Habitat Islands on the Aegean Islands (Greece): Elevational Gradient of Chasmophytic Diversity, Endemism, Phytogeographical Patterns and need for Monitoring and Conservation
Abstract
1. Introduction
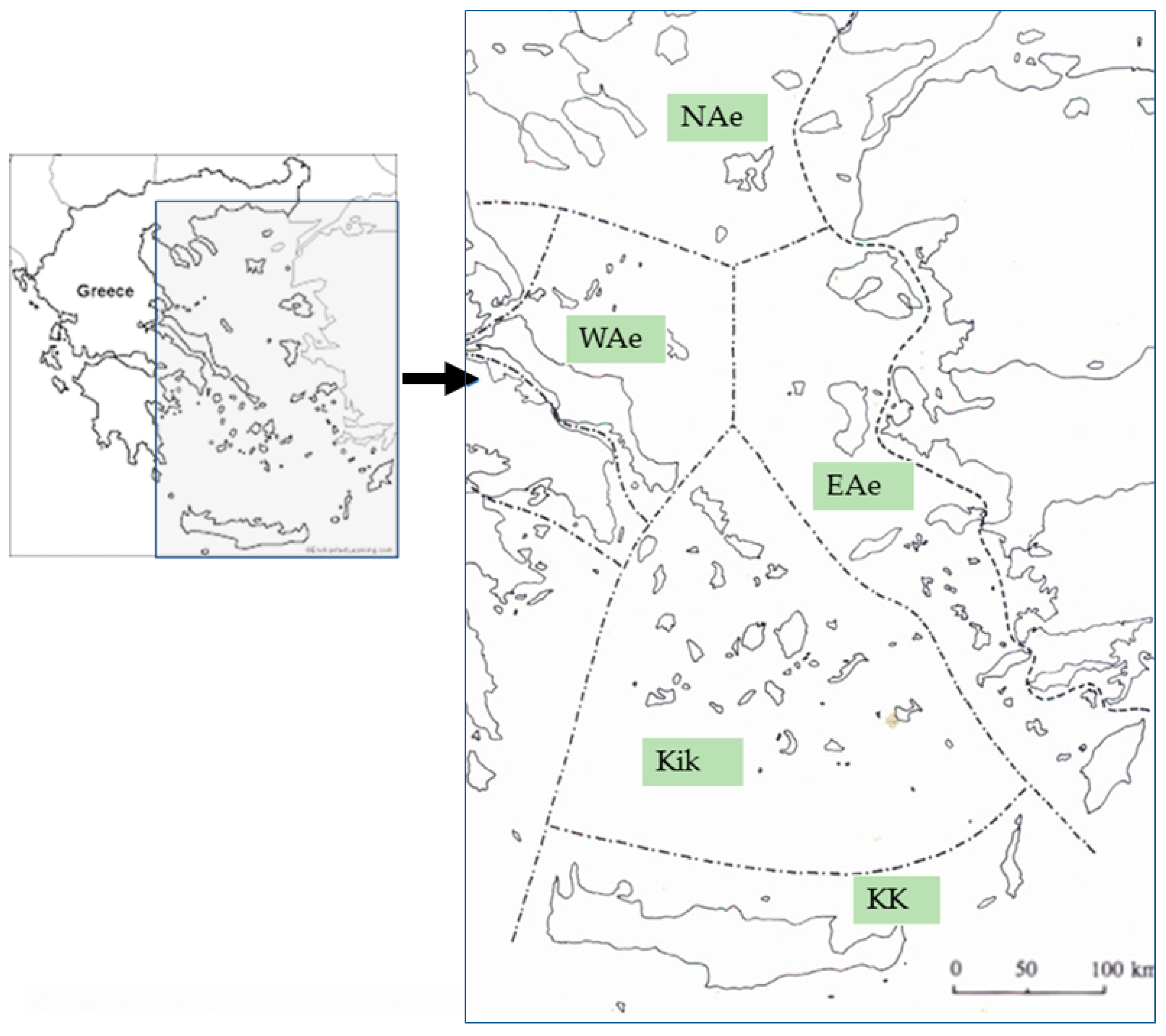
2. Materials and Methods
3. Results
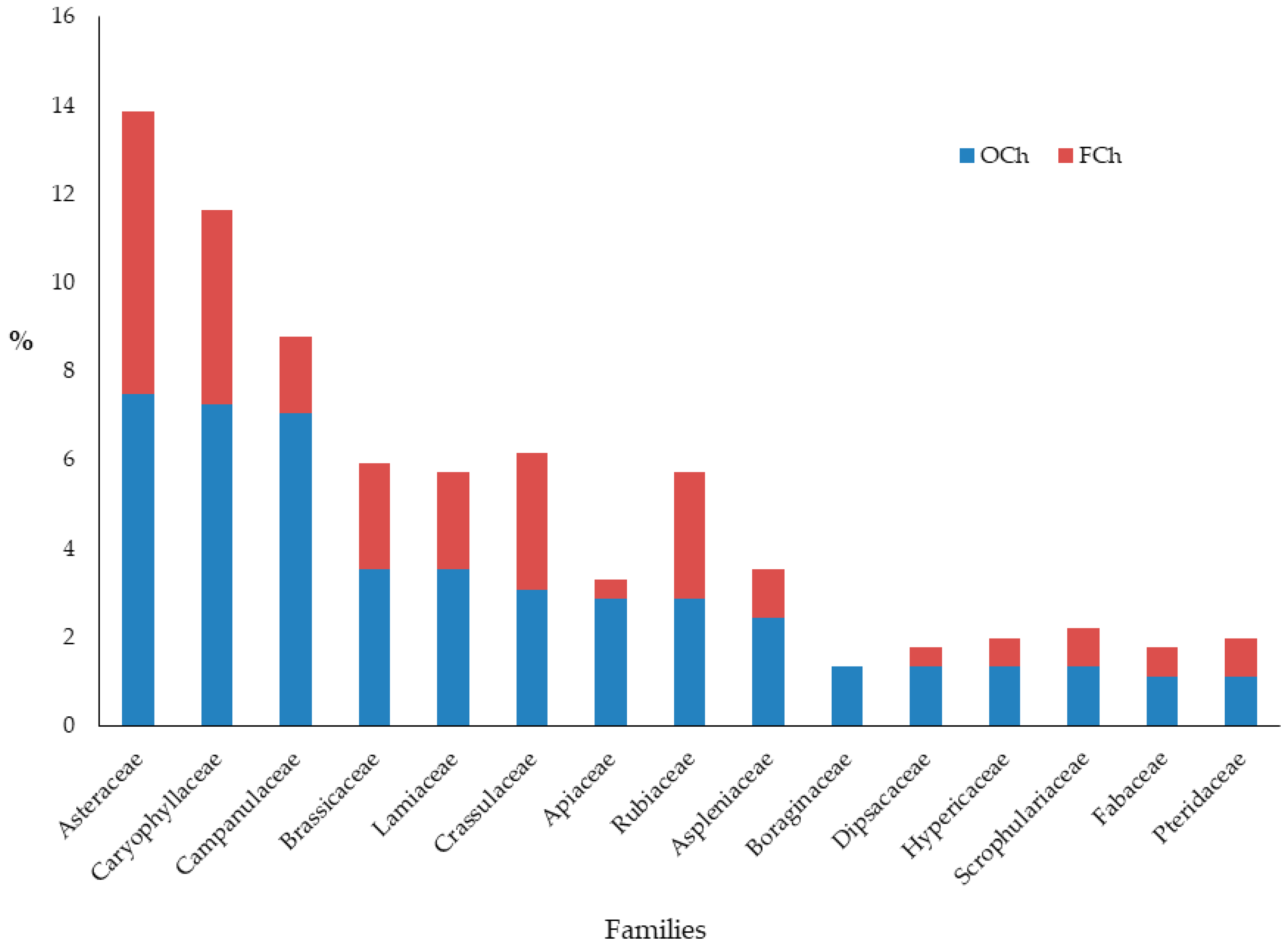
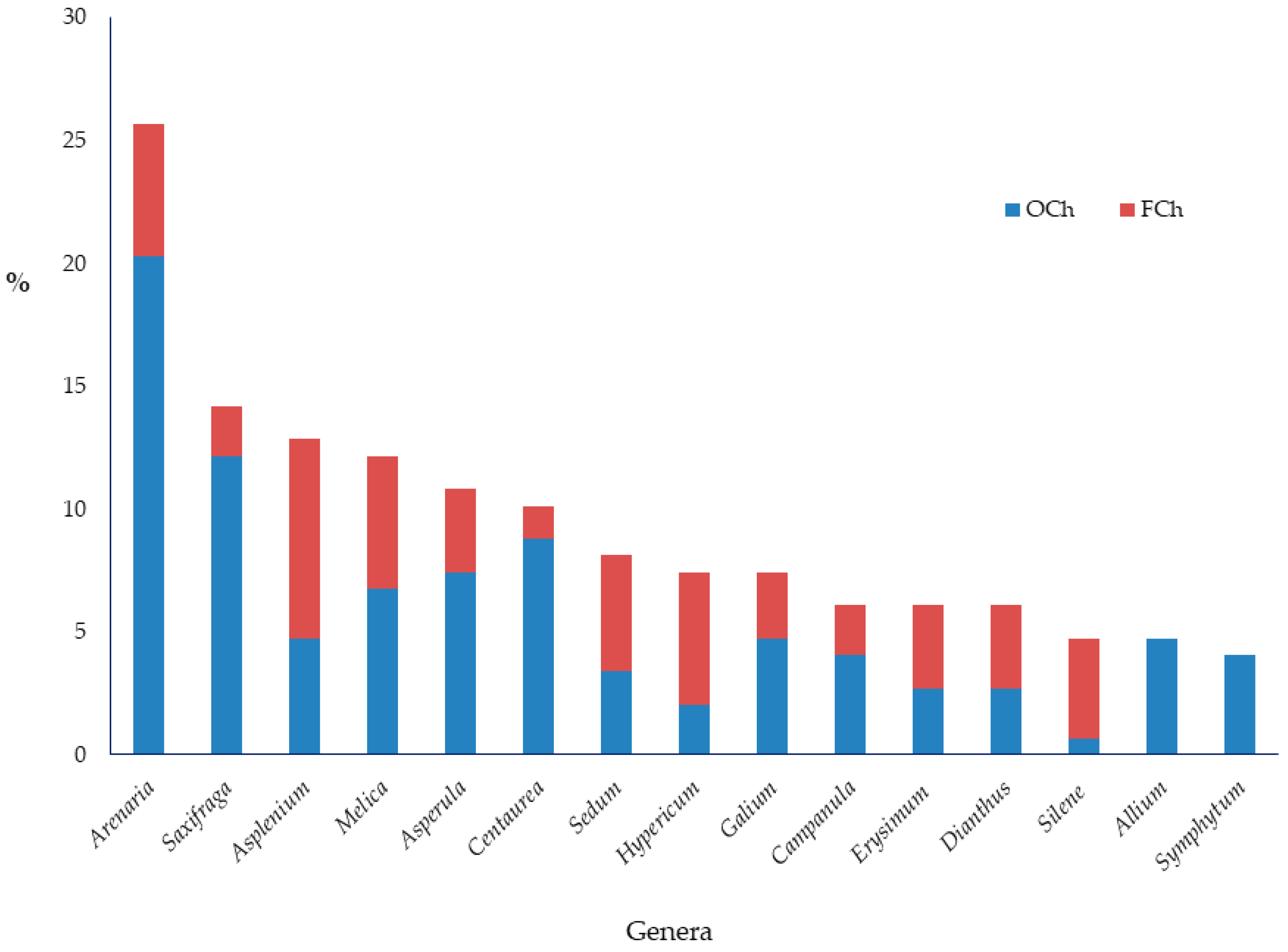
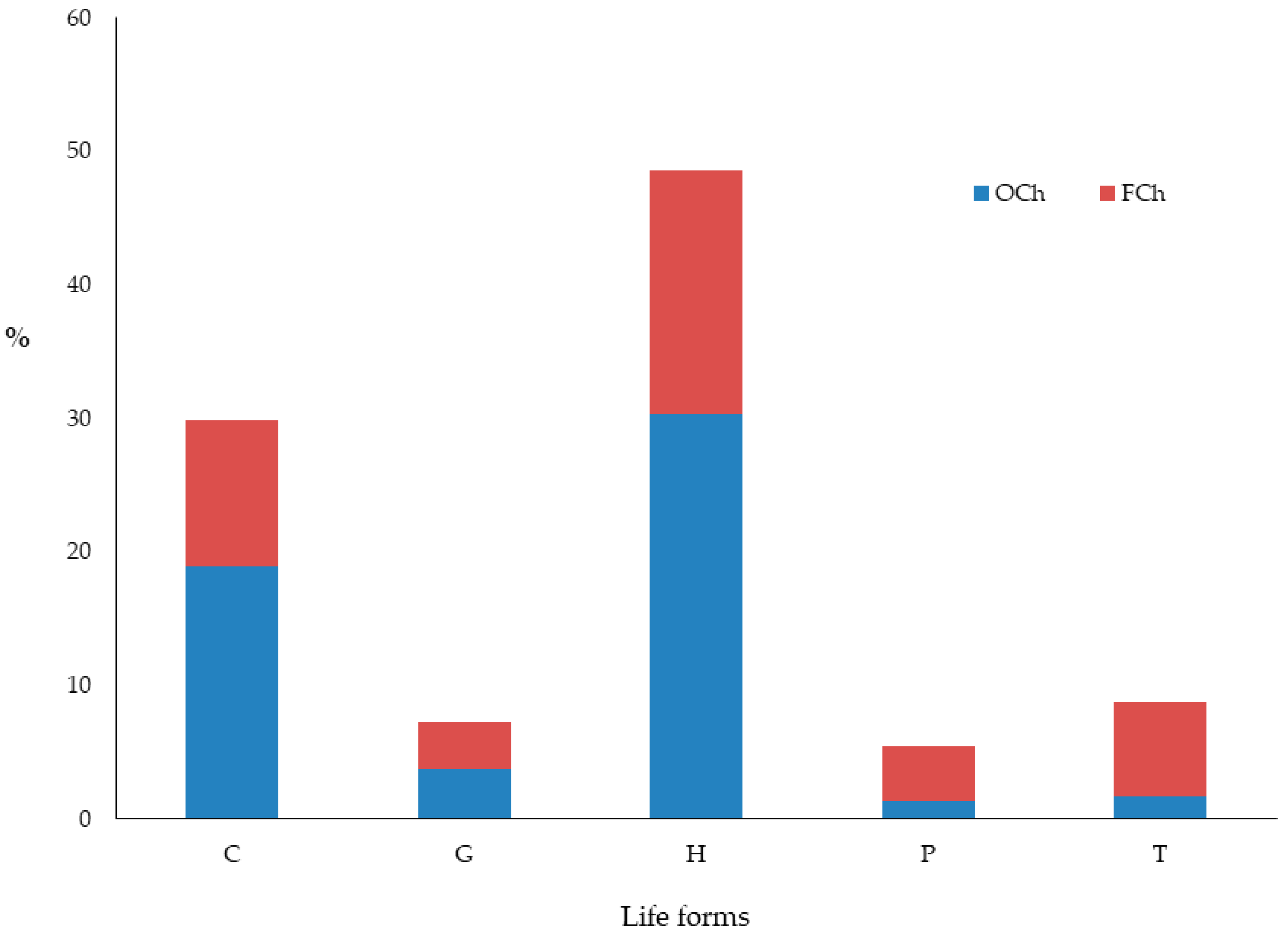
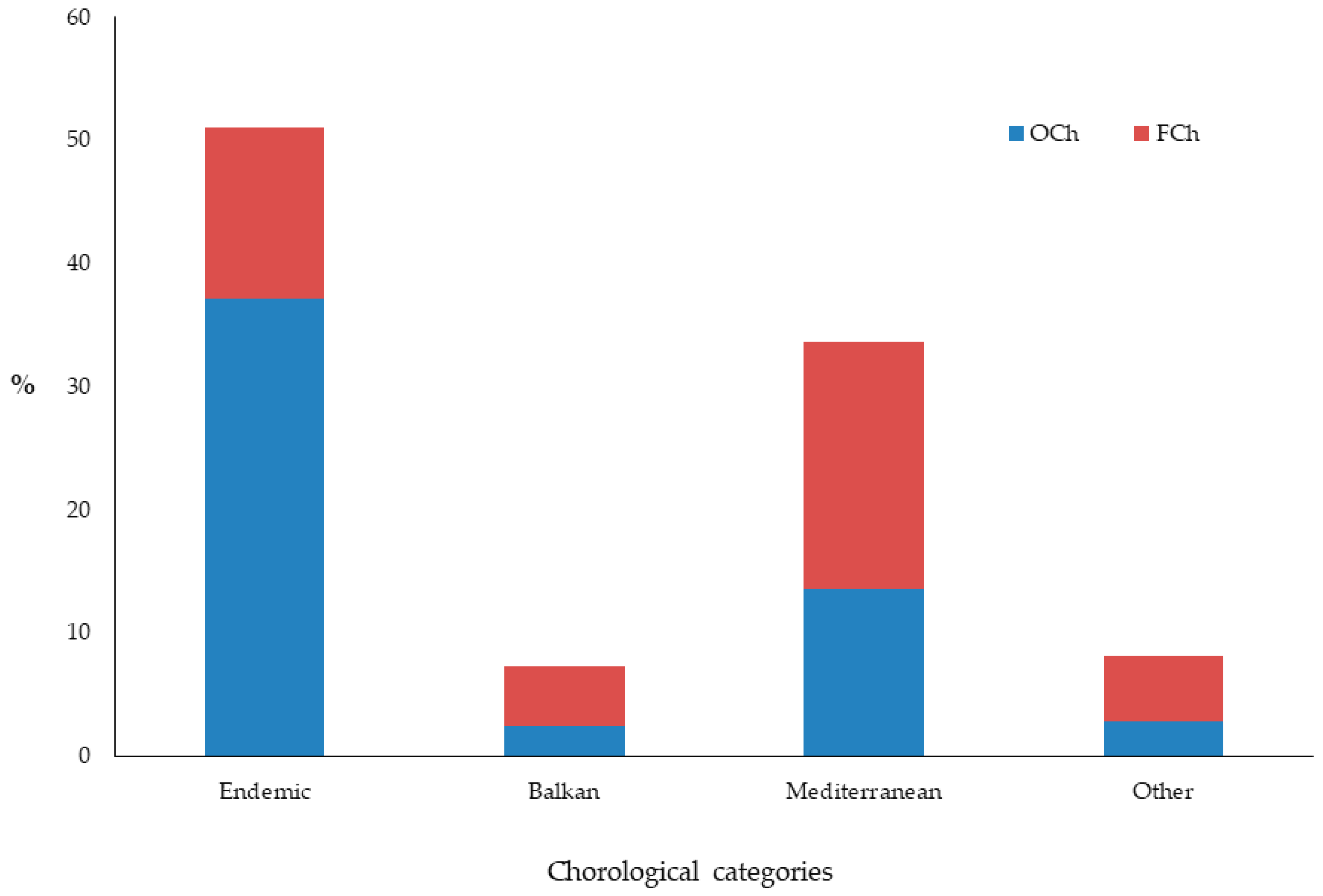
3.1. Chasmophytic Flora of the Aegean, Species Richness, Life Forms, and Chorology
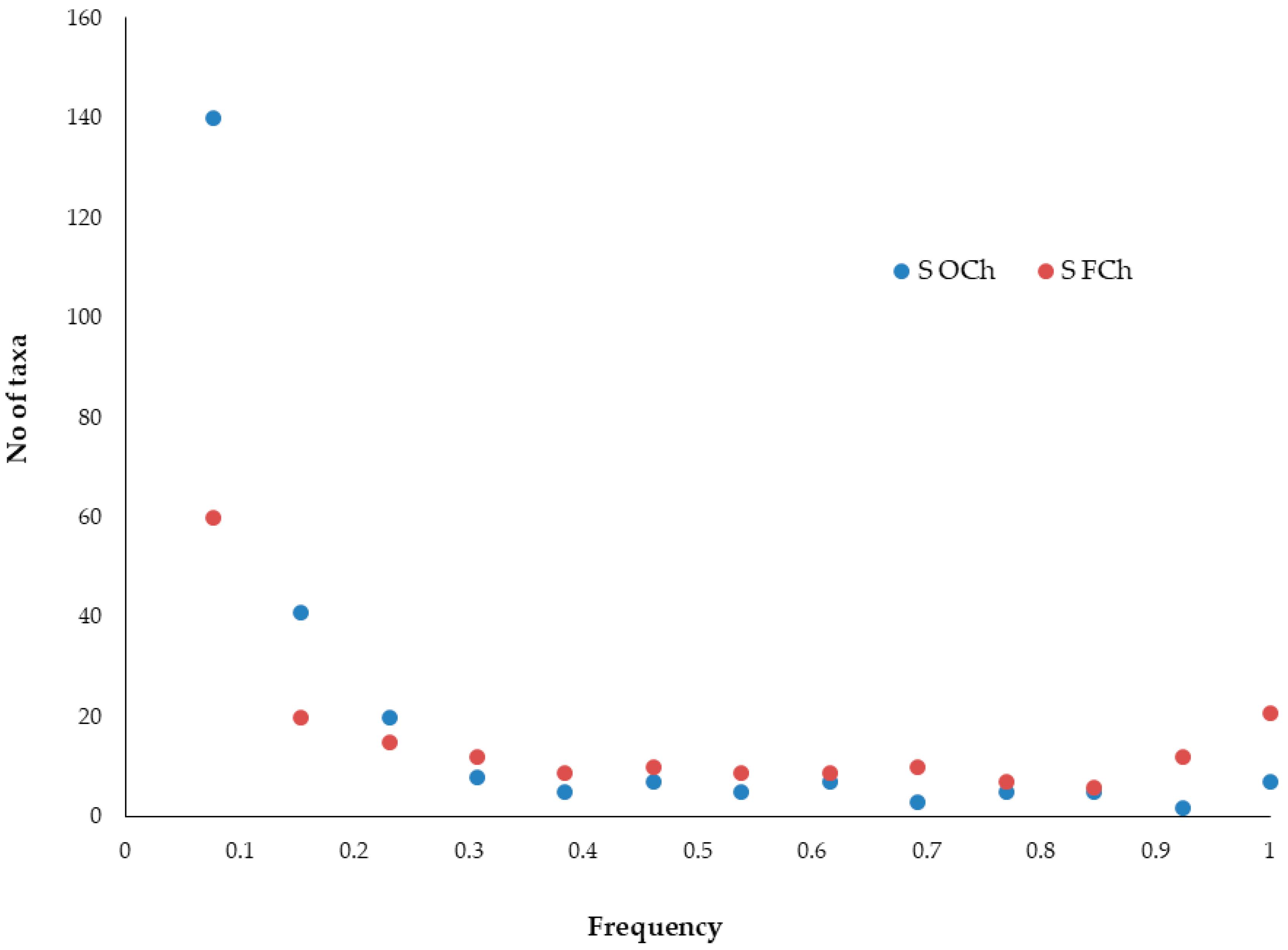
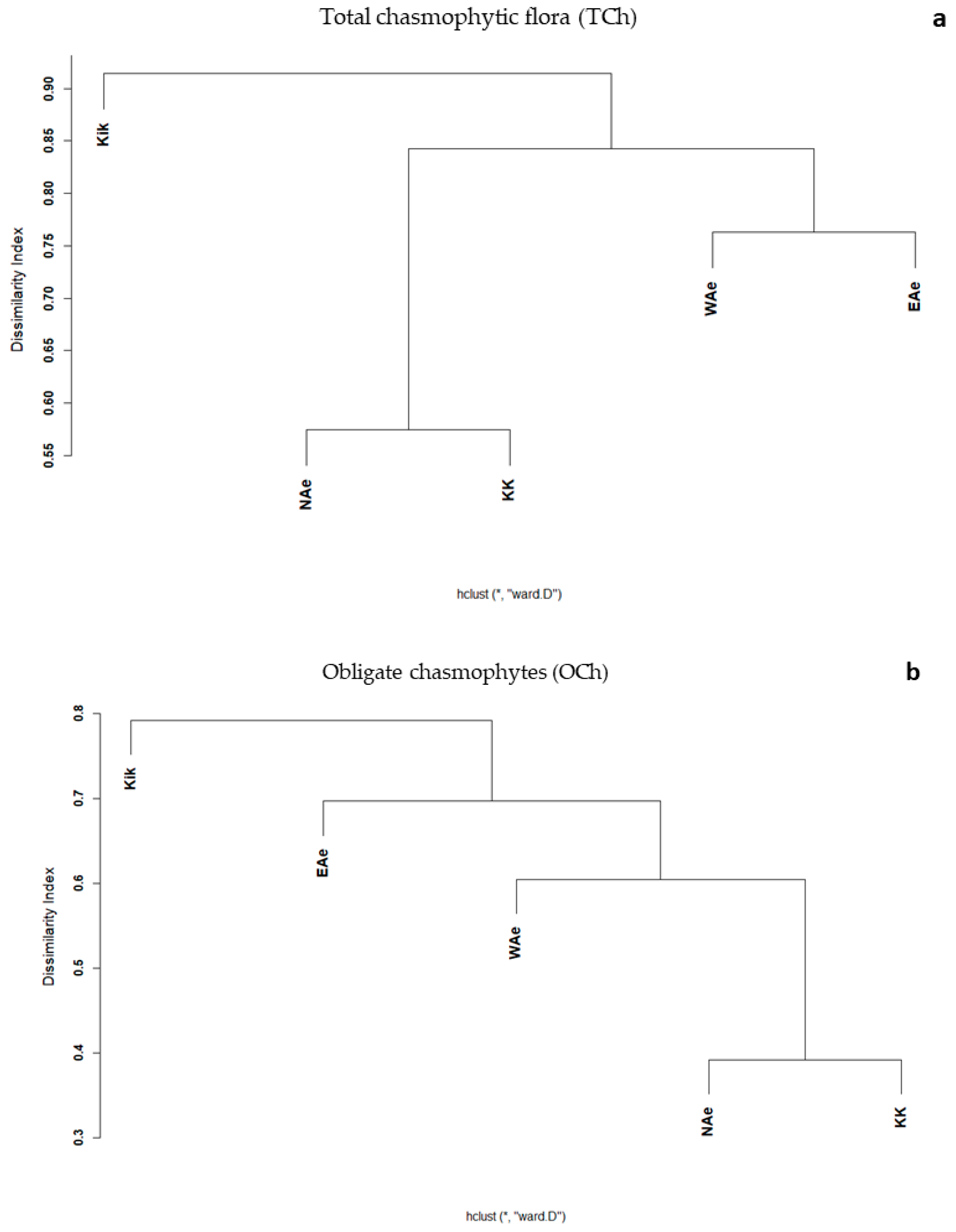
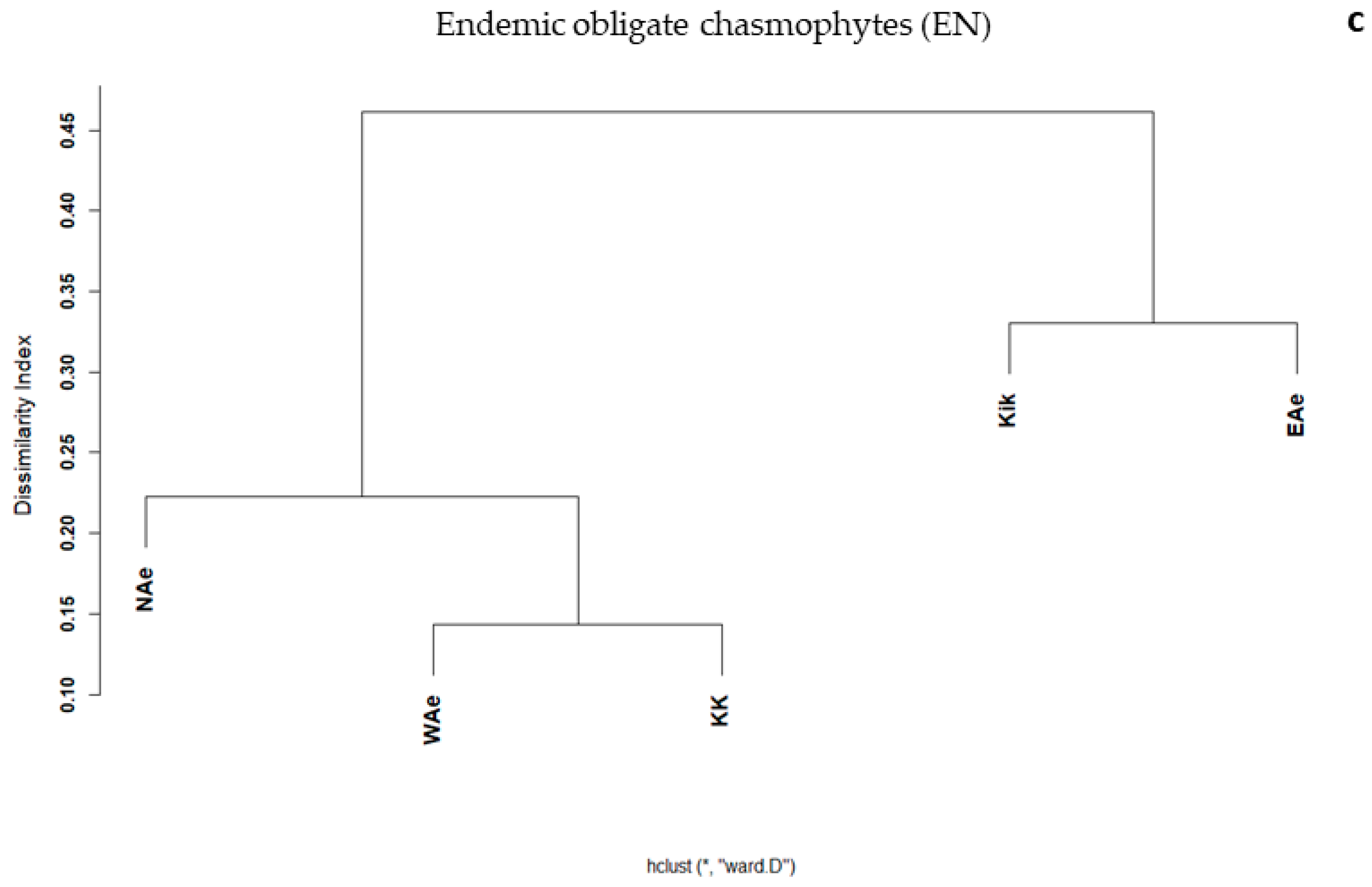
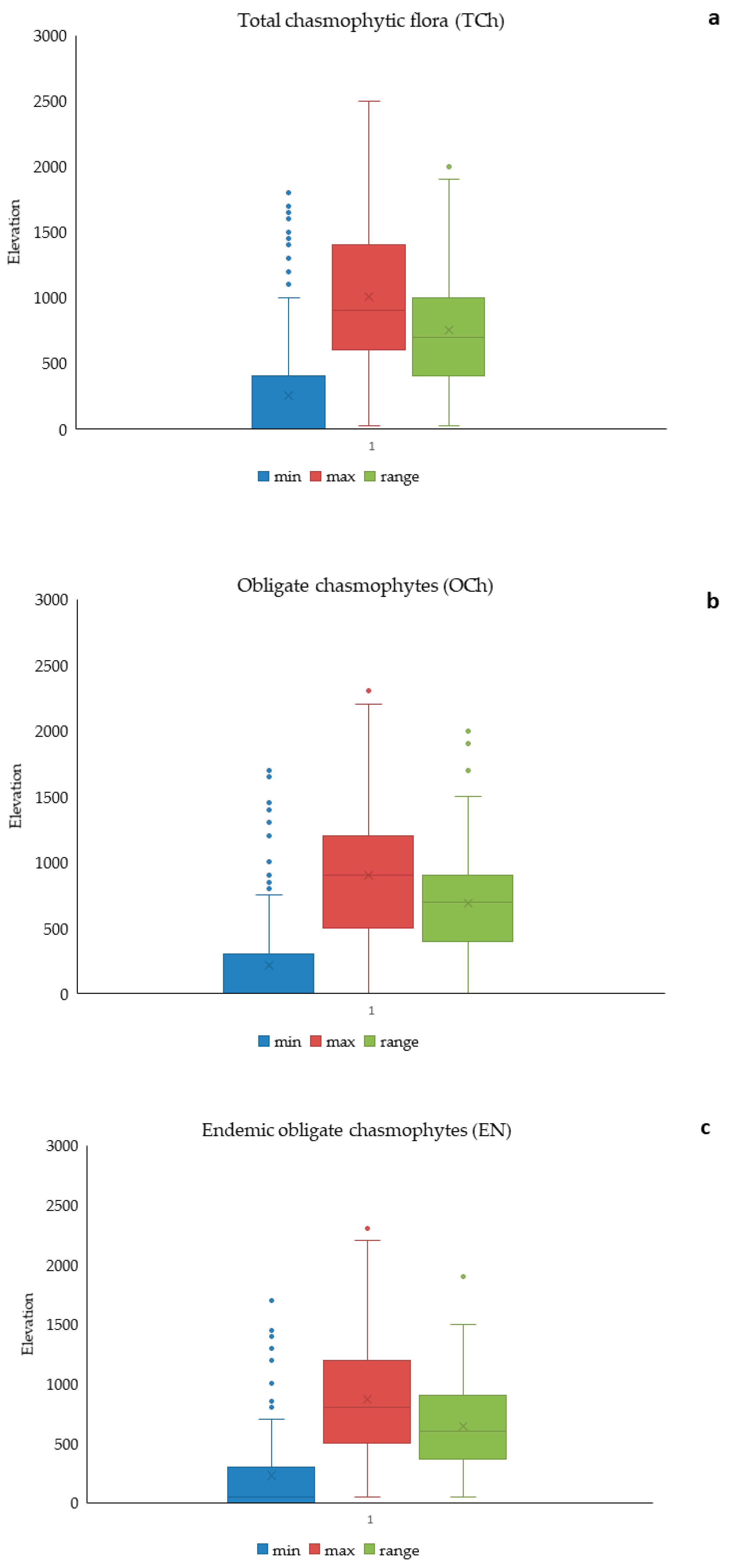
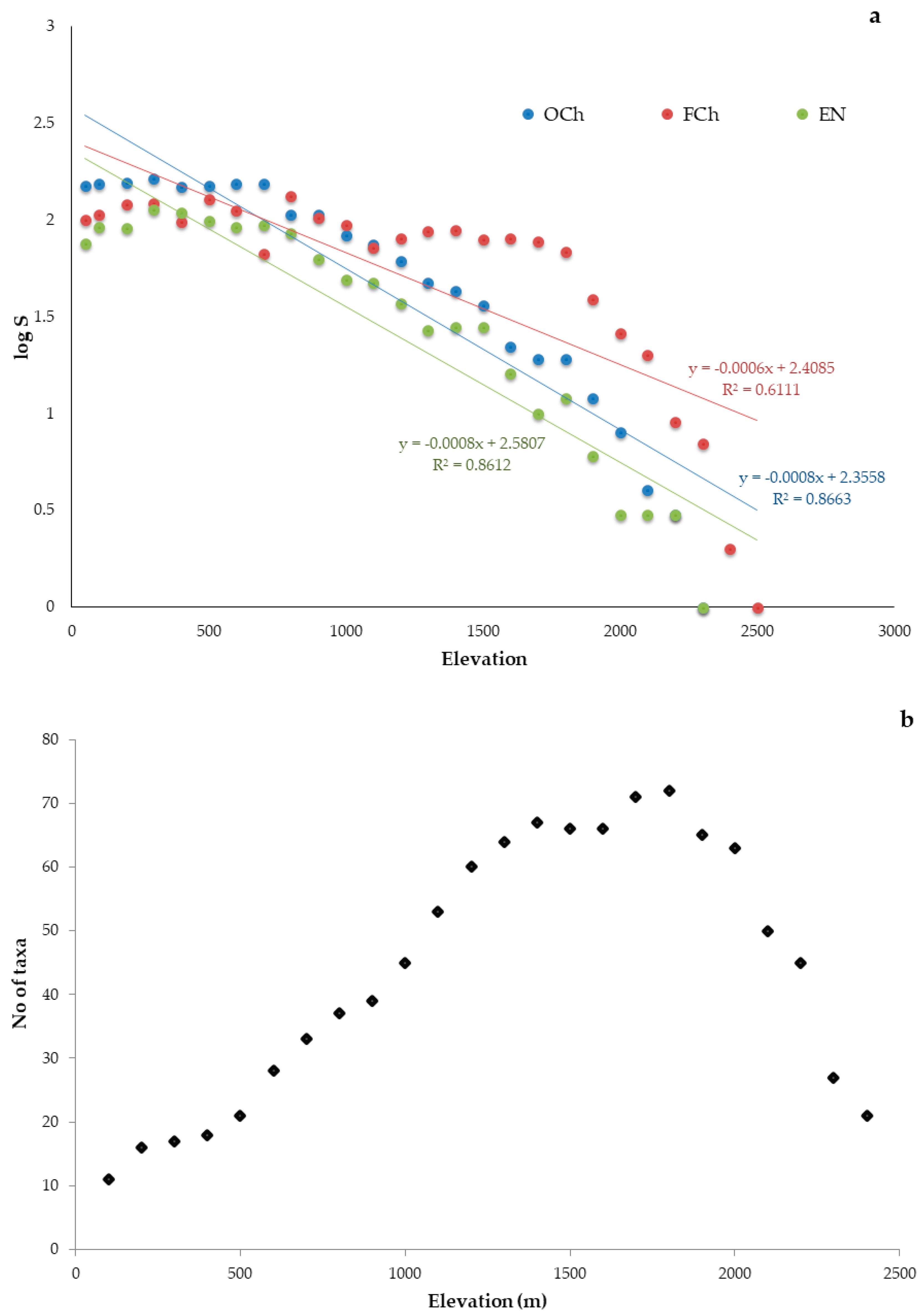
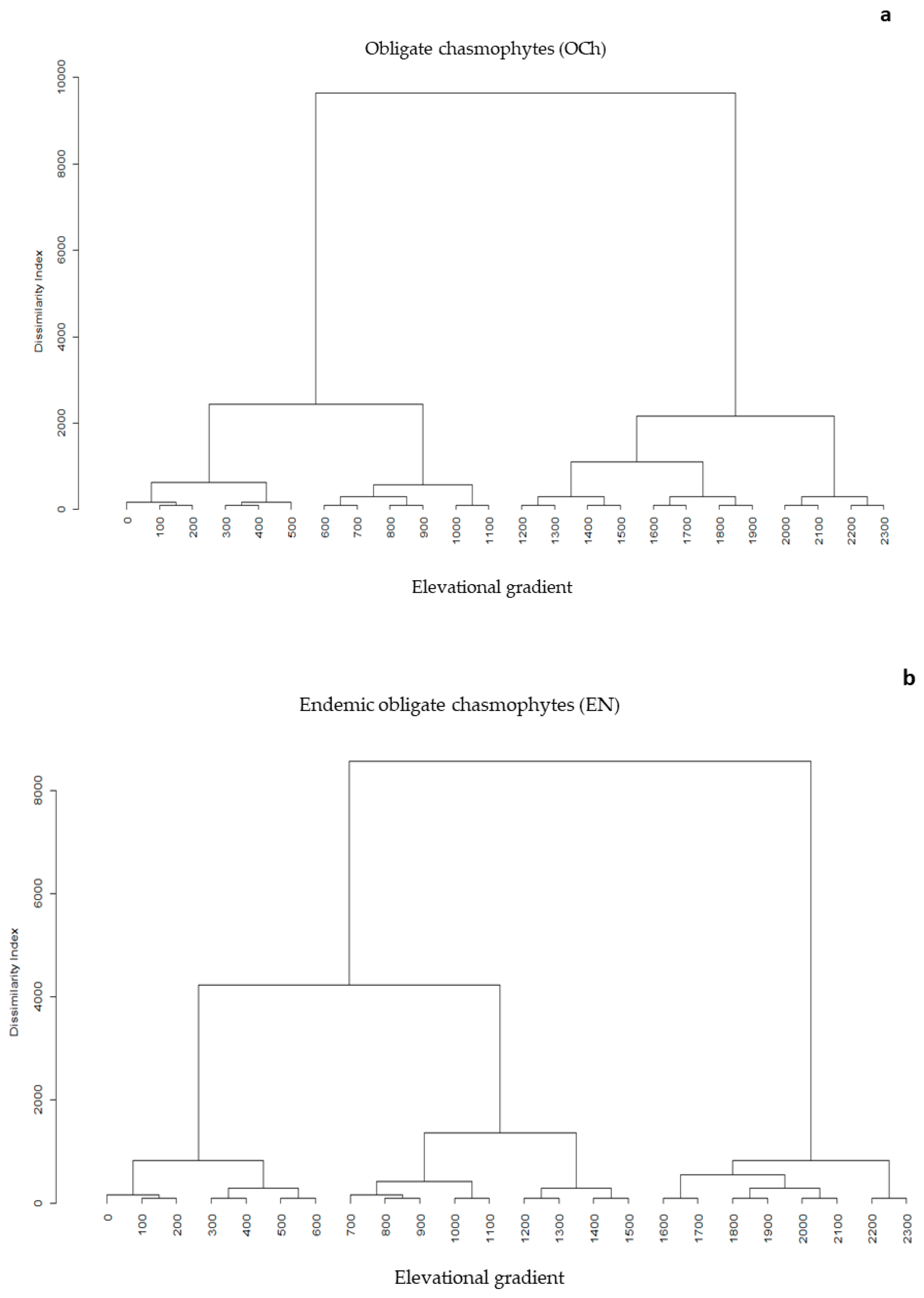
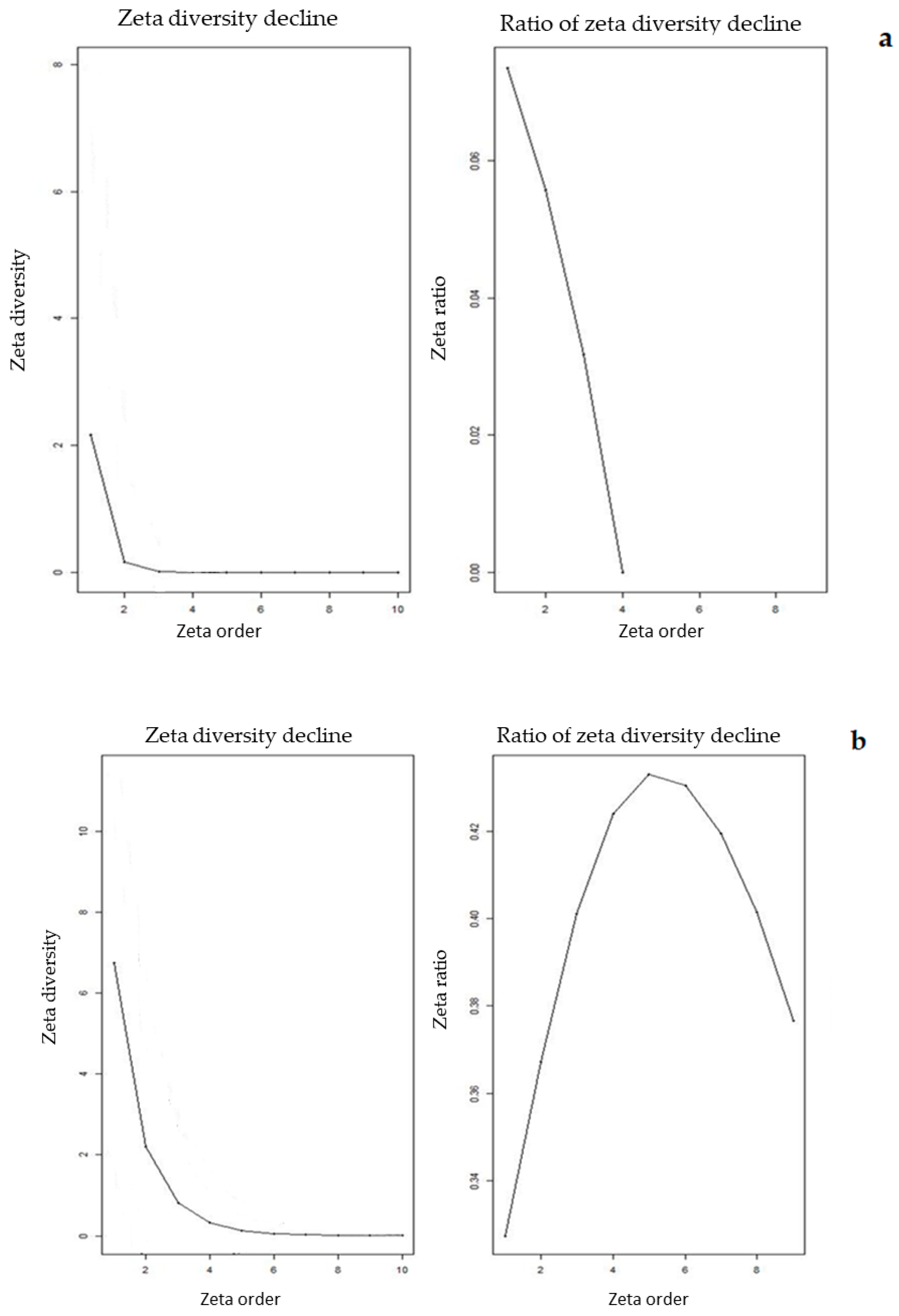
3.2. Elevational Gradients and Spatial Turnover of Chasmophytic Diversity
4. Discussion
4.1. Chasmophytic Flora of the Aegean, Species Richness, Life Forms, and Chorology
4.2. Elevational Gradients and Spatial Turnover of Chasmophytic Diversity: Monitoring Insights
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Médail, F.; Quézel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J.; Bodiou, J.Y.; Bœuf, G. The Mediterranean Region: Biological Diversity in Space and Time, 2nd ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Triantis, K.A.; Mylonas, M. Greek islands, biology. In Encyclopedia of Islands; Gillespie, R., Glague, D.A., Eds.; University of California Press: Berkeley, CA, USA, 2009; pp. 388–392. [Google Scholar]
