Algal Epibionts as Co-Engineers in Mussel Beds: Effects on Abiotic Conditions and Mobile Interstitial Invertebrates
Abstract
1. Introduction
- the effects of Porphyra cover on desiccation and temperature above and underneath the mussel bed—we compared experimentally created Porphyra patches with exposed mussel bed areas late in the Porphyra growing season (February–March), when temperatures are high but algal cover declines (<25%);
- the effects Porphyra cover on interstitial mussel bed invertebrates—we conducted short-term, Porphyra-removal experiments during December–January, when Porphyra cover peaks and desiccation and heat are likely maximum (see above); and
- the occurrence of Porphyra-related patchiness in interstitial invertebrate assemblages—we sampled Porphyra-covered and exposed mussel bed areas that naturally occur late in the Porphyra growing season, when algal cover is low and patchy (see (a)).
2. Materials and Methods
2.1. Study Sites and Organisms
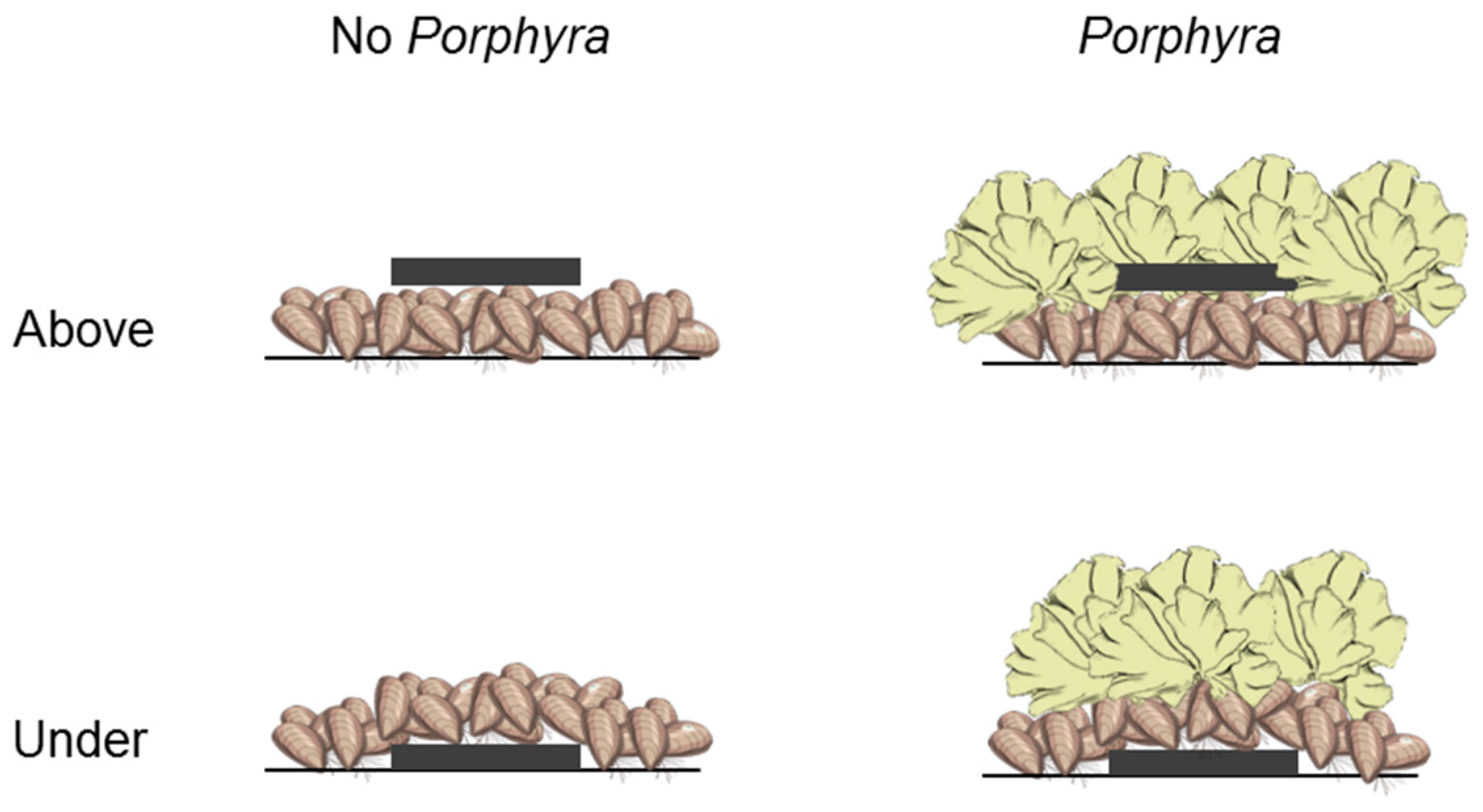
2.2. Effects of Porphyra Cover on Desiccation and Temperature
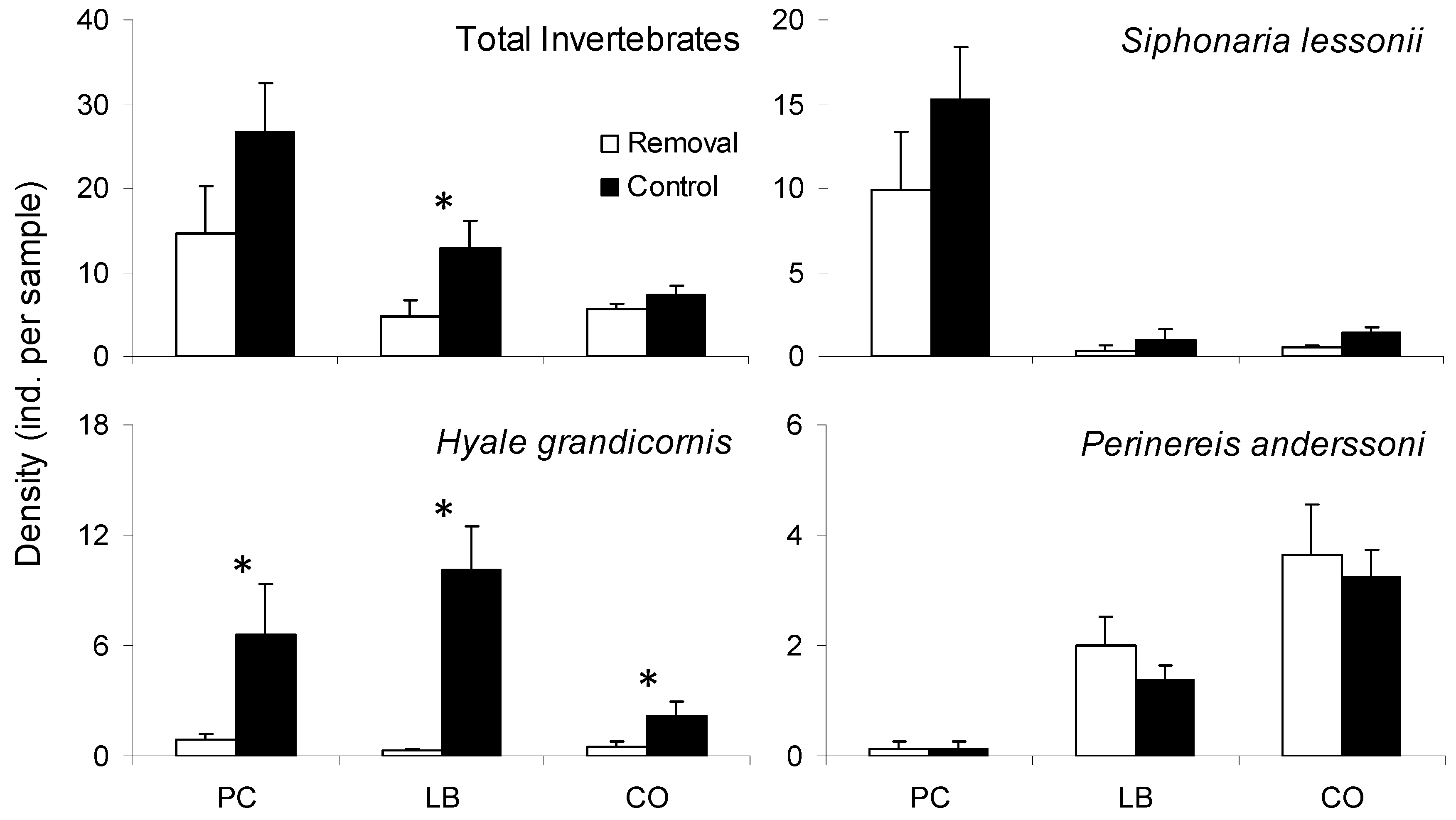
2.3. Effects of Porphyra Cover on Interstitial Mussel Bed Invertebrates
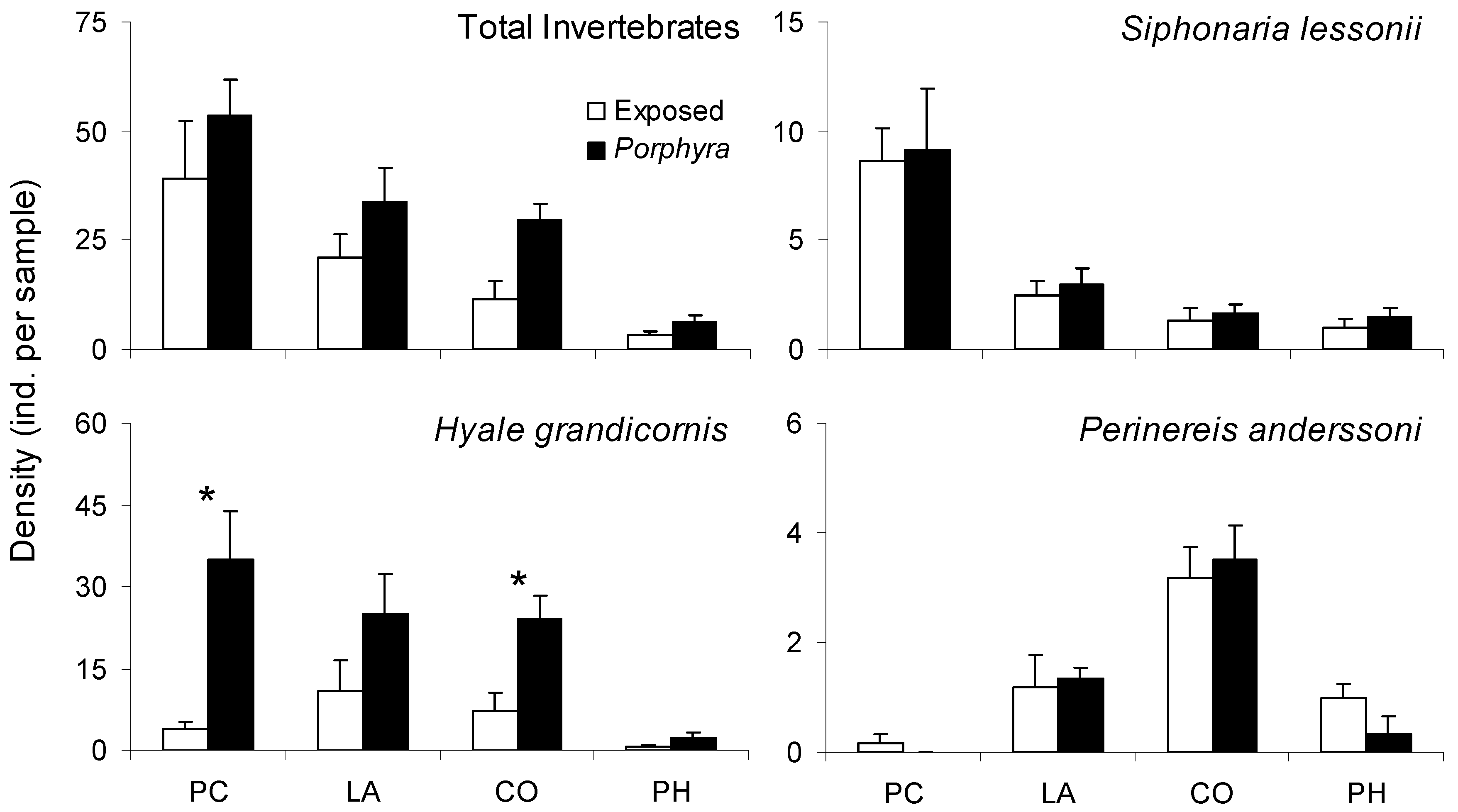
2.4. Porphyra-Related Patchiness in Interstitial Invertebrate Distribution
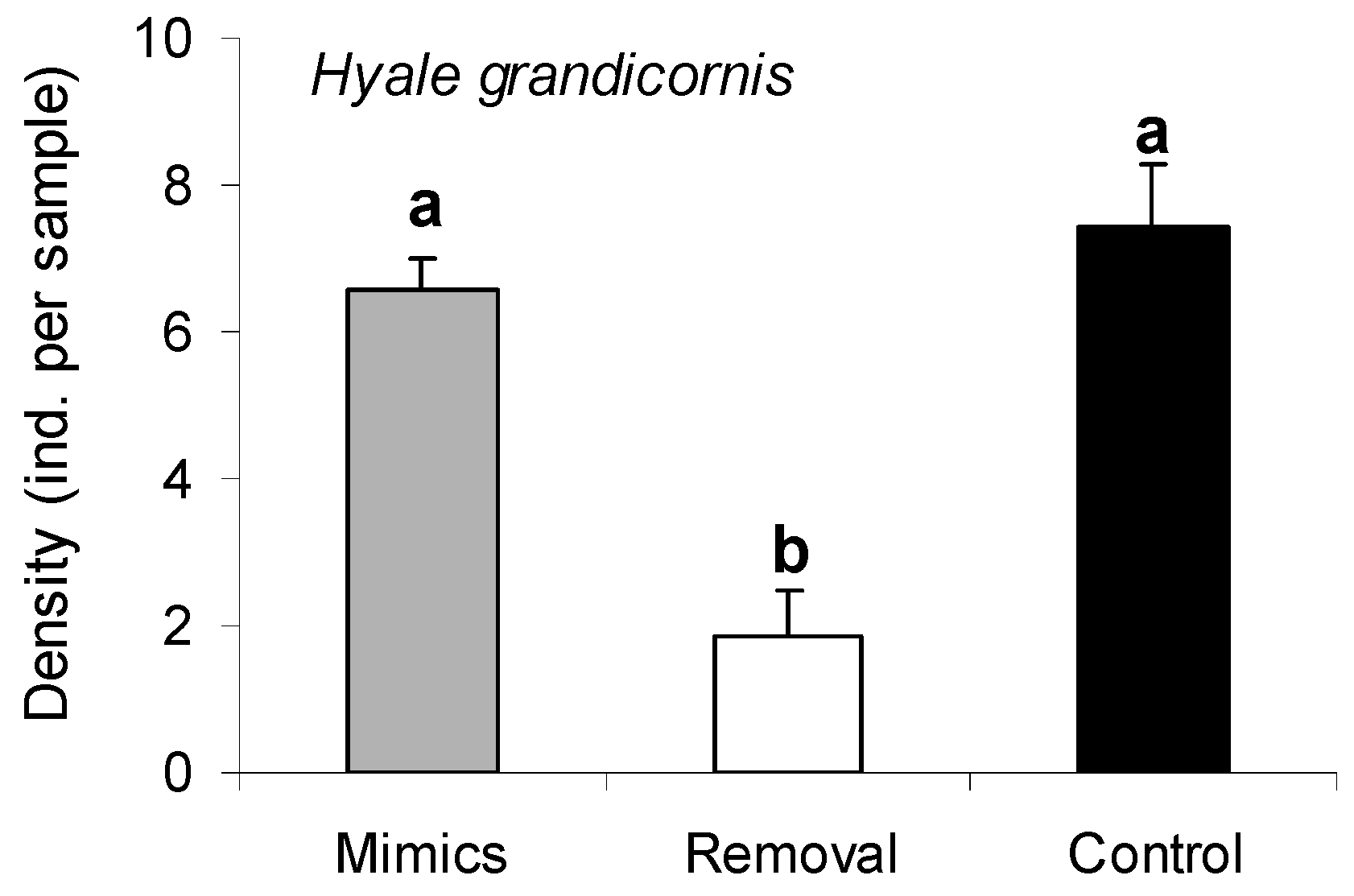
2.5. Responses of a Dominant Grazer to Porphyra Thalli and Structural Porphyra Mimics
3. Results
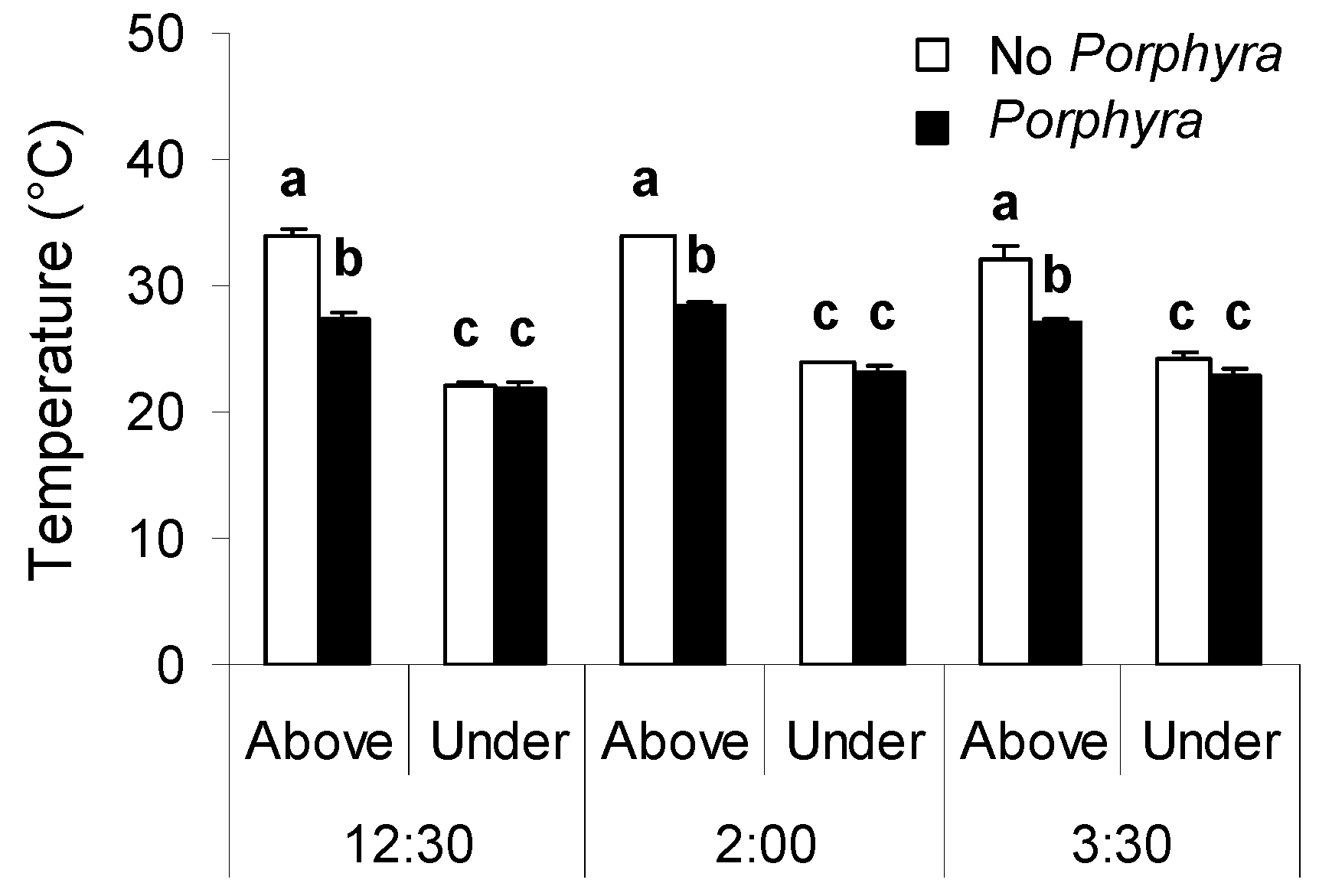
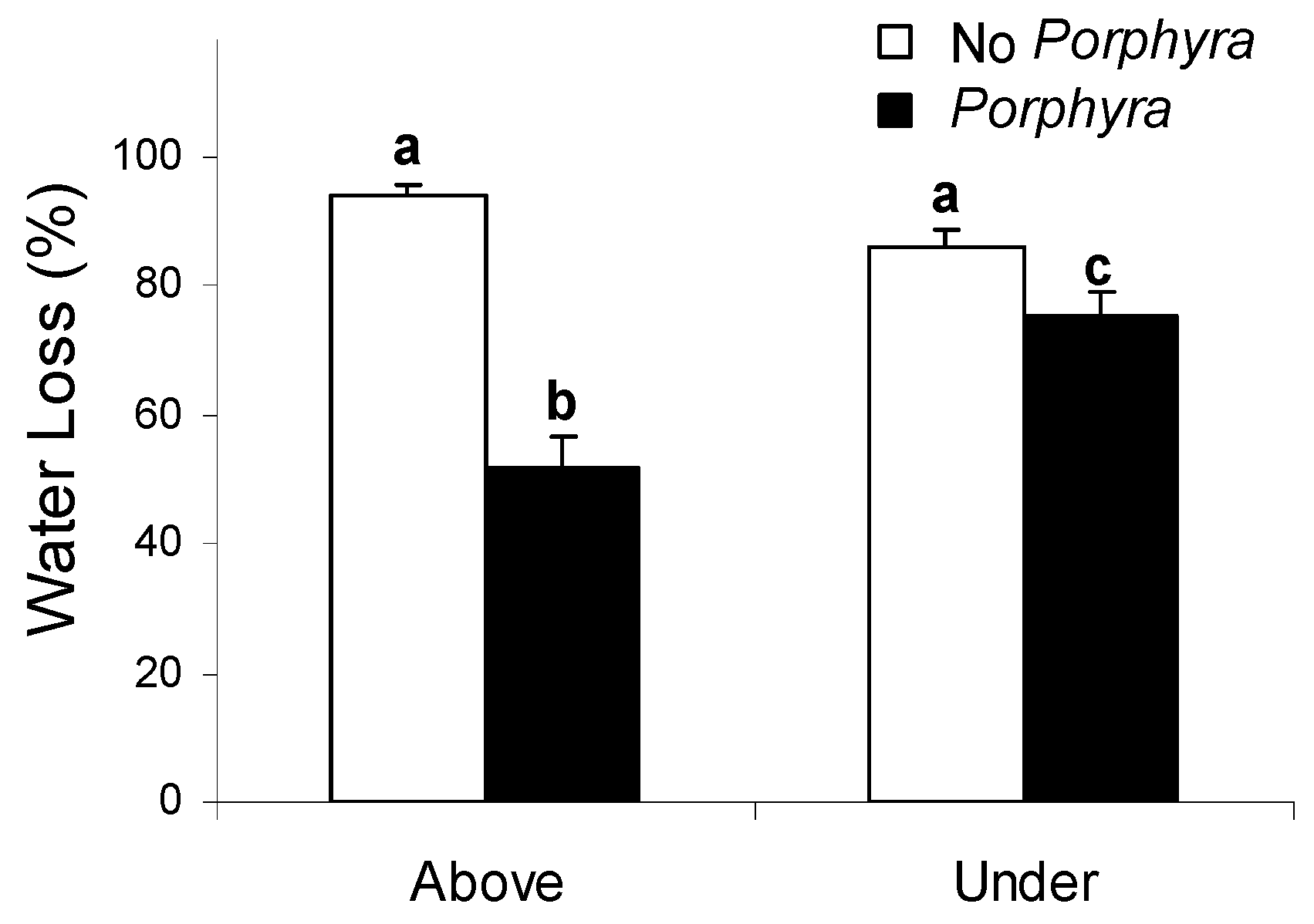
3.1. Effects of Porphyra Cover on Desiccation and Temperature
3.2. Effects of Porphyra Cover on Interstitial Mussel Bed Invertebrates
3.3. Porphyra-Related Patchiness in Interstitial Invertebrate Distribution
3.4. Responses of a Dominant Grazer to Porphyra Thalli and Structural Porphyra Mimics
4. Discussion
4.1. Effects of Porphyra Cover on Desiccation and Temperature
4.2. Mobile Invertebrate Responses to Porphyra Cover
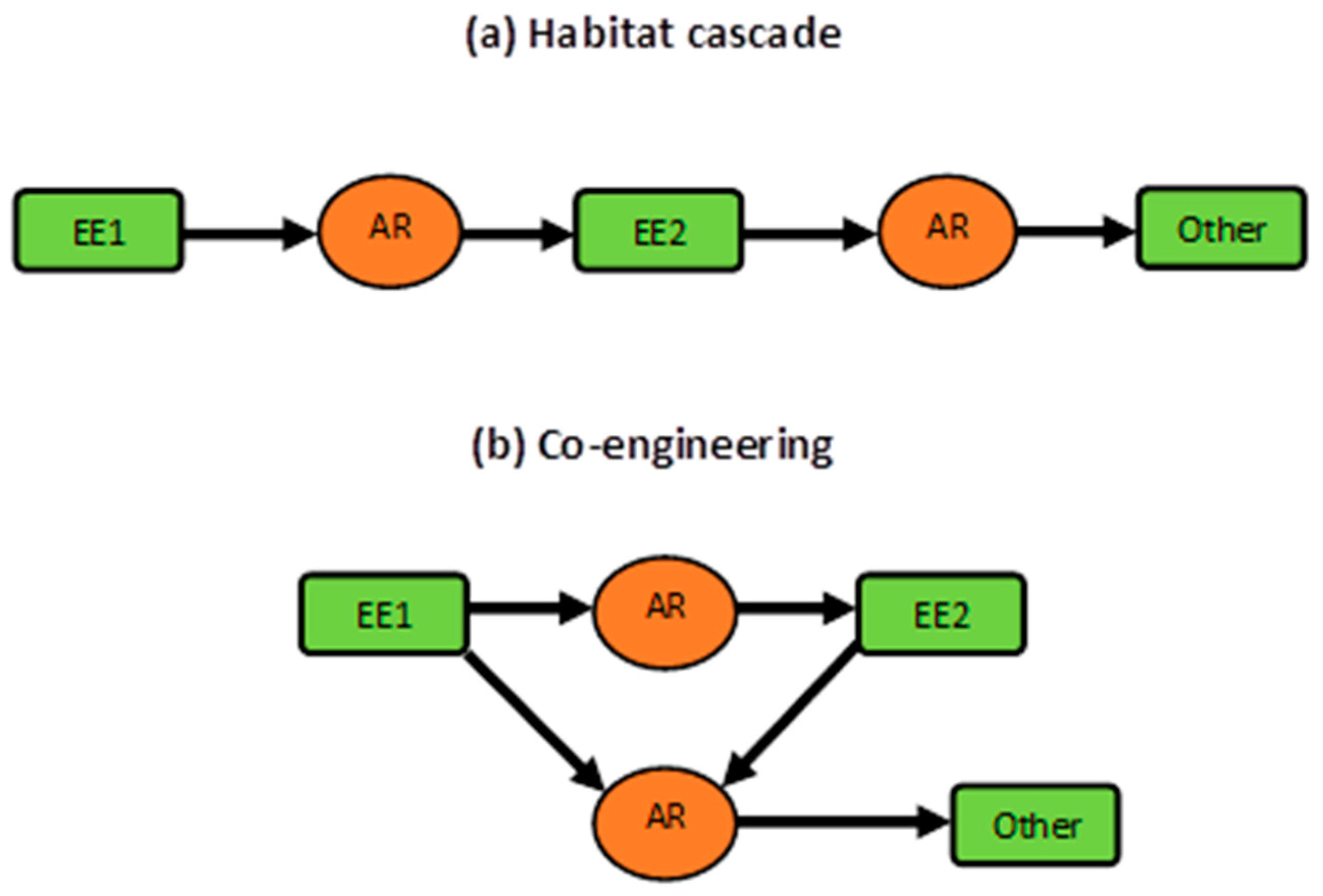
4.3. Concluding Remarks: Co-engineering, Habitat Cascades, and More
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Jones, C.G.; Gutiérrez, J.L. On the purpose, meaning, and usage of the ecosystem engineering concept. In Ecosystem Engineers: Plants to Protists; Cuddington, K., Byers, J.E., Wilson, W.G., Hastings, A., Eds.; Academic Press: New York, NY, USA, 2007; pp. 3–24. [Google Scholar]
