Abstract
We analyze the structure of diameter, richness, and diversity of the forests in the upper limit of the great Amazon basin located in the Ecuadorian territory of the Cordilleras del Cóndor and Cutucú. Our hypothesis was that the forests of the eastern mountain ranges are not homogeneous, but rather present differences in their structure, richness, and floristic diversity. Our main objective was to classify the types of forests based on the characteristics of the diameter structure and the species composition of the Amazonian forests of the eastern mountain ranges in southern Ecuador, and we determined the influence of critical edaphic, environmental, and geomorphological factors, For this we installed eight permanent plots of one hectare in homogeneous and well preserved forest stands, four plots in the province of Zamora Chinchipe and four in the province of Morona Santiago. We identified and measured all trees >10 cm at chest height and for each plot, soil samples, as well as environmental and slope data were taken. We performed an non-metric multidimensional scaling analysis (NMDS) analysis to evaluate changes in climatic and geomorphological gradients, and used the CCA analysis to assess the relationship between the composition of the species at the plot level and the edapho-climatic variables. Finally, we modeled the change in diversity ad species (Fisher’s alpha) in relation to climatic, altitudinal, and geomorphological gradients using a GLM. We determined the existence of two different types of forest, the first called Terra Firme, characterized by the presence of a greater number of species and individuals per plot as compared to the second type of forest called Tepuy or Sandstone forest. Species richness was negatively correlated with the phosphorus content of the soil and the pH, annual average temperature, annual rainfall, and altitude. Terra Firme forests, settled in more stable and nutrient-rich climatic areas, were more diverse and Sandstone forests are poor in nutrients and develop in areas with greater seasonality.
1. Introduction
Ecuador is considered one of the 17 mega-diverse countries of the world [1]; the diversity of plants throughout its territory being a consequence of distribution patterns and geodynamic processes which have been studied during the last decades. The Ecuadorian Amazon is widely known for its great diversity of plants and animals and the determined peaks of diversity have been recognized worldwide [2,3]. Regretfully, anthropic pressure, determined by changes in land use and deforestation, make it one of the most endangered ecosystems, and therefore a priority for conservation [4].
In recent decades, the forests of the Ecuadorian Amazon have undergone high rates of deforestation and fragmentation [5], which modifies environmental conditions at local and regional levels (higher temperatures, changes in precipitation regimes, and increased CO2 concentrations). These ecosystems are home to one of the highest concentrations of vascular plant species [6], but most studies are limited to the floristic diversity of these ecosystems [7,8], without relating this richness and diversity to environmental, geomorphological factors, and soil [9], nor with altitude patterns and specific edaphic-environmental factors such as precipitation, seasonality, soil fertility, and topography [10,11,12]. Others noted that climatic variables (for example, the amount of annual rainfall) are not good estimators of wealth [13].
In Ecuador, two mountain ranges border the great Amazonian basin at its western limit, the Cordillera del Cóndor and the Cutucú. These two cordilleras are located in the southeastern part of Ecuador, on its border with Peru, being the largest in the country, after the main mountain range of the Andes. They are characterized by having white sandstone plateaus with an acidic nature, similar to those of the northeastern mountains of South America, typical of the Precambrian shield of the Guianas. Additionally, they are located in close vicinity to the Huancabamba depression, the lowest point of the Andes. As a result, clouds that form on the western slope slide through this depression, and then deposit their moisture load on these ridges, pushed by the prevailing easterly winds [14].
Previous studies conducted in Cordillera del Cóndor [8,14] have revealed a high floristic richness, similar in magnitude to other areas of the Neotropics. Along the Condor and Cutucú mountain ranges there are several small sandstone islands, where countless species endemic to this type of environment grow. These areas show geological characteristics similar to those of the Venezuelan Guyana massif, and their vegetation presents specific characters and adaptations [8].
Studies conducted both in Ecuador and Peru document a high species richness; in the case of species above 10 cm DBH, with the average density of 253 to 300 species per hectare [11,12,13,15,16]. Although the flora and vegetation of some specific sites in the Cutucú and Cóndor mountain ranges is known in detail [17,18,19], no studies have been carried out regarding the factors that determine the richness and diversity in these mountain ranges.
In this context, the main objective of this study was to determine the forest types from the cordilleras of southeastern Ecuador and to determine which environmental factors affect the diversity and structure of these forests. For this, we proposed the following two questions: (1) Is the forest of the eastern mountain ranges of southern Ecuador homogeneous? and (2) What edapho-climatic or other factors influence the richness, structure, and floristic composition of these forests?
2. Methods
2.1. Study Area
The study was carried out in four localities of the Amazonian forests of the Cóndor and Cutucú mountain ranges, in the southeast of Ecuador, located between 700 and 1650 m, in the provinces of Morona Santiago in the towns of Wusui (plots 5 and 6) and Koankos (plots 7 and 8) and Zamora Chinchipe in the localities of Nangaritza (plots 1 and 2) and Wawaime (plots 3 and 4). Each plot was preliminarily installed in a different type of forest at the province level and locality level. Data of location an altitude are in Supplementary Table S1 (Figure 1).
