9-Oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic Acid
Abstract
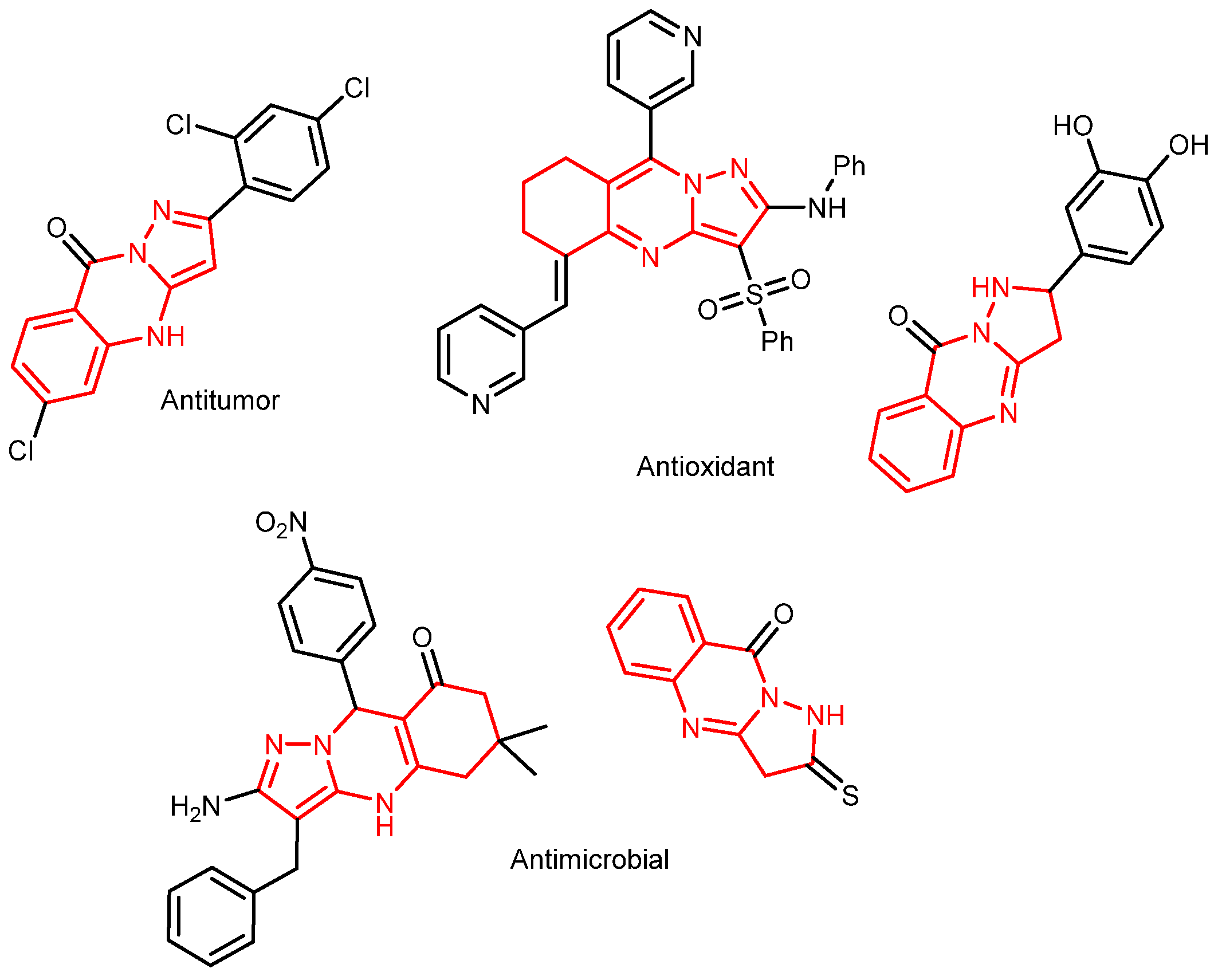
1. Introduction
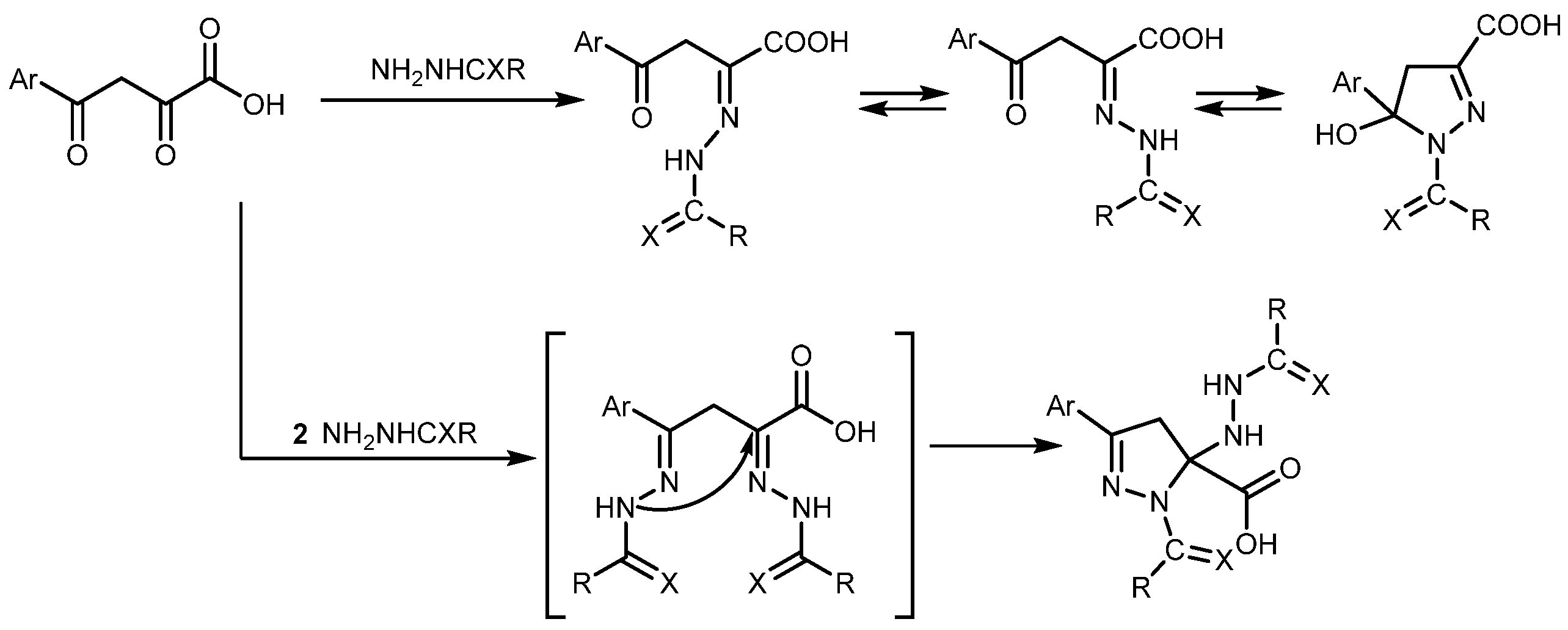
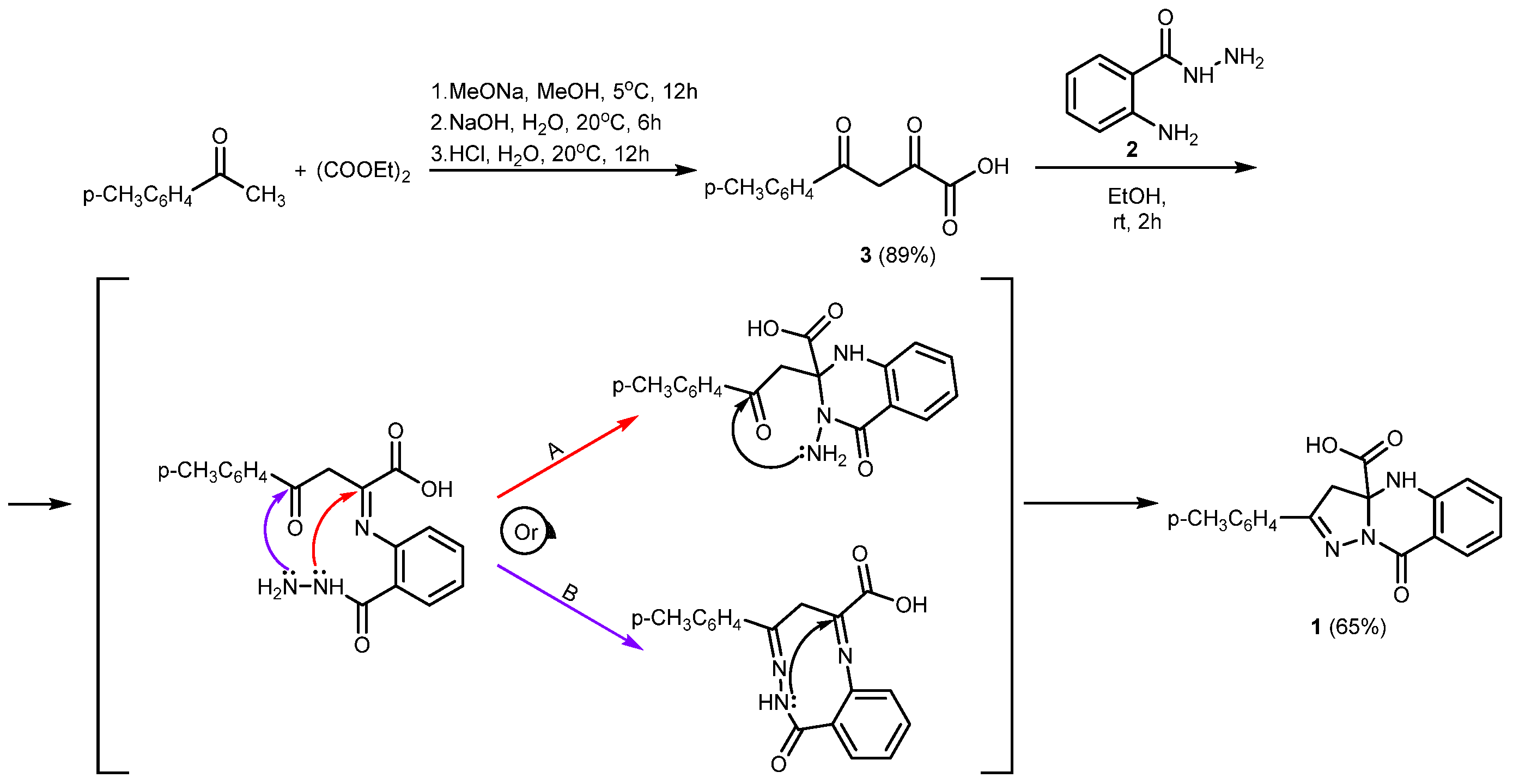
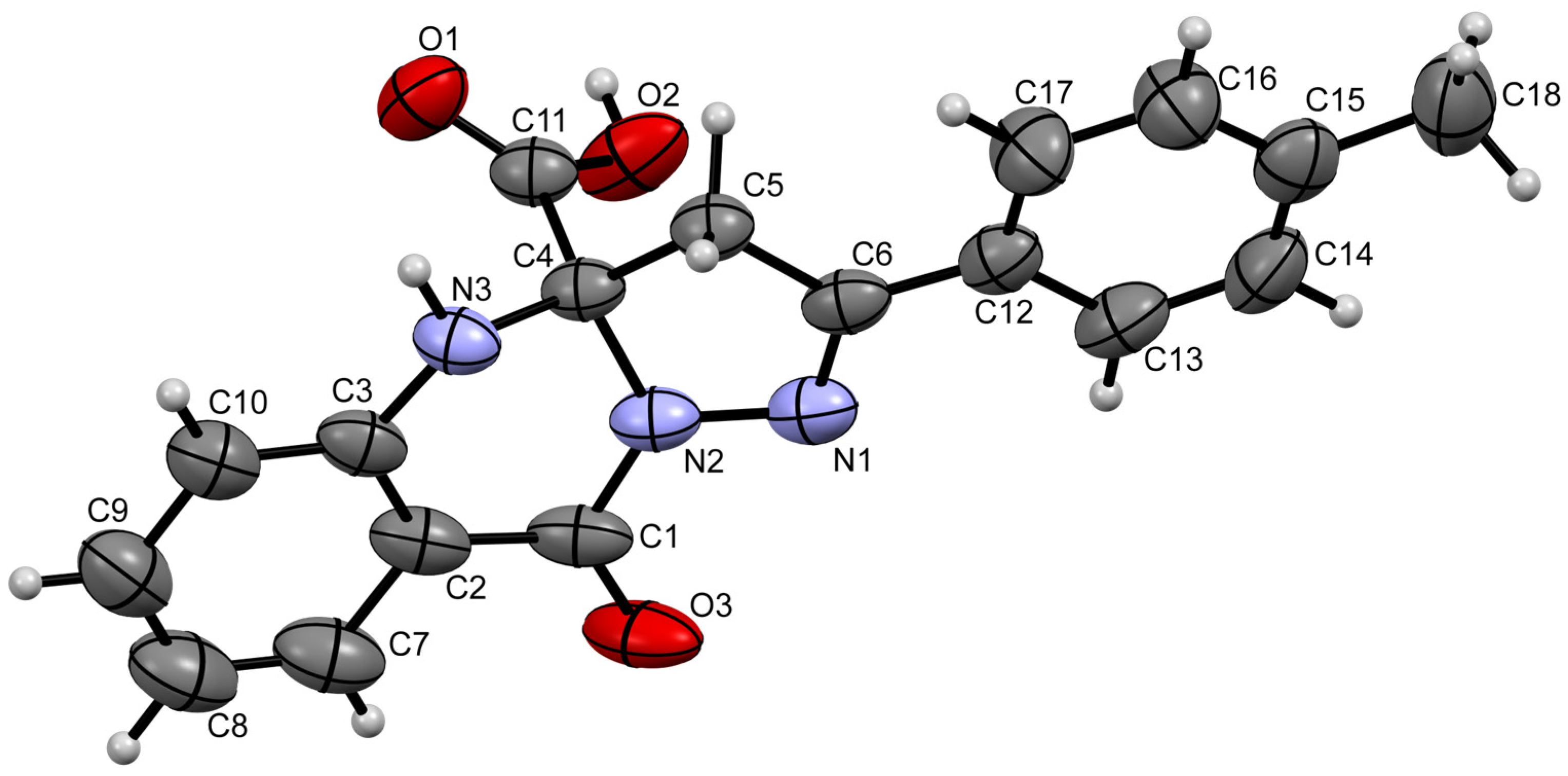
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. 9-Oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic Acid 1
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sanad, S.M.H.; Mekky, A.E.M.; Ahmed, A.A.M. Pyrazolo[5,1-b]quinazolines and Their Bis-analogues Linked to Different Spacers: Regioselective Synthesis, Antibacterial Screening and SwissADME Prediction Study. ChemistrySelect 2023, 8, e202300171. [Google Scholar] [CrossRef]
