How Does an Invasive Cyprinid Benefit from the Hydrological Disturbance of Mediterranean Temporary Streams?
Abstract
1. Introduction
2. Materials and Methods
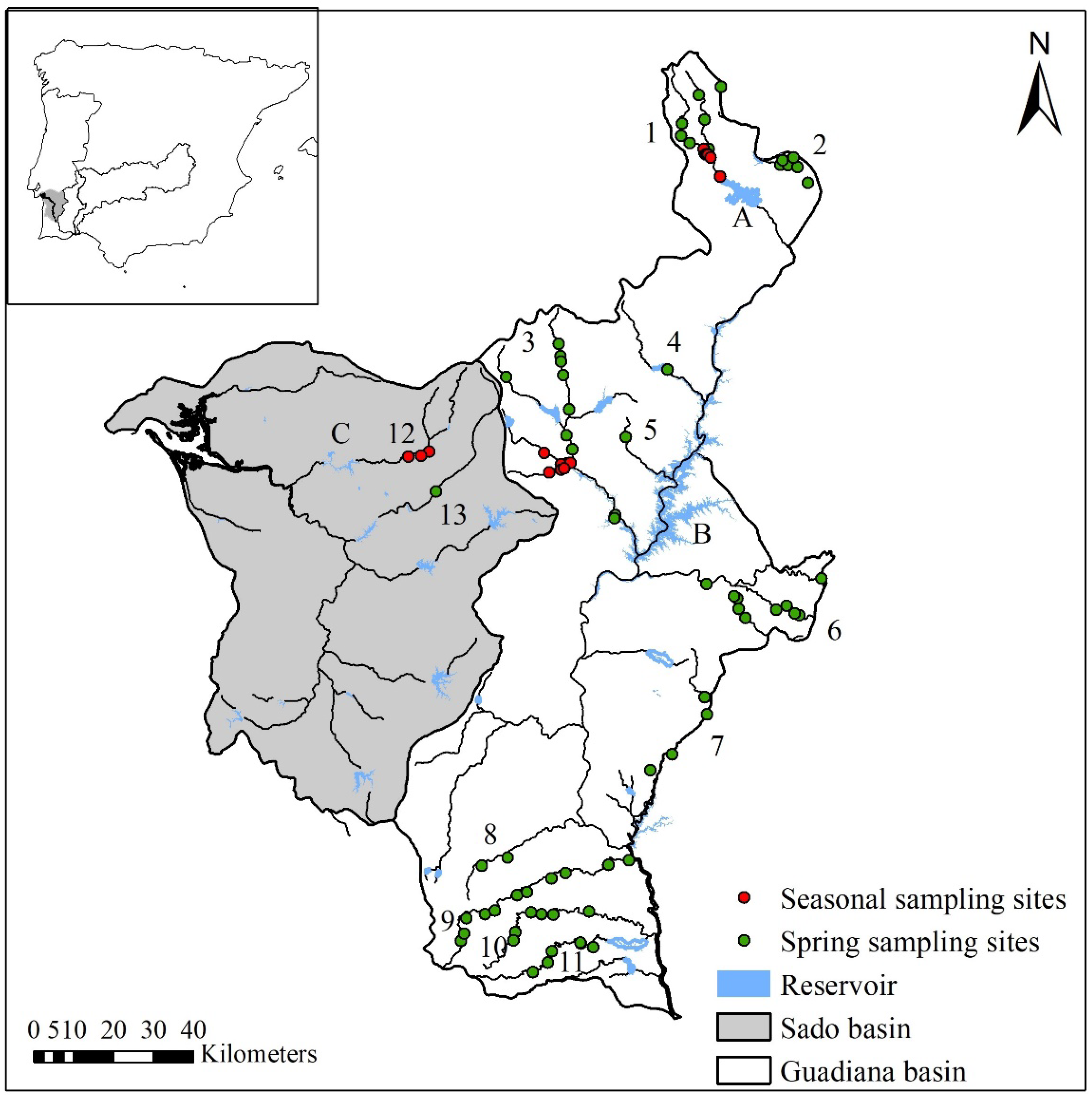
2.1. Study Area
2.2. Fish Sampling and Data Collection
2.3. Data Analysis
2.3.1. Spring Samples
2.3.2. Seasonal Samples
3. Results
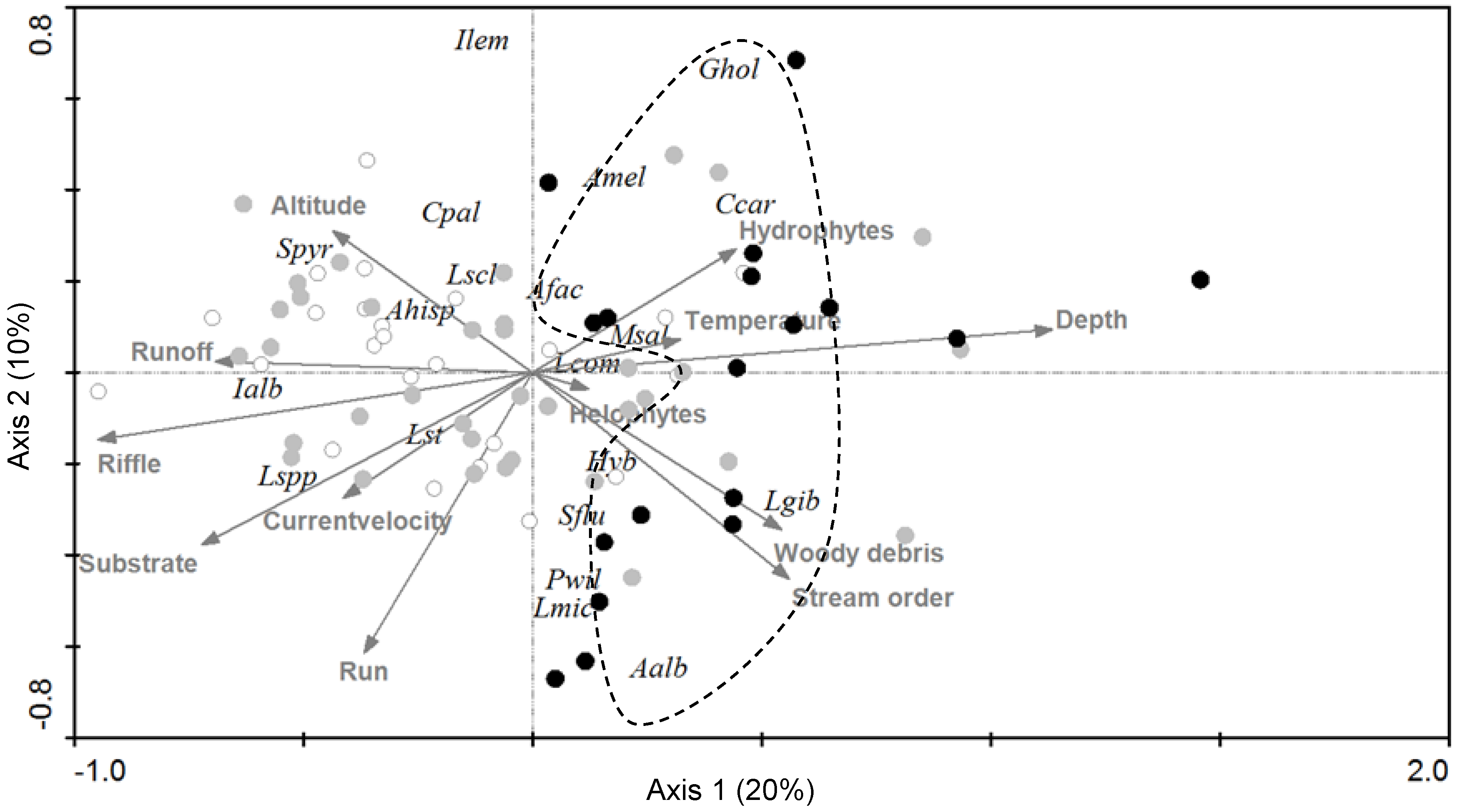
3.1. Fish Species, Environmental Factors, and Anthropogenic Disturbance
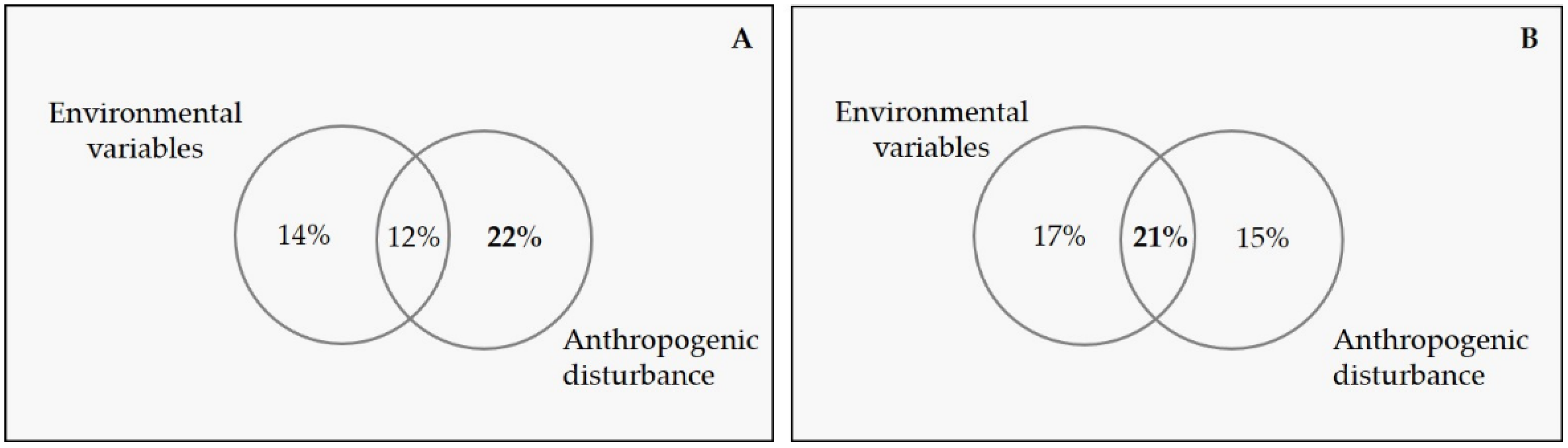
3.2. Relative Importance of Environmental Variables and Anthropogenic Disturbance
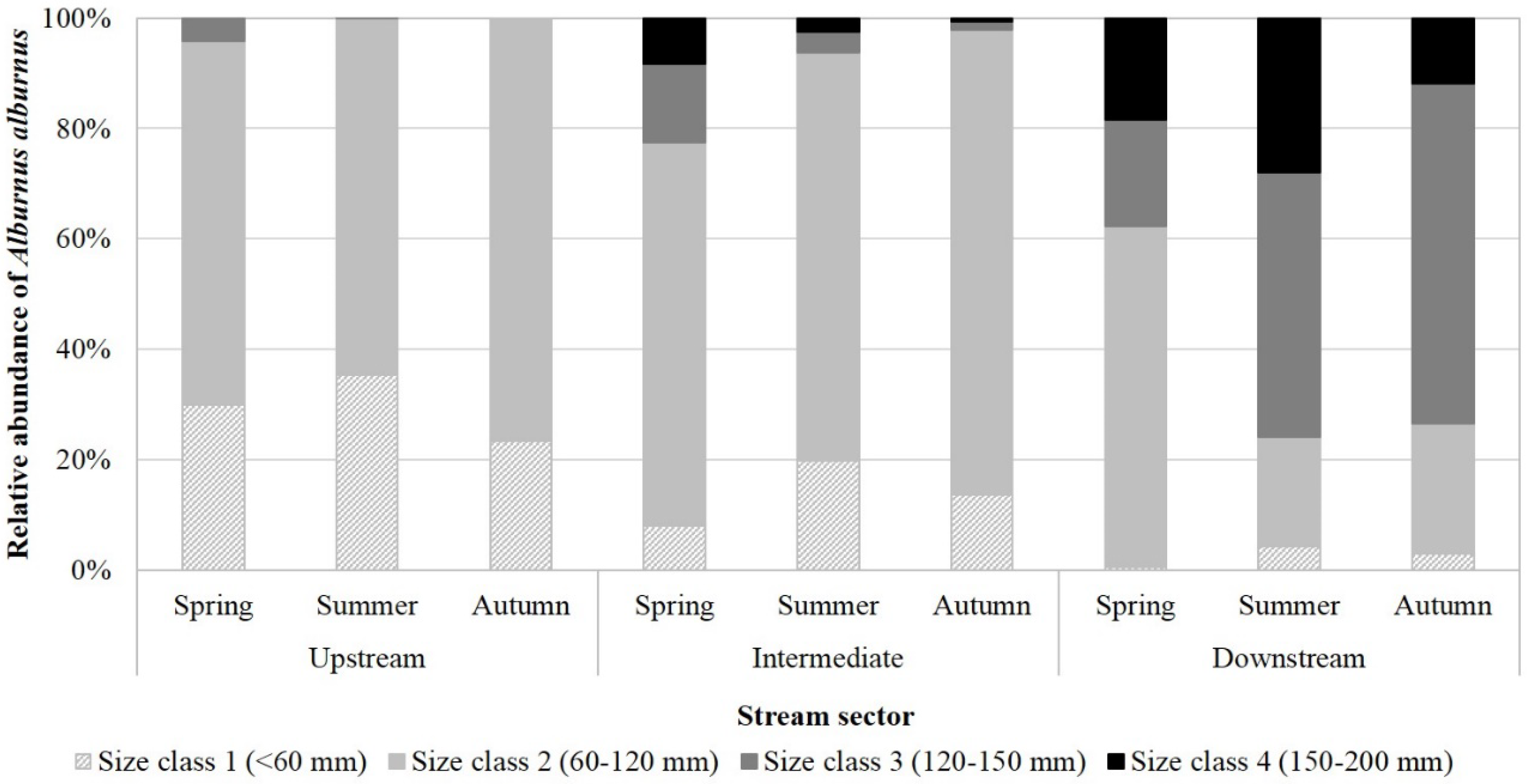
3.3. Spatiotemporal Distribution of A. alburnus
4. Discussion
4.1. Patterns and Drivers of Spatial Distribution
4.2. Possible Seasonal Movements in Temporary Streams
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variables | Description | Assessment Scale | Score | Criteria | Methods |
|---|---|---|---|---|---|
| Land use | Impact of farming/forestry practices | River segment | 5 | >40% Agricultural use (intensive agriculture), very severe impact (rice field) | Local expert assessment complemented with Corine Land Cover (2000, 2006) * |
| 4 | >40% Strong impact (area with strong forestry, including clearcuts) | ||||
| 3 | <40% Moderate impact (subsistence gardens, pastures) | ||||
| 2 | <40% Small impact (cork and holm oaks, high-growth forest) | ||||
| 1 | <10% No significant impacts (natural forest and bush) | ||||
| Land cover and bankface characterization | Local | 5 | Irrigated crops and/or high stocking | ||
| 4 | Horticultural crops, semi-intensive grazing | ||||
| 3 | Extensive cultures (e.g., pastures, cereal crops, pine, eucalyptus), extensive grazing | ||||
| 2 | Cork and holm oaks | ||||
| 1 | Natural | ||||
| Urban area | Impact of urban areas | River segment | 5 | Very severe (location near a city with basic sanitation needs) | Local expert assessment complemented with Corine Land Cover (2000, 2006) * |
| 4 | Town | ||||
| 3 | Village | ||||
| 2 | Hamlet | ||||
| 1 | Negligible (isolated dwellings) | ||||
| Riparian vegetation | Deviation from the natural state of the riparian zone | River segment | 5 | Lack of riparian shrubs and trees (only the presence of annual plants) | Local expert assessment |
| 4 | Fragmented vegetation with bushes and/or the presence of reed | ||||
| 3 | Second replacement step (dominance of dense brushwood) | ||||
| 2 | First replacement step (presence of shrub or tree strata with some level of preservation). | ||||
| 1 | Potential vegetation (presence of shrub and tree strata according to the geo-series) | ||||
| Morphological condition | Deviation from the natural state of the stream bed and banks | Local | 5 | Transverse and longitudinal profile of the channel completely changed, with very few habitats | Local expert assessment |
| 4 | Channelized sector, missing most of the natural habitats | ||||
| 3 | Channelized sector, missing some types of natural habitats, but maintaining much of the shape of the natural channel | ||||