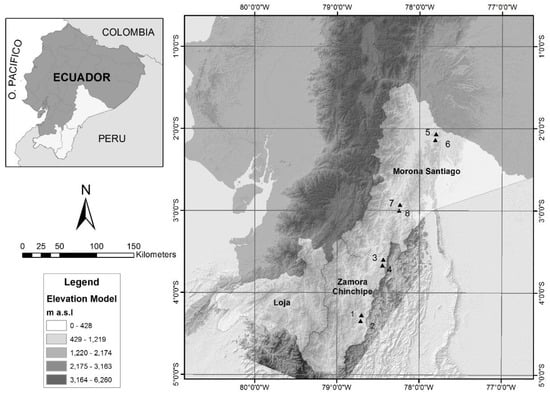
Figure 1.
Location of the sampling plots.
2.2. Plot Installation
In each locality, two permanent one hectare plots were established. Each of these 8 plots was subdivided into 25 subplots of 20 × 20 m. For each subplot, the geographical position, elevation, and slope data were recorded. All individuals equal to or greater than 10 cm in diameter at breast height (DBH) were sampled. This is a standardized methodology that has been applied in other regions of the Neotropics, including Ecuador [20,21,22].
The collected plants were identified in the Herbarium of the Museum of Natural Sciences in Quito-Ecuador (QCNE) and the Herbarium of the National University of Loja (LOJA). For the nomenclature of the species, the catalogs of the flora of Ecuador and Peru were used [23,24,25]. The similarity between each pair of plots was calculated using the abundance of each species per plot.
To characterize the forests of different localities, structural and floristic parameters were calculated using the equations proposed by [26,27,28].
2.3. Climatic and Edaphic Variables
From each plot, soil samples were collected from the four extremes and one from the center; the five samples were then homogenized and conserved for later analysis. All the laboratory analyzes were performed in AGROBIOLAB (Quito) with standardized protocols for each variable analyzed. The edaphic-climatic variables used were: acidity (pH), electrical conductivity (EC), organic matter (MO), aluminum plus hydrogen (AL + H), boron (B), calcium (Ca), cation exchange capacity (CEC), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), sodium (Na), total nitrogen (NH4), phosphorus (P), sulfur (SO4),and zinc (Zn).
The bioclimatic data were extracted from Worldclim [29]. The climatic variables that we used were average annual temperature (°C), isothermality (°C), seasonality of temperature (°C), annual range of temperature (°C), annual total precipitation (mm), precipitation of the driest month (mm), seasonality of precipitation (mm), and precipitation of the warmest quarter (mm). The slope of the terrain (%) was measured with a clinometer and the altitude (m a.s.l.) with an altimeter
2.4. Statistical Analysis
All analyses were performed using the software R 2.14.0. For alpha diversity, we combined PCA and the generalized linear models (GLM) to obtain predictive models for richness calculated at the plot level with other climate and edaphic factors as predictors. The new variables from PCA are suitable to use as predictors in a regression equation since they remove possible effects of multicollinearity [30]. Using the function glm of software R [31], a saturated model was constructed with the 14 explanatory variables cited above. The relationship between the composition of species and the measured edapho-climatic variables was analyzed by canonical correspondence analysis [32]. Preliminarily, an indirect canonical correspondence analysis (DCA) was performed, the results indicated that the standard deviation values allow a canonical correspondence analysis (CCA) [33,34]. Using the step () function, an optimal model was constructed based on the AIC (Akaike information criterion) statistic [35,36]. The variance inflation factor (VIF) was calculated to eliminate redundant variables in the final model, considering VIF > 20 as the threshold from which one variable was considered highly correlated with the others in the model.
We performed a fitted non-metric multidimensional scaling analysis (NMDS), in which the variables identified in the CCA were used to interpret the ordering. For the CCA and NMDS analyses, the abundance values were transformed using Hellinger distance, in order to avoid an exaggerated influence of the rare species [37]. The NMDS was calculated using the Euclidean distance and the CCA and NMDS analyses were completed using the “vegan” package [38]. We used SIMPER similarity percentage analysis to know which species are responsible for the similarity or dissimilarity between floristic groups using the same package.
3. Results
3.1. Alpha Diversity and Forest Structure
A total of 5771 trees >10 cm DBH were sampled, belonging to 76 families and 517 species (430 species in the Terra Firme Forest and 216 species in the Tepuy Forest). The most diverse family is Fabaceae with 51 species and it is the one that contributes in both types of forest, with the largest number of species (relative diversity RD = 9.9%), followed by Rubiaceae 40 ssp. (7.7%), Euphorbiaceae and Lauraceae with 36 ssp. (7%), Moraceae with 27 ssp. (5.2%), Sapotaceae with 19 ssp. (3.7%), Clusiaceae and Melastomataceae with 18 ssp. (3.5%), Myristicaceae with 15 ssp. (2.9%), Burseraceae and Cecropiaceae with 14 ssp (2.7%), Annonaceae and Myrtaceae with 13 ssp. (2.5%), and Flacourtiaceae 11 ssp. (2.1%) and Sapindaceae with 10 ssp. (1.9%). The rest of the 55 families have between one and 10 species (0.2–1.7%) (Supplementary Table S2).
The total basal area of the sampled trees reached 185.4 m2 (130 m2 in the Terra Firme Forest and 53.5 m2 in the Tepuy Forest). The family with the highest value of the basal area is Myristicaceae with 17.6 m2 (9.59% of the total), followed by Moraceae with 15.8 m2 (8.52%), Sapotaceae with 14.53 m2 (7.84%), Fabaceae with 12.1 m2 (6.52%), Euphorbiaceae with 11.9 m2 (6.44%), Arecaceae 11.9 m2 (6.41%), Rubiaceae with 10.7 m2 (5.8%) and Lauraceae with 10.1 m2 (5.4%). There were 23 families that had between 1 and 8 m2 (0.61–4%), while the rest of families had lower values (0.1–0.53%).