- Strid, A. Phytogeographia Aegaea and the Flora Hellenica Database. Annalen Naturhistorischen Museums in Wien 1996, 98 (Suppl. B), 279–289. [Google Scholar]
- Sfenthourakis, S.; Triantis, K.A. The Aegean archipelago: A natural laboratory of evolution, ecology and civilisations. J. Biol. Res. Thessalon. 2017, 24, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Panitsa, M.; Kagiampaki, A.; Kougioumoutzis, K. Plant diversity and biogeography of the Aegean archipelago: A new synthesis. In Biogeography and Biodiversity of the Aegean; In Moysis, M., Pafilis, P., Parmakelis, A., Poulakakis, N., Sfenthourakis, S., Triantis, K., Eds.; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2018. [Google Scholar]
- Kougioumoutzis, K.; Tiniakou, A. Ecological factors and plant species diversity in the South Aegean Volcanic Arc and other central Aegean Islands. Plant Ecol. Divers. 2014, 8, 173–186. [Google Scholar] [CrossRef]
- Uotila, P. Fifty years of mapping the Balkan flora for Atlas Florae Europaeae. Bot. Serbica 2017, 41, 163–175. [Google Scholar]
- Kougioumoutzis, K.; Valli, A.T.; Georgopoulou, E.; Simaiakis, S.M.; Triantis, A.; Trigas, P. Network biogeography of a complex island system: The Aegean Archipelago revisited. J. Biogeogr. 2017, 44, 651–660. [Google Scholar] [CrossRef]
- Strid, A. Atlas of the Aegean Flora; Part 1: Text & Plates; Botanischer Garten und Botanisches Museum Berlin-Dahlem: Berlin, Germany, 2016; Volume 33, pp. 1–1578. [Google Scholar]
- Strid, A. Atlas of the Aegean Flora; Part 2: Maps; Botanischer Garten und Botanisches Museum Berlin Dahlem: Berlin, Germany, 2016. [Google Scholar]