| 2 | Poorly changed sector, close to the natural mosaic of habitats. | ||||
| 1 | Morphological changes absent or negligible | ||||
| Sediment load | Deviation from the natural sediment load (both carried in the water column and deposited on the riverbed) | River segment and local | 5 | >75% of coarse particles of the stream bed are covered with fine sediments (sand, silt, clay) | Local expert assessment |
| 4 | 50–75% of coarse particles of the stream bed are covered with fine sediments (sand, silt, clay) | ||||
| 3 | 25–50% of coarse particles of the stream bed are covered with fine sediments (sand, silt, clay) | ||||
| 2 | 5–25% of coarse particles of the bed are covered with fine sediments (sand, silt, clay) | ||||
| 1 | <5% of coarse particles of the stream bed are covered with fine sediments (sand, silt, clay) | ||||
| Hydrological regime | Deviation from the natural hydrological regime (flow pattern and/or quantity). Includes all sources of hydrologic alteration, such as significant water abstraction. | Local (classes regarding flow pattern) | 5 | <50% and strong deviation from the natural variability of the flow regime | Local expert assessment complemented with information from gauging stations (SNIRH) ** |
| 4 | <50% and moderate deviation from the natural variability of the flow regime | ||||
| 3 | >50% and duration of flood periods close to the natural | ||||
| 2 | >75% and duration of flood periods close to the natural | ||||
| 1 | >90% and normal duration of natural flood periods | ||||
| Local (classes regarding mean annual discharge) | 5 | <10% of mean annual discharge | |||
| 4 | <15% of mean annual discharge | ||||
| 3 | >15% of mean annual discharge | ||||
| 2 | >30% of mean annual discharge | ||||
| 1 | >90% of mean annual discharge | ||||
| Toxic and acidification levels | Deviation from the natural state of toxicity conditions, including acidification, and oxygen levels | Local | 5 | Constant for long periods (months) or frequent occurrence of strong deviations from natural conditions (e.g., pH < 5.0, DO < 30%) | Local expert assessment complemented with information from gauging stations (SNIRH) ** |
| 4 | Constant for long periods (months) or frequent occurrence of strong deviations from natural conditions (e.g., pH < 5.5, DO < 30–50%) | ||||
| 3 | Occasional deviations (single measurements or episodic) in relation to natural conditions (e.g., pH < 5.5, DO < 30–50%) | ||||
| 2 | Occasional deviations (single measurements or episodic) in relation to natural conditions (e.g., pH < 6.0) | ||||
| 1 | Conditions within the normal range of variation | ||||
| Organic and nutrient loads | Deviation from the normal values of BOD, COD, ammonium, nitrate, and phosphate concentrations | Local | 5 | >20% of values in classes D or E | SNIRH ** (classification of water quality for multiple uses, according to the guidelines from the Water National Institute), complemented with local expert assessment |
| 4 | >10% of values in classes D or E | ||||
| 3 | >10% of values in class C | ||||