- Haggam, R.A.; Soylem, E.A.; Assy, M.G.; Arastiedy, M.F. Synthesis and Biological Activities of Some Condensed Oxazine and Pyrimidine Derivatives: Cyclization, Ring Transformation and Functionalization of Oxazine. Curr. Sci. 2018, 115, 1893–1903. [Google Scholar] [CrossRef]
- Hussein, M.A. Synthesis, anti-inflammatory, and structure antioxidant activity relationship of novel 4-quinazoline. Med. Chem. Res. 2013, 22, 4641–4653. [Google Scholar] [CrossRef]
- Fadda, A.A.; Refat, H.M.; Mohamed, N.A.; AbdelAal, M.T. Synthesis and Antioxidant of Some New Pyrazolo[1,5-a]pyrimidine, Pyrazolo[5,1-b]quinazoline and Imidazo[1,2-b]pyrazole Derivatives Incorporating Phenylsulfonyl Moiety. Lett. Appl. NanoBioSci 2021, 10, 2414–2428. [Google Scholar] [CrossRef]
- Kapoor, T.M. Pyrazoloquinazolinone Antitumor Agents. WO Patent WO2018213712A1, 22 November 2018. [Google Scholar]
- Igidov, S.N.; Turyshev, A.; Makhmudov, R.R.; Shipilovskikh, D.A.; Igidov, N.M.; Shipilovskikh, S.A. Synthesis, Intramolecular Cyclization, and Analgesic Activity of Substituted 2-[2-(Furancarbonyl)hydrazinylydene]-4-oxobutanoic Acids. Russ. J. Gen. Chem. 2022, 92, 1629–1636. [Google Scholar] [CrossRef]
- Lipin, D.V.; Denisova, E.I.; Devyatkin, I.O.; Okoneshnikova, E.A.; Shipilovskikh, D.A.; Makhmudov, R.R.; Igidov, N.M.; Shipilovskikh, S.A. Recyclization of 3-(Thiophen-2-yl)imino-3H-furan-2-ones under the Action of Cyanoacetic Acid Derivatives. Russ. J. Gen. Chem. 2020, 91, 809. [Google Scholar] [CrossRef]
- Andreeva, A.A.; Dmitriev, M.V.; Maslivets, A.N. 3-(4-Bromophenyl)-1-carbamothioyl-5-(2-carbamothioylhydrazinyl)-4,5-dihydro-1H-pyrazole-5-carboxylic Acid. Molbank 2024, 2024, M1757. [Google Scholar] [CrossRef]