The five species with the highest basal area were Chrysophyllum sanguineolentum (Sapotaceae) with 10.5 m2 (5.7% of relative dominance), followed by Otoba glycycarpa (Myristicaceae) with 9.1 m2 (4.9%), Pseudolmedia laevigata (Moraceae) with 4.9 m2 (2.7%), Chimarrhis hookeri (Rubiaceae) with 4.7 m2 (2.5%) and Wettinia maynensis (Arecaceae) with 4.5 m2 (2.4%).
The five species with the highest number of individuals were Wettinia maynensis with 321 (5.6% of the total measured individuals), followed by Chrysophyllum sanguineolentum with 260 individuals (4.5%), Pseudolmedia laevigata with 175 (3.03%), Euterpe catinga with 158 individuals (2.7%) and Alchornea grandiflora with 142 individuals (2.5%). There were 179 species (3%) that were represented by a single individual.
The GLM showed that principal component PC1 represented by calcium, potassium, isotermalidad, pH, and factors related with precipitation (e.g., annual precipitation) were positively correlated with species richness. On the contrary PC2 represented by iron, sodium, manganese, copper, and mean annual temperature was negatively correlated with species richness (Table 1).

Table 1.
Results of generalized models of total species richness. Coef = coefficient of variation in the model. SD, standard deviation and P, significance of the model. (p < 0.05).
3.2. Species Turnover
The fitted NMDS analysis separates the plots into two groups. The first group, the Terra Firme Forest, consisted of plots 1–3–5–7. The second group, the Tepuy forest or white Sandstone forest, was formed by plots 2–4–6–8 (Figure 2). All soil variables that were included in the adjusted NMDS analysis except P and Fe significantly influenced the grouping of the plots, although with a low correlation. The annual average temperature and precipitation, and altitude were factors that contributed significantly contributed to the groupings (Table 2).
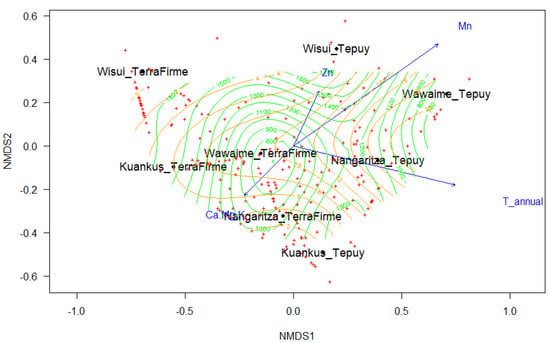
Figure 2.
The non-metric multidimensional scaling analysis (NMDS) analysis shows the differentiation between the Terra Firme and Tepuy plots, the green surface represents the elevation, and the orange SO4.

Table 2.
Factors and vectors that significantly influenced the grouping of the forest plots of the eastern mountain ranges of southern Ecuador.
The Terra Firme forests are characterized by a greater number of individuals and a greater number of species per unit area, (3009 DAP individuals ≥ 10 cm were recorded in an area of 40,000 m2 with an average of 151 species per hectare) as compared with the Tepuy forests (2651 individuals and an average of 89 species). There were no significant differences in the basal area values between the diameter classes of the Terra Firme forests and the Tepuy Forests (Class I Kruskal–Wallis, degrees of freedom (df) = 44, χ2 = 44.6, p > 0.05; Class II, df = 31, χ2 = 27.9, p > 0.05; Class III, df = 11, χ2 = 10.1, p > 0.05; Class IV, df = 7, χ2 = 8.22, p > 0.05; Class V, df = 3, χ2 = 4.3, p > 0.05; and Class VI, df = 2, χ2 = 2.4, p > 0.05).
The Terra Firme plots were more diverse than the plots in that of the Tepuy forest (Table 3). In each forest, the ecologically dominant species was determined, a list of the three most important species from each of the sampled sites can be found in the Supplementary Table S3.

Table 3.
Descriptive values of the attributes of the forests in each plot.
Species richness had a negative correlation with the average annual temperature, phosphorus, pH, annual precipitation, and altitude. Only calcium concentration shows a positive relationship with species richness that was higher in the Terra Firme forests as compared with the Tepuy forests, where soil characteristics (Terra Firme vs. Tepuy) such as pH (x = 4.9 ± 0.7 vs. x = 4.1 ± 0.3), calcium (x = 4.9 ± 1.3 vs. x = 0.9 ± 0.22), and phosphorus (x = 5.9 ± 1.54 vs. x = 10.6 ± 3.29) had different values. Altitude (x = 904 ± 188 vs. x = 1440 ± 207.4), precipitation annual average (x = 2683.5 ± 112.6 vs. x = 2787.5 ± 116.7) and the annual average temperature (x = 21.7 ± 1.3 vs. x = 20.5 ± 0.8) also had different values.
Regarding the diametric structure of the forests (Table 4), the Terra Firme forests have significantly more trees in the first four diametric classes (10–40 cm DAP) than the Tepuy forests, except in Wuisui and Kuankus, where the forests of Tepuy have more individuals of the first diametric class than the forests of Terra Firme. The trees of the last two diametric classes (50 -> 50 cm DBH) disappear in the Tepuy forests, except in Wuisui, where there was only one individual belonging to diametric class VI (Figure 3).