| 2 | No obvious or too small signs of eutrophication and organic loading | ||||
| 1 | No signs of eutrophication and organic loading | ||||
| Artificial lentic water bodies | Impact related to the presence of artificial lentic water bodies upstream and/or downstream of the site (upstream change in thermal and flow regimes; downstream invasion by exotic species of lentic character) | Local | 5 | Local immediately downstream of a large reservoir or within the influence area of its backwater | SNIRH ** and available cartography |
| 4 | Local immediately downstream of a mini-hydro or within the influence area of its backwater | ||||
| 3 | Local downstream of a massive standing water body or within the influence area of the reservoir | ||||
| 2 | Local downstream of a mini-hydro or within the influence area of its backwater | ||||
| 1 | No influence of reservoirs | ||||
| Connectivity | Impact of artificial barriers to fish migration | River basin and segment | 5 | Permanent artificial barrier | SNIRH **, available cartography, documental data and local expert assessment |
| 4 | Occasional passage of some species | ||||
| 3 | Passage of certain species or only in certain years | ||||
| 2 | Passage of most species in most years | ||||
| 1 | No barriers or existence of an effective pass-through device |
References
- Gasith, A.; Resh, V.H. Streams in Mediterranean climate regions: Abiotic influences and biotic responses to predictable seasonal events. Annu. Rev. Ecol. Syst. 1999, 30, 51–81. [Google Scholar] [CrossRef]
- Poff, N.L.; Ward, J.V. Implications of streamflow variability and predictability for lotic community structure: A regional analysis of streamflow patterns. Can. J. Fish. Aquat. Sci. 1989, 46, 1805–1818. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D. Functional organization of stream fish assemblages in relation to hydrologic variability. Ecology 1995, 76, 606–627. [Google Scholar] [CrossRef]
- Richter, B.D.; Mathews, R.; Harrison, D.L.; Wigington, R. Ecologically sustainable water management: Managing river flows for ecological integrity. Ecol. Appl. 2003, 13, 206–224. [Google Scholar] [CrossRef]
- Marchetti, M.P.; Moyle, P.B. Effects of flow regime and habitat structure on fish assemblages in a regulated California stream. Ecol. Appl. 2001, 11, 530–539. [Google Scholar] [CrossRef]
- Magalhães, M.F.; Batalha, D.C.; Collares-Pereira, M.J. Gradients in stream fish assemblages across a Mediterranean landscape: Contributions of environmental factors and spatial structure. Freshw. Biol. 2002, 47, 1015–1031. [Google Scholar] [CrossRef]
- Magalhães, M.F.; Beja, P.; Schlosser, I.J.; Collares-Pereira, M.J. Effects of multi-year droughts on fish assemblages of seasonally drying Mediterranean streams. Freshw. Biol. 2007, 52, 1494–1510. [Google Scholar] [CrossRef]
- Bernardo, J.M.; Ilhéu, M.; Matono, P.; Costa, A.M. Interannual variation of fish assemblage structure in a Mediterranean river: Implications of streamflow on the dominance of native or exotic species. River Res. Appl. 2003, 19, 1–12. [Google Scholar] [CrossRef]
