Synthesis of a Stable Long-Wavelength Fluorescent BODIPY FL-NAADP Conjugate
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of 3
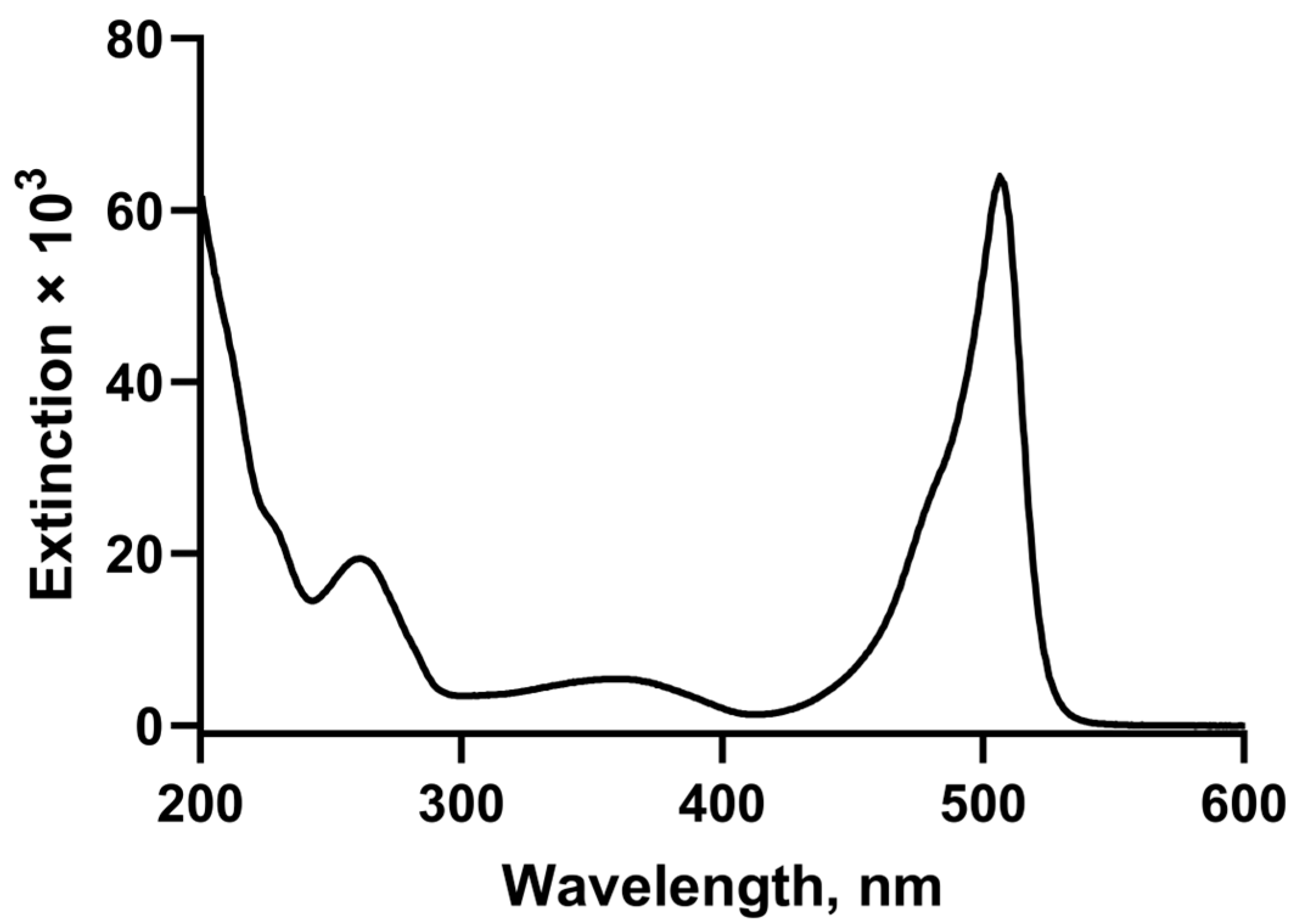
2.2. Spectroscopic Properties of 3
3. Materials and Methods
3.1. General Procedures
3.2. Preparative Anion-Exchange HPLC
3.3. MALDI Analysis
3.4. Determination of UV/Visible Extinction Coefficients
3.5. Analytical HPLC Ion-Exchange Chromatography
3.6. 5-(3-Aminopropyl)-NAADP (1)
3.7. BODIPY FL-NAADP (3)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEAE-cellulose | Diethylaminoethyl-substituted cellulose for anion exchange |
| DHB | Dihydroxybenzoate |
| BODIPY FL | Refers to CAS registry # 165599-63-3 |
| DMSO | Dimethyl sulfoxide |
| NAADP | Nicotinic acid adenine dinucleotide phosphate |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| NHS | N-hydroxysuccinimide |
| PBS | Phosphate-buffered saline solution |
| TFA | Trifluoroacetic acid |
| TPCs | Two-pore channels |
References
- Galione, A. A primer of NAADP-mediated Ca(2+) signalling: From sea urchin eggs to mammalian cells. Cell Calcium 2015, 58, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Function and dysfunction of two-pore channels. Sci. Signal. 2015, 8, re7. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, G.S.; Brailoiu, E.; He, S.; Unterwald, E.M.; Patel, S.; Slama, J.T.; Walseth, T.F.; Marchant, J.S. Essential requirement for JPT2 in NAADP-evoked Ca(2+) signaling. Sci. Signal. 2021, 14, eabd5605. [Google Scholar] [CrossRef] [PubMed]
- Roggenkamp, H.G.; Khansahib, I.; Hernandz, C.; Zhang, Y.; Lodygin, D.; Krüger, A.; Gu, F.; Möckl, F.; Löhndorf, A.; Wolters, V.; et al. HN1L/JPT2: A signaling protein that connects NAADP generation to Ca2+ microdomain formation. Sci. Signal. 2021, 14, eabd5647. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Shah, K.; Yan, J. Lsm12 is an NAADP receptor and a two-pore channel regulatory protein required for calcium mobilization from acidic organelles. Nat. Commun. 2021, 12, 4739. [Google Scholar] [CrossRef]
- Marchant, J.S.; Gunaratne, G.S.; Cai, X.; Slama, J.T.; Patel, S. NAADP-binding proteins find their identity. Trends Biochem. Sci. 2022, 47, 235–249. [Google Scholar] [CrossRef]