- Jones, C.G.; Gutiérrez, J.L.; Byers, J.E.; Crooks, J.A.; Lambrinos, J.G.; Talley, T.S. A framework for understanding physical ecosystem engineering by organisms. Oikos 2010, 119, 1862–1869. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Wernberg, T.; Altieri, A.; Tuya, F.; Gulbransen, D.; McGlathery, K.J.; Holmer, M.; Silliman, B.R. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr. Comp. Biol. 2010, 50, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.J.; Byers, J.E.; Marcek, B.J.; Gribben, P.E. Density-dependent facilitation cascades determine epifaunal community structure in temperate Australian mangroves. Ecology 2012, 93, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Gribben, P.E.; Angelini, C.; Altieri, A.H.; Bishop, M.J.; Thomsen, M.S.; Bulleri, F. Facilitation cascades in marine ecosystems: A synthesis and future directions. Oceanogr. Mar. Biol. Ann. Rev. 2019, in press. [Google Scholar]
- Altieri, A.H.; Silliman, B.R.; Bertness, M.D. Hierarchical organization via a facilitation cascade in intertidal cordgrass bed communities. Am. Nat. 2007, 169, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Gribben, P.E.; Byers, J.E.; Clements, M.; McKenzie, L.A.; Steinberg, P.D.; Wright, J.T. Behavioural interactions between ecosystem engineers control community species richness. Ecol. Lett. 2009, 12, 1127–1136. [Google Scholar] [CrossRef]
- Angelini, C.; Silliman, B.R. Secondary foundation species as drivers of trophic and functional diversity: Evidence from a tree-epiphyte system. Ecology 2014, 95, 185–196. [Google Scholar] [CrossRef]
- Yakovis, E.; Artemieva, A. Cockles, barnacles and ascidians compose a subtidal facilitation cascade with multiple hierarchical levels of foundation species. Sci. Rep. 2017, 7, 237. [Google Scholar] [CrossRef]
- Ward, J.M.; Ricciardi, A. Community-level effects of co-occurring native and exotic ecosystem engineers. Freshw. Biol. 2010, 55, 1803–1817. [Google Scholar] [CrossRef]
- Passarelli, C.; Olivier, F.; Paterson, D.M.; Meziane, T.; Hubas, C. Organisms as cooperative ecosystem engineers in intertidal flats. J. Sea Res. 2014, 92, 92–101. [Google Scholar] [CrossRef]
- Angelini, C.; van der Heide, T.; Griffin, J.N.; Morton, J.P.; Derksen-Hooijberg, M.; Lamers, L.P.; Smolders, A.J.P.; Silliman, B.R. Foundation species’ overlap enhances biodiversity and multifunctionality from the patch to landscape scale in southeastern United States salt marshes. Proc. R. Soc. B 2015, 282, 20150421. [Google Scholar] [CrossRef]
- Suchanek, T.H. Mussels and their role in structuring rocky shore communities. In The Ecology of Rocky Coasts; Moore, P.G., Seed, R., Eds.; Hodder & Stoughton Press: London, UK, 1985; pp. 70–96. [Google Scholar]
- Gutiérrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribarne, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Jones, C.G.; Byers, J.E.; Arkema, K.K.; Berkenbusch, K.; Committo, J.A.; Duarte, C.M.; Hacker, S.D.; Hendriks, I.E.; Hogarth, P.J.; et al. Physical ecosystem engineers and the functioning of estuaries and coasts. In Volume 7: Functioning of Estuaries and Coastal Ecosystems; Heip, C.H.R., Philippart, C.J.M., Middelburg, J.J., Eds.; In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 53–81. [Google Scholar]
- Sousa, R.; Gutiérrez, J.L.; Aldridge, D. Non-indigenous invasive bivalves as ecosystem engineers. Biol. Invasions 2009, 11, 2367–2385. [Google Scholar] [CrossRef]
- Borthagaray, A.I.; Carranza, A. Mussels as ecosystem engineers: Their contribution to species richness in a rocky littoral community. Acta Oecol. 2007, 31, 243–250. [Google Scholar] [CrossRef]
- Palomo, M.G.; People, J.; Chapman, M.G.; Underwood, A.J. Separating the effects of physical and biological aspects of mussel beds on their associated assemblages. Mar. Ecol. Prog. Ser. 2007, 344, 131–142. [Google Scholar] [CrossRef]
- Silliman, B.R.; Bertness, M.D.; Altieri, A.H.; Griffin, J.N.; Bazterrica, M.C.; Hidalgo, F.J.; Crain, C.M.; Reyna, M.V. Whole-community facilitation regulates biodiversity on Patagonian rocky shores. PLoS ONE 2011, 6, e24502. [Google Scholar] [CrossRef]
- Bagur, M.; Gutiérrez, J.L.; Arribas, L.P.; Palomo, M.G. Complementary influences of co-occurring physical ecosystem engineers on species richness: Insights from a Patagonian rocky shore. Biodivers. Conserv. 2016, 25, 2787–2802. [Google Scholar] [CrossRef]
- Lohse, D.P. The importance of secondary substratum in a rocky intertidal community. J. Exp. Mar. Biol. Ecol. 1993, 166, 1–17. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Noda, T. Effects of mussels on competitively inferior species: Competitive exclusion to facilitation. Mar. Ecol. Prog. Ser. 2004, 276, 293–298. [Google Scholar] [CrossRef]
- Sutherland, J.E.; Lindstrom, S.C.; Nelson, W.A.; Brodie, J.; Lynch, M.D.; Hwang, M.S.; Choi, H.G.; Miyata, M.; Kikuchi, N.; Oliveira, M.C.; et al. A new look at an ancient order: Generic revision of the Bangiales (Rhodophyta). J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef] [PubMed]
- Santelices, B.; Martínez, E. Effects of filter-feeders and grazers on algal settlement and growth in mussel beds. J. Exp. Mar. Biol. Ecol. 1988, 118, 281–306. [Google Scholar] [CrossRef]
- Aquilino, K.M.; Bracken, M.E.; Faubel, M.N.; Stachowicz, J.J. Local-scale nutrient regeneration facilitates seaweed growth on wave-exposed rocky shores in an upwelling system. Limnol. Oceanogr. 2009, 54, 309–317. [Google Scholar] [CrossRef]