Table 4.
Comparison of the number of individuals in the determined diametric classes, in each locality and in each type of forest, bold values show significant differences.
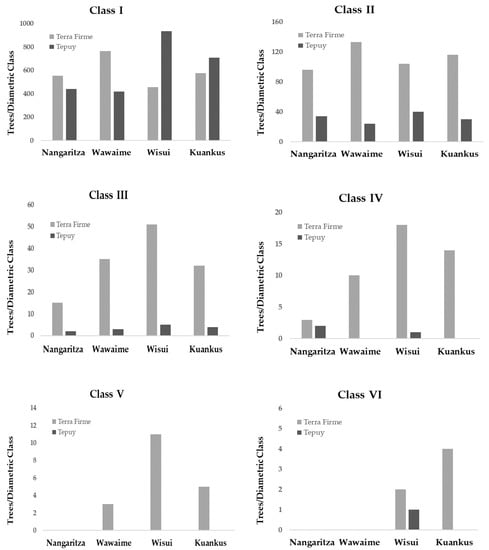
Figure 3.
Total number of trees in each diametric class, by site and type of forest.
The SIMPER analysis shows that the plots of the same province and forest type have greater similarity than the plots of the same forest located in another province (Table 5). There are also species which are characteristic of each plot, contributing to the dissimilarity between the plots. The list of the species and their percentage of contribution to the dissimilarity between each forest can be found in the Supplementary Table S4.

Table 5.
Dissimilarity values between the different types and sampling areas.
The canonical ordering analysis (CCA) shows that the eigenvalues associated with the restricted axes (EV1 = 0.8342 and EV2 = 0.6893) are greater than those associated with the unrestricted ones (EV1 = 0.4869 and EV2 = 0.4519), and therefore the use of CCA is appropriate in this case, although the variance explained by the model is low (Figure 4).
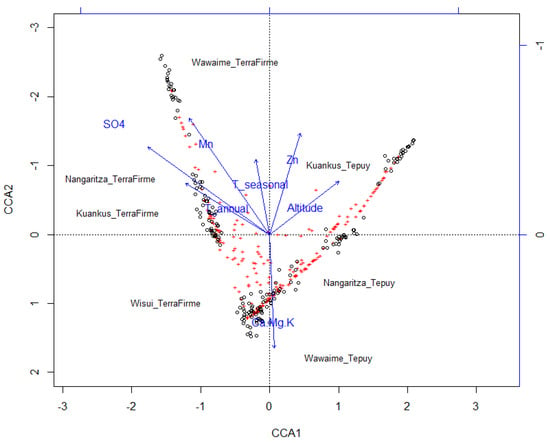
Figure 4.
The canonical ordering analysis (CCA) ordination showing grouping at subplot level. N = 200 subplots of 400 m2.
4. Discussion
Our results show that the forests from the Cóndor and Cutucú mountain ranges group into two distinct forest types, the Terra Firme forest and the Sandstone or Tepuy forest, and the differentiation is due to climatic, environmental, topographic, and edaphic factors.
Condit et al., 1996 [39] point out the close relationship that exists between abundance and richness, that is, given the same area, a habitat with a larger number of individuals should contain more species than one with a smaller number of individuals. Similar studies conducted in Ecuador and Peru document between 253 to 300 species of DBH ≥10 cm per hectare [3,19], which agrees with our results and refutes the claim that lowland Amazonian forests are richer than the forests found along the base of the Andes mountain range [40].
The ecologically important species were also different between the two types of forest. In the TF forest, the best represented species were Chrysophyllum sanguineolentum (Sapotaceae), Otoba glycycarpa (Myristicaceae), and Wettinia maynensis (Arecaceae), whereas in the Tepuy forests the most abundant and frequent species was Alchornea grandiflora (Euphorbiaceae). In forests similar to those studied here, Huamantupa 2009 [41] found that the five most abundant species were Iriartea deltoidea (Arecaceae), Pseudolmedia laevis (Moraceae), Otoba glycycarpa (Myristicaceae), Astrocaryum murumuru (Arecaceae), and Leonia glycicarpa (Violaceae). Other studies in Amazonian forests of Ecuador, Peru, and Colombia mentioned Lecythidaceae, Myristicaceae, Arecaceae, and Moraceae as dominating this type of ecosystem [9,12,19,42].
The diversity pattern in our study areas was very similar to that found in other Amazonian forests. Other authors, such as [13,43], noted that forests on white sands have a low diversity as compared with forests of Terra Firme. In our study, the presence of a greater number of species in the Terra Firme forests was mainly due to edaphic factors. In different ecosystems (lowland and Montane Forests), species diversity and tree height decrease as altitude increases [10,44,45,46,47], which agrees with our observations. In addition, factors such as increased precipitation, lack of seasonality in temperature, and natural fertility of soils have been considered determinants in the number of species that a certain system is able to sustain [10,48].
Other factors we found to negatively affect the species richness were the level of pH and the concentrations of phosphorus and calcium in the soil. Studies in other ecosystems indicate that the availability of nutrients in the soil is an important factor affecting the richness of tree species [45,47,49]. Our results indicate a similar pattern occurs in the two studied ecosystems, where the richness in the Tepuy forests is reduced due to soils with high levels of homogeneity, acidic pH, and poor nutrients. A high richness of arboreal species is related to high levels of soil heterogeneity [50,51].
