [CrossRef]
- Harms, N.; Shearer, J.; Cronin, J.T.; Gaskin, J.F. Geographic and genetic variation in susceptibility of Butomus umbellatus to foliar fungal pathogens. Biol. Invasions 2019, 1–14. [Google Scholar] [CrossRef]
- Cao, L.; Berent, L.; Fusaro, A. Butomus umbellatus L. Available online: https://nas.er.usgs.gov/queries/greatLakes/FactSheet.aspx?SpeciesID=1100&Potential=N&Type=0&HUCNumber= (accessed on 21 November 2019).
- Parkinson, H.; Mangold, J.; Dupuis, V.; Rice, P. Biology, Ecology and Mangament of Flowering Rush (Butomus umbellatus); Montana State University: Bozeman, MT, USA, 2010; pp. 1–12. [Google Scholar]
- Lesica, P.; Lavin, M.; Stickney, P.F. Manual of Montana Vascular Plants; BRIT Press: Ft. Worth, TX, USA, 2012. [Google Scholar]
- Jacobs, J.; Mangold, J.; Parkinson, H.; Dupuis, V.; Rice, P. Ecology and management of flowering rush (Butomus umbellatus L.); Invasive Species Technical Note No. MT-33; United States Department of Agriculture: Washington, DC, USA; Natural Resources Conservation Service: Washington, DC, USA, 2011.
- Roach, D.A.; Wulff, R.D. Maternal Effects in Plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Leger, E.A.; Espeland, E.K. PERSPECTIVE: Coevolution between native and invasive plant competitors: Implications for invasive species management. Evol. Appl. 2010, 3, 169–178. [Google Scholar] [CrossRef]
- Smart, R.M.; Barko, J.W. Laboratory culture of submersed freshwater macrophytes on natural sediments. Aquat. Bot. 1985, 21, 251–263. [Google Scholar] [CrossRef]
- Bose, R.C.; Clatworthy, W.H.; Shrikhande, S.S. Tables of Partially Balanced Designs with Two Associate Classes; North Carolina State University: Raleigh, NC, USA, 1954. [Google Scholar]
- Armas, C.; Ordiales, R.; Pugnaire, F.I. Measuring Plant Interactions: A New Comparative Index. Ecology 2004, 85, 2682–2686. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Sanhueza, A.K.; Torres-Mellado, G.; Casanova-Katny, A. Competition between native Antarctic vascular plants and invasive Poa annua changes with temperature and soil nitrogen availability. Biol. Invasions 2018, 20, 1597–1610. [Google Scholar] [CrossRef]
- Madsen, J.D.; Sartain, B.; Turnage, G.; Marko, M. Management of flowering rush in the Detroit Lakes, Minnesota. J. Aquat. Plant Manag. 2016, 54, 61–67. [Google Scholar]
- Turnage, G.; Madsen, J.D.; Wersal, R.M.; Byrd, J.D. Simulated mechanical control of flowering rush (Butomus umbellatus) under mesocosm conditions. Invasive Plant Sci. Manag. 2019, 12, 120–123. [Google Scholar] [CrossRef]
- Bhattarai, G.P.; Meyerson, L.A.; Anderson, J.; Cummings, D.; Allen, W.J.; Cronin, J.T. Biogeography of a plant invasion: Genetic variation and plasticity in latitudinal clines for traits related to herbivory. Ecol. Monogr. 2017, 87, 57–75. [Google Scholar] [CrossRef]
- Bhattarai, G.P.; Meyerson, L.A.; Cronin, J.T. Geographic variation in apparent competition between native and invasive Phragmites australis. Ecology 2017, 98, 349–358. [Google Scholar] [CrossRef]
- Gillard, M.; Grewell, B.J.; Futrell, C.J.; Deleu, C.; Thiébaut, G. Germination and Seedling Growth of Water Primroses: A Cross Experiment between Two Invaded Ranges with Contrasting Climates. Front. Plant Sci. 2017, 8, 1677. [Google Scholar] [CrossRef] [PubMed]