- O’Connor, N.E. Shore exposure affects mussel population structure and mediates the effect of epibiotic algae on mussel survival in SW Ireland. Estuar. Coast. Shelf Sci. 2010, 87, 83–91. [Google Scholar] [CrossRef]
- Bertness, M.D.; Leonard, G.H.; Levine, J.M.; Schmidt, P.R.; Ingraham, A.O. Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 1999, 80, 2711–2726. [Google Scholar] [CrossRef]
- Kelaher, B.P. Influence of physical characteristics of coralline turf on associated macrofaunal assemblages. Mar. Ecol. Prog. Ser. 2002, 232, 141–148. [Google Scholar] [CrossRef]
- Umanzor, S.; Ladah, L.; Calderon-Aguilera, L.E.; Zertuche-González, J.A. Intertidal macroalgae influence macroinvertebrate distribution across stress scenarios. Mar. Ecol. Prog. Ser. 2017, 584, 67–77. [Google Scholar] [CrossRef]
- Pocklington, J.B.; Jenkins, S.R.; Bellgrove, A.; Keough, M.J.; O’Hara, T.D.; Masterson-Algar, P.E.; Hawkins, S.J. Disturbance alters ecosystem engineering by a canopy-forming alga. J. Mar. Biol. Assoc. UK 2018, 98, 687–698. [Google Scholar] [CrossRef]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37. [Google Scholar] [CrossRef]
- Penchaszadeh, P.E. Ecología de la comunidad del mejillín (Brachidontes rodriguezii D’Orb.) en el mediolitoral rocoso de Mar del Plata, Argentina, el proceso de recolonización. Physis 1973, 32, 51–64. [Google Scholar]
- Gutiérrez, J.L.; Palomo, M.G. Increased algal fouling on mussels with barnacle epibionts: A fouling cascade. J. Sea Res. 2016, 112, 49–54. [Google Scholar] [CrossRef]
- Arribas, L.P.; Bagur, M.; Klein, E.; Penchaszadeh, P.E.; Palomo, M.G. Geographic distribution of two mussel species and associated assemblages along the northern Argentinean coast. Aquat. Biol. 2013, 18, 91–103. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Palomo, M.G.; Bagur, M.; Arribas, L.P.; Soria, S.A. Wave action limits crowding in an intertidal mussel. Mar. Ecol. Prog. Ser. 2015, 518, 153–163. [Google Scholar] [CrossRef]
- Adami, M.L.; Tablado, A.; Lopez-Gappa, J. Spatial and temporal variability in intertidal assemblages dominated by the mussel Brachidontes rodriguezii (d’Orbigny, 1846). Hydrobiologia 2004, 520, 49–59. [Google Scholar] [CrossRef]
- Gutiérrez, J.L.; Bagur, M.; Arribas, L.P.; Palomo, M.G. Does rock type account for variation in mussel attachment strength? A test with Brachidontes rodriguezii in the Southwestern Atlantic. Helgol. Mar. Res. 2018, 72, 10. [Google Scholar] [CrossRef]
- Arribas, L.P.; Bagur, M.; Gutiérrez, J.L.; Palomo, M.G. Matching spatial scales of variation in mussel recruitment and adult densities across southwestern Atlantic rocky shores. J. Sea Res. 2015, 95, 16–21. [Google Scholar] [CrossRef]