- Bernofsky, C. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP+). Methods Enzym. 1980, 66, 105–112. [Google Scholar]
- Jain, P.; Slama, J.T.; Perez-Haddock, L.A.; Walseth, T.F. Nicotinic acid adenine dinucleotide phosphate analogues containing substituted nicotinic acid: Effect of modification on Ca(2+) release. J. Med. Chem. 2010, 53, 7599–7612. [Google Scholar] [CrossRef]
- Lee, H.C.; Aarhus, R. Structural determinants of nicotinic acid adenine dinucleotide phosphate important for its calcium-mobilizing activity. J. Biol. Chem. 1997, 272, 20378–20383. [Google Scholar] [CrossRef]
- Trabbic, C.J.; Zhang, F.; Walseth, T.F.; Slama, J.T. Nicotinic acid adenine dinucleotide phosphate analogues substituted on the nicotinic acid and adenine ribosides. effects on receptor mediated Ca2+ release. J. Med. Chem. 2015, 58, 3593–3610. [Google Scholar] [CrossRef]
- Krukenberg, S.; Möckl, F.; Weiß, M.; Dekiert, P.; Hofmann, M.; Gerlach, F.; Winterberg, K.J.; Kovacevic, D.; Khansahib, I.; Troost, B. MASTER-NAADP: A membrane permeable precursor of the Ca2+ mobilizing second messenger NAADP. Nat. Commun. 2024, 15, 8008. [Google Scholar] [CrossRef]
- Parkesh, R.; Lewis, A.M.; Aley, P.K.; Arredouani, A.; Rossi, S.; Tavares, R.; Vasudevan, S.R.; Rosen, D.; Galione, A.; Dowden, J. Cell-permeant NAADP: A novel chemical tool enabling the study of Ca2+ signalling in intact cells. Cell Calcium 2008, 43, 531–538. [Google Scholar] [CrossRef]
- Lee, H.C.; Aarhus, R.; Gee, K.R.; Kestner, T. Caged nicotinic acid adenine dinucleotide phosphate: Synthesis and use. J. Biol. Chem. 1997, 272, 4172–4178. [Google Scholar] [CrossRef]
- Parkesh, R.; Vasudevan, S.R.; Berry, A.; Galione, A.; Dowden, J.; Churchill, G.C. Chemo-enzymatic synthesis and biological evaluation of photolabile nicotinic acid adenine dinuclotide phosphate (NAADP+). Org. Biomol. Chem. 2007, 5, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Aarhus, R. Fluorescent analogs of NAADP with calcium mobilizing activity. Biochim. Biophys. Acta 1998, 