- Grewell, B.J.; Thomason, M.J.S.; Futrell, C.J.; Iannucci, M.; Drenovsky, R.E. Trait responses of invasive aquatic macrophyte congeners: Colonizing diploid outperforms polyploid. AoB Plants 2016, 8. [Google Scholar] [CrossRef]
- Castillo, J.M.; Grewell, B.J.; Pickart, A.; Bortolus, A.; Peña, C.; Figueroa, E.; Sytsma, M. Phenotypic plasticity of invasive Spartina densiflora (Poaceae) along a broad latitudinal gradient on the Pacific Coast of North America. Am. J. Bot. 2014, 101, 448–458. [Google Scholar] [CrossRef]
- Grewell, B.J.; Castillo, J.M.; Thomason, M.J.S.; Drenovsky, R.E. Phenotypic plasticity and population differentiation in response to salinity in the invasive cordgrass Spartina densiflora. Biol. Invasions 2016, 18, 2175–2187. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhou, F.; Pan, X.Y.; van Kleunen, M.; Liu, M.; Li, B. Evolution of increased intraspecific competitive ability following introduction: The importance of relatedness among genotypes. J. Ecol. 2019, 107, 387–395. [Google Scholar] [CrossRef]
- Liu, L.; Dong, B.C.; Alpert, P.; Yu, F.H. Effects of soil substrate heterogeneity and moisture on interspecific competition between Alternanthera philoxeroidesand four native species. J. Plant Ecol. 2016, 10, 528–537. [Google Scholar]
- Liu, M.; Zhou, F.; Pan, X.Y.; Zhang, Z.J.; Traw, M.B.; Li, B. Specificity of herbivore-induced responses in an invasive species, Alternanthera philoxeroides (alligator weed). Ecol. Evol. 2018, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Boot, R.G.A.; Van Der Aart, P.J.M. The relation between above- and belowground biomass allocation patterns and competitive ability. Oecologia 1991, 87, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.J.F.; Casper, B.B. Investigating the relationship between neighbor root biomass and belowground competition: Field evidence for symmetric competition belowground. Oikos 2000, 90, 311–320. [Google Scholar] [CrossRef]
- Rudak, A.; Wódkiewicz, M.; Znój, A.; Chwedorzewska, K.J.; Galera, H. Plastic biomass allocation as a trait increasing the invasiveness of annual bluegrass (Poa annua L.) in Antarctica. Polar Biol. 2018, 42, 149–157. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Ren, G.Q.; Li, Q.; Li, Y.; Li, J.; Adomako, M.O.; Dai, Z.C.; Li, G.L.; Wan, L.Y.; Zhang, B.; Zou, C.B.; et al. The enhancement of root biomass increases the competitiveness of an invasive plant against a co-occurring native plant under elevated nitrogen deposition. Flora 2019, 261, 151486. [Google Scholar] [CrossRef]
- Harms, N.E.; Cronin, J.T.; Gaskin, J.F. Increased ploidy in the invasive range of Butomus umbellatus L. is not associated with higher phenotypic plasticity to N and P. in review. 2020. [Google Scholar]
- Sterck, F.J.; Clark, D.B.; Clark, D.A.; Bongers, F. Light fluctuations, crown traits, and response delays for tree saplings in a Costa Rican lowland rain forest. J. Trop. Ecol. 1999, 15, 83–95. [Google Scholar] [CrossRef]
- Bargeron, C.T.; Moorhead, D.J. EDDMapS—Early detection and distribution mapping system for the southeast exotic pest plant council. Wildland Weeds 2007, 10, 4–8. [Google Scholar]