- Adami, M.L.; Pastorino, G.; Orensanz, J.M. Phenotypic differentiation of ecologically significant Brachidontes species co-occurring in intertidal mussel beds from the Southwestern Atlantic. Malacologia 2013, 56, 59–67. [Google Scholar] [CrossRef]
- Trovant, B.; Ruzzante, D.E.; Basso, N.G.; Orensanz, J.M. Distinctness, phylogenetic relations and biogeography of intertidal mussels (Brachidontes, Mytilidae) from the south-western Atlantic. J. Mar. Biol. Assoc. UK 2013, 93, 1843–1855. [Google Scholar] [CrossRef]
- López Gappa, J.J.; Tablado, A.; Magaldi, N.H. Influence of sewage pollution on a rocky intertidal community dominated by the mytilid Brachidontes rodriguezii. Mar. Ecol. Prog. Ser. 1990, 63, 163–175. [Google Scholar] [CrossRef]
- Boraso, A.; Zaixso, J.M. Algas marinas bentónicas. In Atlas de Sensibilidad Ambiental de la Costa y el Mar Argentino; Boltovskoy, D., Ed.; Secretaría de Ambiente y Desarrollo Sustentable: Buenos Aires, Argentina, 2011; pp. 1–28. [Google Scholar]
- Varela-Álvarez, E.; Stengel, D.B.; Guiry, M.D. Seasonal growth and phenotypic variation in Porphyra linearis (Rhodophyta) populations on the west coast of Ireland. J. Phycol. 2007, 43, 90–100. [Google Scholar] [CrossRef]
- Becherucci, M.E.; Benavides, H.; Vallarino, E.A. Effect of taxonomic aggregation in macroalgae assemblages in a rocky shore of Mar del Plata, Argentina, Southwest Atlantic Ocean. Thalassas 2014, 30, 9–20. [Google Scholar]
- Van Alstyne, K.L.; Olson, T.K. Estimating variation in surface emissivities of intertidal macroalgae using an infrared thermometer and the effects on temperature measurements. Mar. Biol. 2014, 161, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J.; Li, W. Applied Linear Statistical Models, 5th ed.; McGraw-Hill/Irwin: New York, NY, USA, 2005. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electr. 2001, 4, 1–9. [Google Scholar]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Allison, P.D. Logistic Regression Using SAS: Theory and Application; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Hacker, S.D.; Steneck, R.S. Habitat architecture and the abundance and body-size-dependent habitat selection of a phytal amphipod. Ecology 1990, 71, 2269–2285. [Google Scholar] [CrossRef]
- Jurgens, L.J.; Gaylord, B. Edge effects reverse facilitation by a widespread foundation species. Sci. Rep. 2016, 6, 37573. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Thomas, D.; Flores, V.; Correa, J.A. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J. Exp. Bot. 2010, 62, 1815–1829. [Google Scholar] [CrossRef]