- Ilhéu, M. Patterns of Habitat Use by Freshwater Fishes in Mediterranean Rivers. Ph.D. Thesis, University of Évora, Évora, Portugal, 2004. [Google Scholar]
- Economidis, P.S. Endangered freshwater fishes of Greece. Biol. Conserv. 1995, 72, 201–211. [Google Scholar] [CrossRef]
- Collares-Pereira, M.J.; Cowx, I.G.; Ribeiro, F.; Rodrigues, J.A.; Rogado, L. Threats imposed by water resources development schemes on the conservation of the endangered fish species in the Guadiana River, Portugal. Fish. Manag. Ecol. 2000, 7, 167–178. [Google Scholar] [CrossRef]
- Moyle, P.B.; Light, T. Biological invasions of freshwater: Empirical rules and assembly theory. Biol. Conserv. 1996, 78, 149–161. [Google Scholar] [CrossRef]
- Bunn, S.E.; Arthington, A.H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manage. 2002, 30, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Kennard, M.J.; Arthington, A.H.; Pusey, B.J.; Harch, B.D. Are alien fish a reliable indicator of river health? Freshw. Biol. 2005, 50, 174–193. [Google Scholar] [CrossRef]
- Gehrke, P.C.; Harris, J.H. Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia. Regul. River 2001, 17, 369–391. [Google Scholar] [CrossRef]
- Filipe, A.F.; Marques, T.; Seabra, S.; Tiago, P.; Ribeiro, F.; Moreira da Costa, L.; Cowx, I.G.; Collares-Pereira, M.J. Selection of priority areas for fish conservation in Guadiana River Basin, Iberian Peninsula. Conserv. Biol. 2004, 18, 189–200. [Google Scholar] [CrossRef]
- Leprieur, F.; Beauchard, O.; Blanchet, S.; Oberdorff, T.; Brosse, S. Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biol. 2008, 6, e28. [Google Scholar] [CrossRef]
- Marr, S.M.; Marchetti, M.P.; Olden, J.D.; García-Berthou, E.; Morgan, D.L.; Arismendi, I.; Day, J.A.; Griffiths, C.L.; Skelton, P.H. Freshwater fish introductions in mediterranean-climate regions: Are there commonalities in the conservation problem? Divers. Distrib. 2010, 16, 606–619. [Google Scholar] [CrossRef]
- Rahel, F.J. Homogenization of fish faunas across the United States. Science 2000, 288, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Marr, S.M.; Olden, J.D.; Leprieur, F.; Arismendi, I.; Ćaleta, M.; Morgan, D.L.; Nocita, A.; Šanda, R.; Tarkan, A.S.; García-Berthou, E. A global assessment of freshwater fish introductions in mediterranean-climate regions. Hydrobiologia 2013, 719, 317–329. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M.; Blanco-Garrido, F.; Prenda, J. Invasive species and habitat degradation in Iberian streams: An analysis of their role in freshwater fish diversity loss. Ecol. Appl. 2011, 21, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Cucherousset, J.; Olden, J.D. Ecological Impacts of Nonnative Freshwater Fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Leunda, P.M. Impacts of non-native fishes on Iberian freshwater ichthyofauna: Current knowledge and gaps. Aquat. Invasions 2010, 5, 239–262. [Google Scholar] [CrossRef]