| Population | Latitude | Longitude | Cytotype | Genotype * |
|---|---|---|---|---|
| Kildeer Pond, OH, USA | 40.710 | −83.369 | Diploid | G4 |
| Springbrook Pond, IL, USA | 41.730 | −88.205 | Diploid | G3 |
| Point Rosa Marsh, MI, USA | 42.576 | −82.805 | Diploid | G4 |
| Oswegatchie River, NY, USA | 44.690 | −75.495 | Diploid | G4 |
| Kenduskeag River, ME, USA | 44.818 | −68.793 | Diploid | G4 |
| Forest Lake, MN, USA | 45.272 | −92.937 | Diploid | G5 |
| Mississippi River, IL, USA | 42.343 | −90.409 | Triploid | G1 |
| Rose Pond, ID, USA | 43.247 | −112.315 | Triploid | G1 |
| Mississquoi River, VT, USA | 44.951 | −73.162 | Triploid | G1 |
| Flathead Lake, MT, USA | 47.697 | −114.071 | Triploid | G1 |
| Factor | Num df | Den df | F | p | |
|---|---|---|---|---|---|
| Submersed | Total biomass | ||||
| Cytotype | 1 | 13.4 | 8.74 | 0.011 | |
| Neighbor presence | 1 | 98 | 1.49 | 0.225 | |
| Cytotype × Neighbor presence | 1 | 98.8 | 0.77 | 0.383 | |
| Initial mass | 1 | 103 | 1.21 | 0.275 | |
| Reproductive biomass | |||||
| Cytotype | 1 | 15.7 | 5.39 | 0.034 | |
| Neighbor presence | 1 | 98.2 | 0.18 | 0.670 | |
| Cytotype × Neighbor presence | 1 | 99.4 | 0.09 | 0.771 | |
| Initial mass | 1 | 90.6 | 0.38 | 0.539 | |
| Shoot:root Ratio | |||||
| Cytotype | 1 | 11.8 | 6.7 | 0.024 | |
| Neighbor presence | 1 | 97.3 | 12.81 | <0.001 | |
| Cytotype × Neighbor presence | 1 | 98.1 | 2.2 | 0.141 | |
| Initial mass | 1 | 105 | 0.59 | 0.445 | |
| Emergent | Total biomass | ||||
| Cytotype | 1 | 10.4 | 20.93 | <0.001 | |
| Neighbor presence | 1 | 107 | 94.28 | <0.001 | |
| Cytotype × Neighbor presence | 1 | 107 | 34.64 | <0.001 | |
| Initial mass | 1 | 109 | 7.44 | 0.007 | |
| Reproductive biomass | |||||
| Cytotype | 1 | 11.5 | 23.81 | <0.001 | |
| Neighbor presence | 1 | 107 | 39.67 | <0.001 | |
| Cytotype × Neighbor presence | 1 | 107 | 23.6 | <0.001 | |
| Initial mass | 1 | 96.1 | 3.93 | 0.05 | |
| Shoot:root Ratio | |||||
| Cytotype | 1 | 11.9 | 4.86 | 0.048 | |
| Neighbor presence | 1 | 106 | 124.8 | <0.001 | |
| Cytotype × Neighbor presence | 1 | 106 | 18.73 | <0.001 | |
| Initial mass | 1 | 94.1 | 4.78 | 0.031 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harms, N.E. Competitive Interactions of Flowering Rush (Butomus umbellatus L.) Cytotypes in Submersed and Emergent Experimental Aquatic Plant Communities. Diversity 2020, 12, 40. https://doi.org/10.3390/d12010040
Harms NE. Competitive Interactions of Flowering Rush (Butomus umbellatus L.) Cytotypes in Submersed and Emergent Experimental Aquatic Plant Communities. Diversity. 2020; 12(1):40. https://doi.org/10.3390/d12010040
Chicago/Turabian StyleHarms, Nathan E. 2020. "Competitive Interactions of Flowering Rush (Butomus umbellatus L.) Cytotypes in Submersed and Emergent Experimental Aquatic Plant Communities" Diversity 12, no. 1: 40. https://doi.org/10.3390/d12010040
APA StyleHarms, N. E. (2020). Competitive Interactions of Flowering Rush (Butomus umbellatus L.) Cytotypes in Submersed and Emergent Experimental Aquatic Plant Communities. Diversity, 12(1), 40. https://doi.org/10.3390/d12010040