- Hodder, R.L. Roadside dry-land planting research in Montana. Highw. Res. Rec. 1970, 335, 29–34. [Google Scholar]
- Kumar, K.V.; Bai, R.K. 2008. Performance study on solar still with enhanced condensation. Desalination 2008, 230, 51–61. [Google Scholar] [CrossRef]
- Khammash, A. A three-dimensional study of sub-foliar condensation in desert rhubarb (Rheum palaestinum, Polygonaceae). Plant Ecol. Evol. 2016, 149, 137–143. [Google Scholar] [CrossRef]
- Joseph, M.M. Tidal rhythm in the feeding activity of the intertidal amphipod Hyale hawaiensis (Dana). Proc. Ind. Natl. Acad. Part B Biol. Sci. 1972, 38, 456–461. [Google Scholar]
- Buschmann, A.H. Intertidal macroalgae as refuge and food for amphipoda in central Chile. Aquat. Bot. 1990, 36, 237–245. [Google Scholar] [CrossRef]
- Griffin, N.J.; Bolton, J.J.; Anderson, R.J. Distribution and population dynamics of Porphyra (Bangiales, Rhodophyta) in the southern Western Cape, South Africa. J. Appl. Phycol. 1999, 11, 429–436. [Google Scholar] [CrossRef]
- Moore, P.G. Organization in simple communities: Observations on the natural history of Hyale nilssoni (Amphipoda) in high littoral seaweeds. In Biology of Benthic Organisms; Keegan, B.F., Boaden, P.J.S., Ceidigh, P.O., Eds.; Pergamon Press: Oxford, UK, 1977; pp. 443–451. [Google Scholar]
- Byers, J.E.; Gribben, P.E.; Yeager, C.; Sotka, E.E. Impacts of an abundant introduced ecosystem engineer within mudflats of the southeastern US coast. Biol. Invasions 2012, 14, 2587–2600. [Google Scholar] [CrossRef]
- Wright, J.T.; Byers, J.E.; DeVore, J.L.; Sotka, E.E. Engineering or food? mechanisms of facilitation by a habitat-forming invasive seaweed. Ecology 2014, 95, 2699–2706. [Google Scholar] [CrossRef]
- Blankley, W.O.; Grindley, J.R. The intertidal and shallow subtidal food web at Marion Island. In Antarctic Nutrient Cycles and Food Webs; Siegfried, W.R., Condy, P.R., Laws, R.M., Eds.; Springer: Berlin, Germany, 1985; pp. 630–636. [Google Scholar]
- Laudien, J.; Wahl, M. Indirect effects of epibiosis on host mortality: Seastar predation on differently fouled mussels. Mar. Ecol. 1999, 20, 35–47. [Google Scholar] [CrossRef]
- Albrecht, A.; Reise, K. Effects of Fucus vesiculosus covering intertidal mussel beds in the Wadden Sea. Helgol. Meeresunters 1994, 48, 243–256. [Google Scholar] [CrossRef]
- Wahl, M.; Schneider Covachã, S.; Saderne, V.; Hiebenthal, C.; Müller, J.D.; Pansch, C.; Sawall, Y. Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol. Oceanogr. 2018, 63, 3–21. [Google Scholar] [CrossRef]