- Andreeva, A.A.; Shklyaev, Y.V.; Maslivets, A.N. Reaction of Aroylpyruvic Acids with 3- and 4-Nitrobenzohydrazides. Synthesis of Pyrazoline-5-carboxylic Acids. Russ. J. Org. Chem. 2025, 61, 1458–1464. [Google Scholar] [CrossRef]
- Peet, N.P.; Huber, E.W. Pyrazoloquinazolines from 2-aminobenzoylhydrazine. Heterocycles 1993, 35, 315–323. [Google Scholar] [CrossRef]
- El-Shaieb, K.M.; Ameen, M.A.; Abdel-Latif, F.F.; Mohamed, A.H. Condensation reactions of 2-aminobenzohydrazide with various carbonyl compounds. Z. Für Naturforschung B 2012, 67, 1144–1150. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.42.74a; Rigaku Oxford Diffraction: Wroclaw, Poland, 2022. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 1 December 2023).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Claisen, L. Ueber die Einführung von Säureradicalen in Ketone. Ber. Dtsch. Chem. Ges. 1887, 20, 2178–2188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, A.A.; Shklyaev, Y.V.; Maslivets, A.N. 9-Oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic Acid. Molbank 2025, 2025, M2105. https://doi.org/10.3390/M2105
Andreeva AA, Shklyaev YV, Maslivets AN. 9-Oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic Acid. Molbank. 2025; 2025(4):M2105. https://doi.org/10.3390/M2105
Chicago/Turabian StyleAndreeva, Anastasia A., Yurii V. Shklyaev, and Andrey N. Maslivets. 2025. "9-Oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic Acid" Molbank 2025, no. 4: M2105. https://doi.org/10.3390/M2105
APA StyleAndreeva, A. A., Shklyaev, Y. V., & Maslivets, A. N. (2025). 9-Oxo-2-(p-tolyl)-4,9-dihydropyrazolo[5,1-b]quinazoline-3a(3H)-carboxylic Acid. Molbank, 2025(4), M2105. https://doi.org/10.3390/M2105