- Larson, D.W.; Matthes, U.; Kelly, P. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Davis, P.H. Cliff vegetation in the eastern Mediterranean. J. Ecol. 1951, 39, 63–93. [Google Scholar] [CrossRef]
- Snogerup, S. Evolutionary and plant geographical aspects of chasmophytic communities. In Plant Life of South-West ASIA; Davis, P.H., Harper, P.C., Hedge, I.C., Eds.; Botanical Society of Edinburgh: Edinburgh, Scotland, 1971; pp. 157–170. [Google Scholar]
- Thompson, J.D. Plant Evolution in the Mediterranean; Oxford University Press Inc.: New York, NY, USA, 2005. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M.; Matthews, T.J.; Borregaard, M.K.; Triantis, K.A. Island biogeography: Taking the long view of nature’s laboratories. Science 2017, 357, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Snogerup, S. The Mediterranean Islands. In Plant conservation in the Mediterranean Area; Gomez Campo, C., Ed.; Springer: Dordrecht, The Netherlands, 1985; Volume 7, pp. 159–173. [Google Scholar]
- Strid, A.; Tan, K. (Eds.) Flora Hellenica; University of Copenhagen, Koeltz Scientific Books: Koenigstein, Germany, 1997; 547p. [Google Scholar]
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Lavergne, S.; Thompson, J.D.; Garnier, E.; Debussche, M. The biology and ecology of narrow endemic and widespread plants: A comparative study of trait variation in 20 congeneric pairs. Oikos 2004, 107, 505–518. [Google Scholar] [CrossRef]