In addition to edaphic factors, it is assumed that the reduction of tree species in the Tepuy forests is due to stressful climatic conditions, such as strong winds, water deficiency, steep slopes, and large rock masses, however, it has been observed that, in other tropical ecosystems, species richness increases in places with high to moderate slopes [24,45,52]. Finally, Escudero 1996 [53] notes that rocky outcrops could significantly reduce the space available for tree species to establish.
In our study, on the one hand, altitude, temperature, and precipitation explained in large part the observed differences in the composition of species. Similar results have been documented in mountain forests and dry forests, where altitude and variables related to water availability are the main determinants of diversity [47,54]. On the other hand, Poulsen et al. 2006 [55] point out that, at the local and regional scale, environmental factors such as altitude, topography, and edaphic parameters are the main drivers of the floristic changes of the vegetation. In addition, environmental changes at different scales in the Amazon basin influence the floristic composition and distribution of plant communities [10,56,57,58,59].
The pH, concentration of cations, nitrogen, phosphorus, copper, and soil organic matter largely explained the changes in the composition of species. As mentioned by Neill 2005 [8], the geological composition of the Cordillera del Cóndor region is key to understanding its floristic composition. This is consistent with similar studies carried out in different tropical ecosystems, where soil parameters play an important role in the determination of species composition [19,60,61,62]. The differences in the composition of species between the Terra Firme and Tepuy forest are mainly due to the fact that the former have a greater amount of nutrients and lower acidity. Homeier et al., 2010 [47] states that there is a significant relationship between tree diversity and soil parameters, such as pH, calcium, nitrogen, phosphorus, magnesium, and potassium. This is important since there is a decrease in the nutrient content and an increase in the acidity of the soil as the altitude increases, which implies a pattern of soil heterogeneity, which conforms to the hypothesis of “habitat heterogeneity” [50,51], which predicts that the heterogeneity of the resources, and, therefore the number of species capable of coexisting, will peak at the sites of availability of intermediate resources, while in the High parts (Tepuy forests) we find forests less rich in species associated with low soil heterogeneity.
Finally, Sollins, in 1998, [63] states that the availability of phosphorus, potassium, calcium, and magnesium significantly influences the composition of species in lowland rainforests, although by integrating other factors such as soil drainage and topography (pending relatively pronounced in the forests of Terra Firme and flat relief in the Tepuy forests in our study) make it necessary to incorporate other factors to understand the distribution of plant wealth. Similar results have been reported in Amazonian forests, where calcium and aluminum have shown a high correlation with species distribution patterns at the regional and local scales, however, microhabitat variability, related to soil heterogeneity, are responsible for the presence of certain species throughout the forest, a factor that we mentioned as important in the determination of groups of plants or types of forest in our study [9,55,58,64,65].
In conclusion, the diversity of the communities of tree species in the Amazonian forests are conditioned by environmental, topographic, and edaphic factors. Altitude, geomorphology, and soil parameters such as acidity, nitrogen, phosphorus, and potassium are responsible for the greatest differences in the composition and species richness of the Terra Firme and Tepuy forests. In the latter, the conditions of a low amount of nutrients and high acidity of the soil allow the establishment of species adapted to living on white sand soils, which are poor in nutrients.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/11/10/196/s1, Table S1: Geographic coordinates and altitude of each monitored permanent plot; Table S2: Values of relative diversity and number of species determined in the forests of southeastern Ecuador; and Table S3: Structural parameters of the three most ecologically important species recorded in each of the plots analyzed in the Terra Firme and Tepuy Forests. DR, relative density; Dom, relative dominance; IVI, importance value index Table S4: Dissimilarity between forest types and sampling sites, species, and abundance values and percentage contribution for dissimilarity are shown.
Author Contributions
Conceptualization, W.Q., Á.B., and J.M.; methodology, W.Q., Á.B., and O.C.; writing—original draft preparation, W.Q., Á.B., J.M., K.C., H.U., I.H., and O.C.; writing—review and editing W.Q., Á.B., and O.C.
Funding
This research received no external funding.