1425, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Jurášek, M.; Dráberová, E.; Řehulka, J.; Gurská, S.; Ivanova, A.; Polishchuk, P.; Ječmeňová, K.; Fähnrich, J.; Marešová, A.; Tauchen, J.; et al. Colchicine-BODIPY Probes: Evidence for the Involvement of Intracellular Membranes in the Targeting of Colchicine to Tubulin. ACS Pharmacol. Transl. Sci. 2025, 8, 1965–1985. [Google Scholar] [CrossRef]
- Lin, W.; Liu, J.; Jeffries, C.; Yang, L.; Lu, Y.; Lee, R.E.; Chen, T. Development of BODIPY FL vindoline as a novel and high-affinity pregnane X receptor fluorescent probe. Bioconjugate Chem. 2014, 25, 1664–1677. [Google Scholar] [CrossRef]
- Stanková, J.; Jurasek, M.; Hajduch, M.; Dzubak, P. Terpenes and Terpenoids conjugated with BODIPYs: An overview of biological and chemical properties. J. Nat. Prod. 2024, 87, 1306–1319. [Google Scholar] [CrossRef]
- Alexson, J.T.; Bodley, J.W.; Walseth, T.F. A volatile liquid chromatography system for nucleotides. Anal. Biochem. 1981, 116, 347–360. [Google Scholar] [CrossRef]
- Ames, B.N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzym. 1966, 8, 115–118. [Google Scholar] [CrossRef]
- Graeff, R.; Liu, Q.; Kriksunov, I.A.; Hao, Q.; Lee, H.C. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J. Biol. Chem. 2006, 281, 28951–28957. [Google Scholar] [CrossRef] [PubMed]
- Munshi, C.; Lee, H.C. High-Level Expression of Recombinant Aplysia ADP-Ribosyl Cyclase in Pichia pastoris by Fermentation. Protein Expr. Purif. 1997, 11, 104–110. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Z.; Slama, J.T. Synthesis of a Stable Long-Wavelength Fluorescent BODIPY FL-NAADP Conjugate. Molbank 2025, 2025, M2085. https://doi.org/10.3390/M2085
Guan Z, Slama JT. Synthesis of a Stable Long-Wavelength Fluorescent BODIPY FL-NAADP Conjugate. Molbank. 2025; 2025(4):M2085. https://doi.org/10.3390/M2085
Chicago/Turabian StyleGuan, Zhong, and James T. Slama. 2025. "Synthesis of a Stable Long-Wavelength Fluorescent BODIPY FL-NAADP Conjugate" Molbank 2025, no. 4: M2085. https://doi.org/10.3390/M2085
APA StyleGuan, Z., & Slama, J. T. (2025). Synthesis of a Stable Long-Wavelength Fluorescent BODIPY FL-NAADP Conjugate. Molbank, 2025(4), M2085. https://doi.org/10.3390/M2085