- Imbert, E.; Youssef, S.; Carbonell, D.; Baumel, A. Do endemic species always have a low competitive ability? A test for two Mediterranean plant species under controlled conditions. J. Plant Ecol. 2012, 5, 305–312. [Google Scholar] [CrossRef]
- Gankin, R.; Major, J. Arctostaphylos myrtifolia, its biology and relationship to the problem of endemism. Ecology 1964, 45, 792–808. [Google Scholar] [CrossRef]
- Panitsa, M.; Kontopanou, A. Diversity of chasmophytes in the vascular flora of Greece: Floristic analysis and phytogeographical patterns. Bot. Serbica 2017, 41, 199–211. [Google Scholar]
- Kypriotakis, Z.; Tzanoudakis, D. Contribution to the study of the Greek insular flora: The chasmophytic flora of Crete. Bocconea 2001, 13, 495–503. [Google Scholar]
- Cattaneo, C.; Grano, M. Contribution to the knowledge of vascular flora on Astypalea Island (Dodecanese, Greece). Phytol. Balc. 2016, 22, 405–417. [Google Scholar]
- Médail, F.; Verlaque, R. Ecological characteristics and rarity of endemic plants from southeast France and Corsica: Implications for biodiversity conservation. Biol. Conserv. 1997, 80, 269–271. [Google Scholar] [CrossRef]
- Runemark, H. The phytogeography of the Central Aegean. Opera Bot. 1971, 30, 20–28. [Google Scholar]
- Escudero, A. Community patterns on exposed cliffs in a Mediterranean calcareous mountain. Vegetatio 1996, 125, 99–110. [Google Scholar] [CrossRef]
- Aronne, G.; Arena, C.; De Micco, V.; Giovanetti, M.; Buonanno, M. Full light and soil drought constrain plant growth in Mediterranean cliffs: The case of Primula palinuri Petagna. Plant Biosyst. 2018, 152, 863–872. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef]