- Elvira, B.; Almodóvar, A. Freshwater fish introductions in Spain: Facts and figures at the beginning of the 21st century. J. Fish Biol. 2001, 59, 323–331. [Google Scholar] [CrossRef]
- Ribeiro, F.; Collares-Pereira, M.J.; Moyle, P.B. Non-native fish in the fresh waters of Portugal, Azores and Madeira Islands: A growing threat to aquatic biodiversity. Fish. Manag. Ecol. 2009, 16, 255–264. [Google Scholar] [CrossRef]
- Freyhof, J.; Kottelat, M. Alburnus alburnus. The IUCN Red List of Threatened Species. 2008. Available online: http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T789A13079658.en (accessed on 2 October 2017).
- Elvira, B. Native and exotic freshwater fishes in Spanish river basins. Freshw. Biol. 1995, 33, 103–108. [Google Scholar] [CrossRef]
- Pérez-Bote, J.L.; Roso, R.; Pula, H.J.; Díaz, F.; López, M.T. Primeras citas de la lucioperca, Sander (= Stizostedion) lucioperca (Linnaeus, 1758) y del alburno, Alburnus alburnus (Linnaeus, 1758) en las cuencas extremeñas de los ríos Tajo y Guadiana, SO de la Península Ibérica. Ann. Biol. 2004, 26, 93–100. [Google Scholar]
- Velasco, J.C. Peces. In Guía de los Peces, Anfibios, Reptiles y Mamíferos de Castilla y León; Velasco, J.C., Lizana, M., Román, J., Delibes, M., Fernández, J., Eds.; Náyade: Medina del Campo, Spain, 2005. [Google Scholar]
- Vinyoles, D.; Robalo, J.I.; de Sostoa, A.; Almodóvar, A.M.; Elvira, B.; Nicola, G.G.; Fernández-Delgado, C.; Santos, S.; Doadrio, I.; Sardá-Palomera, F.; et al. Spread of the alien bleak Alburnus alburnus (Linnaeus, 1758) (Actinopterygii, Cyprinidae) in the Iberian Peninsula: The role of reservoirs. Graellsia 2007, 63, 101–110. [Google Scholar] [CrossRef]
- Brabrand, A. Distribution of fish and food of roach Rutilus rutilus, bleak Alburnus alburnus, bream Abramis brama and ruffe Acerina cernua in Lake Vansjo Southeast Norway. Fauna 1983, 36, 57–64. [Google Scholar]
- Almeida, D.; Stefanoudis, P.V.; Fletcher, D.H.; Rangel, C.; da Silva, E. Population traits of invasive bleak Alburnus alburnus between different habitats in Iberian fresh waters. Limnologica 2014, 46, 70–76. [Google Scholar] [CrossRef]
- Masó, G.; Latorre, D.; Tarkan, A.S.; Vila-Gispert, A.; Almeida, D. Inter-population plasticity in growth and reproduction of invasive bleak, Alburnus alburnus (Cyprinidae, Actinopterygii), in northeastern Iberian Peninsula. Folia Zool. 2016, 65, 10–14. [Google Scholar] [CrossRef]
- Myers, J.H.; Simberloff, D.A.; Kuris, A.M.; Carey, J. Eradication revisited: Dealing with exotic species. Trends Ecol. Evol. 2000, 15, 316–320. [Google Scholar] [CrossRef]
- Agência Portuguesa Do Ambiente. Atlas do Ambiente Digital. Available online: http://sniambapambiente.pt/ (accessed on 27 October 2017).
- Miranda, P.; Coelho, F.S.; Tomé, A.R.; Valente, M.A. 20th century Portuguese climate and climate scenarios. In Climate Change in Portugal: Scenarios, Impacts and Adaptation Measures—SIAM Project; Santos, F.D., Forbes, K., Moniz, R., Eds.; Gradiva: Lisboa, Portugal, 2002; pp. 23–84. Available online: http://www.siam.fc.ul.pt/SIAM_Book (accessed on 20 September 2017).