Acknowledgments
W.Q., K.C., and H.U. thank the Universidad Estatal Amazónica for their support in concluding the manuscript; O.C. and A.B. thank the UTPL for their support in the culmination of the manuscript. We thank Diana Szeckely for her help in language revision and Galo Guamán (UTPL) for helping with the GIS analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, H.; Singh, A.; Kant, S.; Zhu, Z.; Waller, E. Integrating Habitat Status, Human Population Pressure, and Protection Status into Biodiversity Conservation Priority Setting. Conserv. Biol. 2005, 19, 1273–1285. [Google Scholar] [CrossRef]
- Guevara Andino, J.E.; Pitman, N.C.A.; ter Steege, H.; Mogollón, H.; Ceron, C.; Palacios, W.; Oleas, N.; Fine, P.V.A. Incorporating Phylogenetic Information for the Definition of Floristic Districts in Hyperdiverse Amazon Forests: Implications for Conservation. Ecol. Evol. 2017, 7, 9639–9650. [Google Scholar] [CrossRef] [PubMed]
- Valencia, R.; Balsley, H.; Paz y Miño, G. High Tree Alpha Diversity in Amazonian Ecuador. Biodivers. Conserv. 1994, 3, 21–28. [Google Scholar] [CrossRef]
- Añazco, M.; Morales, M.; Palacios, W.; Vega, E.; Cuesta, A. Sector Forestal Ecuatoriano: Propuestas para una gestión forestal sostenible; Serie Investigación y Sistematización No. 8; Programa Regional ECOBONA-INTERCOOPERATION: Quito, Ecuador, 2010. [Google Scholar]
- Malhi, Y.; Phillips, O.L.; Lloyd, J.; Baker, T.; Wright, J.; Almeida, S.; Arroyo, L.; Frederiksen, T.; Grace, G.; Higuchi, N.; et al. An international Network to Monitor the Structure, Composition and Dynamics of Amazonian Forests (RAINFOR). J. Veg. Sci. 2002, 13, 439–450. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.M.; Graham, D.J.; Webster, A.L.; Pimm, S.A.; Bookbinder, M.P.; Ledec, G.C. A Conservation Assessment of the Terrestrial Ecoregions of Latin America and the Caribbean; The World Bank/World Wildlife Fund: Washington, DC, USA, 1995. [Google Scholar]
- Duque, A.; Cárdenas, D.; Rodríguez, N. Dominancia Florística y Variabilidad Estructural en Bosques de Tierra Firme en el Noroccidente de la Amazonía Colombiana. Caldasia 2003, 25, 139–152. [Google Scholar]
- Neill, D.A. Cordillera del Cóndor: Botanical Treasures between the Andes and the Amazon. Plant Talk 2005, 41, 17–21. [Google Scholar]
- Silva, J.; Montoya, A.; López, D.; Hurtado, H. Variación florística de especies arbóreas a escala local en un bosque de tierra firme en la Amazonia colombiana. Acta Amaz. 2010, 40, 179–188. [Google Scholar] [CrossRef]
- Gentry, H.A. Tree Species Richness of Upper Amazonian Forests. Proc. Natl. Acad. Sci. USA 1988, 85, 156–159. [Google Scholar] [CrossRef]
- Phillips, O.L.; Hall, P.; Gentry, A.H.; Sawyer, S.A.; Vasquez, R. Dynamics and Species Richness of Tropical Rain Forests. Proc. Natl. Acad. Sci. USA 1994, 91, 2805–2809. [Google Scholar] [CrossRef]
- Valencia, R.; Foster, R.B.; Villa, G.; Condit, R.; Svenning, J.C.; Hernández, C.; Romeleroux, K.; Losos, E.; Magard, E.; Basley, H. Tree Species Distributions and Local Habitat Variation in the Amazon: Large Forest Plot in Eastern Ecuador. J. Ecol. 2004, 92, 214–229. [Google Scholar] [CrossRef]
- ter Steege, H.; Sabatier, D.; Casterllanos, H.; Van Andel, T.; Duivenvoorden, J.; De Oliveira, A.; Ek, R.; Lilwak, R.; Maas, P.; Mori, S. An Analysis of the Floristic Composition and Diversity of Amazonian Forests Including Those of the Guiana Shield. J. Trop. Ecol. 2000, 16, 801–828. [Google Scholar] [CrossRef]
- Fabregas, G. The Cordillera del Cóndor region of Ecuador and Peru: A Biological Assessment; Schulenberg, T.S., Awbrey, K., Eds.; RAP Working Papers 7; Consernation International: Washington, DC, USA, 1997; pp. 1–231. [Google Scholar]
- Vásquez, R.; Phillips, O. Allpahuayo: Floristics, Structure, and Dynamics of a High.Diversity Forest in Amazonian Perú. Ann. Mo. Bot. Gard. 2000, 87, 499–527. [Google Scholar] [CrossRef]
- Pitman, N.C.A.; Terborgh, J.W.; Silman, M.R.; Percy-Núñez, V.; Neill, D.A.; Ceron, C.E.; Palacios, W.A.; Aulestia, M. Dominance and Distribution of Tree Species in Upper Amazonian Terra Firme Forests. Ecology 2001, 82, 2101–2117. [Google Scholar] [CrossRef]
- Ulloa, C.; Neill, D. Phainantha Shuariorum (Melastomataceae), una especie nueva de la Cordillera del Cóndor, Ecuador, disyunta de un género guayanés. Novon 2006, 16, 281–285. [Google Scholar] [CrossRef]
- Guayasamin, J.M.; Bonaccorso, E. (Eds.) Evaluación Ecológica Rápida de la Biodiversidad de los Tepuyes de la Cuenca Alta del Río Nangaritza, Cordillera del Cóndor, Ecuador; Conservación Internacional: Quito, Ecuador, 2011. [Google Scholar]
- Neill, D.; Asanza, M. Lozania nunkui (Lacistemataceae), A New Species from the Sandstone Plateaus of the Cordillera del Cóndor in Ecuador and Peru. Novon 2012, 22, 207–211. [Google Scholar] [CrossRef]