- Horvat, I.; Glavač, V.; Ellenberg, H. Vegetation Südosteuropas; G. Fischer: Stuttgart, Germany, 1974; 768p. [Google Scholar]
- Zaffran, J. Contributions à la Flore et à la Végétation de la Crète; de l’Université de Provence: Marseille, Germany, 1990; p. 615. [Google Scholar]
- Dimopoulos, P.; Sykora, K.V.; Mucina, L.; Georgiadis, T. The high-rank syntaxa of the rock-cliff and scree vegetation of the mainland Greece and Crete. Folia Geobot Phytotax 1997, 32, 313–334. [Google Scholar] [CrossRef]
- Bergmeier, E. The vegetation of the high mountains of Crete—A revision and multivariate analysis. Phytocoenologia 2002, 32, 205–249. [Google Scholar] [CrossRef]
- Bergmeier, E.; Dimopoulos, P.; Mucina, L. Validation of some alliances of the Aegean chasmophytic vegetation of the Asplenietea trichomanis. Lazaroa 2011, 32, 183–186. [Google Scholar]
- Trigas, P.; Panitsa, M.; Tsiftsis, S. Elevational Gradient of Vascular Plant Species Richness and Endemism in Crete—Effect of Post-Isolation Mountain Uplift on a Continental Island System. PLoS ONE 2013, 8, e59425. [Google Scholar] [CrossRef] [PubMed]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 1–10. [Google Scholar]
- Steinbauer, M.J.; Field, R.; Grytnes, J.A.; Trigas, P.; Ah-Peng, C.; Attorre, F.; Birks, H.J.B.; Borges, P.A.; Cardoso, P.; Chou, C.H.; et al. Topography-driven isolation, speciation and a global increase of endemism with elevation. Glob. Ecol. Biogeogr. 2016, 25, 1097–1107. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Irl, S.D.; Beierkuhnlein, C. Elevation-driven ecological isolation promotes diversification on Mediterranean islands. Acta Oecol. 2013, 47, 52–56. [Google Scholar] [CrossRef]