- Berkenbusch, K.; Rowden, A.A. Ecosystem engineering—Moving away from ‘just-so’ stories. N. Z. J. Ecol. 2003, 27, 67–73. [Google Scholar]
- Procheş, Ş.; Marshall, D.J. Epiphytic algal cover and sediment deposition as determinants of arthropod distribution and abundance on mangrove pneumatophores. J. Mar. Biol. Assoc. UK 2002, 82, 937–942. [Google Scholar] [CrossRef]
- Pereira-Filho, G.H.; de Cerqueira Veras, P.; Francini-Filho, R.B.; de Moura, R.L.; Pinheiro, H.T.; Gibran, F.Z.; Matheus, Z.; Neves, L.M.; Amado-Filho, G.M. Effects of the sand tilefish Malacanthus plumieri on the structure and dynamics of a rhodolith bed in the Fernando de Noronha Archipelago, tropical West Atlantic. Mar. Ecol. Prog. Ser. 2015, 541, 65–73. [Google Scholar] [CrossRef]
| Site | Latitude (S) | Longitude (W) | Rock Type | Field Activities |
|---|---|---|---|---|
| Punta Cantera | 38°04′51″ | 57°32′08″ | OO | 2.3, 2.4 |
| Los Acantilados | 38°07′28″ | 57°35′56″ | PL | 2.4 |
| Las Brusquitas | 38°14′43″ | 57°46′33″ | PL | 2.2, 2.3, 2.5 |
| Copacabana | 38°14′50″ | 57°46′50″ | PL | 2.3, 2.4 |
| Punta Hermengo | 38°17′14″ | 57°50′12″ | PL | 2.4 |
| Source | MS | df | F | p |
|---|---|---|---|---|
| Between-subjects | ||||
| Porphyra cover | 178.92 | 1 | 107.58 | <0.001 * |
| Position | 992.35 | 1 | 596.65 | <0.001 * |
| Porphyra cover X Position | 108.78 | 1 | 65.41 | <0.001 * |
| Subject (i.e., plots) | 1.66 | 20 | ||
| Within-subjects | ||||
| Time | 6.32 | 2 | 5.46 | 0.008 * |
| Porphyra cover X Time | 0.22 | 2 | 0.19 | 0.825 |
| Position X Time | 10.95 | 2 | 9.47 | <0.001 * |
| Porphyra cover X Position X Time | 1.99 | 2 | 1.72 | 0.192 |
| Residual | 1.16 | 40 |
| Source | MS | df | F | p |
|---|---|---|---|---|
| Porphyra cover | 461.35 | 1 | 60.32 | <0.001 * |
| Position | 31.97 | 1 | 4.96 | 0.038 * |
| Porphyra cover X Position | 149.76 | 1 | 19.58 | <0.001 * |
| Initial Water Content | 18.36 | 1 | 2.40 | 0.138 |
| Residual | 7.65 | 19 |
| Source | MS | df | F | p |
|---|---|---|---|---|
| Treatment | 1.36 | 1 | 9.99 | <0.001 * |
| Site/Date | 2.39 | 2 | 17.63 | <0.001 * |
| Treatment X Site/Date | 0.77 | 2 | 2.83 | 0.008 * |
| Residual | 5.70 | 42 |
| Response Variable Explanatory Variables | Deviance | df | p | D2 |
|---|---|---|---|---|
| Total Invertebrates | ||||
| Treatment | 28.45 | 46 | 0.980 | 0.20 |
| Site/Date | 24.04 | 46 | 0.998 | 0.32 |
| Treatment X Site/Date | 12.70 | 42 | >0.999 | 0.64 |
| Intercept | 35.41 | 47 | 0.892 | |
| Siphonaria lessonii | ||||
| Treatment | 93.61 | 46 | <0.001 * | 0.02 |
| Site/Date | 35.01 | 46 | 0.881 | 0.64 |