- WWF Mediterranean Programme. Water Footprint in Portugal—An Analysis of the External Footprint and Consumption; Technical Report; WWF Mediterranean Programme. 2011. Available online: www.wwf.pt (accessed on 20 September 2017).
- Matono, P.; Sousa, D.; Ilheu, M. Effects of land use intensification on fish assemblages in Mediterranean climate streams. Environ. Manage. 2013, 52, 1213–1229. [Google Scholar] [CrossRef] [PubMed]
- Sistema Nacional de Informação de Recursos Hídricos. Available online: http://snirh.pt (accessed on 20 September 2017).
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Cabral, M.J.; Almeida, J.; Almeida, P.R.; Dellinger, T.R.; Ferrand de Almeida, N.; Oliveira, M.E.; Palmeirim, J.M.; Queiroz, A.I.; Rogado, L.; Santos-Reis, M. Livro Vermelho dos Vertebrados de Portugal; Instituto da Conservação da Natureza: Lisboa, Portugal, 2005. [Google Scholar]
- Smith, K.G.; Darwall, W.R.T. The Status and Distribution of Freshwater Fish Endemic to the Mediterranean Basin; IUCN: Gland, Switzerland; Cambridge, UK, 2006. [Google Scholar]
- Hermoso, V.; Clavero, M. Threatening processes and conservation management of endemic freshwater fish in the Mediterranean basin: A review. Mar. Freshw. Res. 2011, 62, 244–254. [Google Scholar] [CrossRef]
- Ilhéu, M.; Matono, P.; Bernardo, J.M.; Costa, A.M.; Miguens, F.; Ribeiro, L.; Oliveira, R.P. Alterações Climáticas e Comunidades Piscícolas de Cursos de Tipo Mediterrânico. Impacte Potencial na Bio-Integridade e Implicações na Avaliação do Estado Ecológico; Relatório Final de Projeto PTDC/AAC-AMB/102541/2008; Universidade de Évora: Évora, Portugal, 2014. [Google Scholar]
- Inag, I.P. Manual Para a Avaliação Biológica da Qualidade da Água em Sistemas Fluviais Segundo a Directiva Quadro da Água Protocolo de Amostragem e Análise Para a Fauna Piscícola; Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional, Instituto da Água, I.P: Lisboa, Portugal, 2008. [Google Scholar]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000. Establishing a Framework for Community Action in the Field of Water Policy; Official Journal of the European Communities L327. 2000. Available online: http://ec.europa.eu/environment/water/water-framework/index_en.html (accessed on 8 June 2016).
- European Union. Directive 2010/63/EU of The European Parliament and of the Council of 22 September 2010 on the Protection of Animals used for Scientific Purposes. Off. J. Eur. Union 2010, L276, 33–79. [Google Scholar]
- Giller, P.S.; Malmqvist, B. The Biology of Streams and Rivers; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Fame Group. Development, Evaluation & Implementation of a Standardised Fish-based Assessment Method for the Ecological Status of European Rivers—A Contribution to the Water Framework Directive; Final Report, Scientific Achievements (Sections 5 & 6) (Co-Ordinator: Stefan Schmutz); Institute for Hydrobiology and Aquatic Ecosystem Management, University of Natural Resources and Applied Life Sciences: Vienna, Austria, 2004; Available online: http://fame.boku.ac.at (accessed on 8 June 2016).
- Matono, P.; Bernardo, J.M.; Costa, A.M.; Ilhéu, M. Fish response to anthropogenic pressures in temporary streams: The importance of environmental drivers. River Res. Appl. 2014, 30, 1281–1295. [Google Scholar] [CrossRef]
- CIS-WFD—Guidance on Establishing Reference Conditions and Ecological Status Class Boundaries for Inland Surface Waters. Final Version, EU Common Implementation Strategy for the Water Framework Directive. 2003. Available online: http://ec.europa.eu/environment/water/water-framework/index_en.html (accessed on 8 June 2016).
- Lorenzen, C.J. Determination of chlorophyll and pheopigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Environmental Federation: Washington, DC, USA, 1998. [Google Scholar]
- Jongman, R.H.G.; ter Braak, C.J.F.; van Tongeren, O.F.R. Data Analysis in Community and Landscape Ecology; Pudoc: Wageningen, The Netherlands, 1987. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4.0); Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Barbosa, A.M.; Brown, J.A.; Jiménez-Valverde, A.; Real, R. modEvA—An R Package for Model Evaluation and Analysis; R Package Version 0.1. 2014. Available online: http://modeva.r-forge.r-project.org/ (accessed on 5 July 2017).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kottelat, M. European Freshwater Fishes; Slovak Academy of Sciences: Štefánikova, Slovakia, 1997. [Google Scholar]
- Bhattacharya, C.G. A simple method of resolution of a distribution into Gaussian components. Biometrics 1967, 23, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Sturges, H.A. The choice of a class interval. J. Am. Stat. Assoc. 1926, 21, 65–66. [Google Scholar] [CrossRef]
- Scott, D.W. Sturges’ rule. WIREs Comp. Stat. 2009, 1, 303–306. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 5 July 2017).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011; Available online: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion (accessed on 5 July 2017).
- Barton, K. MuMIn: Multi-model Inference; R Package Version 1.4. 2017. Available online: http://r-forge.r-project.org/projects/mumin/ (accessed on 5 July 2017).