- Nikolić, T.; Antonić, O.; Alegro, A.L.; Dobrović, I.; Bogdanović, S.; Liber, Z.; Rešetnik, I. Plant species diversity of Adriatic islands: An introductory survey. Plant Biosyst. 2008, 142, 435–445. [Google Scholar] [CrossRef]
- Fois, M.; Fenu, G.; Cañadas, E.M.; Bacchetta, G. Disentangling the influence of environmental and anthropogenic factors on the distribution of endemic vascular plants in Sardinia. PLoS ONE 2017, 12, e0182539. [Google Scholar] [CrossRef]
- Valli, A.T.; Kougioumoutzis, K.; Iliadou, E.; Panitsa, M.; Trigas, P. Determinants of alpha and beta vascular plant diversity in Mediterranean island systems: The Ionian islands, Greece. Nord. J. Bot. 2019, 37, e02156. [Google Scholar] [CrossRef]
- Cellinese, N.; Smith, S.A.; Edwards, E.J.; Kim, S.T.; Haberle, R.C.; Avramakis, M.; Donoghue, M.J. Historical biogeography of the endemic Campanulaceae of Crete. J. Biogeogr. 2009, 36, 1253–1269. [Google Scholar] [CrossRef]
- Bilz, M.; Kell, S.P.; Maxted, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Phitos, D.; Strid, A.; Snogerup, S.; Greuter, W. (Eds.) The Red Data Book of Rare and Threatened Plants of Greece; Worldwide Fund for Nature: Athens, Greece, 1995. [Google Scholar]
- Phitos, D.; Konstantinidis, T.; Kamari, G. (Eds.) The Red Data Book of Rare and Threatened Plants of Greece (A–D); Hellenic Botanical Society: Patras, Greece, 2009. [Google Scholar]