- Dallmeier, F. Long-Term Monitoring of Biological Diversity in Tropical Forest Areas-Methods for Establishment and Inventory of Permanent Plots; United Nations Educational, Scientific and Cultural Organization: Paris, France, 1992. [Google Scholar]
- Campbell, P.; Comiskey, J.; Alonso, A.; Dallmeier, F.; Nuñez, P.; Beltran, H.; Baldeon, S.; Nauray, W.; Colina, R.D.L.; Acurio, L.; et al. Modified Whittaker Plots as An Assessment and Monitoring Tool for Vegetation in A Lowland Tropical Rainforest. Environ. Monit. Assess. 2002, 76, 19–41. [Google Scholar] [CrossRef]
- Condit, R.S.; Ashton, M.S.; Balslev, H.; Brokaw, N.V.L.; Bunyavejchewin, S.; Chuyong, G.B.; Co, L.; Dattaraja, H.S.; Davies, S.J.; Esufali, S.; et al. Tropical Tree A-Diversity: Results from A Worldwide Network of Large Plots. Biologiske Skri. 2005, 55, 565–582. [Google Scholar]
- Brako, L.; Zarucchi, J.L. Catálogo de las Angiospermas y Gimnospermas del Perú. Monogr. Syst. Bot. Mo. Bot. Gard. 1993, 45, 1–1286. [Google Scholar]
- Jørgensen, P.M.; León-Yánez, S. (Eds.) Catalogue of the Vascular Plants of Ecuador. Monogr. Syst. Bot. Mo. Bot. Gard. 1999, 75, 1–1181. [Google Scholar]
- León-Yánez, S.; Valencia, R.; Pitman, N.; Endara, L.; Ulloa, C.; Navarrete, H. (Eds.) Libro rojo de las plantas endémicas del Ecuador, 2nd ed.; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2011. [Google Scholar]
- Cabrera, O.; Benítez, Á.; Cumbicus, N.; Naranjo, C.; Ramón, P.; Tinitana, F.; Escudero, A. Geomorphology and Altitude Effects on the Diversity and Structure of the Vanishing Montane Forest of Southern Ecuador. Diversity 2019, 11, 32. [Google Scholar] [CrossRef]
- Madsen, J.E.; Øllgaard, B. Floristic Composition, Structure, and Dynamics of An Upper Montane rain Forest iSouthern Ecuador. Nord. J. Bot. 1994, 14, 403–423. [Google Scholar] [CrossRef]
- Magurran, A.E. Why diversity? In Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; pp. 1–5. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Mccullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; CRC Press/Chapman and Hall: Boca Raton, FL, USA, 1989. [Google Scholar]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. [Google Scholar]
- Hill, M.O.; Gauch, H.G. Detrended Correspondence Analysis: An Improved Ordination Technique. Vegetation 1980, 42, 47–58. [Google Scholar] [CrossRef]
- ter Braak, C.J.F. Canonical Correspondence Analysis, A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Legendre, P.; Anderson, M.J. Distance-Based Redundancy Analysis: Testing Multispecies Responses in Multifactorial Ecological Experiments. Ecol Monogr 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Oksanen, J. Constrained Ordination: Tutorial with R and Vegan. 2012. Available online: http://cc.oulu.fi/~jarioksa/opetus/metodi/sessio2.pdf (accessed on 31 May 2019).
- Cayuela, L.; Golicher, D.J.; Rey Benayas, J.M.; González-Espinosa, M.; Ramírez-Marcial, N. Fragmentation, Disturbance and Tree Diversity Conservation in Tropical Montane Forests. J. Appl. Ecol. 2006, 43, 1172–1181. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Oksanen, M.J. Package ‘Vegan’. Community Ecology Package; Version, 2(9). 2013. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 12 October 2018).
- Condit, R.; Hubbell, S.P.; Lafrankie, J.V.; Sukumar, R.; Manokaran, N.; Foster, R.B.; Ashton, P.S. Species-Area and Species-Individual Relationships for Tropical Trees: A Comparison of Three 50-ha Plots. J. Ecol. 1996, 84, 549–562. [Google Scholar] [CrossRef]
- Gentry, A.H. Floristic Similarities and Differences between Southern Central America and Upper and Central Amazonia. In Four Neotropical Rainforests; Gentry, A.H., Ed.; Yale University Press: New Haven, CT, USA, 1990; pp. 141–157. [Google Scholar]
- Huamantupa, I. Análisis de la composición arbórea en los bosques amazónicos de tierra firme en la base de los Andes. Tesis de Maestría, Universidad Internacional Menéndez Pelayo, Madrid, Spain, 2009. [Google Scholar]
- Condit, R.; Pitman, N.; Leigh, E.G.; Chave, J.; Terborgh, J.; Foster, R.B.; Nuñez, P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Betadiversity in Tropical Forest Trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef]
- Fine, P.V.A.; Villacorta, R.; Pitman, N.; Mesones, I.; Kembel, W. A Floristic Study of the White-Sand Forests of Peru. Ann. Mo. Bot. Gard. 2010, 97, 283–305. [Google Scholar] [CrossRef]
- Lieberman, D.; Lieberman, M.; Peralta, R.; Hartshorn, G. Tropical Forest Structure and Composition on A Large-Scale Altitudinal Gradient in Costa Rica. J. Ecol. 1996, 84, 137–152. [Google Scholar] [CrossRef]