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Arthington, A.H.; Hamlet, S.; Bluhdorn, D.R. The role of habitat disturbance in the establishment of introduced warm-water fishes in Australia. In Introduced and Translocated Fishes and Their Ecological Effect. Bureau of Rural Resources Proceedings No. 8; Pollard, D.A., Ed.; Australian Government Publishing Service: Canberra, Australia, 1990; pp. 61–66. [Google Scholar]
- Ross, R.; Lellis, W.A.; Bennett, R.M.; Johnson, C.S. Landscape determinants of non-indigenous fish invasions. Biol. Invasions 2001, 3, 347–361. [Google Scholar] [CrossRef]
- Meador, M.R.; Brown, L.R.; Short, T. Relations between introduced fish and environmental Conditions at large geographic scales. Ecol. Indic. 2003, 3, 81–92. [Google Scholar] [CrossRef]
- Ilhéu, M.; Matono, P.; Bernardo, J.M. Invasibility of Mediterranean-Climate Rivers by Non-Native Fish: The Importance of Environmental Drivers and Human Pressures. PLoS ONE 2014, 9, e109694. [Google Scholar] [CrossRef] [PubMed]
- Lepart, J.; Debussche, M. Human Impact on Landscape Patterning: Mediterranean Examples. In Landscape Boundaries, Ecological Studies (Analysis and Synthesis); Hansen, A.J., di Castri, F., Eds.; Springer: New York, NY, USA, 1992; Volume 92, pp. 76–106. [Google Scholar]
- Davis, M.; Grime, J.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Belote, R.T.; Jones, R.H.; Hood, S.M.; Wender, B. Diversity-invasibility across an experimental disturbance gradient in Appalachian forests. Ecology 2008, 89, 183–192. [Google Scholar] [CrossRef] [PubMed]
- González, A.L.; Kominoski, J.S.; Danger, M.; Ishida, S.; Iwai, N.; Rubach, A. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 2010, 119, 779–790. [Google Scholar] [CrossRef]
- Clavero, M.; Blanco-Garrido, F.; Prenda, J. Fish fauna in Iberian Mediterranean basins: Biodiversity, introduced species and damming impacts. Aquat. Conserv. 2004, 14, 575–585. [Google Scholar] [CrossRef]
- Niemela, J.; Spence, J.R. Distribution and abundance of an exotic ground beetle (Carabidae): A test of community impact. Oikos 1991, 62, 351–359. [Google Scholar] [CrossRef]
- Townsend, C.R. Invasion biology and ecological impacts of brown trout Salmo trutta in New Zealand. Biol. Conserv. 1996, 78, 13–22. [Google Scholar] [CrossRef]
- McIntosh, A.R. Habitat and size-related variations in exotic trout impacts on native galaxiid fishes in New Zealand streams. Can. J. Fish. Aquat. Sci. 2000, 57, 2140–2151. [Google Scholar] [CrossRef]
- Baltz, D.M.; Moyle, P.B. Invasion resistance to introduced species by a native assemblage of California stream fishes. Ecol. Appl. 1993, 3, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.R. Individual, population, community and ecosystem consequences of a fish invader in New Zealand streams. Conserv. Biol. 2003, 17, 38–47. [Google Scholar] [CrossRef]
- Chown, S.L.; Gremmen, N.J.M.; Gaston, K.J. Ecological biogeography of southern ocean islands: Species–area relationships, human impacts, and conservation. Am. Nat. 1998, 152, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.W.; Irwin, R.E. Linking economic activities to the distribution of exotic plants. Proc. Natl. Acad. Sci. USA 2004, 101, 17725–17730. [Google Scholar] [CrossRef] [PubMed]
- Meyerson, L.A.; Mooney, H.A. Invasive alien species in an era of globalization. Front. Ecol. Environ. 2007, 5, 199–208. [Google Scholar] [CrossRef]
- Matono, P.; da Silva, J.; Bernardo, J.M.; Costa, A.M.; Ilhéu, M. Patterns of Habitat Use of the Endangered Saramugo, Anaecypris hispanica, and the Invasive Bleak, Alburnus alburnus in Mediterranean Temporary Rivers: Potential Negative Interactions. Unpublished work. 2018. [Google Scholar]
- Godinho, F.N.; Ferreira, M.T.; Cortes, R.V. Composition and spatial organization of fish assemblages in the lower Guadiana basin, southern Iberia. Ecol. Freshw. Fish 1997, 6, 134–143. [Google Scholar] [CrossRef]
- Havel, J.E.; Lee, C.E.; Vander Zanden, M.J. Do reservoirs facilitate invasions into landscapes? BioScience 2005, 55, 518–525. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Olden, J.D.; Vander Zanden, M.J. Dam invaders: Impoundments facilitate biological invasions into freshwaters. Front. Ecol. Environ. 2008, 6, 357–363. [Google Scholar] [CrossRef]
- Welcomme, R.L. River Fisheries; FAO Technical Paper 262; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- Power, M.E. Predator avoidance by grazing fishes in temperate and tropical streams: Importance of stream depth and prey size. In Predation: Direct and Indirect Impacts on Aquatic Communities; Kerfoot, W.C., Sih, A., Eds.; University of New England Press: Hanover, NH, USA, 1987; pp. 333–353. [Google Scholar]
- Schlosser, I.J. A conceptual framework for fish communities in small warmwater streams. In Community and Evolutionary Ecology of North American Stream Fishes; Matthews, W.J., Heins, D.C., Eds.; University of Oklahoma Press: Norman, OK, USA, 1987; pp. 17–24. [Google Scholar]
- Moore, K.M.S.; Gregory, S.V. Summer habitat utilization and ecology of cutthroat trout fry (Salmo clarki) in Cascade Mountain streams. Can. J. Fish. Aquat. Sci. 1988, 45, 1921–1930. [Google Scholar] [CrossRef]