- Phitos, D.; Konstantinidis, T.; Kamari, G. (Eds.) The Red Data Book of Rare and Threatened Plants of Greece (E–Z); Hellenic Botanical Society: Patras, Greece, 2009. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece. An Annotated Checklist; Englera, 31; Botanischer Garten und Botanisches Museum Berlin-Dahlem: Berlin, Germany, 2013. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Community Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Latombe, G.; Hui, C.; McGeoch, M.A. Multi-site generalised dissimilarity modelling: Using zeta diversity to differentiate drivers of turnover in rare and widespread species. Methods Ecol. Evol. 2017, 8, 431–442. [Google Scholar] [CrossRef]
- Hui, C.; McGeoch, M.A. Zeta diversity as a concept and metric that unifies incidence-based biodiversity patterns. Am. Nat. 2014, 184, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Latombe, G.; McGeoch, M.A.; Nipperess, D.; Hui, C. Zetadiv: Functions to Compute Compositional Turnover Using Zeta Diversity. R Package Version 1.0. 2017. Available online: https://cran.r-project.org/package=zetadiv (accessed on 17 May 2017).
- Directive, H. Council Directive 92/43/ EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 7–50. [Google Scholar]
- IUCN The IUCN Red List of Threatened Species. Version 2019-3. 2019. Available online: http://www.iucnredlist.org (accessed on 10 December 2019).
- Pasta, S.; Perez-Graber, A.; Fazan, L.; de Montmollin, B. (Eds.) The Top 50 Mediterranean Island Plants Update 2017; E-Book and Online; IUCN/SSC/Mediterranean Plant Specialist Group: Neuchâtel, Switzerland, 2017; p. 144. Available online: http://top50.iucn-mpsg.org (accessed on 31 December 2017).