- Aiba, S.; Kitayama, K. Structure, Composition and Species Diversity in An Altitude-Substrate Matrix of Rain Forest Tree Communities on Mount Kinabalu, Borneo. Plant. Ecol. 1999, 140, 139–157. [Google Scholar] [CrossRef]
- Kessler, M. The Elevational Gradient of Andean Plant Endemism: Varying Influences of Taxon-Specific Traits and Topography at Different Taxonomic Levels. J. Biogeogr. 2002, 29, 1159–1165. [Google Scholar] [CrossRef]
- Homeier, J.; Breckle, S.; Gunter, S.; Rollembeck, R.; Leuschner, C. Tree Diversity, Forest Structure and Productivity along Altitudinal and Topographical Gradients in a Species-Rich Ecuadorian Montane Rain Forest. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Clinebell, R.R.; Phillips, O.L.; Gentry, A.H.; Stark, N.; Zuuring, H. Prediction of Neotropical Tree and Liana Species Richness from Soil and Climatic Data. Biodivers. Conserv. 1995, 4, 56–90. [Google Scholar] [CrossRef]
- Givnish, T.J. On the Causes of Gradients in Tropical Tree Diversity. J. Ecol. 1999, 87, 193–210. [Google Scholar] [CrossRef]
- Tilman, D.; Pacala, S. The Maintenance of Species Diversity in Plant Communities. Species Diversity in Ecological Communities; University of Chicago Press: Chicago, IL, USA, 1993. [Google Scholar]
- Rajaniemi, T.K. Explaining Productivity-Diversity Relationships in Plants. Oikos 2003, 101, 449–457. [Google Scholar] [CrossRef]
- Slik, J.F.; Raes, N.; Aiba, S.I.; Brearley, F.Q.; Cannon, C.H.; Meijaard, E.; Nagamasu, H.; Nilus, R.; Paoli, G.; Poulsen, A.; et al. Environmental Correlates for Tropical Tree Diversity and Distribution Patterns in Borneo. Divers. Distrib. 2009, 15, 523–532. [Google Scholar] [CrossRef]
- Escudero, A. Community Patterns on Exposed Cliffs in a Mediterranean Calcareous Mountain. Vegetatio 1996, 125, 99–110. [Google Scholar] [CrossRef]
- Espinosa, C.I.; Cabrera, O.; Luzuriaga, A.L.; Escudero, A. What Factors Affect Diversity and Species Composition of Endangered Tumbesian Dry Forests in Southern Ecuador? Biotropica 2011, 43, 15–22. [Google Scholar] [CrossRef]
- Poulsen, A.D.; Tuomisto, H.; Baslev, H. Edaphic and Floristic Variation a 1-ha Plot of Lowland Amazonian Rain Forest. Biotropica 2006, 38, 468–478. [Google Scholar] [CrossRef]
- Duivenvoorden, J.F.; Lips, J.M. A Land-Ecological Study of Soils, Vegetation, and Plant Diversity in Colombian Amazonia; Tropenbos Foundation: Wageningen, The Netherlands, 1995. [Google Scholar]
- Toumisto, H.; Ruokolainen, K.; Kalliola, R.; Linna, A.; Danjoy, W.; Rodriguez, M. Dissecting Amazonian Biodiversity. Science 1995, 269, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H.; Ruokolainen, K.; Yli-Halla, M. Dispersal, Environment, and Floristic Variation of Western Amazonian Forests. Science 2003, 299, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Sanchez, M.; Cavelier, J.; Duivenvoorden, J.F. Different Floristic Patterns of Woody Understorey and Canopy Plants in Colombian Amazonia. J. Trop. Ecol. 2002, 18, 499–525. [Google Scholar] [CrossRef]
- Phillips, O.L.; Nuñez, P.; Vargas, A.; Monteagudo, A.; Peñacruz, A.; Chuspezans, M.E.; Galiano, W.; Sanchez, M.; Yli-Halla, M.; Rose, S. Habitat Association among Amazonian Tree Species: A Landscape Scale Approach. J. Ecol. 2003, 91, 757–775. [Google Scholar] [CrossRef]
- ter Steege, H.; Pitman, N.C.A.; Phillips, O.L.; Chave, J.; Sabatier, D.; Duque, A.; Molino, J.F.; Prevost, M.-F.; Spichiger, R.; Castellanos, H.; et al. Continental-Scale Patterns of Canopy Tree Composition and Function across Amazonia. Nature 2006, 443, 444–447. [Google Scholar] [CrossRef]
- Chytry, M.; Danihelka, J.; Kubesova, S.; Lustyk, P.; Ermakov, N.; Hajek, M.; Hajkova, P.; Koci, M.; Otypkova, Z.; Rolecek, J.; et al. Diversity of Forest Vegetation across a Strong Gradient of Climatic Continentally: Western Sayan Mountains, Southern Siberia. Plant Ecol. 2008, 196, 61–83. [Google Scholar] [CrossRef]
- Sollins, P. Factors Influencing Species Composition in Tropical Lowland Rain Forest: Does Soil Matter? Ecology 1998, 79, 23–30. [Google Scholar] [CrossRef]
- Duque, A.; Sanchez, M.; Cavelier, J.; Duivenvoorden, J.F.; Mirana, P.; Mirana, J.; Mtapi, A. 2001 Relación Bosque-Ambiente en el Medio Caquetá, Amazonia colombiana. In Evaluación de recursos vegetales no maderables en la Amazonia noroccidental; Duivenvoorden, J., Balslev, H., Cavelier, J., Grandez, C., Tuomisto, H., Valencia, R., Eds.; Fluid Phase Equilibria 2001; Caracterización, Ambiental; IBED, Universiteit van Amsterdam: Amsterdam, The Netherlands, 2001; pp. 23–30. [Google Scholar]
- Jones, M.M.; Tuomisto, H.; Olivas, P.C. Differences in the Degree of Environmental Control on Large and Small Tropical Plants: Just a Sampling Effect? J. Ecol. 2008, 96, 367–377. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).