| Treatment X Site/Date | 31.09 | 42 | 0.892 | 0.68 |
| Intercept | 96.00 | 47 | <0.001 * | |
| Hyale grandicornis | ||||
| Treatment | 44.99 | 46 | 0.514 | 0.46 |
| Site/Date | 73.53 | 46 | 0.006 * | 0.12 |
| Treatment X Site/Date | 35.67 | 42 | 0.744 | 0.57 |
| Intercept | 83.91 | 47 | <0.001 * | |
| Perinereis anderssoni | ||||
| Treatment | 47.01 | 46 | 0.431 | <0.01 |
| Site/Date | 19.34 | 46 | >0.999 | 0.59 |
| Treatment X Site/Date | 18.95 | 42 | 0.999 | 0.60 |
| Intercept | 47.29 | 47 | 0.461 |
| Source | MS | df | F | p |
|---|---|---|---|---|
| Treatment | 1.39 | 3 | 8.78 | <0.001 * |
| Site/Date | 1.19 | 1 | 7.51 | <0.001 * |
| Treatment X Site/Date | 0.30 | 3 | 1.91 | 0.024 * |
| Residual | 0.16 | 40 |
| Response Variable Explanatory Variables | Deviance | df | p | D2 |
|---|---|---|---|---|
| Total Invertebrates | ||||
| Patch Type | 42.02 | 46 | 0.640 | 0.06 |
| Site/Date | 19.98 | 46 | >0.999 | 0.55 |
| Patch Type X Site/Date | 15.88 | 40 | >0.999 | 0.64 |
| Intercept | 44.84 | 47 | 0.562 | |
| Siphonaria lessonii | ||||
| Patch Type | 42.09 | 46 | 0.637 | 0.14 |
| Site/Date | 18.40 | 46 | >0.999 | 0.62 |
| Patch Type X Site/Date | 17.96 | 40 | 0.999 | 0.63 |
| Intercept | 48.78 | 47 | 0.401 | |
| Hyale grandicornis | ||||
| Patch Type | 69.90 | 46 | 0.013 * | 0.21 |
| Site/Date | 58.01 | 46 | 0.110 | 0.34 |
| Patch Type X Site/Date | 38.99 | 40 | 0.516 | 0.56 |
| Intercept | 88.27 | 47 | <0.001 * | |
| Perinereis anderssoni | ||||
| Patch Type | 47.01 | 46 | 0.431 | 0.01 |
| Site/Date | 19.33 | 46 | >0.999 | 0.59 |
| Patch Type X Site/Date | 18.95 | 40 | 0.998 | 0.60 |
| Intercept | 47.29 | 47 | 0.461 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, J.L.; Bagur, M.; Palomo, M.G. Algal Epibionts as Co-Engineers in Mussel Beds: Effects on Abiotic Conditions and Mobile Interstitial Invertebrates. Diversity 2019, 11, 17. https://doi.org/10.3390/d11020017
Gutiérrez JL, Bagur M, Palomo MG. Algal Epibionts as Co-Engineers in Mussel Beds: Effects on Abiotic Conditions and Mobile Interstitial Invertebrates. Diversity. 2019; 11(2):17. https://doi.org/10.3390/d11020017
Chicago/Turabian StyleGutiérrez, Jorge L., María Bagur, and M. Gabriela Palomo. 2019. "Algal Epibionts as Co-Engineers in Mussel Beds: Effects on Abiotic Conditions and Mobile Interstitial Invertebrates" Diversity 11, no. 2: 17. https://doi.org/10.3390/d11020017
APA StyleGutiérrez, J. L., Bagur, M., & Palomo, M. G. (2019). Algal Epibionts as Co-Engineers in Mussel Beds: Effects on Abiotic Conditions and Mobile Interstitial Invertebrates. Diversity, 11(2), 17. https://doi.org/10.3390/d11020017