- Steinbauer, M.J.; Otto, R.; Naranjo-Cigala, A.; Beierkuhnlein, C.; Fernández-Palacios, J.M. Increase of island endemism with altitude—Speciation processes on oceanic islands. Ecography 2012, 35, 23–32. [Google Scholar] [CrossRef]
- Vogiatzakis, I. Mediterranean Mountain Environments; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Zhao, Y.J.; Gong, X. Diversity and conservation of plant species in dry valleys, southwest 498 China. Biodivers. Conserv. 2015, 24, 2611–2623. [Google Scholar] [CrossRef]
- Cutts, V.; Katal, N.; Löwer, C.; Algar, A.C.; Steinbauer, M.J.; Irl, S.D.; Beierkuhnlein, C.; Field, R. The effect of small-scale topography on patterns of endemism within islands. Front. Biogeogr. 2019, 11, e43737. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece. An Annotated Checklist. Willdenowia (Supplement) 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Jeanmonod, D.; Naciri, Y.; Schlüssel, A.; Gamisans, J. Floristic analyses of the Corsican flora: Biogeographical origin and endemism. Candollea 2015, 70, 21–42. [Google Scholar] [CrossRef]
- Bacchetta, G.; Casti, M.; Mossa, L. New ecological and distributive data on the rupestrian flora of Sardinia. J. Bot. Soc. Bot. Fr. 2007, 38, 73–83. [Google Scholar]
- Beard, J.S.; Chapman, A.R.; Gioia, P. Species richness and endemism in the western Australian flora. J. Biogeogr. 2000, 27, 1257–1268. [Google Scholar] [CrossRef]
- Wagenitz, G. Centaurea in South-West Asia: Patterns of distribution and diversity. Proc. R. Soc. Edinb. 1986, 89, 11–21. [Google Scholar] [CrossRef]
- Hellwig, F.H. Centaureinae (Asteraceae) in the Mediterranean history of ecogeographical radiation. Plant Syst. Evol. 2004, 246, 137–162. [Google Scholar] [CrossRef]
- Trigas, P.; Iatrou, G.; Karetsos, G. Species diversity, endemism and conservation of the family Caryophyllaceae in Greece. Biodivers. Conserv. 2007, 16, 357–376. [Google Scholar] [CrossRef]
- Heywood, V.H.; Brummitt, R.K.; Culham, A.; Seberg, O. Flowering Plant Families of the World; Kew Royal Botanic Gardens: Kew, UK, 2007. [Google Scholar]
- Eddie, W.M.M.; Shulkina, T.; Gaskin, J.; Haberle, R.C.; Jansen, R.K. Phylogeny of Campanulaceae S. Str. Inferred from Its Sequences of Nuclear Ribosomal DNA. Ann. Mo. Bot. Gard. 2003, 90, 554–575. [Google Scholar] [CrossRef]
- Georghiou, K.; Delipetrou, P. Patterns and traits of the endemic plants of Greece. Bot. J. Linn. Soc. 2010, 162, 130–422. [Google Scholar] [CrossRef]
- Carlsson, B.A.; Karlsson, P.S.; Svensson, B.M. Alpine and subalpine vegetation. Acta Phytogeogr. Suec. 1999, 84, 75–90. [Google Scholar]
- Körner, C. Alpine plant life. In Functional Plant Ecology of High Mountain Ecosystems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Lazarina, M.; Charalampopoulos, A.; Psaralexi, M.; Krigas, N.; Michailidou, D.E.; Kallimanis, A.S.; Sgardelis, S.P. Diversity Patterns of Different Life Forms of Plants along an Elevational Gradient in Crete, Greece. Diversity 2019, 11, 200. [Google Scholar] [CrossRef]
- Vogiatzakis, I.; Griffiths, G.H.; Mannion, A.M. Environmental factors and vegetation composition, Lefka Ori massif, Crete, S. Aegean. Glob. Ecol. Biogeogr. 2003, 12, 131–146. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Simaiakis, S.M.; Tiniakou, A. Network biogeographical analysis of the central Aegean archipelago. J. Biogeogr. 2014, 41, 1848–1858. [Google Scholar] [CrossRef]
- Kallimanis, A.S.; Panitsa, M.; Bergmeier, E.; Dimopoulos, P. Examining the relationship between total species richness and single island palaeo- and neo-endemics. Acta Oecol. 2011, 37, 65–70. [Google Scholar] [CrossRef]
- Thomas, G.H.; Orme, C.D.L.; Davies, R.G.; Olson, V.A.; Bennett, P.M.; Gaston, K.J.; Owens, I.P.F.; Blackburn, T.M. Regional variation in the historical components of global avian 486 species richness. Glob. Ecol. Biogeogr. 2008, 17, 340–351. [Google Scholar] [CrossRef]
- Verboom, G.A.; Bergh, N.G.; Haiden, S.A.; Hoffmann, V.; Britton, M.N. Topography as a 488 driver of diversification in the Cape Floristic Region of South Africa. New Phytol. 2015, 207, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Ree, R.H. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl. Acad. Sci. USA 2017, 114, 3444–3496. [Google Scholar] [CrossRef]
- Gentili, R.; Bacchetta, G.; Fenu, G.; Cogoni, D.; Abeli, T.; Rossi, G.; Salvatore, M.C.; Baroni, C.; Citterio, S. From cold to warm stage refugia for boreo-alpine plants in southern European and Mediterranean mountains: The last chance to survive or an opportunity for speciation? Biodiversity 2015, 16, 247–261. [Google Scholar] [CrossRef]
- De Castro Godinho, M.B.; Da Silva, F.R. The influence of riverine barriers, climate, and 423 topography on the biogeographic regionalization of Amazonian anurans. Sci. Rep. 2018, 8, 3427. [Google Scholar] [CrossRef]
- Harrison, S.; Noss, R. Endemism hotspots are linked to stable climatic refugia. Ann. Bot. 2017, 119, 207–214. [Google Scholar] [CrossRef]
- Rechinger, K.H. Der Endemismus in der griechischen Flora. Rev. Roumaine Biol. Ser. Bot. 1965, 10, 135–138. [Google Scholar]
- Panitsa, M.; Trigas, P.; Iatrou, G.; Sfenthourakis, S. Factors affecting plant species richness and endemism on land-bridge islands—An example from the East Aegean archipelago. Acta Oecol. 2010, 36, 431–437. [Google Scholar] [CrossRef]
- Lazarina, M.; Kallimanis, S.; Dimopoulos, P.; Psaralexi, M.; Michailidou, D.E.; Sgardelis, S. Patterns and drivers of species richness and turnover of neo-endemic and palaeo-endemic vascular plants in a Mediterranean hotspot: The case of Crete, Greece. J. Biol. Res. Thessalon. 2019, 26, 12–25. [Google Scholar] [CrossRef]
- Tomaselli, M.; Foggi, B.; Carbognani, M.; Gennai, M.; Petraglia, A. The rock-face vegetation in the northern Apennines and neighbouring mountain areas, from the coastline to the highest summits. Phytocoenologia 2019, 49, 7–70. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Grytnes, J.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel competitors shape species’ responses to climate change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Cardoso, P.; Kreft, H.; Whittaker, R.J.; Fattorini, S.; Emerson, B.C.; Gil, A.; Gillespie, R.G.; Matthews, T.J.; Santos, A.M.; et al. Global Island Monitoring Scheme (GIMS): A proposal for the long-term coordinated survey and monitoring of native island forest biota. Biodivers. Conserv. 2018, 27, 2567. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. GLORIA: A global observation research initiative in alpine environments. Mt. Res. Dev. 2000, 20, 190–191. [Google Scholar] [CrossRef]
- Alexander, J.M.; Kueffer, C.; Daehler, C.C.; Edwards, P.J.; Pauchard, A.; Seipel, T. MIREM consortium, Assembly of non-native floras along elevational gradients explained by directional ecological filtering. Proc. Natl. Acad. Sci. USA 2011, 108, 656–661. [Google Scholar] [CrossRef]
- Fernandez-Calzado, R.; Ghosn, D.; Gottfried, M.; Kazakis, G.; Molero Mesa, J.; Pauli, H.; Merzouki, A. Patterns of endemism along an elevation gradient in Sierra Nevada (Spain) and Lefka Ori (Crete, Greece). Pirineos. Revista de Ecología de Montaña 2003, 168, 7–24. [Google Scholar] [CrossRef]
- Porro, F.; Tomaselli, M.; Abeli, T.; Gandini, M.; Gualmini, M.; Orsenigo, S.; Petraglia, A.; Rossi, G.; Carbognani, M. Could plant diversity metrics explain climate-driven vegetation changes on mountain summits of the GLORIA network? Biodivers. Conserv. 2019, 28, 3575–3596. [Google Scholar] [CrossRef]
- European Commission. Doc. Hab.12-04/06; European Commission: Brussels, Belgium, 2012; Available online: https://ec.europa.eu/environment/nature/natura2000/management/docs/commission_note/commission_note2_EN.pdf (accessed on 17 January 2019).
- Crain, B.; Cuervo, S.; Ana, M.; White, J.; Steinberg, S. Conservation ecology of rare plants within complex local habitat networks. Oryx 2015, 49, 696–703. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontopanou, A.; Panitsa, M. Habitat Islands on the Aegean Islands (Greece): Elevational Gradient of Chasmophytic Diversity, Endemism, Phytogeographical Patterns and need for Monitoring and Conservation. Diversity 2020, 12, 33. https://doi.org/10.3390/d12010033
Kontopanou A, Panitsa M. Habitat Islands on the Aegean Islands (Greece): Elevational Gradient of Chasmophytic Diversity, Endemism, Phytogeographical Patterns and need for Monitoring and Conservation. Diversity. 2020; 12(1):33. https://doi.org/10.3390/d12010033
Chicago/Turabian StyleKontopanou, Anna, and Maria Panitsa. 2020. "Habitat Islands on the Aegean Islands (Greece): Elevational Gradient of Chasmophytic Diversity, Endemism, Phytogeographical Patterns and need for Monitoring and Conservation" Diversity 12, no. 1: 33. https://doi.org/10.3390/d12010033
APA StyleKontopanou, A., & Panitsa, M. (2020). Habitat Islands on the Aegean Islands (Greece): Elevational Gradient of Chasmophytic Diversity, Endemism, Phytogeographical Patterns and need for Monitoring and Conservation. Diversity, 12(1), 33. https://doi.org/10.3390/d12010033