- Pires, A.M.; Cowx, I.G.; Coelho, M.M. Seasonal changes in fish community structure of intermittent streams in the middle reaches of the Guadiana basin, Portugal. J. Fish Biol. 1999, 54, 235–249. [Google Scholar] [CrossRef]
- Sakai, A.K.; Allendorf, F.W.; Holt, J.S.; Lodge, D.M.; Molofsky, J.; With, K.A.; Baughman, S.; Cabin, R.J.; Cohen, J.E.; Ellstrand, N.C.; et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 2001, 32, 305–332. [Google Scholar] [CrossRef]
- Hladík, M.; Kubecka, J. Fish migration between a temperate reservoir and its main tributary. Hydrobiologia 2003, 504, 251–266. [Google Scholar] [CrossRef]
- Kotusz, J.; Witkowski, A.; Baran, M.; Błachuta, J. Fish migrations in a large lowland river (Odra R., Poland) based on fish pass observations. Folia Zool. 2006, 55, 386–398. [Google Scholar]
- Ilhéu, M.; Matono, P.; da Silva, J.; Sousa-Santos, C.; Venade, D.; Emídio, M.; Jines, C.; Bernardo, J.M.; Costa, A.M.; Sousa, D.; et al. Ação A4—Estudo Sobre o Impacte de Alburmo (Alburnus alburnus) Sobre as Populações de Saramugo; Relatório Final da Ação A4 do Projeto LIFE 13 NAT/PT/000786; Universidade de Évora: Évora, Portugal, 2016. [Google Scholar]
- Reichard, M.; Jurajda, P.; Ondračkovaá, M. Interannual variability in seasonal dynamics and species composition of drifting young-of-the-year fishes in two European lowland rivers. J. Fish Biol. 2002, 60, 87–101. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Elsevier Academic Press: London, UK, 2001. [Google Scholar]
- Vøllestad, L.A. Resource partitioning of roach Rutilus rutilus and bleak Alburnus alburnus in two eutrophic lakes in SE Norway. Ecography 1985, 8, 88–92. [Google Scholar] [CrossRef]
- Taylor, C.M.; Warren, M.L. Dynamics in species composition of stream fish assemblages: Environmental variability and nested subsets. Ecology 2000, 82, 2320–2330. [Google Scholar] [CrossRef]
- Bernardo, J.M.; Costa, A.M.; Matono, P.; da Silva, J. Ação A5—Estudo de Mecanismos de Controlo de Dispersão de Alburno: Barreiras à Progressão de Alburno; Relatório Final da Ação A5 do Projeto LIFE 13 NAT/PT/000786; Universidade de Évora: Évora, Portugal, 2017. [Google Scholar]
- Starrs, T.; Starrs, D.; Lintermans, M.; Fulton, C.J. Assessing upstream invasion risk in alien freshwater fishes based on intrinsic variations in swimming speed performance. Ecol. Freshw. Fish 2017, 26, 75–86. [Google Scholar] [CrossRef]
- Caetano, M.; Nunes, V.; Nunes, A. CORINE Land Cover 2006 for Continental Portugal; Instituto Geográfico Português: Lisboa, Portugal, 2009. [Google Scholar]
| Best Models | Model-Averaged Coefficients | Relative Variable Importance (RI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response Variable | Distribution | Link Function | Model Type | AICc | ΔAIC | wAIC | Predictive Variables | Estimate | Std. Error | z Value | Pr (>IzI) | |
| Occurrence (presence-absence) | Binomial | Logit | Env | 77.400 | 0 | 0.620 | Woody debris | 2.184 | 0.791 | 2.763 | 0.006 | 1 |
| Mean water depth | 7.226 | 2.824 | 2.559 | 0.011 | 1 | |||||||
| Run | 3.003 | 1.139 | 2.635 | 0.008 | 1 | |||||||
| Dist | 67.490 | 0 | 0.080 | Sediment load | 3.524 | 1.033 | 3.411 | 0.001 | 1 | |||
| Hydrological regime | 2.821 | 1.180 | 2.390 | 0.017 | 0.85 | |||||||
| Env + Dist | 59.340 | 0 | 0.400 | Sediment load | 3.837 | 1.345 | 2.853 | 0.004 | 1 | |||
| Hydrological regime | 3.480 | 1.314 | 2.648 | 0.008 | 1 | |||||||
| Mean water depth | 6.765 | 3.319 | 2.039 | 0.042 | 0.81 | |||||||
| Run | 4.205 | 1.500 | 2.803 | 0.005 | 1 | |||||||
| Abundance (density) | Poisson | Log | Env | 143.020 | 0 | 0.280 | Woody debris | 0.895 | 0.430 | 2.079 | 0.038 | 0.77 |
| Shadow | 1.635 | 0.527 | 3.103 | 0.002 | 1 | |||||||
| Mean water depth | 2.589 | 1.010 | 2.563 | 0.010 | 0.73 | |||||||
| Run | 1.047 | 0.568 | 1.843 | 0.049 | 0.57 | |||||||
| Stream order | 1.402 | 0.730 | 1.922 | 0.047 | 0.87 | |||||||
| Dist | 143.140 | 0 | 0.190 | Sediment load | 2.129 | 0.514 | 4.141 | <0.001 | 1 | |||
| Lentic water bodies | 1.281 | 0.354 | 3.622 | <0.001 | 1 | |||||||
| Env + Dist | 123.460 | 0 | 0.240 | Sediment load | 2.057 | 0.627 | 3.282 | 0.001 | 1 | |||
| Lentic water bodies | 1.280 | 0.373 | 3.428 | 0.001 | 1 | |||||||
| Shadow | 1.666 | 0.547 | 3.049 | 0.002 | 1 | |||||||
| Mean water depth | 2.020 | 1.141 | 1.769 | 0.050 | 0.44 | |||||||
| Run | 1.163 | 0.610 | 1.906 | 0.048 | 0.55 | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matono, P.; Da Silva, J.; Ilhéu, M. How Does an Invasive Cyprinid Benefit from the Hydrological Disturbance of Mediterranean Temporary Streams? Diversity 2018, 10, 47. https://doi.org/10.3390/d10020047
Matono P, Da Silva J, Ilhéu M. How Does an Invasive Cyprinid Benefit from the Hydrological Disturbance of Mediterranean Temporary Streams? Diversity. 2018; 10(2):47. https://doi.org/10.3390/d10020047
Chicago/Turabian StyleMatono, Paula, Janine Da Silva, and Maria Ilhéu. 2018. "How Does an Invasive Cyprinid Benefit from the Hydrological Disturbance of Mediterranean Temporary Streams?" Diversity 10, no. 2: 47. https://doi.org/10.3390/d10020047
APA StyleMatono, P., Da Silva, J., & Ilhéu, M. (2018). How Does an Invasive Cyprinid Benefit from the Hydrological Disturbance of Mediterranean Temporary Streams? Diversity, 10(2), 47. https://doi.org/10.3390/d10020047




